Introduction
Arginase activity has been investigated in several conditions including trauma states, cancer, chronic wounds, pregnancy and diabetes. In atopic diseases, including asthma and allergic rhinitis, arginase research has been more limited and focused more towards another product of arginine metabolism, nitric oxide.
L-Arginine is a conditionally essential amino acid whose necessity is based on both developmental age as well as health status. It is processed via two main pathways which may be co-expressed in the same cell: 1) through arginase (Arg I or Arg II) to create urea and L-ornithine, and 2) through nitric oxide synthase (nNOS, iNOS, or eNOS) to form nitric oxide (NO) and L-citrulline. These catabolic pathways are differentially regulated by induction via different cytokine milieus: Arginase I expression is induced by Th2 cytokines (IL-4, IL-13, and TGF-β while iNOS activity is induced by Th1 cytokines (IFN-γ, IL-1 and TNF) (1). In either pathway, however, limitation in availability of L-arginine substrate will suppress enzymatic activity to some extent.
Though not previously investigated in atopic dermatitis, the issue of arginine bioavailability has been studied in other atopic diseases such as asthma and allergic rhinitis. Asthmatic patients have been found to have transiently elevated serum arginase activity during acute asthma exacerbation (2), while animal models of allergic asthma have demonstrated a similar finding after allergen challenge (3,4). High arginase activity was postulated to result in a low level of plasma L-arginine which compromises the body’s ability to synthesize nitric oxide, a potent vasodilator, and contributes to airway hypersensitivity (5). Moreover, high doses of L-arginine have been shown to decrease airway hyperresponsiveness and inflammation (6). Similarly to the results seen in asthmatic patients, in patients with allergic rhinitis, arginase I expression in nasal mucosa was also found to be elevated after allergen challenge (7), though serum arginase was not notably changed (8).
Given these findings in other atopic diseases, this exploratory study was initiated in order to investigate the role of arginase in children with atopic dermatitis, measuring both arginase activity as well as serum arginine levels. Human arginase I is localized to the granules of polymorphonuclear (PMN) cells and found to be constitutively expressed (9). For this reason, this study not only looked into serum L-arginine levels, but also the arginase I activity within the patient’s granulocytes in order to determine if there was a difference in activity level at the source. Surprisingly, we found decreased levels of arginase I activity in the granulocytes of our atopic dermatitis patients coupled with low arginase I protein and some elevation of L-arginine within the serum as well. These results, in contrast to the other atopic diseases investigated, imply a different involvement of arginase I in the inflammatory mechanism of atopic dermatitis.
Methods
Patients
Fifteen pediatric patients with a history of atopic dermatitis, requiring therapy of daily moisturization, corticosteroids, and/or immunomodulators, with current clinical manifestations of varying severity were enrolled in the study. Atopic dermatitis was defined by a chronic, xerotic, excoriative, pruritic, and/or lichenified skin condition which was treated by the Division of Allergy/Immunology at the Louisiana State University Health Sciences Center. Most patients also had previously confirmed sensitizations to foods and/or inhalants. Each patient’s dermatitis was documented, including location and percent of body surface area (BSA) involved, and a mild, moderate, or severe rating was assigned by the examiner for stratification purposes. Patients who showed: >15% BSA involvement, no active infections, and no antibiotic use within three months of sample collection were enrolled in the study. (See Table 1) At the time of enrollment, 9 patients were receiving topical corticosteroids regularly, 5 patients were receiving topical calcineurin inhibitors regularly, 7 patients were on antihistamines, and 6 patients were on leukotriene inhibitors. The mean age of patients was 4.8 years with a range of 1–13 years. Nine patients were male and 6 were female with 8 Caucasian patients, 5 African American patients, and 2 Hispanic patients.
Table 1.
Clinical and laboratory profile of atopic dermatitis patients.
| N | Age (yrs) |
Sex | Ethnicity | Sensitizations | Total IgE (IU/ml) |
Absolute Eosinophil count |
Severity |
|---|---|---|---|---|---|---|---|
| 1 | 6 | F | W | inhalants | 58 | 400 | mild-mod |
| 2 | 9 | M | W | foods | 1838 | ND | mild-mod |
| 3 | 4 | M | B | inhalants, foods | 1803 | 520 | mild |
| 4 | 5 | M | H | foods | 159 | 320 | mild |
| 5 | 7 | M | W | inhalants | 75.3 | 980 | mod |
| 6 | 1 | M | B | inhalants | 44.2 | 120 | mod |
| 7 | 2 | F | B | foods | 181 | 330 | mild |
| 8 | 1 | F | H | foods | 1480 | 360 | mod-severe |
| 9 | 4 | F | W | inhalants, foods | 908 | 760 | severe |
| 10 | 13 | M | B | ND | 373 | 630 | mild |
| 11 | 7 | F | W | inhalants, foods | 104 | 110 | mild |
| 12 | 1 | M | W | ND | 137 | 210 | mild |
| 13 | 6 | M | B | inhalants, foods | 5760 | 1580 | severe |
| 14 | 4 | F | W | inhalants, foods | 4106 | 300 | mod |
| 15 | 2 | M | W | foods | 214 | 320 | mild |
ND= Not done
Six age-matched control patients were also enrolled at the time of the study who had no personal or family history of atopy. Patients with a history of recurrent infections, skin disorders, or chronic medical conditions were excluded from participating as a control. Their mean age was 5.8 with a range of 1–14 years. This group was 5 males, 1 female with an equal distribution of Caucasians and African Americans.
At time of enrollment, blood was collected from each of the patients for determination of arginase activity, arginase protein quantitation, and amino acid levels.
Isolation of PMNs
Polymorphonuclear cells from patients and healthy donors and patients were isolated by dextran sedimentation. Briefly, peripheral blood was centrifuged over Ficoll-Hypaque (GE Biosciences, Uppsala, Sweden), after which PBMC were collected and the rest of the cells were resuspended in 3% dextran for 60 minutes at 37°C and 5% CO2. The supernatants were then collected and centrifuged 5 minutes at 1600 rpm at 4°C. Red blood cells were lysed by hypotonic lysis. PMN purity was determined by Giemsa staining and by flow cytometry staining of CD11b+/CD14− and ranged between 97–100%.
Arginase I Activity Assay
Granulocyte cell extracts from patients and controls were tested for arginase activity by measuring the conversion of a known quantity of L-arginine to L-ornithine and urea. Briefly, 25µg of protein from cell lysates were added to 25 µl of Tris-HCl (50 mM; pH 7.5) containing 10 mM MnCl2. This mixture was heated at 55–60°C for 10 min to activate arginase. Then, a solution containing 150 µL carbonate buffer (100 mM) (Sigma) and 50 µl L-Arg (100 mM) was added and incubated at 37°C for 20 min. The hydrolysis reaction from L-Arg to L-ornithine was identified by a colorimetric assay after the addition of ninhydrin solution and incubation at 95°C for 1 h. In addition, the hydrolysis reaction from L-Arg to urea was detected with diacetyl monoxime (Sigma) and incubation at 95°C for 10 min.
MMP-9 and MPO expression
Granule contents which co-localize with arginase I were identified in order to monitor for degranulation of storage granules. Cell extracts were suspended in lysis buffer (50mM HEPES, 150mM NaCl, 5mM EDTA, 1mM NaVO4, and 0.5% Triton) containing 50 µg/ml aprotinin, 50 µg/ml leupeptin, 100µg/ml trypsin-chymotrypsin inhibitor, and 2mM PMSF. Lysates were centrifuged at 3000 × g for 10 min at 4°C. The expression of MMP-9, MPO and GAPDH was detected by immunoblot using 25 µg of cell extracts. Cytoplasmic extracts were electrophoresed in 10% Tris-Glycine gels (Novex, San Diego, CA), transferred to PVDF membranes and immunoblotted with the appropriate antibodies. The reactions were detected using the ECL kit (Amersham).
Serum Arginase I protein levels
Arginase I levels were tested in the serum of healthy volunteers donors and atopic dermatitis patients using an ELISA kit (BioVendor, Candler, NC).
Serum levels of L-arginine
L-Arg concentration in serum samples were measured by HPLC-ECD using an ESA-CoulArray Model 540 (ESA Inc; Chelmsford, MA) with an 80 × 3.2 Column with 120A pore size. Briefly, serum samples were deproteinized in methanol. After centrifugation at 6000 × g for 10 min at 4°C, the supernatant was derivatized with 0.2 M OPA/BME (o-phtaldialdehyde containing 7 mM µ-mercaptoethanol). Fifty microliters of the sample were injected into the column. The retention time for L-Arg was 10.2 min.
Statistics
Statistical analysis was done using GraphPad Prism 3.0 (Graph Pad software, San Diego, CA). Differences between the groups were determined by Students’ t test and Anova.
Results
Decreased Arginase I activity in granulocytes of patients with atopic dermatitis
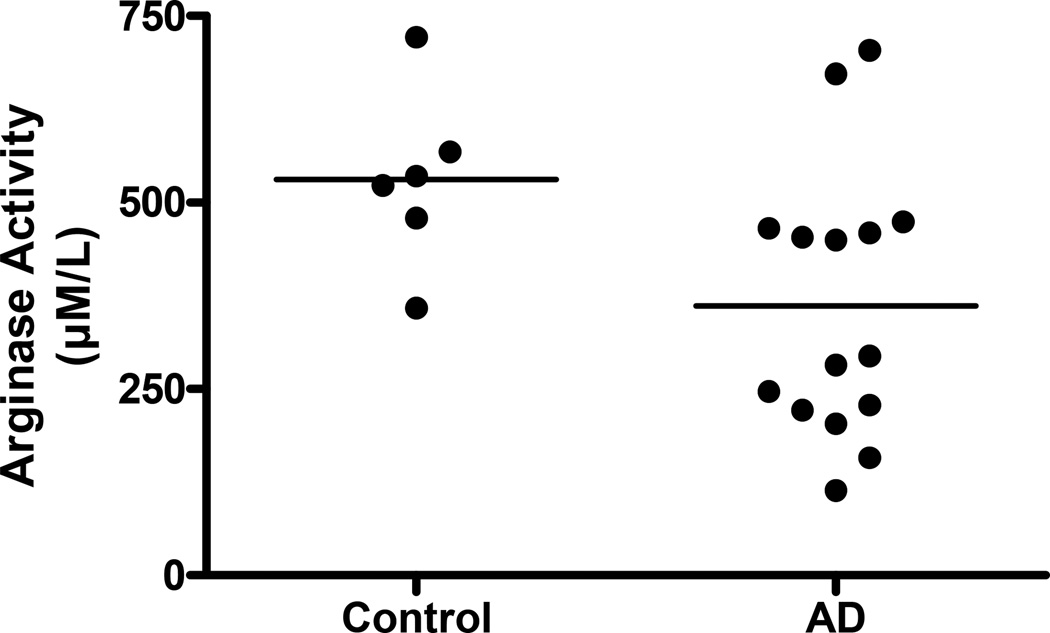
Arginase I activity found within polymorphonuclear cells of atopic dermatitis patients was significantly decreased when compared to patients without atopic disease (361.8 +/− 46.38nM vs 530.9 +/− 48.44, p< 0.01). (Figure 1)
Figure 1. Decreased arginase activity is found in granuloctyes of patients with atopic dermatitis.
Granulocyte lysates were tested for arginase activity by measuring the production of L-ornithine via a colorimetric assay. (P < 0.01, Student’s t test)
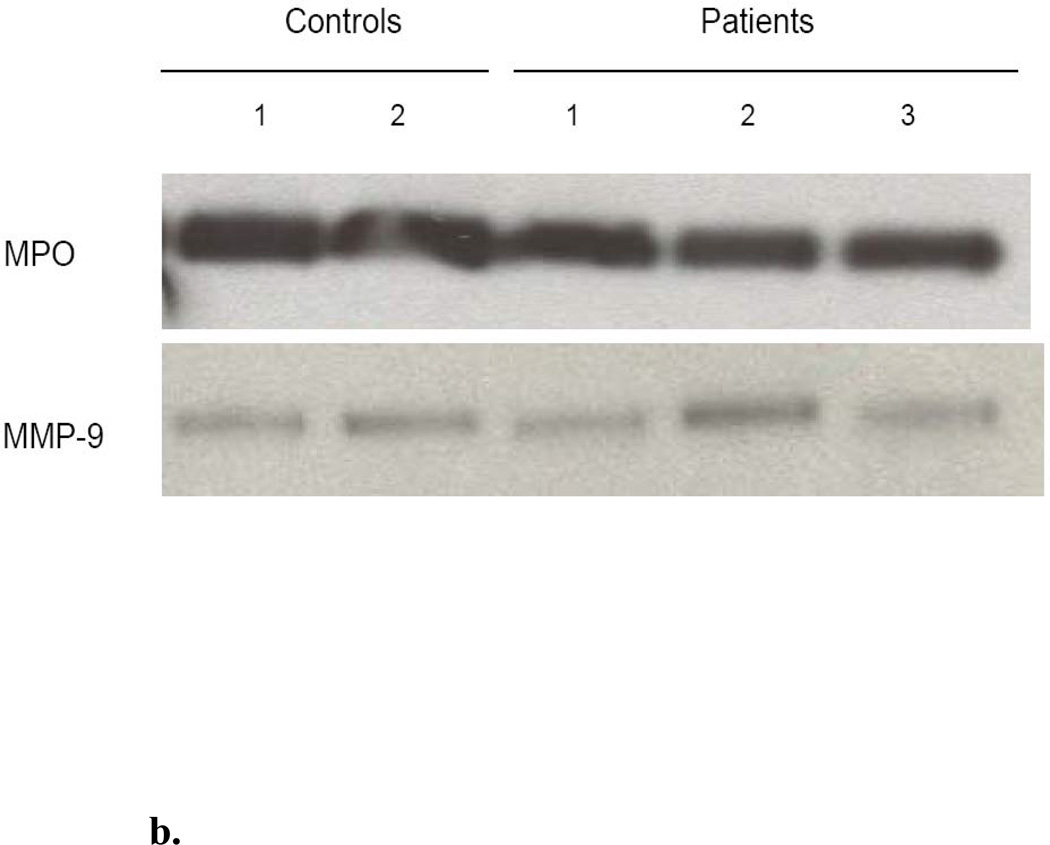
b. Decrease in Arginase I activity in granulocytes is not due to degranulation and release. Western blot analysis reveals comparable levels of MPO and MMP-9 in the cells of both controls and patients with AD, implying lack of degranulation. This suggests that the decrease of arginase I activity in the cells is not due to protein release into the extracellular space.
Arginase I is thought to be stored in either the gelatinase (10) or azurophilic (9) granules of PMNs, which also contain Myeloperoxidase (MPO) and Metalloproteinase 9 (MMP-9), respectively. In order to assess degranulation of these two granule types, the intracellular protein expression of MMP-9 and MPO was determined by Western blot. The enzymes were found to be present in equal amounts in representative samples (3 patients and 2 controls), implying lack of degranulation of the PMNs. (Figure 1b)
Decreased arginase I protein in the serum of patients with atopic dermatitis
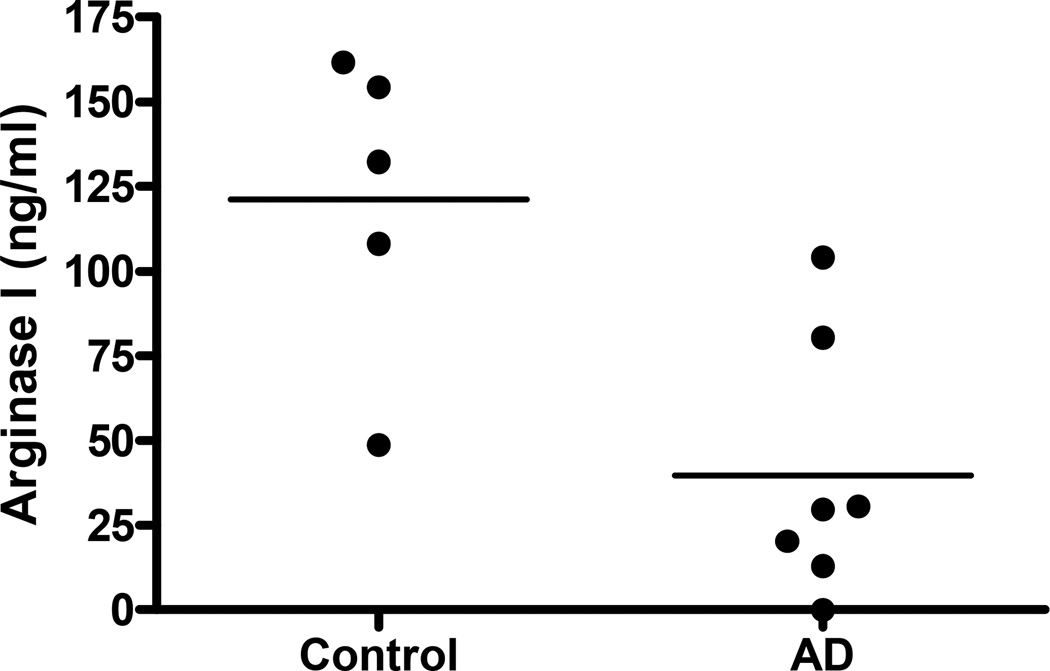
Because the decrease in granulocyte arginase might be a result of degranulation of the PMNs with release of arginase into the local environment, the patients’ serum levels of arginase I were compared to controls. In congruence with the decreased intracellular activity level, there was also a notable decrease in the level of arginase I protein in the serum of patients with atopic dermatitis. (39.7µM +/− 14.35 vs 121.1 µM +/− 20.33, p < 0.01) (Figure 2)
Figure 2. Decreased arginase I protein found in the plasma of patients with atopic dermatitis.
Plasma samples of 7 patients with atopic dermatitis and 5 controls were analyzed for arginase I protein via ELISA. (P < 0.01)
Increase in L-arginine in serum of patients with atopic dermatitis
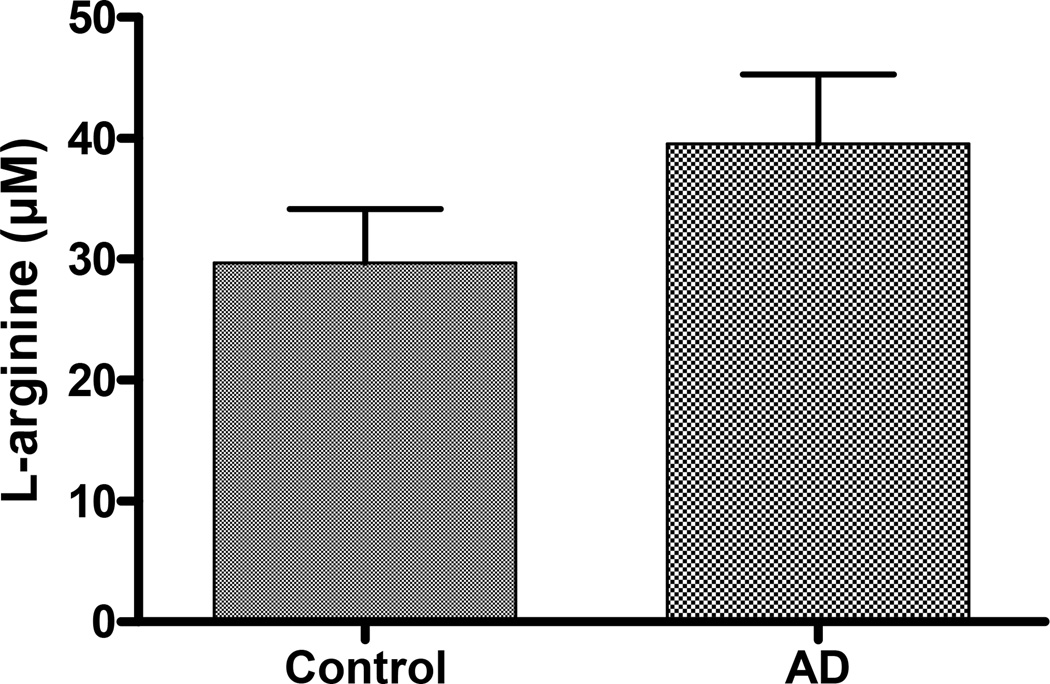
Consistent with the findings of decreased arginase protein in patients with atopic dermatitis, there was also a trend towards higher levels of L-arginine when compared to controls. (Figure 3)
Figure 3. Increased levels of L-arginine are found in the plasma of patients with atopic dermatitis.
Assessment of L-arginine levels of 7 patients and 6 controls via HPLC shows a trend toward lower levels of arginine in controls compared to atopic dermatitis patients
Discussion
This is the first report of arginase activity in the setting of atopic dermatitis (AD). In contrast to other atopic diseases studied, our patients with AD showed lower levels of arginase activity in their granulocytes and lower arginase I protein expression in their serum. This was not due to degranulation and dilution in the serum, as we noted the other PMN granule proteins were notably intact, but is likely due to decreased production or increased degradation. These results are further supported by the slightly higher levels of plasma L-arginine in comparison to controls as well, though these levels did not reach statistical significance.
In the complex pathophysiology of acute AD, the Th2 cytokine milieu predominates in the early phase of disease, while as the process becomes chronic, the cytokines become skewed towards the Th1 balance with IFN-γ and IL-12 being the predominant effectors (11). This change has importance in relation to arginine metabolism as these Th1 cytokines found in chronic AD, specifically IFN-γ, could skew processing towards the NOS pathway causing increased production of NO. In mechanisms of wound repair, NO in large quantities has been shown to have deleterious effects on wound healing (12). In AD patients, this could contribute to impaired skin barrier healing as well.
Additionally, the seeming disparity between our results and those of the previously investigated atopic diseases may be due to this same Th1 cytokine skewing. While Th2 cytokines dominate both asthma and allergic rhinitis, presumably causing an increase in arginase I expression and activity, the Th1-dominated chronic AD milieu may have the opposite effect. Furthermore, based on this, there may be a differential of expression based on the chronicity of the disease as well.
Continued stimulation of the iNOS pathway also leads to downregulation of arginase I. Primary cultured keratinocytes, when exposed to Th1 cytokines IFN-γ, TNF-β and IL-1β show an increased expression of iNOS along with a decreased arginase I mRNA expression of approximately 45% (13). In mouse models of AD-like lesions, skin showed increased iNOS expression and serum samples were noted to have increased nitrites compared to controls (14). Additionally, even after epicutaneous sensitization, iNOS knockout mice do not show the normally-induced symptoms of swelling/scratching, nor increased nitrite levels, as control mice show (15). More specifically to AD in humans, serum nitrate levels were found to be elevated in pediatric atopic dermatitis patients and were related to both severity of disease as well as eosinophil count (16). Elevated urinary nitrite levels were also correlated with atopic dermatitis diagnosis as well (17), though severity was not consistently correlated by other groups. In our patients, arginase activity did not show a correlation in severity or IgE level though there was some consistency with absolute eosinophil count. (Data not shown.) However, the continued stimulation of the iNOS system which is found in AD patients could account for the decreased levels of arginase I protein seen in our patients and be a factor in the pathophysiology of AD, as the nitrites produced would induce further breakdown and inability to heal. These findings are corroborated by the successful use of AD treatments such as topical hydrocortisone (18) and FK506 (19), which both inhibit iNOS mRNA expression. Additionally, a small study also showed an 80% reduction in itching and 60% reduction in erythema with daily application of NOS inhibitor Nω-nitro-L-arginine in 1% petrolatum (20).
Suppression of the arginase I pathway can also have direct effects on wound healing pathways. Less processing of arginine into L-ornithine, which is further metabolized to proline via ornithine aminotransferase and to polyamines via ornithine decarboxylase, could result in impaired collagen production and cell proliferation, respectively (21). Recently, researchers showed that local arginase I deficiency induced delay in wound healing though an altered inflammatory response and abnormal matrix deposition (22). This issue is also impactful for our patients with AD who often have barrier function defects associated with impaired healing (23).
Recent research has focused on the filaggrin protein, which is one of the structural components of the epidermis. Mutations in filaggrin have also been associated with AD (24). Upon degradation, filaggrin releases free amino acids, including arginine, which are processed into natural moisturizing factors in the skin (25). Arginase I then hydrolyzes this arginine into ornithine and urea. Urea is known to enhance hydration in the stratum corneum and has been used in topical form to improve skin barrier function and enhance antimicrobial peptides (26). Recent proteomic profiling of patients with AD found a significant decrease in expression of arginase I in skin samples (27). The authors posited that decreased expression of arginase I might also decrease urea generation as well. This finding, in congruence with ours, may also give insight into another problem with the epidermal barrier in atopic dermatitis- low arginase I expression would decrease urea production, thereby causing barrier hydration dysfunction.
While this early research has begun to link the iNOS/arginase pathway to atopic dermatitis, there are still many issues which will need to be elucidated. First, expansion of patient numbers will clarify differences between patients based on severity stratification. Secondly, the mechanism of action for the decreased arginase I enzyme expression found in these patients will need to be further investigated based on pre- and post-transcriptional analyses. Finally, direct measurement of both iNOS and arginase I activity in the AD skin microenvironment (both acute and chronic) may help to clarify the local interactions between these two enzymes.
In summary, this exploratory study has identified a role for arginase I in atopic dermatitis. In this disease state, overproduction of iNOS may be contributing to the suppression of the arginase system, causing both impaired wound healing and barrier dysfunction. Further research into the causes of iNOS overexpression and resultant arginase I suppression may lead to therapeutic interventions in the future.
Acknowledgments
Financial support: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature Rev Imm. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 2.Morris CR, Poluakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM. Decreased Arginine Bioavailability and Increased Serum Arginase Activity in Asthma. Am J Resp Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 3.Maarsingh H, Zaagsma J, Meurs H. Arginine homeostasis in allergic asthma. Eur J Pharm. 2008;585:375–384. doi: 10.1016/j.ejphar.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann N, King NE, Laporte J, et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Inv. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meurs H, McKay S, Maarsingh H, et al. Increased arginase activity underlies allergen-induced deficiency of cNOS-derived nitric oxide and airway hyperresponsiveness. Brit J Pharm. 2002;136:391–398. doi: 10.1038/sj.bjp.0704725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabalirajan U, Ahmad T, Leishangthem GD, et al. Beneficial effects of high dose L-arginine on airway hyperresponsiveness and airway inflammation in a murine model of asthma. J All Clin Immunol. 2010;125:626–635. doi: 10.1016/j.jaci.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 7.Cho WS, Kim TH, Kim KH, et al. Increased expression of Arginase I and II in nasal mucosa. Laryngoscope. 2011;121(2):236–240. doi: 10.1002/lary.21288. [DOI] [PubMed] [Google Scholar]
- 8.Unal M, Eskandari HG, Erçetin N, Doğruer ZN, Pata YS. Serum nitrite/nitrate and arginase levels in patients with allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2007;69(2):113–115. doi: 10.1159/000097842. [DOI] [PubMed] [Google Scholar]
- 9.Munder M, Mollinedo F, Calafat J, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–2556. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen LC, Theilgaard-Mönch K, Christensen EI, Borregaard N. Arginase I is expressed in myelocytes/metamyelocytes and localized in gelatinase granules of human neutrophils. Blood. 2007;109:3084–3087. doi: 10.1182/blood-2006-06-032599. [DOI] [PubMed] [Google Scholar]
- 11.Beiber T. Atopic Dermatitis. Ann Dermatol. 2010;22(2):125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizk M, Witte MB, Barbul A. Nitric Oxide and Wound Healing. World J Surg. 2004;28:301–306. doi: 10.1007/s00268-003-7396-7. [DOI] [PubMed] [Google Scholar]
- 13.Bruch-Gerharz D, Schnorr O. Arginase I overexpression in psoriasis: limitation of inducible nitric oxide synthase activity as a molecular mechanism for keratinocyte hyperproliferation. Am J Pathol. 2003;162:203–211. doi: 10.1016/S0002-9440(10)63811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo M, Kambayashi Y, Takemoto K, Okuda J, Muto M, Ogino K. Reactive nitrogen species formation in eosinophils and imbalance in nitric oxide metabolism are involved in atopic dermatitis-like skin lesions in NC/Nga mice. Free Radical Res. 2005;39(7):719–727. doi: 10.1080/10715760500139260. [DOI] [PubMed] [Google Scholar]
- 15.Orita K, Hiramoto K, Kobayashi H, Ishii M, Sekiyama A, Inoue M. Inducible nitric oxide synthase (iNOS) and α-melanocyte-stimulating hormones of iNOS origin play important roles in the allergic reactions of atopic dermatitis in mice. Exper Derm. 2011;20:911–914. doi: 10.1111/j.1600-0625.2011.01360.x. [DOI] [PubMed] [Google Scholar]
- 16.Taniuchi S, Kojima T, Hara Mt K, et al. Increased serum nitrate levels in infants with atopic dermatitis. Allergy. 2001;56:693–695. doi: 10.1034/j.1398-9995.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakai K, Yoneda K, Maeda R, et al. Urinary biomarker of oxidative stress in patients with psoriasis vulgaris and atopic dermatitis. J Eur Acad of Derm Vener. 2009;23:1405–1408. doi: 10.1111/j.1468-3083.2009.03327.x. [DOI] [PubMed] [Google Scholar]
- 18.Radomski MW, Palmer RMJ, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Nat Acad Sci. 1990;87:10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan CC, Kao YH, Huang SM, Yu HS, Chen GS. FK506 independently upregulates transforming growth factor b and downregulates inducible nitric oxide synthase in cultured human keratinocytes: possible mechanisms of how tacrolimus ointment interacts with atopic skin. Brit J Derm. 2004;151:679–684. doi: 10.1111/j.1365-2133.2004.06109.x. [DOI] [PubMed] [Google Scholar]
- 20.Morita H, Semma M, Hori M, Kitano Y. Clinical Application of Nitric Oxide Synthase Inhibitor for Atopic Dermatitis. Inter J Derm. 1995;34:294–295. doi: 10.1111/j.1365-4362.1995.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 21.Witte MB, Barbul A. Arginine physiology and its implication for wound healing. Wound Rep Regen. 2003;11:419–423. doi: 10.1046/j.1524-475x.2003.11605.x. [DOI] [PubMed] [Google Scholar]
- 22.Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. Local Arginase 1 Activity Is Required for Cutaneous Wound Healing. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.164. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boguniewicz M, Leung D. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAleer M, Irvine A. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol. 2013;131:280–291. doi: 10.1016/j.jaci.2012.12.668. [DOI] [PubMed] [Google Scholar]
- 25.Candi E, Schmidt R, Melino G. The cornified envelope: A model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 26.Grether-Beck S, Felsner I, Brenden H, et al. Urea uptake enhances barrier function and antimicrobial defense in humans by regulating epidermal gene expression. J Invest Dermatol. 2012;132(6):1561–72. doi: 10.1038/jid.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broccardo CJ, Mahaffey S, Schwarz J, et al. Comparative proteomic profiling of patients with atopic dermatitis based on history of eczema herpeticum infection and Staphylococcus aureus colonization. J Allergy Clin Immunol. 2011;127:186–193. doi: 10.1016/j.jaci.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]