Abstract
Background
Few studies have examined the association between obesity and markers of kidney injury in a chronic kidney disease population. We hypothesized that obesity is independently associated with proteinuria, a marker of chronic kidney disease progression.
Study Design
Observational cross-sectional analysis.
Setting & Participants
Post hoc analysis of baseline data for 652 participants in the African American Study of Kidney Disease (AASK).
Predictors
Obesity, determined using body mass index (BMI).
Measurements & Outcomes
Urine total protein–creatinine ratio and albumin-creatinine ratio measured in 24-hour urine collections.
Results
AASK participants had a mean age of 60.2 ± 10.2 years and serum creatinine level of 2.3 ± 1.5 mg/dL; 61.3% were men. Mean BMI was 31.4 ± 7.0 kg/m2. Approximately 70% of participants had a daily urine total protein excretion rate <300 mg/d. In linear regression analyses adjusted for sex, each 2-kg/m2 increase in BMI was associated with a 6.7% (95% CI, 3.2-10.4) and 9.4% (95% CI, 4.9-14.1) increase in urine total protein–creatinine and urine albumin-creatinine ratios, respectively. In multivari-able models adjusting for age, sex, systolic blood pressure, serum glucose level, uric acid level, and creatinine level, each 2-kg/m2 increase in BMI was associated with a 3.5% (95% CI, 0.4-6.7) and 5.6% (95% CI, 1.5-9.9) increase in proteinuria and albuminuria, respectively. The interaction between older age and BMI was statistically significant, indicating that this relationship was driven by younger AASK participants.
Limitations
May not generalize to other populations; cross-sectional analysis precludes statements regarding causality.
Conclusions
BMI is associated independently with urine total protein and albumin excretion in African Americans with hypertensive nephrosclerosis, particularly in younger patients.
Index Words: Obesity, body mass index, chronic kidney disease, proteinuria, hypertension
Obesity is a growing public health concern. Several observational studies have implicated obesity as an independent risk factor for the onset and progression of chronic kidney disease (CKD).1,2 The mechanism(s) underlying the association between obesity and CKD has not been elucidated. However, studies of mice and humans suggest that adipokines (cytokines derived from fat cells) may alter glomerular permeability to proteins, leading to proteinuria,3,4 which is a powerful predictor of progression of CKD. In the African American Study of Kidney Disease and Hypertension (AASK), baseline (and follow-up) proteinuria was a better predictor of subsequent decrease in kidney function than baseline glomerular filtration rate (GFR).5-7 Thus, correlates of baseline proteinuria are of special interest in identifying those at risk of progression of hypertensive nephrosclerosis. We hypothesized that obesity is associated independently with proteinuria. To test this hypothesis, we examined obesity and factors known to be independent predictors of proteinuria in 652 participants in the AASK Cohort Study. This study was a prospective observational follow-up study of participants previously enrolled in the AASK clinical trial (designated NCT00582777 in the ClinicalTrials.gov registry).
Methods
Study Population
The AASK Cohort Study was a National Institutes of Health–sponsored, large-scale, 5-year, multicenter, prospective observational study that followed the AASK clinical trial. Briefly, to be eligible for enrollment in the AASK clinical trial, study participants had to be African American (by declaration), be 21 years or older, have diastolic blood pressure (BP) ≥95 mm Hg, and have a measured GFR using renal clearance of iothalamate in the range of 25-65 mL/min/ 1.73 m2. All study participants had a measured GFR at entry to the clinical trial, which occurred between 1994 and 1997.7 The AASK was a randomized double-blind controlled trial that used a 2×3 factorial design. The trial compared 2 levels of BP control (mean arterial pressure <92 mm Hg and 102-107 mm Hg) and 3 antihypertensive regimens (metoprolol, amlodipine, and ramipril). Additional antihypertensives were added, and doses of these add-on medications were adjusted as needed to achieve BP control levels throughout the study. The AASK cohort began enrolling study participants in July 2002, approximately 6 months after completion of the AASK clinical trial.8 Briefly, all AASK trial study participants who were alive and not on renal replacement therapy were invited to participate in the cohort study. A total of 691 African American men and women were recruited by the 21 centers to participate in the AASK cohort study and were followed up for up to 5 years to identify factors beyond BP that are important in the progression of hypertensive nephrosclerosis. This report is restricted to 652 of the 691 AASK cohort study participants who provided a 24-hour urine specimen for creatinine, total protein, and albumin measurement and in whom body mass index (BMI) was calculated at the baseline visit. The institutional review board approved the study protocol, and written informed consent was obtained at each participating institution.
Study Procedures
We collected demographic, clinical, and laboratory data at a baseline clinic visit for all AASK cohort study participants using standardized study-specific forms. Demographic information including age, sex, body weight and height, and employment status was collected and entered into a secure website for transmission to the data coordinating center. A 24-hour urine sample and fasting morning blood sample were obtained during the baseline evaluation period of the study. Study participants were instructed to collect 24-hour urine samples using standard procedures identical to those used in the AASK clinical trial. Of 691 participants, 39 were missing either a baseline 24-hour urine sample or BMI calculation. Systolic BP and high-density lipoprotein (HDL) cholesterol levels were higher in the 39 excluded persons than in participants who provided BMI values and 24-hour urine samples. However, reflecting the relatively small proportion of excluded patients, there were no major differences in baseline characteristics between the full cohort and the subgroup of 652 included in the report.
Measurements
BMI was calculated as body weight in kilograms divided by height in meters squared. Serum chemistry tests, including serum urea nitrogen, serum creatinine, electrolytes, total and HDL cholesterol, uric acid, and glucose, were performed using an autoanalyzer in the central laboratory of the data coordinating center (The Cleveland Clinic). Urine albumin was measured using nephelometry, and urine total protein, using pyrogallol red. All measurements were performed in duplicate. Urine creatinine was measured using an autoanalyzer by means of the modified Jaffé reaction. Median interassay coefficients of variation for urine total protein, urine creatinine, and urine total protein–creatinine ratio were 3.10, 0.58, and 3.11, respectively. Urine total protein and albumin indices were calculated from measured values obtained from 24-hour urine specimens and expressed as the ratio of total protein to creatinine in milligrams of total protein per gram of creatinine and in milligrams of albumin per gram of creatinine.
Statistical Analysis
We produced descriptive summaries (mean ± standard deviation) of baseline characteristics for the entire group and for the BMI categories <25, 25-29, 30-35, and >35 kg/m2 as estimates of those with normal, overweight, obese, and very obese BMI (Table 1). Distributions of indices based on urine total protein and urine albumin (including total protein–creatinine and albumin-creatinine ratios) were summarized separately for men and women using mean ± standard deviation and 25th, 50th, and 75th percentiles. These descriptive analyses were stratified by sex because of prior studies indicating sex differences in relationships among different indices of proteinuria.9-12
Table 1. Baseline Characteristics of Cohort Population by BMI Category.
| BMI (kg/m2) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Baseline Variables | All Patients (N = 652) | <25(n = 114) | 25-30 (n = 200) | 30-35 (n = 156) | >35 (n = 182) |
| Age (y)a | 60.2 ± 10.2 | 63.0 ± 9.95 | 61.1 ± 10.1 | 60.7 ± 10.2 | 57.2 ± 9.91 |
| SCr (mg/dL) | 2.27 ± 1.46 | 2.44 ± 2.08 | 2.21 ± 1.38 | 2.18 ± 1.17 | 2.31 ± 1.29 |
| Systolic BP (mm Hg) | 135 ± 21.6 | 136 ± 23.6 | 134 ± 21.2 | 136 ± 22.7 | 137 ± 19.6 |
| Diastolic BP (mm Hg) | 80.4 ± 12.3 | 79.3 ±11.5 | 79.9 ± 13.0 | 80.8 ± 13.0 | 81.4 ± 11.4 |
| Total cholesterol (mg/dL) | 201 ± 45.3 | 200 ± 51.4 | 199 ± 44.4 | 204 ± 42.4 | 202 ± 45.0 |
| HDL cholesterol (mg/dL)a | 47.2 ± 15.2 | 51.8 ± 18.6 | 48.7 ± 15.7 | 46.0 ±14.1 | 43.9 ± 12.0 |
| Non-HDL cholesterol (mg/dL) | 154 ± 43.5 | 148 ± 50.5 | 150 ± 42.3 | 158 ± 39.7 | 158 ± 42.8 |
| Triglycerides (mg/dL)a | 144 ± 90.4 | 127 ± 83.6 | 142 ±103 | 141 ± 73.8 | 158 ± 91.1 |
| Uric acid (mg/dL) | 8.78 ± 2.17 | 8.45 ± 2.20 | 8.63 ± 2.22 | 8.85 ± 2.17 | 9.09 ± 2.06 |
| BMI (kg/m2)a | 31.4 ± 7.02 | 22.4 ± 1.98 | 27.6 ± 1.39 | 32.4 ± 1.40 | 40.4 ± 4.80 |
| Serum glucose (mg/dL) | 101 ± 34.2 | 94.8 ± 21.0 | 101 ± 44.3 | 101 ± 26.1 | 106 ± 33.7 |
Note: Results are expressed as mean ± standard deviation. Conversion factors for units: SCr in mg/dL to μmol/L, ×88.4; total, HDL, and non-HDL cholesterol in mg/dL to mmol/L, ×0.02586; triglycerides in mg/dL to mmol/L, ×0.01129; uric acid in mg/dL to μmol/L, ×59.48; glucose in mg/dL to mmol/L, ×0.5551.
Abbreviations: BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; SCr, serum creatinine.
Significant difference (P < 0.05) among mean levels in the 4 groups using analysis of variance.
Simple linear regression analyses were used to relate log-transformed urine total protein–creatinine ratio and urine albumin-creatinine ratio to sex. Separate regression analyses then were performed to relate the 2 log-transformed proteinuria indices to other individual baseline factors (BMI, age, log-transformed serum creatinine level, HDL cholesterol level, total cholesterol level, non-HDL cholesterol level, triglyceride level, uric acid level, systolic BP, diastolic BP, and serum glucose level) while controlling for sex. These factors were selected before statistical analyses based on previously observed or hypothesized relationships of these factors with proteinuria. The decision to log transform serum creatinine values was made after examination of nonparametric smoothing spline regression curves that showed approximately linear relationships of the log-transformed urine total protein–creatinine and urine albumin-creatinine indices with log-transformed serum creatinine level, but not with the untransformed serum creatinine values.
In sensitivity analyses, we extended each of the indicated regression analyses to test for the presence of an interaction between sex and the regression coefficients relating the log-transformed proteinuria indices to each of the other designated baseline factors. These pairwise interactions were tested by estimating regression coefficients relating proteinuria indices to baseline factors separately for men and women, then dividing the differences by the square root of the sum of the squared standard errors for men and women and comparing the resulting statistic with the standard normal distribution.
Multiple linear regression analysis was used to relate log-transformed urine total protein–creatinine and urine albumin-creatinine indices to BMI after covariate adjustment for 3 prespecified baseline factors (ie, age, sex, and log-transformed serum creatinine level), which were viewed as potential confounders for the effects of BMI on indices of proteinuria. Three additional baseline factors (fasting serum glucose level, BP, and uric acid level) were viewed as both potential confounding factors and potential intermediate variables on causal pathways between BMI and proteinuria. An expanded set of multiple linear regression analyses was performed in which these 3 factors were added as additional covariates to the original multiple regressions. For both the basic and expanded regression models, potential interactions between sex or BMI with each of the other baseline factors included in the multiple regression analyses were examined by applying a forward stepwise variable selection procedure to pairwise interaction terms between sex or BMI and each other covariate in multiple regression models that included each of the individual covariates. Only the interaction of BMI with age met the criterion for significance and was added to the model. The resulting multivariate models are presented in the final table with each of the main-effect variables being standardized to have a mean value of zero. Based on this standardization, regression coefficients for the main effects of age and BMI each indicate the effects of these factors evaluated at the mean level of the other factor.
For both the basic and expanded regression models, potential interactions between BMI and each of the other baseline factors included in the multiple regression analyses were examined by applying forward stepwise variable selection procedure to pairwise interaction terms between BMI and each covariate after each of the individual covariates was entered in the model as a main effect.
In all analyses, the effect of each baseline factor was expressed as percentage of change in the geometric mean of urine total protein–creatinine ratio or urine albumin-creatinine ratio per increment in the baseline factor. Diastolic BP was excluded from multivariable models because of its high correlation with systolic BP. The shapes of the relationships of the log-transformed urine total protein–creatinine and urine albumin-creatinine ratios with baseline BMI were examined further by relating the log-transformed proteinuria indices to BMI quartiles after adjusting for the expanded set of baseline covariates. Because a significant interaction was identified between BMI and age, these analyses were performed after stratifying by age group (age at the median of ≤61 years or >61 years). Statistical significance was inferred for P < 0.05, without adjustment for multiple comparisons. Standard regression diagnostics, including visual inspection of partial residual plots and examination of smoothing splines for each predictor variable, were used to investigate potential violations of the assumptions of regression analysis, including the assumed linearity of the relationships and the absence of major heteroscedasticity.
To evaluate the possibility that 24-hour urine collections during the baseline assessment may have represented severe over- or undercollection, we compared urine creatinine level (milligrams per day) at that assessment with the average of all 24-hour urine creatinine measurements obtained during either the 3 years before the cohort study or the first 3 years of the cohort study. Approximately 8 (median value) urine creatinine measurements per participant were used in this analysis. Six baseline measurements were identified as outliers, with deviations of at least 963 (women) or 2,176 mg/d (men) from the median 24-hour urine creatinine excretion during this 6-year period. The main analyses of this report were repeated after excluding these measurements and provided results similar to those obtained including all available data. Therefore, the analyses reported here include the complete data set.
Results
Baseline Characteristics
Study participants were middle aged and 39% were women (Table 1). Most study participants had increased BMI (>25 kg/m2). Those in the highest BMI category were slightly younger than those with lower BMI. As anticipated, average systolic and diastolic BPs were within the reference ranges, and average serum creatinine level was increased across categories of BMI, reflecting the nature of the study population. Mean fasting serum glucose level was in the high-normal range, non-HDL cholesterol level was moderately increased, and mean serum triglyceride level was in the reference range. Mean serum uric acid level was higher than normal. There was a trend for increased BP and glucose, triglyceride, uric acid, and non-HDL cholesterol levels and a trend toward decreased HDL cholesterol level with increasing BMI.
Urine Creatinine, Total Protein, and Albumin Excretion Rates
Urine creatinine excretion was approximately symmetrically distributed and, as expected, higher in men compared with women (Table 2). In men, 25th-75th percentiles of urine creatinine index (creatinine per body weight per day) were 15-23 mg/kg/d, and for women, the corresponding values were approximately 12-18 mg/kg/d. Overall, these data combined with sensitivity analyses (see Methods) suggest that 24-hour collections were adequate for quantification of 24-hour urine creatinine, protein, and albumin excretion.
Table 2. Summary of Baseline Urine Indices of Cohort Population by Sex.
| All Patients (N = 652) | Men(n = 400) | Women (n = 252) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean ± SD | Median (25th-75th percentiles) | Mean ± SD | Median (25th-75th percentiles) | Mean ± SD | Median (25th-75th percentiles) | |
| Urine Cr (mg/d)a | 1,596 ± 855 | 1,502 (1,122-1,987) | 1,833 ± 956 | 1,756 (1,355-2,198) | 1,218 ± 456 | 1,163 (894-1,465) |
| Urine Cr/weight (mg/kg/d)a | 18 ± 10 | 17 (13-21) | 19 ± 11 | 19 (15-23) | 15 ± 5 | 14 (12-18) |
| Urine protein (mg/d) | 533 ± 1,067 | 91 (40-461) | 536 ± 1,044 | 90 (40-491) | 529 ± 1,105 | 94 (39-439) |
| UPCR (mg/g)a | 377 ±821 | 63 (29-325) | 340 ± 762 | 55 (23-301) | 436 ± 905 | 79 (36-350) |
| Urine Alb (mg/d)a | 178 ± 464 | 10 (5-88) | 180 ± 448 | 12 (5-103) | 174 ± 489 | 7 (4-75) |
| UACR (mg/g) | 123 ± 336 | 7 (3-57) | 113 ± 296 | 7 (3-48) | 139 ± 391 | 7 (4-66) |
| Urine Alb/protein (%)a | 21 ± 17 | 16 (9-26) | 23 ± 18 | 16 (11-29) | 19 ± 17 | 14 (8-22) |
Abbreviations: Alb, albumin; Cr, creatinine; SD, standard deviation; UACR, urine albumin-creatinine ratio; UPCR, urine total protein–creatinine ratio.
Significant difference (P < 0.05) between women and men using the Wilcoxon Mann-Whitney test.
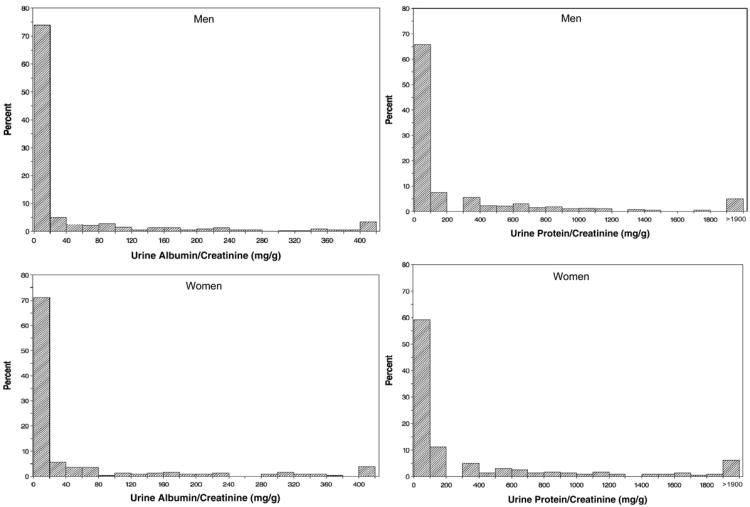
Urine total protein excretion rate was increased overall and numerically higher in men compared with women. Distributions of urine total protein–creatinine and albumin-creatinine ratios by sex are shown separately for men and women in Fig 1. Both urine total protein–creatinine and albumin-creatinine ratios varied considerably in both male and female study participants (Fig 1; Table 2), and their distributions showed marked positive skewness. Overall, approximately 70% of AASK cohort enrollees had a daily urine total protein excretion rate <300 mg/d; this is similar to the 67.2% previously reported for the larger group of enrollees in the AASK trial.7 The geometric mean of urine total protein–creatinine ratio was 41.5% (95% confidence interval [CI], 10.6-81.0) higher in women than men (Table 2). Distributions of urine albumin-creatinine ratios generally were similar between men and women. The fraction of urine total protein contributed by albumin was about 20%.
Figure 1.
Histograms of urine albumin-creatinine ratio (left-hand panels) and urine total protein–creatinine ratio (right-hand panels) in men (top) and women (bottom) in the AASK (African American Study of Kidney Disease and Hypertension) cohort.
Associations of Urine Total Protein–Creatinine and Urine Albumin-Creatinine Ratios With Baseline Covariates
After adjustment for sex only, age, BMI, serum creatinine level, diastolic BP, systolic BP, and uric acid level were each significantly associated with both urine total protein–creatinine and albumin-creatinine ratios (Table 3). Of baseline factors considered in Table 3, we observed a significant interaction with sex only for serum triglyceride level (P = 0.03) on log-transformed urine total protein–creatinine ratio. Each 50-mg/dL increase in serum triglyceride level was associated with a 17.0% (95% CI, 5.8-29.3) increase in urine total protein–creatinine ratio (P = 0.002) for women (n = 221), but only a 0.1% (95% CI, −9.1 to 10.2) increase for men (P = 0.9; n = 357).
Table 3. Univariate Relationship of UPCR and UACR With Baseline Variables.
| Variable | UPCR (mg/g) | UACR (mg/g) | ||
|---|---|---|---|---|
|
|
|
|||
| % Change (95% CI) | P | % Change (95% CI) | P | |
| Unadjusted | ||||
| Female sex | 41.5 (10.9 to 81.0) | <0.001 | 17.0 (-13.8 to 58.9) | 0.3 |
| Adjusted for sex | ||||
| Age (/10 y) | −34.4 (−41.4 to −26.5) | <0.001 | −37.0 (−45.3 to −27.4) | <0.001 |
| BMI (/2 kg/m2) | 6.7 (3.2 to 10.4) | <0.001 | 9.4 (4.9 to 14.1) | <0.001 |
| Diastolic BP (/10 mm Hg) | 34.1 (22.0 to 47.5) | <0.001 | 41.1 (25.4 to 58.8) | <0.001 |
| Systolic BP (/10 mm Hg) | 16.9 (10.7 to 23.6) | <0.001 | 20.9 (13.0 to 29.5) | <0.001 |
| SCr (/doubling) | 257.9 (201.9 to 324.4) | <0.001 | 260.7 (188.7 to 350.8) | <0.001 |
| Total cholesterol (/20 mg/dL) | −0.5 (−5.7 to 5.1) | 0.9 | −0.2 (−6.8 to 6.8) | 0.9 |
| HDL cholesterol (/5 mg/dL) | −2.3 (−6.2 to 2.0) | 0.3 | −2.2 (−7.1 to 3.1) | 0.4 |
| Total-HDL cholesterol (/20 mg/dL) | 0.6 (−4.9 to 6.3) | 0.8 | 0.8 (−6.0 to 8.0) | 0.8 |
| Triglycerides (/50 mg/dL) | 5.4 (—1.7 to 13.0) | 0.1 | 5.9 (−2.9 to 15.6) | 0.2 |
| Uric acid (/1 mg/dL) | 10.6 (4.6 to 16.9) | <0.001 | 7.7 (0.5 to 15.5) | 0.04 |
| Serum glucose (/10 mg/dL) | 2.3 (—1.2 to 6.0) | 0.2 | 4.3 (−0.1 to 9.0) | 0.04 |
Note: Percentages of differences in geometric mean UPCRs or UACRs associated with the indicated increases in the respective predictor variables in univariate analyses adjusting for sex. Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; total, HDL, and non-HDL cholesterol in mg/dL to mmol/L, ×0.02586; triglycerides in mg/dL to mmol/L, ×0.01129; uric acid in mg/dL to μmol/L, ×59.48; glucose in mg/dL to mmol/L, ×0.5551.
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; HDL, high-density lipoprotein; SCr, serum creatinine; UACR, urine albumin-creatinine ratio; UPCR, urine total protein–creatinine ratio.
In multivariable analysis, age, serum glucose level, log-transformed serum creatinine level, systolic BP, and BMI were independently associated with one or both indices (Table 4). Statistically significant interactions were found between BMI and age, such that associations of log-transformed urine total protein–creatinine and urine albumin-creatinine indices with BMI were more pronounced in younger than older participants.
Table 4. Multivariable Models Relating UPCR and UACR to Baseline Variables.
| UPCR (mg/g) (n = 649) | UACR (mg/g) (n = 648) | |||
|---|---|---|---|---|
|
|
|
|||
| % Changea (95% CI) | P | % Changea (95% CI) | P | |
| Model Using Selected Set of Baseline Variables | ||||
|
|
||||
| Age (/10 y) | −20.8 (−28.6 to −12.1) | <0.001 | −22.8 (−32.6 to −-11.5) | <0.001 |
| BMI (/2 kg/m2) | 3.5 (0.4 to 6.7) | 0.03 | 5.6 (1.5 to 9.9) | 0.007 |
| Female sex | 89.1 (52.8 to 134.0) | <0.001 | 53.1 (15.8 to 102.5) | 0.003 |
| SCr (/doubling) | 227.2 (176.3 to 287.4) | <0.001 | 226.0 (161.2 to 306.9) | <0.001 |
| BMI & age interaction | −4.9 (−7.5 to −2.1) | <0.001 | −6.3 (−9.7 to −2.8) | <0.001 |
| Model Using Expanded Set of Baseline Variables | ||||
|
|
||||
| Age (/10 y) | −23.8 (−31.0 to −15.8) | <0.001 | −26.6 (−35.6 to −16.3) | <0.001 |
| BMI (/2 kg/m2) | 3.6 (0.5 to 6.7) | 0.02 | 5.6 (1.6 to 9.8) | 0.006 |
| Female sex | 69.7 (37.9 to 108.9) | <0.001 | 33.9 (2.0 to 75.9) | 0.04 |
| SCr (/doubling) | 245.9 (190.8 to 311.5) | <0.001 | 261.8 (188.0 to 354.5) | <0.001 |
| Serum glucose (/10 mg/dL) | 3.6 (0.7 to 6.6) | 0.02 | 5.7 (1.8 to 9.7) | 0.004 |
| Systolic BP (/10 mm Hg) | 17.7 (12.5 to 23.2) | <0.001 | 21.8 (14.7 to 29.3) | <0.001 |
| Uric acid (/1 mg/dL) | −5.0 (−9.5 to −0.2) | 0.04 | −8.5 (−14.2 to −2.4) | 0.007 |
| BMI & age interaction | −4.1 (−6.7 to —1.5) | 0.002 | −5.5 (−8.8 to −2.1) | 0.002 |
Note: Conversion factors for units: SCr in mg/dL to μmol/L, ×88.4; uric acid in mg/dL to μmol/L, ×59.48; glucose in mg/dL to mmol/L, ×0.5551.
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; SCr, serum creatinine; UACR, urine albumin-creatinine ratio; UPCR, urine total protein–creatinine ratio.
Percentage of differences in geometric mean UPCRs or UACRs associated with the indicated increases in the listed predictor variables in multivariable analysis.
Association Between Urine Total Protein Excretion and BMI
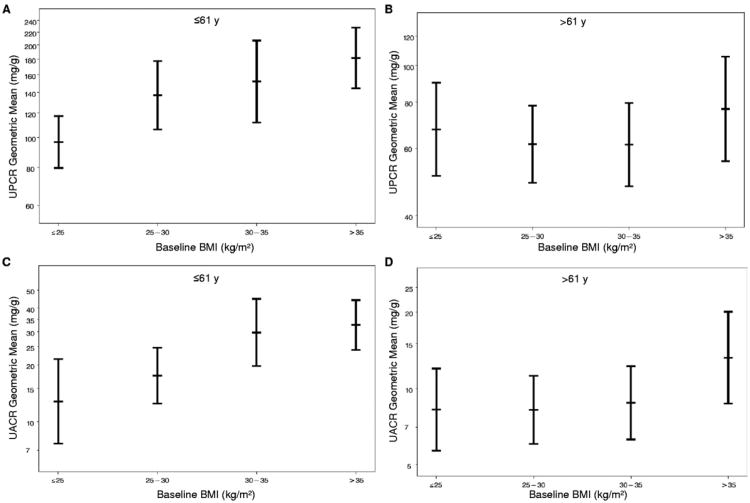
Approximately 50% of the study population had a BMI within the obese range (>30 kg/m2; Fig 2). As noted, both urine total protein–creatinine and urine albumin-creatinine ratios were associated independently with BMI in the full cohort in both regression models shown in Table 4. Under the expanded regression models, at a mean age of 60 years, each 2-kg/m2 increment in BMI was associated with a 3.5% (95% CI, 0.4-6.7; P = 0.03) increment in geometric mean urine total protein–creatinine ratio and a 5.6% (95% CI, 1.5-9.9; P = 0.007) increment in geometric mean urine albumin-creatinine ratio. Because of the interactions between BMI and age, these estimated effects, expressed as percentages of increase in geometric mean levels per 2 kg/m2, increased by 4.9% and 6.3% for each decade younger than 60 years for urine total protein–creatinine and urine albumin-creatinine ratios, respectively.
Figure 2.
Relationships between body mass index (BMI) and (A, B) geometric mean urine total protein–creatinine ratio (UPCR) and (C, D) urine albumin-creatinine ratio (UACR) in patients (A, C) 61 years or younger and (B, D) older than 61 years. Shown are adjusted geometric mean values and 95% confidence intervals, controlling for age, sex, blood pressure, serum creatinine level, serum uric acid level, and fasting serum glucose level.
As shown in Fig 2A and C, in patients younger than the median age of 61 years, there was a graded increase in adjusted geometric mean urine total protein–creatinine and urine albumin-creatinine ratios with increasing BMI after controlling for the other baseline factors listed in the expanded regression model shown in Table 4. This relationship was not apparent for the subgroup older than 61 years (Fig 2B and D).
Discussion
The principal new finding in this study is the independent association between BMI and urine total protein and urine albumin excretion in African Americans with hypertensive nephrosclero-sis. We found that BMI was related independently to both urine total protein–creatinine and albumin-creatinine ratios, and that higher urine total protein–creatinine and urine albumin-creatinine ratios were observed in those with the highest BMI. This association was independent of traditional factors previously observed or hypothesized to be related to proteinuria, including BP, level of kidney function, glycemia, and hyperuricemia. In addition, we found that this association was particularly evident in individuals younger than 61 years. This finding raises the possibility that obesity is a risk factor for proteinuria and albuminuria in hypertensive nephrosclerosis and may have a role in the development and progression of kidney disease, particularly in younger patients.
Recent studies suggest that people with metabolic syndrome are at higher risk of nephropathy. Cross-sectional analyses of NHANES III (Third National Health and Nutrition Examination Survey) data identified an association between metabolic syndrome and BMI and risk of CKD.1,2 Also, the Framingham investigators reported an independent association between BMI and pro–teinuria.13 In contrast, longitudinal data from the CHS (Cardiovascular Health Study) and the ARIC (Atherosclerosis Risk in Communities) Study indicated that waist-to-hip ratio, but not BMI, was associated with incident CKD.14 A weakness of all the mentioned studies was the use of urine dipstick or spot urine samples to detect or quantify total protein or albumin. In contrast, our study used 24-hour urine samples to measure urine total protein, albumin, and creatinine excretion and calculate urine total protein–creatinine and urine albumin-creatinine ratios.
In the AASK, we previously reported a high prevalence of metabolic syndrome and that metabolic syndrome was associated with proteinuria and progression of kidney disease in African Americans with established CKD. However, after controlling for proteinuria, metabolic syndrome was not associated independently with CKD progression.15 In the present study, we evaluated a population of individuals with known CKD to identify factors that may be associated with proteinuria, a known predictor of kidney disease progression in hypertensive nephrosclerosis. We found that after controlling for level of kidney function and other components of metabolic syndrome (glucose, triglyceride, and HDL cholesterol levels and BP), increased BMI was associated strongly with both albuminuria and proteinuria. We also found a significant interaction between BMI, proteinuria, and age, such that the relationship between BMI and protein–uria was stronger in younger compared with older participants. The precise explanation for this interaction is not clear. Both increased and decreased BMI have been associated with incident CKD in Asian population-based studies.16 In the US population, higher BMI has been associated with increased risk of end-stage renal disease independent of age.2 It is of interest that increased BMI is associated independently with proteinuria in younger individuals in Japan.17 We speculate that the stronger association between BMI and proteinuria in younger participants in the AASK cohort may be caused by differences in adipokine secretion and/or visceral body fat, the latter having a stronger association with albuminuria.18 Additional studies are needed to better understand the important interaction between age and BMI on proteinuria shown in our analysis.
Obesity has been associated with heavy pro-teinuria in those with focal sclerosis and glomerulomegaly.19-21 However, apart from a preliminary report suggesting that overweight habitus is an independent risk factor for proteinuria in kidney transplant recipients, we are unaware of reports of obesity as a risk factor for proteinuria and albuminuria in the general population or patients with CKD.19 We found a graded increase in the magnitude of urine total protein– creatinine and urine albumin-creatinine ratios and BMI (Fig 2). In addition, we found an independent association between albuminuria and BMI when BMI was evaluated as a continuous variable (Table 4).
Possible mechanisms by which obesity could cause proteinuria include alteration in podocyte structure or function, glomerular capillary hypertension, and adipocyte-derived cytokines. The latter have been purported to increase in glomerular capillary permeability to proteins and enhance renal fibrosis.22-24 Recent studies suggest that adipokines, such as adiponectin, may increase glomerular permeability to plasma proteins, leading to proteinuria.3,4 In addition, obesity has been associated with transforming growth factor β, a cytokine that contributes to proteinuria, renal fibrosis, and progressive kidney disease.25
Our study describes the association between BMI and proteinuria; however, it cannot establish a causal link between them. Still, in separate models, we assessed the impact of covariates that could be in the causal pathway for both obesity and proteinuria, including glucose, uric acid, and BP. Hypertension, hyperglycemia, and insulin resistance are associated with obesity and albuminuria.26-32 In addition, in patients with diabetes, hyperuricemia is associated with metabolic syndrome, albuminuria, and uric acid stone formation.33-35 After taking these factors into account, we found that BMI remained associated independently with both proteinuria and albuminuria in our study population.
Limitations of our study include that the analysis was cross-sectional in nature and involved a single measurement of urine total protein and albumin. Because urine total protein can vary from day to day, repeated measurements may provide better precision for assessing the relationships between proteinuria and factors considered in this study. However, prior reports of analyses of proteinuria and outcomes in the AASK indicate that total protein–creatinine ratio is a strong predictor of end-stage renal disease.7,36 Study participants were selected on the basis of a clinical diagnosis of CKD with decreased GFR; therefore, the findings may not be generalizable to populations with hypertension and GFR in the reference range. In addition, because most study participants had very low levels of urine total protein and albumin, the data may be limited in applicability to the general population. In this regard, it will be important to further explore the nonalbumin component of urine total proteins to determine whether better protein biomarkers of kidney disease prediction and progression can be identified, particularly in those with kidney disease that progresses to end stage. Finally, we did not measure waist circumference or waist-to-hip ratio in our study, a potential independent risk factor for CKD, as discussed.
In conclusion, we found that BMI was associated independently with urine total protein–creatinine and albumin-creatinine ratios in African Americans with hypertensive nephrosclerosis, particularly in younger patients. An important observation is that obese individuals (BMI >30 kg/m2) and especially those with BMI >35 kg/m2 have higher urine total protein and albumin excretion rates. This finding raises the possibility that obesity may be causally related to kidney injury and could represent a modifiable risk factor for the development and progression of CKD. The association between obesity and proteinuria and albuminuria requires further investigation.
Acknowledgments
The AASK Collaborative Group deeply appreciates the impressive and sustained commitment of AASK participants and staff.
The AASK Collaborative Group is composed of the following individuals, listed by center, who participated in the conduct of AASK. Case Western Reserve University: Jackson T. Wright Jr, MD, PhD (Principal Investigator), Mahboob Rahman, MD (Study Coordinator), Renee Dancie, CMA, Louise Strauss, RN; Emory University: Janice Lea, MD (Principal Investigator), Beth Wilkening, PA-C (Study Coordinator), Arlene Chapman, MD, Diane Watkins, MA; Harbor-UCLA Medical Center: Joel D. Kopple, MD (Principal Investigator), Linda Miladinovich, RN (Study Coordinator), Jooree Choi, MD, Patricia Oleskie, Connie Secules; Harlem Hospital Center: Velvie Pogue, MD (Principal Investigator), Donna Dowie, MD (Study Coordinator), Herman Anderson, MD, Leroy Herbert, MD, Robeta Locko, MD, Hazeline Nurse, MD, Jen-Tse Cheng, MD, Fred Darkwa, Victoria Dowdy, RN, Beverly Nicholas; Howard University: Otelio Randall, MD (Principal Investigator), Tamrat Retta, MD, PhD, Shichen Xu (Study Coordinator), MD, Mulueme-bet Ketete, MD, Debra Ordor, RN, Carl Tilghman, RN; Johns Hopkins University: Edgar Miller, MD, PhD (Principal Investigator), Brad Astor, PhD, MPH, MS, Charalett Diggs, RN (Study Coordinator), Jeanne Charleston, RN, Charles Harris, Thomas Shields, BS, Lawrence Appel, MD, MPH (Steering Committee Chair); Charles R. Drew University: Keith Norris, MD (Principal Investigator), David Martins, MD, Melba Miller, RN (Study Coordinator), Holly Howell, BA, Laurice Pitts, LVN; Medical University of South Carolina: DeAnna Cheek, MD (Principal Investigator), Deborah Brooks, MSN, RN (Study Coordinator); Me-harry Medical College: Marquetta Faulkner, MD (Principal Investigator), Olufemi Adeyele, MD, Karen Phillips, RN (Study Coordinator), Ginger Sanford, RN, Cynthia Weaver, MT; Morehouse School of Medicine: William Cleveland, MD (Principal Investigator), Kimberly Chapman, BS, Winifred Smith, MPH (Study Coordinator), Sherald Glover; Mount Sinai School of Medicine and University of Massachusetts: Robert Phillips, MD, PhD (Principal Investigator), Michael Lipkowitz, MD, Mohammed Rafey, MD, Avril Gabriel, RN, MPA (Study Coordinator), Eileen Condren, Natasha Coke; Ohio State University: Lee Hebert, MD (Principal Investigator), Ganesh Shidham, MD, Leena Hire-math, PhD (Study Coordinator), Stephanie Justice, RN; University of Chicago: George Bakris, MD (Principal Investigator), James Lash, MD, Linda Fondren, RN, BSN (Study Coordinator), Louise Bagnuolo, RN, NP, Janet Cohan, RN, MSN, Anne Frydrych, RN, MSN; University of Alabama, Birmingham: Stephen Rostand, MD (Principal Investigator), Denyse Thornley-Brown, MD, Beverly Key, RN (Study Coordinator); University of California, San Diego: Francis B. Gabbai, MD (Principal Investigator), Daniel T. O'Connor, MD, Brenda Thomas, LVN (Study Coordinator); University of Florida: C. Craig Tisher, MD (Principal Investigator), Geraldine Bichier, MD, Cipriano Sarmiento, RN (Study Coordinator), Amado Diaz, RN, Carol Gordon; University of Miami: Gabriel Contreras, MD (Principal Investigator), Jacques Bourgoignie, MD, Dollie Florence-Green, MD, Jorge Junco (Study Coordinator), Jacqueline Vassallo; University of Michigan: Kenneth Jamerson, MD (Principal Investigator), Akinlou Ojo, MD, Tonya Corbin, MD, Denise Cornish-Zirker, RN, ADN (Study Coordinator), Tanya Graham, MA, Wendy Bloembergen, MD; University of Southern California: Shaul Massry, MD (Principal Investigator), Miroslav Smogorzewski, MD; Annie Richardson, LVN (Study Coordinator), Laurice Pitts, LVN; University of Texas Southwestern Medical Center, Dallas: Robert Toto, MD (Principal Investigator), Gail Peterson, MD, Rames Saxena, MD, PhD, Tammy Lightfoot, RN (Study Coordinator), Sherry-Ann Blackstone, RN, Carlos Loreto, RN; Vanderbilt University: Julie Lewis, MD (Principal Investigator), Gerald Schulman, MD, Mo Sika, PhD (Study Coordinator), Sandy McLeroy, MS, RD; National Institute of Diabetes and Digestive and Kidney Diseases: Lawrence. Y. Agodoa, MD, Josephine. P. Briggs, MD, John. W. Kusek, PhD; Cleveland Clinic Foundation (Data Coordinating Center): Jennifer Gassman, PhD, Gerald Beck, PhD, Tom Greene, PhD, Bo Hu, PhD, Karen Brittain (Study Coordinator), Susan Sherer, BS, Laurie Tua-son, MS, Cynthia Kendrick, BS, Sharon Bi, MCIS, Harvey Litowitz, MS, Xianyou Liu, MCIC, Xuelei Wang, MS, Kimberly Wiggins, AAB, Cheryl A. Tatum, Nancy Patterson; Central Biochemistry Laboratory: Frederick Van Lente, PhD, Joan Waletzky, MS, Cathy O'Laughlin, MLT (ASCP), LaChauna Burton, BS.
Support: This project was supported by National Institutes of Health (NIH) grants 5-K24-DK002818-05, 5-U01-DK45386-15S1, and P30-DK079328 from the National Institute of Diabetes and Digestive and Kidney Diseases, grant M01-RR000633 from the NIH National Center for Research Resources, funding from the NIH National Center for Minority Health and Health Disparities, and King-Monarch Pharmaceuticals.
Footnotes
A list of the participants in the AASK Collaborative Research Group appears in the Acknowledgements.
Because the Editor-in-Chief recused himself from consideration of this manuscript, the Deputy Editor (Daniel E. Weiner, MD, MS) served as Acting Editor-in-Chief. Details of the journal's procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140(3):167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 3.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118(5):1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahima RS. Linking adiponectin to proteinuria. J Clin Invest. 2008;118(5):1619–1622. doi: 10.1172/JCI35655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American Study of Kidney Disease and Hypertension. Arch Intern Med. 2005;165(8):947–953. doi: 10.1001/archinte.165.8.947. [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 7.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 8.Appel LJ, Middleton J, Miller ER, III, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14(7 suppl 2):S166–172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 9.Jones CA, Francis ME, Eberhardt MS, et al. Microalbu-minuria in the US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39(3):445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 10.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16(1):180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 11.Kollerits B, Fliser D, Heid IM, Ritz E, Kronenberg F. Gender-specific association of adiponectin as a predictor of progression of chronic kidney disease: the Mild to Moderate Kidney Disease Study. Kidney Int. 2007;71(12):1279–1286. doi: 10.1038/sj.ki.5002191. [DOI] [PubMed] [Google Scholar]
- 12.Xu R, Zhang L, Zhang P, et al. Gender-specific reference value of urine albumin-creatinine ratio in healthy Chinese adults: results of the Beijing CKD survey. Clin Chim Acta. 2008;398(1-2):125–129. doi: 10.1016/j.cca.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Foster MC, Hwang SJ, Larson MG, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008;52(1):39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsayed EF, Sarnak MJ, Tighiouart H, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52(1):29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lea J, Cheek D, Thornley-Brown D, et al. Metabolic syndrome, proteinuria, and the risk of progressive CKD in hypertensive African Americans. Am J Kidney Dis. 2008;51(5):732–740. doi: 10.1053/j.ajkd.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez SP, McClellan W, Port FK, Hsu SI. Risk factors for proteinuria in a large, multiracial, southeast Asian population. J Am Soc Nephrol. 2002;13(7):1907–1917. doi: 10.1097/01.asn.0000018406.20282.c8. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita K, Yasuda G, Shouda M, Umemura S. Evaluation of renal function and proteinuria based on mass health examinations in young Japanese obese adults. Clin Exp Nephrol. 2009;13(4):316–324. doi: 10.1007/s10157-009-0164-8. [DOI] [PubMed] [Google Scholar]
- 18.Tamba S, Nakatsuji H, Kishida K, et al. Relationship between visceral fat accumulation and urinary albumin-creatinine ratio in middle-aged Japanese men. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2010.02.037. published online ahead of print March 4, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Chew-Wong A, Ron O, Ricalde G, Parra A, Romo L, Reyes-Acevedo R. Overweight in kidney transplant recipients is associated with an increased risk of proteinuria and hypertension [abstract] J Am Soc Nephrol. 2001;12(Program and Abstracts Issue):883A. [Google Scholar]
- 20.Fernandez-De-Castro J, Bernal M, Ayuzo C, Ramos F. Massive obesity and heavy proteinuria in a young patient. Report of a case [abstract] J Am Soc Nephrol. 2001;12(Program and Abstracts Issue):100A. [Google Scholar]
- 21.Chen HM, Li SJ, Chen HP, Wang QW, Li LS, Liu ZH. Obesity-related glomerulopathy in China: a case series of 90 patients. Am J Kidney Dis. 2008;52(1):58–65. doi: 10.1053/j.ajkd.2008.02.303. [DOI] [PubMed] [Google Scholar]
- 22.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12(6):1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 23.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2(3):550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Shahinfar S, Keane WF, et al. Importance of baseline distribution of proteinuria in renal outcomes trials: lessons from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) Study. J Am Soc Nephrol. 2005;16(6):1775–1780. doi: 10.1681/ASN.2004080632. [DOI] [PubMed] [Google Scholar]
- 25.Torun D, Ozelsancak R, Turan I, Micozkadioglu H, Sezer S, Ozdemir FN. The relationship between obesity and transforming growth factor beta on renal damage in essential hypertension. Int Heart J. 2007;48(6):733–741. doi: 10.1536/ihj.48.733. [DOI] [PubMed] [Google Scholar]
- 26.Hoehner CM, Greenlund KJ, Rith-Najarian S, Casper ML, McClellan WM. Association of the insulin resistance syndrome and microalbuminuria among nondiabetic native Americans. The Inter-Tribal Heart Project. J Am Soc Nephrol. 2002;13(6):1626–1634. doi: 10.1097/01.asn.0000015762.92814.85. [DOI] [PubMed] [Google Scholar]
- 27.Manco M, Ciampalini P, Devito R, Vania A, Cappa M, Nobili V. Albuminuria and insulin resistance in children with biopsy proven non-alcoholic fatty liver disease. Pediatr Nephrol. 2009;24(6):1211–1217. doi: 10.1007/s00467-009-1134-9. [DOI] [PubMed] [Google Scholar]
- 28.Mykkanen L, Zaccaro DJ, O'Leary DH, Howard G, Robbins DC, Haffner SM. Microalbuminuria and carotid artery intima-media thickness in nondiabetic and NIDDM subjects. The Insulin Resistance Atherosclerosis Study (IRAS) Stroke. 1997;28(9):1710–1716. doi: 10.1161/01.str.28.9.1710. [DOI] [PubMed] [Google Scholar]
- 29.Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55(5):1456–1462. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Zhang P, Wang F, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis. 2008;51(3):373–384. doi: 10.1053/j.ajkd.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Savage S, Nagel NJ, Estacio RO, Lukken N, Schrier RW. Clinical factors associated with urinary albumin excretion in type II diabetes. Am J Kidney Dis. 1995;25(6):836–844. doi: 10.1016/0272-6386(95)90565-0. [DOI] [PubMed] [Google Scholar]
- 32.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 33.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004;13(2):181–189. doi: 10.1097/00041552-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RJ, Segal MS, Srinivas T, et al. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16(7):1909–1919. doi: 10.1681/ASN.2005010063. [DOI] [PubMed] [Google Scholar]
- 35.Chen N, Wang W, Huang Y, et al. Community-based study on CKD subjects and the associated risk factors. Nephrol Dial Transplant. 2009;24(7):2117–2123. doi: 10.1093/ndt/gfn767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285(21):2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]