Abstract
Background
The number of cardiac implantable electronic device (CIED) recalls and advisories has increased over the past three decades, yet no consensus exists on how to best manage patients with these CIEDs partially because rates of complications from prophylactic replacement are unknown.
Objective
To establish rates of complications when recalled CIED generators are replaced prophylactically
Methods
We searched MEDLINE and Cochrane Controlled Trials Register for reports of prophylactic replacement of recalled CIED generators. Studies with < 20 subjects were excluded. We then conducted a meta-analysis of qualifying studies to determine the rates of mortality, reoperation, and combined major complications.
Results
We identified 7 citations meeting our inclusion criteria and reporting ≥1 endpoint of interest. Four were single center; three were multicenter. Six studies collected data retrospectively (n=1213) and one prospectively (n=222). Using a random effects model to combine data from all included studies, the rate of major complications was 2.5% (95% CI 1.0–4.5%). Combining data from 6 studies reporting mortality and reoperation, the rates were 0.5% (95% CI 0.1–0.9%) and 2.5% (95% CI 0.8–4.5%), respectively.
Conclusions
Prophylactic replacement of recalled CIED generators is associated with a low mortality rate but non-trivial rates of other major complications similar to those reported when CIED generators are replaced for other reasons. Thus, when considering replacing a recalled CIED generator, known risks of elective generator replacement likely apply and can be weighed against risks associated with device failure.
Keywords: recall, cardiac implantable electronic devices, complications, mortality
Introduction
Cardiac implantable electronic devices (CIEDs) including pacemakers (PM), implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices, all have an inherent rate of failure. When an unforeseen failure mechanism or rate of failure is identified after a device has been approved, the FDA may employ an advisory or recall typically in cooperation with the device manufacturer. During the last three decades, partly due to the increasing complexity of CIEDs, there has been an increase in the number and rate of PM and ICD advisories and recalls.(1,2)
When these device problems cannot be addressed through noninvasive software updates, providers must consider how to best manage patients with advisory or recalled CIEDs in situ. Options include intensified monitoring with intervention only if and when there is evidence of generator malfunction or failure versus prophylactic generator replacement. This consideration depends on the suspected failure rate and mechanism and potential outcomes of failure along with patient characteristics and preferences. To date, there is no consensus on how to best manage patients with recalled generators in situ due, in part, to a paucity of information about the risk of prophylactic replacement of these generators.
Therefore, we sought to perform a systematic review and meta-analysis of observational studies to more accurately estimate the risk of complications associated with prophylactic replacement of CIED generators under FDA advisory or recall.
Methods
Search Strategy
An expert reference librarian designed and conducted an electronic search strategy with input from the primary investigator. The initial search was implemented in PubMed (September, 2014) using a combination of medical subject headings (MeSH) and keywords to combine the subjects of CIEDs, FDA recall or advisory, and complications from CIED replacement procedures. After this initial search, terms were translated and a similar search was employed in the Cochrane Database (Appendix I). The search was limited to English language. The bibliographies of selected full-length manuscripts were reviewed manually to identify any additional relevant references not captured in our search.
Eligibility
Any study that systematically reported complications from the prophylactic replacement of advisory or recalled CIEDs were eligible for inclusion. Studies were excluded if they had fewer than twenty subjects.
Extraction
All screening decisions were made and tracked in a DistillerSR database (Evidence Partners Inc., Manotick, ON, Canada) by two investigators (E.P.Z. and D.P.). Extracted data included patient characteristics, combined major complications, and mortality. Disagreements were resolved by consensus. We evaluated the strength of evidence using approaches described by the Agency for Healthcare Research and Quality (AHRQ) and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group. (3,4)
Endpoints
The primary endpoint of interest was combined major complications. Other endpoints included mortality and reoperation/pocket revision.
Overall combined major complications
The endpoint of combined major complications was defined variably among included studies (Table 1). In some cases, this represented complications detailed in the manuscript which for the purpose of this paper were combined by the primary investigator for an overall rate.
Table 1.
Definition of combined major complications by study
| Author, Year | Definition |
|---|---|
| Moore, 2009(14) | Death + any complication requiring reoperation (infection, bleeding/hematoma, system malfunction) |
| Amin, 2008(9) | Death + any complication associated with device replacement |
| Mahajan, 2008(23) | Death + any complication associated with reoperation |
| Costea, 2008(15) | Death + any complication requiring reoperation (bleeding/hematoma, lead damage, device “protrusion”) + stroke |
| Kapa, 2007(12) | Any complication requiring intervention or reoperation up to 60 days post procedure |
| Hauser, 2006(11) | Death |
| Gould, 2006(24) | Death + any complication requiring reoperation (infection, bleeding, system malfunction, pain) |
Mortality
Death as a complication of generator replacement was defined as occurring during the operation or in the immediate postoperative period (less than thirty days post procedure)
Reoperation/Pocket Revision
Reoperation and/or pocket revision as a complication of CIED generator change was defined as any complication leading to an unexpected reoperation or revision of the pocket. In some cases a definition was not explicitly provided. In other cases, this endpoint represented complications that clearly resulted in reoperation and/or pocket revision which for the purpose of this analysis were combined by the investigators. These included but were not limited to: bleeding into the CIED header requiring revision, hematoma, system malfunction, pocket infection requiring extraction, lead damage requiring revision, and site pain requiring reoperation.
Data Analysis
Most meta-analyses are calculated using standard meta-analysis software such as the Comprehensive Meta Analysis program.(5) However these programs use normal approximations which are not appropriate for very small counts. Many of the counts in the studies included in our analyses are either 0 or 1. This problem was discussed by Hasselblad et al.(6) For the particular endpoints in this study, it is important to base the calculations on the binomial distribution because that is the distribution of the individuals study rates.
The calculation of a fixed effects estimate for a series of independent binomial distributions is estimated from the pooled numerators and denominators. The logical random-effects model is the beta-binomial distribution. (7) This distribution can be fitted to the observed counts using the FAST*PRO software.(8)
Results
Search Results
Our search identified 142 abstracts which were reviewed for inclusion and exclusion criteria (Figure 1). Among this group of abstracts, 91 were excluded due to irrelevance to our topic of interest. The full manuscripts for the remaining 51 studies were retrieved for detailed review. Following full text review, 44 were excluded as follows: unrelated to recall/advisory (n=3), did not report clinical outcomes of interest (18), related only to recall/advisory leads (8), sample less than 20 subjects (3), editorial/comment/case report only (11), and duplicate data (1). Seven studies representing 1435 patients remained for inclusion in our meta-analysis representing 1435 unique patients. Table 2 summarizes results from the 7 studies that examined at least one of the following complications after prophylactic replacement of a recalled or advisory CIED generator: overall combined major complications, mortality, and pocket revision/reoperation.
Figure 1.
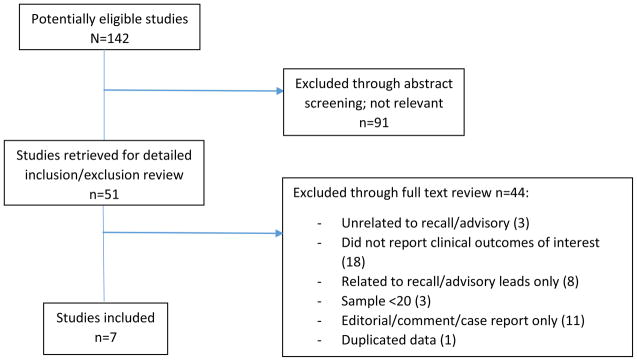
QUOROM diagram
Table 2.
Study Characteristics
| Author, Year | Study Sites | Location | Study Design | Overall number of subjects | Follow up after revision (mean) | Meandwell time in years (standard deviation) | Combined major complications n (%) | Mortality n (%) | Reoperation/pocket revision n (%) | Quality** |
|---|---|---|---|---|---|---|---|---|---|---|
| Moore, 2009(14) | Single | US | retrospective | 237 | 198 days | 2.6 (1.3) | 4 (1.69) | 0 | 4 (1.69) | Fair |
| Amin, 2008(9) | Single | US | retrospective | 57 | NR | NR | 0 | 0 | 0 | Poor |
| Mahajan, 2008(23) | Multi | US | retrospective | 89 | NR | 3.1 (1.3) | 2 (2.25) | 1 (1.12) | 1 (1.12) | Poor |
| Costea, 2008(15) | Single | US | retrospective | 222 | 3 months* | NR | 6 (2.70) | 0 | 4 (1.80) | Fair |
| Kapa, 2007(12) | Single | US | retrospective | 162 | 60 days * | NR | 1 (0.62) | NR | 1 (0.62) | Poor |
| Hauser, 2006(11) | Multi | US | retrospective | 135 | NR | 1.7 (0.8) | 1 (0.74) | 1 (0.74) | NR | Poor |
| Gould, 2006(24) | Multi | Non-US | retrospective | 533 | 2.7 months | 2.2 (1.0) | 33 (6.19) | 2 (0.38) | 31 (0.75) | Fair |
Baseline Characteristics
Six of the seven included studies reported data collected retrospectively (9–14), and one reported prospectively collected data (15). Three were multicenter (10,11,13) and four were single center (9,12,14,15). All but one reported experience within the US only(10), however in all cases, advisories and recalls were issued by the FDA rather than a local or international regulator of medical devices. Six of seven studies reported the distribution of CIED type, and in these studies, more than 90% of CIEDs replaced prophylactically were ICDs. (9–11,13–15) Cardiac resynchronization devices with or without an ICD represented only 2% of CIEDs in the six studies reporting device type. Three studies reported a mean patient age – 64, 67, and 68 years, respectively.(10,14,15) Three of the remaining studies reported outcomes in adult patients without specifying an actual mean or median age (9,11,12) whereas Mahajan et al described outcomes in pediatric and patients with congenital heart disease.(13)
Four studies specifically reported the number of patients who were pacemaker dependent – 26% (n=62), 21% (n=112), 49% (n=28), and 19% (n=41), respectively. (9,10,14,15) In the case of Gould et al and Moore et al, pacemaker dependency was one qualifying condition that led to prophylactic replacement. Three studies reported the number of ICD patients with primary vs secondary prevention devices. (10,14,15) The percentage of primary prevention ICDs among ICD patients in these three studies were 34, 84, and 67%, respectively. These same three studies reported the percentage of women included: 23, 29, and 24%, respectively. No studies, however, reported complications based on the subgroups of women, pacemaker dependent patients or those with a primary prevention device.
Other patient characteristics including race, comorbidities, measure of heart failure severity, device indication (primary vs secondary prevention), and health status were reported in insufficient amount and detail to warrant inclusion in our meta-analysis as potential modifying factors.
Using the aforementioned guidelines on a scale of poor, fair, good, and excellent, 3 of 7 included studies were judged to be of fair quality(10,14,15); and 4 were poor(9,11–13). The primary reason that these studies were “good” or worse was the observational design and the lack of controlling for bias. In all studies, the inclusion and exclusion criteria were applied uniformly across groups. Moreover, the interventions/exposures in all studies were defined consistently and reliably. However, in some cases the outcomes and follow up were not well defined leading to a downgrading of quality.
Combined Overall Complications
All seven studies reported a rate of combined major complications or reported complications with sufficient detail that complication rates could be added to arrive at a combined total. Rate of combined major complications ranged from 0.00–6.52% with an overall estimate of 2.60% (95% CI 1.05–4.46%) (Table 3 and Figure 2). There was evidence of significant heterogeneity (χ2=25.340, 6 degrees of freedom, p=0.0003).
Table 3.
Results before and after removing Mahajan et al from the meta-analysis
| Endpoint | Including Mahajan et al (2008) | Excluding Mahajan et al (2008) | ||||||
|---|---|---|---|---|---|---|---|---|
| Point estimate | 95% CI | Heterogeneity measures | Point estimate | 95% CI | Heterogeneity measures | |||
| χ2 | p-value | χ2 | p-value | |||||
| Combined/overall | 2.6% | 1.1–4.5% | 25.340 | 0.0003 | 2.6% | 0.9–4.8% | 25.146 | 0.0001 |
| Mortality | 0.47% | 0.1–0.9% | 3.414 | 0.6364 | 0.4% | 0.1–1.1% | 1.871 | 0.7595 |
| Reoperation/pocket revision | 2.5% | 0.9–4.5% | 22.568 | 0.0004 | 2.7% | 0.8–5.1% | 20.399 | 0.0004 |
Figure 2.
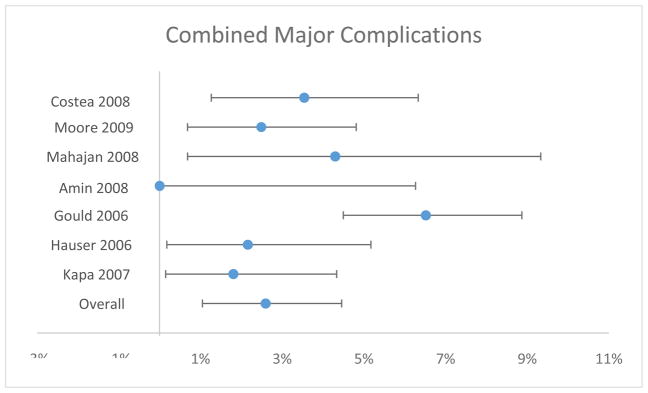
Combined Major Complications
Mortality
Six of the seven included studies reported the rate of death following prophylactic replacement of CIEDs in response to FDA advisory or recall.(9–11,13–15) In all six studies, there was a total of 4 deaths representing a rate of death ranging from 0.00 to 2.16%. Using random effects meta-analysis, the overall point estimate for death rate was 0.47% (95% CI 0.13–0.91%) (Table 3 and Figure 3). There was not significant heterogeneity in this endpoint (χ2=3.4143, 5 degrees of freedom, p=0.6364).
Figure 3.
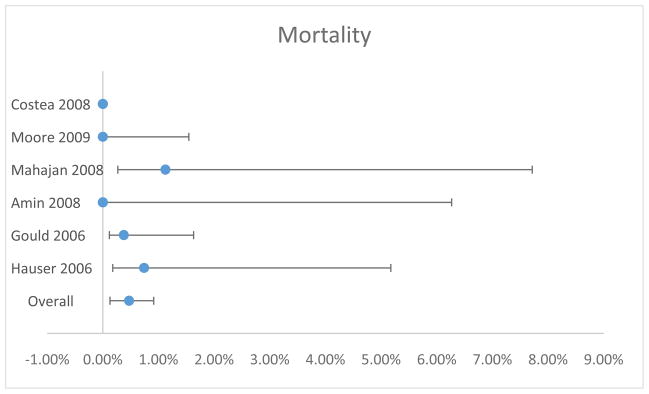
Mortality
Reoperation/Pocket Revision
The rate of reoperation and/or pocket revision was reported in six of seven studies. The rate of this complication ranged from 0.00 to 6.15%. The overall estimate was 2.51% (95% CI 0.87–4.53%) (Table 3 and Figure 4). Significant heterogeneity was identified (χ2=22.568, 5 degrees of freedom, p=0.0004).
Figure 4.
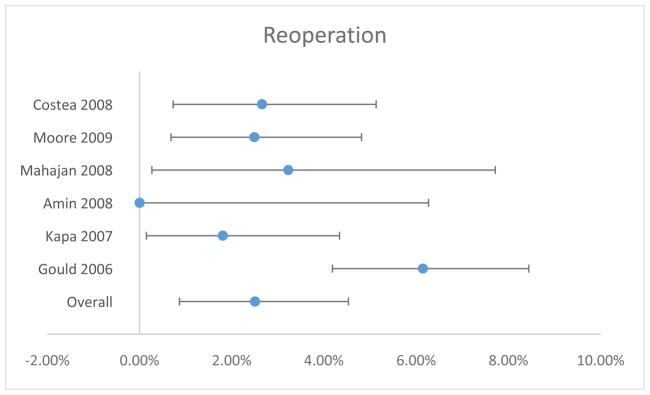
Reoperation/Pocket Revision
Sensitivity Analysis
As noted above, Mahajan et al described outcomes in pediatric and congenital patients undergoing CIED generator replacement. (13) While these groups of patients were not excluded from our meta-analysis a priori, it is conceivable that outcomes in these patients may be significantly different from an adult, non-congenital heart disease cohort. As such, we repeated a random effects meta-analysis for the three outcomes above with this study removed (Table 3). In no instance did the results or heterogeneity measures change significantly.
Discussion
This meta-analysis has four major findings. First there is only one prospective study examining prophylactic generator replacement in response to FDA recall and no randomized controlled trials (RCTs) on this topic. Secondly, rates of other complications are not insignificant. Thirdly, the rate of death from prophylactic replacement of recalled CIED generators is low (OR: 0.47%). Finally, and most importantly, the rates of complications reported in this meta-analysis in the setting of prophylactic replacement of generators in response to FDA recall, are similar to rates reported when CIED generators are electively replaced for other reasons.
Among six studies examining mortality, there were 4 deaths reported. Mahajan et al reported one death “following complications related to device revision”.(13) Hauser et al reported one death “associated with the prophylactic replacement of a recalled device” and the death “was caused by short-circuiting during shock delivery.”(11) Lastly, Gould et al, reported two deaths—one due to RV perforation and one due to overwhelming sepsis one week postoperatively despite system extraction. (10) Because of these small numbers, even with meta-analysis, the confidence interval is quite wide.
While rates of death were very low in this meta-analysis, they should still be seriously considered when deciding on how to manage recalled devices. This careful approach is further supported by the non-trivial rates of other complications. We found an overall combined major complications rate of 2.60%. This complication rate is comparable to the rate of complications seen when CIED generators are electively replaced for other reasons (e.g., end of expected battery life). (16–19) For example, a large registry based report of complications from generator replacement for a variety of indications including device advisory/recall reported a combined complication rate of 4%.(16) In a different analysis of the Canadian experience, 2.5% of patients experienced a major complication from generator replacement. (18)
Critical to the consideration of prophylactic CIED generator replacement in response to FDA advisory or recall is the expected device failure rate. However, at the time of advisory, the failure rate is often not definitively known. Part of the reason for this uncertainty is the imperfect medical device post market safety surveillance system which is known to underreport device failures. (20) Six of the seven studies in our meta-analysis reported the manufacturer and/or models that were replaced due to advisory/recall (9,10,12–15), and only in one case were the number of replacements for each generator model reported. (15) For those that did report the relevant advisories/recalls, the majority of devices were part of the Guidant Prizm and Contak Renewal or Medtronic Marquis recalls – both in 2005; a small number were related to generators manufactured by St. Jude Medical and/or ELA with recalls in 2005 and 2001, respectively (Table 4). (9,10,12–15) This pattern is reflective of the recalls implemented during or just prior to the study period represented (2006–2009). The average dwell time was reported in four studies (Table 2) and ranged from 1.7 to 3.1 years, and this also reflects the fact that most of the relevant device advisories took place in 2005. (10,11,13,14) Dwell time from advisory/recall to replacement in the included studies is unknown. Failure rates of these devices based on product performance reports, physician communications, and other published reports ranged mostly from <0.01% to 0.1% per year; the ELA Alto ICD had a 2.6% per year risk of failure (Table 4).
Table 4.
Summary of major device advisories, failure mechanisms, and yearly failure rates relevant to meta-analyzed studies
| Manufacturer/Device | Date of Advisory (month/year) | Failure mechanism | Risk of failure, %/year(10,12) |
|---|---|---|---|
| Medtronic Marquis | 2/05 | Accelerated battery depletion | 0.01 |
| Guidant Ventak Prizm 2 DR ICD | 6/05 | Short circuit caused by wire insulation problem | 0.1 |
| Guidant Ventak Prizm AVT, Vitality AVT, and Contak Renewal AVT ICDs | 6/05 | Random memory error limiting delivery of therapies | 0.0095 |
| Guidant Contak Renewal 3, 4, Renewal 3, 4 AVT, and Renewal RF ICDs | 6/05 | Magnetic switch faulty impairing delivery of therapies | 0.009 |
| St Jude Photon DR, Photon Micro VR/DR, and Atlas VR/DR ICDs | 10/05 | Memory chip affected by atmospheric radiation, impairs pacing & therapy delivery | 0.167 |
| ELA Alto ICD | 8/01 | Migration of metal impairing pacing and delivery of therapies | 0.1–2.6 |
Clinical Implications
Given the rising number of generator recalls, physicians increasingly face more difficult decisions on how to best manage patients with recalled generators in situ. This decision is made even more difficult by a paucity of existing literature on this topic. Our analysis shows that prophylactic generator replacement in response to FDA advisory or recall has a low mortality rate and a similar rate of major complications to generator replacement procedures performed electively for other reasons such as battery depletion. However, the management decision for patients with a recalled generator in situ should be individualized based on patient characteristics, patient preferences, operator/site experience, and the expected rate of device failure. For example, the weighing of procedural complication risks changes when concomitant lead revision is planned or when the risk of device malfunction is high as in the case of pacing dependency or a secondary prevention ICD. Fortunately, software that are downloaded to devices to enhance early detection of failures or to mitigate the adverse effects of potential failures are becoming more wide-spread.
Limitations
Our meta-analysis has some limitations. Although not mentioned by the included studies, we cannot completely rule out that generator revisions also included lead related procedures. The addition of a lead procedure would likely inflate the complication rate. (16) There are other characteristics of the procedure and the patients which are unknown and may impact complications including, for example, the number of previous CIED-related procedures or comorbid conditions (e.g., diabetes). (19,21,22) Studies included in our analysis were from 2006–2009. Generator change in response to these recalls may or may not be representative of modern or future CIED generator recalls. This is highlighted by the fact that only a very small percentage of CIEDs in our analysis were CRTs and CRT generator replacements have been associated with greater risk of complications. (16) Event rates in our analysis were small with many incidences of 0 or 1 in individual studies which makes our point estimates somewhat imprecise. Lastly, there was evidence of significant heterogeneity. This is not surprising since the included studies were mostly retrospective observational studies with varying sample sizes, locations, definitions of complications, and length of follow up.
Conclusion
In summary, through meta-analysis of relevant studies of prophylactic replacement of advisory or recalled CIED generators, we found that the rate of complications is not insignificant. The rate of non-fatal complications in this setting does not appear to be meaningfully different from the rate of complications when CIED generators are electively replaced for other reasons. As such, when considering prophylactic CIED generator replacement in response to an advisory or recall, providers should consider patient characteristics, patient preferences, and device characteristics including mechanism of failure and remaining battery life in the context of an expectation for a low rate of procedural complications, as well as the presence of software that could lead to early detection of failure or that could mitigate the potential effects of a failure. Future prospective studies are needed to more clearly delineate the risks associated with prophylactic replacement of modern advisory or recalled CIED generators.
Clinical Perspectives.
While the rate and number of CIED generator advisories and recalls has increased over the past three decades, the rate of complications from replacing these devices is largely unknown. In this meta-analysis of seven reports of prophylactic replacement of advisory or recalled CIED generators, we report the rate of mortality and complications associated with prophylactic replacement. Mortality occurred in 4 of 1273 (0.3%) patients. Reoperation or pocket revision occurred at a higher rate of 2.51%, and overall complications occurred at a rate of 2.60%. These complication rates are similar to those that occur in the setting of elective CIED generator replacement for other reasons. Thus, providers can incorporate these findings into a discussion with patients about prophylactic replacement of an advisory or recalled CIED generator.
Acknowledgments
Financial Support: Dr. Zeitler was funded by National Institutes of Health (NIH) T-32 training grant #2 T32 HL69749-11 A1. However, no relationships exist related to the analysis presented.
The authors wish to thank Megan M Chobot, MSLS, for her assistance in devising the search strategy and for facilitating use of DistillerSR in conducting this review and meta-analysis.
Glossary of Abbreviations
- CIED
cardiac implantable electronic device
- FDA
United States Food and Drug Administration
- ICD
implantable cardioverter defibrillator
- CRT
cardiac resynchronization therapy
- PM
pacemaker
Appendix I. Search Strategy
Keywords: advisory OR recall, pacemaker, implantable cardioverter defibrillator OR ICD, cardiac resynchronization therapy OR CRT, complication, replacement, FDA.
| PubMed: | |
|---|---|
| Set # | Terms |
| #1 | “Defibrillators, Implantable”[MeSH] OR “ICD”[tiab] OR “implantable cardioverter defibrillator”[tiab] OR “Pacemaker, Artificial”[MeSH] OR “pacemaker” OR “Cardiac Resynchronization Therapy Devices”[MeSH] OR “CRT”[tiab] OR “cardiac resynchronization therapy”[tiab] |
| #2 | “Medical Device Recalls”[MeSH] OR “Safety-Based Medical Device Withdrawals”[MeSH] OR “advisory” [tiab] OR “recall”[tiab] OR FDA[tiab] |
| #3 | “Intraoperative Complications” [MeSH] OR “Postoperative Complications”[MeSH] OR “complication” [tiab] OR “complications” [tiab] OR “Mortality” [MeSH] OR “mortality” [tiab] or “death” [tiab] OR “infection” [MeSH] OR “infection” [tiab] OR “Hemorrhage” [MeSH] OR “hemorrhage” [tiab] OR “bleeding” [tiab] OR “bleed” [tiab] OR “Hematoma” [MeSH] OR “hematoma” [tiab] OR “Reoperation” [MeSH] OR “reoperation” [tiab] OR “pocket revision” [tiab] OR ((“Reoperation” [MeSH] OR “reoperation” [tiab] OR “pocket revision” [tiab]) AND (“Anxiety” [MeSH] OR “Stress, Psychological” [MeSH] OR “anxiety” OR “emotional” [tiab] OR “stress” [tiab])) |
| #4 | #1 AND #2 AND #3 |
| Limits: English | |
| Cochrane: | |
|---|---|
| Set # | Terms |
| #1 | MeSH descriptor: [Defibrillators, Implantable] explode all trees |
| #2 | MeSH descriptor: [Pacemaker, Artificial] explode all trees |
| #3 | #1 or #2 or (ICD):ti,ab,kw or (implantable defibrillator):ti,ab,kw or (pacemaker):ti,ab,kw |
| Limit: Cochrane Reviews | |
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- 1.Maisel WH, Sweeney MO, Stevenson WG, Ellison KE, Epstein LM. Recalls and safety alerts involving pacemakers and implantable cardioverter-defibrillator generators. JAMA. 2001;286:793–9. doi: 10.1001/jama.286.7.793. [DOI] [PubMed] [Google Scholar]
- 2.Maisel WH, Stevenson WG, Epstein LM. Changing trends in pacemaker and implantable cardioverter defibrillator generator advisories. Pacing and clinical electrophysiology : PACE. 2002;25:1670–8. doi: 10.1046/j.1460-9592.2002.01670.x. [DOI] [PubMed] [Google Scholar]
- 3.Owens DK, Lohr KN, Atkins D, Treadwell JR, Reston JT, Bass EB, Chang S, Helfand M. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions--agency for healthcare research and quality and the effective health-care program. Journal of clinical epidemiology. 2010;63:513–23. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. Journal of clinical epidemiology. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta Analysis, Version 2. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 6.Hasselblad V, Mosteller F, Littenberg B, Chalmers TC, Hunink MG, Turner JA, Morton SC, Diehr P, Wong JB, Powe NR. A survey of current problems in meta-analysis. Discussion from the Agency for Health Care Policy and Research inter-PORT Work Group on Literature Review/Meta-Analysis. Medical care. 1995;33:202–20. [PubMed] [Google Scholar]
- 7.Skellam JG. A probability distribution derived from the binomial distribution by regarding the probability of success as variable between sets of trials. Journal of the Royal Statistical Society. 1948;10:257–261. [Google Scholar]
- 8.Eddy D, Hasselblad V. FAST*PRO Software for Meta analysis by the Confidence Profile Method. Academic Press; 1992. [Google Scholar]
- 9.Amin MS, Wood MA, Shepard RK, Kalahasty G, Ellenbogen KA. Clinical judgment versus decision analysis for managing device advisories. Pacing and clinical electrophysiology : PACE. 2008;31:1236–40. doi: 10.1111/j.1540-8159.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- 10.Gould PA, Krahn AD Canadian Heart Rhythm Society Working Group on Device A. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006;295:1907–11. doi: 10.1001/jama.295.16.1907. [DOI] [PubMed] [Google Scholar]
- 11.Hauser RG, Hayes DL, Epstein AE, Cannom DS, Vlay SC, Song SL, Tyers GF. Multicenter experience with failed and recalled implantable cardioverter-defibrillator pulse generators. Heart Rhythm. 2006;3:640–4. doi: 10.1016/j.hrthm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Kapa S, Hyberger L, Rea RF, Hayes DL. Complication risk with pulse generator change: implications when reacting to a device advisory or recall. Pacing and clinical electrophysiology : PACE. 2007;30:730–3. doi: 10.1111/j.1540-8159.2007.00742.x. [DOI] [PubMed] [Google Scholar]
- 13.Mahajan T, Dubin AM, Atkins DL, Bradley DJ, Shannon KM, Erickson CC, Franklin WH, Cecchin F, Berul CI. Impact of manufacturer advisories and FDA recalls of implantable cardioverter defibrillator generators in pediatric and congenital heart disease patients. Journal of cardiovascular electrophysiology. 2008;19:1270–4. doi: 10.1111/j.1540-8167.2008.01259.x. [DOI] [PubMed] [Google Scholar]
- 14.Moore JW, 3rd, Barrington W, Bazaz R, Jain S, Nemec J, Ngwu O, Schwartzman D, Shalaby A, Saba S. Complications of replacing implantable devices in response to advisories: a single center experience. International journal of cardiology. 2009;134:42–6. doi: 10.1016/j.ijcard.2007.12.070. [DOI] [PubMed] [Google Scholar]
- 15.Costea A, Rardon DP, Padanilam BJ, Fogel RI, Prystowsky EN. Complications associated with generator replacement in response to device advisories. Journal of cardiovascular electrophysiology. 2008;19:266–9. doi: 10.1111/j.1540-8167.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 16.Poole JE, Gleva MJ, Mela T, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–61. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 17.Palmisano P, Accogli M, Zaccaria M, Luzzi G, Nacci F, Anaclerio M, Favale S. Rate, causes, and impact on patient outcome of implantable device complications requiring surgical revision: large population survey from two centres in Italy. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2013;15:531–40. doi: 10.1093/europace/eus337. [DOI] [PubMed] [Google Scholar]
- 18.Krahn AD, Lee DS, Birnie D, et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. Circulation Arrhythmia and electrophysiology. 2011;4:136–42. doi: 10.1161/CIRCEP.110.959791. [DOI] [PubMed] [Google Scholar]
- 19.Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186–94. doi: 10.1093/eurheartj/eht511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resnic FS, Normand SL. Postmarketing surveillance of medical devices--filling in the gaps. The New England journal of medicine. 2012;366:875–7. doi: 10.1056/NEJMp1114865. [DOI] [PubMed] [Google Scholar]
- 21.Mittal S, Shaw RE, Michel K, Palekar R, Arshad A, Musat D, Preminger M, Sichrovsky T, Steinberg JS. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm. 2014;11:595–601. doi: 10.1016/j.hrthm.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Nery PB, Fernandes R, Nair GM, Sumner GL, Ribas CS, Menon SM, Wang X, Krahn AD, Morillo CA, Connolly SJ, Healey JS. Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. Journal of cardiovascular electrophysiology. 2010;21:786–90. doi: 10.1111/j.1540-8167.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 23.Mahajan T, Dubin AM, Atkins DL, Bradley DJ, Shannon KM, Erickson CC, Franklin WH, Cecchin F, Berul CI Members of the P, Congenital Electrophysiology S. Impact of manufacturer advisories and FDA recalls of implantable cardioverter defibrillator generators in pediatric and congenital heart disease patients. Journal of cardiovascular electrophysiology. 2008;19:1270–4. doi: 10.1111/j.1540-8167.2008.01259.x. [DOI] [PubMed] [Google Scholar]
- 24.Gould PA, Krahn AD. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. Jama. 2006;295:1907–11. doi: 10.1001/jama.295.16.1907. [DOI] [PubMed] [Google Scholar]


