Abstract
The endocannabinoid ligand 2-arachidonoylglycerol (2-AG) is inactivated primarily by monoacylglycerol lipase (MAGL). We have shown recently that chronic treatments with MAGL inhibitor JZL184 produce antidepressant- and anxiolytic-like effects in a chronic unpredictable stress (CUS) model of depression in mice. However, the underlying mechanisms remain poorly understood. Adult hippocampal neurogenesis has been implicated in animal models of anxiety and depression and behavioral effects of antidepressants. We tested whether CUS and chronic JZL184 treatments affected adult neurogenesis and synaptic plasticity in the dentate gyrus (DG) of mouse hippocampus. We report that CUS induced depressive-like behaviors and decreased the number of bromodeoxyuridine (BrdU)-labeled neural progenitor cells and doublecortin-positive immature neurons in the DG, while chronic JZL184 treatments prevented these behavioral and cellular deficits. We also investigated the effects of CUS and chronic JZL184 on a form long-term potentiation (LTP) in the DG known to be neurogenesis-dependent. CUS impaired LTP induction, whereas chronic JZL184 treatments restored LTP in CUS-exposed mice. These results suggest that enhanced adult neurogenesis and long-term synaptic plasticity in the DG of the hippocampus might contribute to antidepressant- and anxiolytic-like behavioral effects of JZL184.
Keywords: chronic unpredictable stress, endocannabinoid, CB1 receptor, neurogenesis, depression
Introduction
Natural cannabis improves mood and ameliorates depressive symptoms in humans (Gruber et al., 1996). Synthetic cannabinoid receptor (CB1) agonists produce anxiolytic- and antidepressant-like effects in animals (Berrendero and Maldonado, 2002; Jiang et al., 2005; Patel and Hillard, 2006; Valjent et al., 2002). Clinical and laboratory observations over the last decade indicate that the endocannabinoid (eCB) system represents a promising target for pharmacotherapy of depression (Bambico and Gobbi, 2008; Hill et al., 2009; Hillard and Liu, 2013). The eCB system has at least two endogenous ligands, anandamide (AEA) and 2-arachidonoylglycerol (2-AG) that activate CB1 receptor (Di Marzo et al., 1998; Piomelli, 2003). Anandamide is hydrolyzed and inactivated by fatty acid amide hydrolase (FAAH), while 2-AG is hydrolyzed primarily by monoacylglycerol lipase (MAGL) (Blankman et al., 2007; Cravatt et al., 1996). FAAH and MAGL inhibitors amplify endogenous eCB activity with temporal and spatial fidelity and should be superior to direct CB1 agonists as therapeutic agents. FAAH and AEA transporter inhibitors produce antidepressant- and anxiolytic-like effects in rodents (Bortolato et al., 2007; Busquets-Garcia et al., 2011; Gobbi et al., 2005; Patel and Hillard, 2006). More recent studies indicate that MAGL inhibitor JZL184 induces anxiolytic-like effects in marble burying (Kinsey et al., 2011), novelty-suppressed feeding (Sumislawski et al., 2011) and elevated zero/plus maze assays (Busquets-Garcia et al., 2011; Sciolino et al., 2011). Using chronic mild unpredictable stress (CUS) as an animal model for depression (Willner et al., 1987), we have shown recently that CUS led to impairment of 2-AG-mediated retrograde synaptic depression in the CA1 region of the hippocampus, while chronic JZL184 treatments prevented CUS-induced depressive-like behaviors via activation of CB1-mammalian target of rapamycin (mTOR) signaling (Zhong et al., 2014). However, the cellular mechanisms that mediate the antidepressant-like effects of MAGL inhibitor JZL184 remain poorly understood.
Adult hippocampal neurogenesis has been implicated in animal models of anxiety and depression and behavioral effects of antidepressants (Hanson et al., 2011; Sahay and Hen, 2007), although this issue remains controversial (Hanson et al., 2011). Adult neurogenesis in the dentate gyrus (DG) of the hippocampus is required for the antidepressant behavioral effects of antidepressants imipramine and fluoxetine (Hanson et al., 2011; Malberg et al., 2000; Sahay and Hen, 2007; Santarelli et al., 2003; Wang et al., 2008). The DG of the hippocampus expresses one of the highest levels of CB1 receptors in the brain (Katona et al., 2006; Monory et al., 2006). Adult neurogenesis in the DG of the hippocampus is impaired in CB1 knockout mice (Jin et al., 2004) and diacylglycerol lipase α (DAGLα) knock-out mice (Gao et al., 2010). DAGL hydrolyses diacylglycerol into 2-AG and is therefore responsible for 2-AG synthesis (Bisogno et al., 2003). Thus, 2-AG-mediated activation of CB1 receptors plays an important role in adult hippocampal neurogenesis. To investigate whether hippocampal neurogenesis is involved in the antidepressant-like effects of JZL184, we examined the effects of CUS and chronic JZL184 treatments on (1) the number of bromodeoxyuridine (BrdU)-labeled neural progenitor cells and doublecortin-positive (DCX+) immature neurons in the DG in control and CUS-exposed mice; (2) a form of long-term potentiation (LTP) in the DG known to be dependent on hippocampal neurogenesis (Saxe et al., 2006; Snyder et al., 2001; Wang et al., 2000). We report that CUS exposure decreased the number of BrdU+ and DCX+ cells and impairs LTP induction in the DG of the hippocampus and chronic JZL184 treatments prevented these deficits. The enhancement of hippocampal neurogenesis and synaptic plasticity might provide a putative mechanism for antidepressant-like behavioral effects of JZL184.
Materials and Methods
Animals
Male C57BL/6J mice (8-10 weeks of age) were purchased from the Jackson Laboratory. Animal maintenance and use were in accordance with protocols approved by the Institutional Animal Care and Use Committee of Medical College of Wisconsin.
CUS paradigm and drug treatment
After one week of initial habituation, mice were subjected to CUS for a total of 5 weeks as we have described (Zhong et al., 2014). The stressors included restraint (1 hour in a soft, flexible plastic cone, DecapiCone, Braintree Scientific, Inc.), inversion of day/night light cycle, cold (in a cold room at 4°C for 1 hour), 45° tilted cage (overnight), cage rotation (20 min), rat bedding (odor, 3 hours), wet bedding (250 ml water added into cage, overnight) and no bedding (overnight), low intensity stroboscopic illumination (10 Hz, overnight), food and water deprivation (overnight), overcrowding (overnight) (Koo and Duman, 2008; Wang et al., 2010; Willner et al., 1987) (Table 1). Unstressed controls were handled only for injections, cage changes and behavioral tests. At the beginning of the third week, CUS-exposed mice and time-matched control mice were given intraperitoneal (i.p.) injections of vehicle (18:1:1 saline:emulphor:ethanol) or JZL184 (8 mg/kg) every two days for 3 weeks before behavior tests. JZL184 was freshly dissolved in saline:emulphor:ethanol vehicle with vigorous sonication.
Table 1.
Experimental schedule for the chronic unpredictable stress (CUS) procedure in mice
| Week | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
|---|---|---|---|---|---|---|---|
| 1 | Cold Wet bedding |
Restraint No bedding |
Light inversion Cage tilt |
Cage rotation Strobe |
Cold Food & water deprivation |
Restraint Overcrowding |
Light inversion Wet bedding |
| 2 | Cold Cage tilt |
Cage rotation Food & water deprivation |
Restraint Wet bedding |
Rat bedding Strobe |
Light inversion No bedding |
Cage rotation Food & water deprivation |
Cold Wet bedding |
| 3 | Rat bedding Strobe |
Restraint Light inversion |
Cage rotation No bedding |
Light inversion Food & water deprivation |
Cold Wet bedding |
Cage tilt Strobe |
Light inversion Overcrowding |
| 4 | Cold No bedding |
Restraint Food & water deprivation |
Cage rotation Strobe |
Rat bedding Light inversion |
Cold Cage tilt |
Restraint Wet bedding |
Cage rotation No bedding |
| 5 | Cold Food & water deprivation |
Cage rotation Strobe |
Light inversion Wet bedding |
Cold Cage tilt |
Cage rotation No bedding |
Light inversion Overcrowding |
Restraint Cage tilt |
Behavior
Open field test (OPT)
Mice were placed individually in one corner of an open field box (50 cm length × 45 cm wide × 30 cm deep) and allowed to freely explore the arena during a 15 min test session. Locomotor activities were recorded using an automated video-tracking system (Mobile Datum, Shanghai, China). Total distance traveled and time spent in the center of the open field during the first 5 min were analyzed. Time in center was defined as the amount of time that was spent in the central 25 cm × 22.5 cm area of the open field.
Forced swim test (FST)
Mice were placed individually into glass cylinders (13 cm diameter, 25 cm tall) filled to a depth of 18 cm with water (25 ± 1°C) for 6 minutes. The time spent immobile during the last 4 min was scored by an observer blind to treatment conditions. Immobility was defined as the cessation of all movements (e.g., climbing, swimming) except those necessary for the mouse to keep its head above water (i.e., floating).
Novelty-suppressed feeding (NSF)
NSF was carried out similar to a published protocol (Santarelli et al., 2003). After fasting for 24 hours, mice were placed individually in one corner of a plastic box (45 cm long × 35 cm wide × 20 cm deep) where five food pellets (regular chow) was placed on a piece of white filter paper (11 cm in diameter) in the center of the box. The latency to feed in the novel environment was measured. Feeding was defined as biting the food with the use of forepaws, not simply sniffing or touching the food. Following the test, mice were transferred to the home cage, and the latency to feed in the home cage was measured.
Immunohistochemistry
Following behavioral tests, mice were administrated with BrdU (4 × 75 mg/kg every 2 hours, Sigma, i.p). Twenty-four hours after BrdU injection, mice were killed and transcardially perfused with 4% paraformaldehyde in 0.1 M PBS supplemented with 4% sucrose. The brains were postfixed overnight in 4% paraformaldehyde and stored in 30% sucrose at 4°C. Serial sections were cut through the entire hippocampus at 40 μm thickness with a cryostat and stored in cryoprotectant solution containing 25% ethylene glycol and 25% glycerol in 0.05 M PBS sodium phosphate buffer (pH 7.4) at 4°C until use.
BrdU staining was based on a published protocol with minor modifications (Wojtowicz and Kee, 2006). Sections were treated with 1% hydrogen peroxide in PBS for 15 min to remove the endogenous peroxidase. DNA denaturation was conducted by incubation with 2 M HCl for 30 minutes at 37 °C. Sections were washed in 0.1 M borate buffer (pH = 8.4) for 5 min and in 0.1 PBS for 5 min. After blocking with 1% BSA-5% goat serum-0.3% Triton X-100 in 0.1 M PBS, sections were incubated with goat anti-BrdU antibody (1:400, Sigma) for 48 hours and then with secondary antibody (1:100; rabbit anti-goat IgG-HRP; Chemicon) for 4 hours. For DCX-staining, sections were treated with 1% hydrogen peroxide and 20% methanol in PBS for 15min. After rinsing in PBS and incubated with blocking solution (3% donkey serum-0.3% Triton X-100 in PBS) for 30 minutes, sections were incubated with guinea pig anti-DCX antibody (1:500; Chemicon) for 48 hour at 4 °C, and then with secondary antibody (1:500; donkey anti-guinea pig IgG-HRP; Chemicon) for 4 hours. All sections were washed in PBS and then in 0.1 M ammonium phosphate buffer (APB, pH 7.0), BrdU- or DCX-immunoreactivity was detected with 0.05% 3,3′-diaminobenzidine (DAB)-0.004% H2O2 in APB.
BrdU- and DCX-positive cells were quantified based on unbiased stereology protocols (Eisch et al., 2000; Koo and Duman, 2008; Wang et al., 2008). Every sixth section throughout the hippocampus was examined. Sections were coded to ensure blind analysis. All BrdU- or DCX-labeled cells in the subgranular zone and the granule cell layer were counted in each section. The number of BrdU-or DCX-labeled cells per section was counted and multiplied by 6 to yield the total number of cells per hippocampus.
Slice preparation and electrophysiology
Mice were anaesthetized by isoflurane inhalation and decapitated. Transverse hippocampal slices (350 μm) were prepared based on methods described in our previous study (Pan et al., 2008). Slices were prepared at 4-6°C in artificial cerebrospinal fluid (ACSF) containing (in mM): 125 NaCl, 3 KCl, 2.5 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose. The ACSF was saturated with 95% O2 and 5% CO2. Slices were recovered for at least 1.5 hour at room temperature before recordings. Field excitatory postsynaptic potentials (fEPSPs) were recorded in the medial molecular layer of the DG, and a bipolar tungsten stimulation electrode (WPI) was placed in the in the middle region of the molecular layer to stimulate the medial perforant pathway (MPP) every 30 s. The stimulation of the MPP was confirmed by paired-pulse depression (PPD, interval 50 ms) of fEPSPs (Wang et al., 2008). The recording pipettes were filled with the 2 M NaCl (1-2 MΩ) and their distance to the stimulating electrode were kept constant (~300 μm). Input/output (I/O) curves were generated using incremental stimulus intensities. For LTP experiments, stable baseline fEPSPs were recorded for 20 min at an intensity that induced ~30% of the maximal evoked response. LTP was induced by high frequency stimulation consisting of four trains of 100 pulses at 100 Hz with 15 s inter-train intervals. The recordings were made using Multiclamp 700B amplifiers. Data acquisition and analysis were performed using digitizer DigiData 1440A and analysis software pClamp 10 (Molecular Devices). Signals were filtered at 2 kHz and sampled at 10 kHz. All recordings were performed at 32 ± 1°C by using an automatic temperature controller (Warner Instrument, Hamden, CT).
Data Analysis and Statistics
All results are expressed as mean ± SEM. Behavioral test results were analyzed with two-way ANOVA followed by Tukey’s post hoc analysis. The F values and group and experimental degrees of freedom are included in the Results. fEPSP slope was normalized to the baseline. LTP (%) was calculated as follows: 100 × [mean fEPSP slope during the final 10 min of recording/ mean baseline fEPSP slope]. Results were considered to be significant at p < 0.05.
Results
Chronic JZL184 treatments produced antidepressant-like behavioral effects
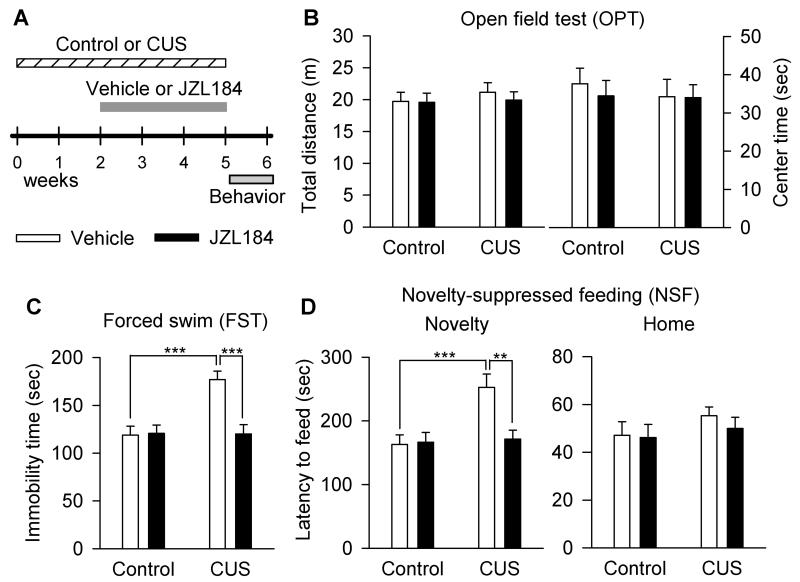
We examined the effect of chronic JZL184 treatments on depression-related behavior in a CUS model of depression. Mice were exposed to CUS for a total of 5 weeks. At the beginning of the third week, CUS-exposed mice and time-matched control mice were given i.p. injections of vehicle or JZL184 (8 mg/kg) every two days for a total of 3 weeks (see Materials and Methods). The dose and treatment time of JZL184 were chosen based on previous studies showing that JZL184 irreversibly inhibits MAGL and produces at least two-fold increase in 2-AG levels in the brain at a dose of 8 mg/kg when dissolved in the vehicle used in this study (Kinsey et al., 2013; Long et al., 2009a; Long et al., 2009b; Sumislawski et al., 2011). Repeated administration of JZL184 at this low-dose does not induce apparent CB1 receptor desensitization and functional tolerance (Kinsey et al., 2013). The time course of stress exposure, drug treatment and behavioral tests is shown in Fig. 1A.
Figure 1.
Chronic JZL184 treatments produced antidepressant-like effects in mice in a CUS model of depression. (A) Time course of the CUS exposure, JZL184 treatment and behavioral tests. (B) Neither CUS nor chronic JZL184 treatments affected the total distance travelled (p > 0.05) and on center time (p > 0.05) in the OPT. (C) CUS significantly increased the immobility time in the FST (***p < 0.001); JZL184 treatment increased the immobility time in CUS-exposed mice (***p < 0.001), but not in control mice. (D) CUS induced a significant increase in the latency to feed in the novel environment in the NSF test (***p < 0.001), while JZL184 treatment decreased the latency to feed in the NSF (**p < 0.01). Neither CUS nor JZL184 treatments affected the latency to feed in the home cage in the NSF test. N = 12 mice/group.
We used an open field test (OPT) to determine whether CUS-exposed mice exhibited abnormalities in general locomotor activity and anxiety-related behavior. Reduced activity in the center of an open field reflects anxiety and depression level in rodents (El Yacoubi et al., 2003). However, neither CUS nor chronic JZL184 administration had any significant effect on the total distance travelled (CUS: F1,44 = 0.38, p < 0.05; JZL184 treatment: F1,44 = 0.23, p > 0.05; CUS × JZL184 treatment: F1,44 = 0.14, p > 0.05) and on center time (CUS: F1,44 = 0.22, p > 0.05; JZL184 treatment: F1,44 = 0.19, p > 0.05; CUS × JZL184 treatment: F1,44 = 0.13, p > 0.05; Fig. 1B).
Forced swim test (FST) is a common behavioral test for detecting depression-like behaviors and screening antidepressants (Porsolt et al., 1977). We examined the effect of chronic JZL184 treatments on the immobility time in the FST in control and CUS-exposed mice. CUS and JZL184 treatment significantly changed the immobility time in the FST (CUS: F1,44 = 10.00, p < 0.01; JZL184 treatment: F1,44 = 9.15, p < 0.01; CUS × JZL184 treatment: F1,44 = 10.40, p < 0.01; Fig. 1C). Post-hoc analysis of these data indicate that JZL184 significantly decreased the immobility time in CUS-exposed mice (p < 0.001), but not in control mice (p > 0.05).
The novelty-suppressed feeding (NSF) test is another behavior paradigm that measures depression and anxiety. In NSF test, a fasting mouse faces the choice between eating food pellets and avoiding the novel environment in an open field. An increase in the latency to feed in the novel environment indicates increased anxiety levels (Santarelli et al., 2003). CUS and JZL184 treatments significantly changed the latency to feed in the novel environment in the NSF test (CUS: F1,44 = 8.29, p < 0.01; JZL184 treatment: F1,44 = 5.60, p < 0.05; CUS × JZL184 treatment: F1,44 = 6.63, p < 0.05; Fig. 1D). Post-hoc analysis indicates that JZL184 significantly decreased the latency to feed in CUS-exposed mice (p < 0.01), but not in control mice (p > 0.05). In contrast, neither CUS nor JZL184 treatments affected the latency to feed in the home cage (CUS: F1,44 = 1.46, p > 0.05; JZL184 treatment: F1,44 = 0.40, p > 0.05; CUS × JZL184 treatment: F1,44 = 0.20, p > 0.05; Fig. 1D). These results indicate that chronic JZL184 treatments prevented CUS-induced depressive-like behaviors.
Chronic JZL184 treatments prevented CUS-induced decrease in the number of BrdU+ and DCX+ cells in the DG
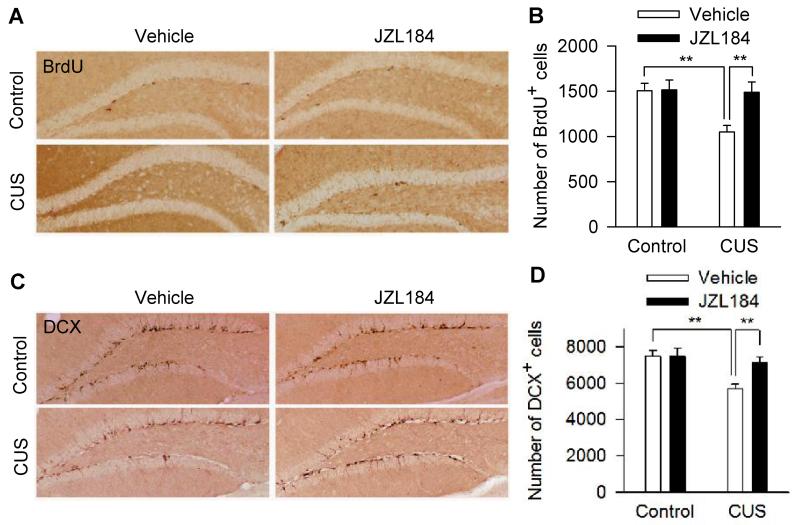
To investigate whether hippocampal neurogenesis is involved in antidepressant-like behavioral effects of JZL184, we examined the effects of chronic JZL184 on the number of cells labeled with BrdU, a marker for neural progenitor cells (Malberg et al., 2000; Takahashi et al., 1992), and cells labeled with DCX, a marker for immature neurons (Brown et al., 2003; Kempermann et al., 2003). A modified stereology protocol was used to count the number of BrdU+ and DCX+ cells throughout the subgranular zone and hilus of the DG (Malberg et al., 2000; Wang et al., 2008; Wojtowicz and Kee, 2006). CUS significantly decreased the number of BrdU+ cells in the DG, while chronic JZL184 increased the number of BrdU+ cells in CUS group, but not in control group (CUS: F1,18 = 6.167, p <0.05; JZL184: F1,18 = 5.361, p < 0.05; CUS × JZL184: F1,18 = 4.877, p < 0.05; control-vehicle vs. CUS-vehicle, p < 0.01; CUS-vehicle vs. CUS-JZL184, p < 0.01; post hoc analysis; Fig. 2A,B). Similarly, CUS decreased the number of DCX+ cells in the DG, while chronic JZL184 increased the number of DCX+ cells in CUS group, but not in control group (CUS: F1,18 = 9.425, p < 0.01; JZL184: F1,18 = 4.529, p < 0.05; CUS × JZL184: F1,18 = 4.494, p < 0.05; control-vehicle vs. CUS-vehicle, p < 0.01; CUS-vehicle vs. CUS-JZL184, p < 0.01; post hoc analysis; Fig. 2C,D). Together, these results indicate that chronic JZL184 treatments prevented CUS-induced impairment of hippocampal neurogenesis.
Figure 2.
Chronic JZL184 treatments prevented CUS-induced decreases in BrdU+ and DCX+ cells in the DG of the hippocampus. (A,C) Representative micrographs of BrdU+ cells (A) and DCX+ cells (C) in the subgranular zone and the granule cell layer of the DG in vehicle or JZL184-treated control and CUS mice (N = 5-6 mice/group). Scale bar, 100 μm. (B,D) Summary data show that CUS significantly decreased the number of BrdU+ cells (B) and DCX+ cells (D) in the DG, which was prevented by chronic JZL184 treatments (**p < 0.01; N = 5-6 mice/group).
Although the number of BrdU+ cells in the DG was reduced in CB1 knockout mice, CB1 antagonists rimonabant and AM 251 paradoxically increased the number of BrdU+ cells in these knockout mice, and these effects were mediated by the vanilloid receptor (Jin et al., 2004). These findings preclude the examination of whether the effects of chronic JZL184 on hippocampal neurogenesis were blocked by the CB1 receptor antagonists.
The effects of CUS and chronic JZL184 treatments on basal synaptic properties and LTP in the DG
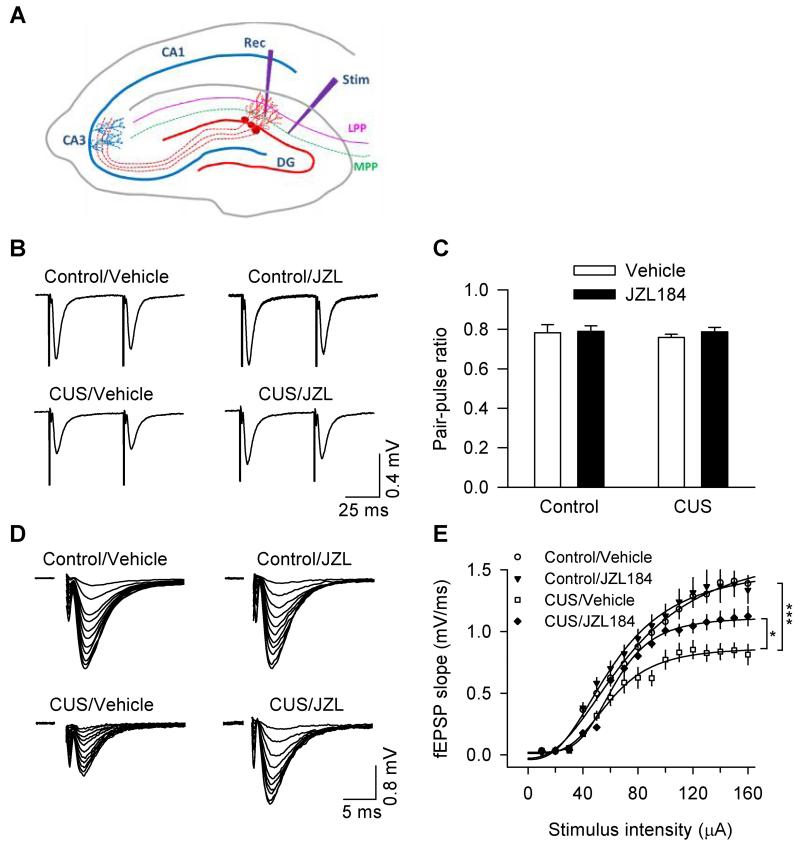
Newborn neurons are functionally integrated into the local neuronal network and participate in synaptic transmission and plasticity (Ming and Song, 2005; van Praag et al., 2002). Having shown that CUS and chronic JZL184 altered the number of newborn neurons, we next examined the impact of these alterations on basic synaptic properties and long-term synaptic plasticity (LTP) in the medial perforant path (MPP) of the DG. Hippocampal slices were prepared from vehicle- or JZL184-treated control and CUS-exposed mice. Field excitatory postsynaptic potentials (fEPSPs) were recorded in the medial molecular layer of the DG, while the MPP was stimulated at 0.033 Hz (Fig. 3A). The stimulation of the MPP was confirmed by paired-pulse depression (PPD) of fEPSPs at 50 ms inter-pulse intervals (Fig. 3B), while stimulation of the LPP showed paired-pulse facilitation of fEPSPs (not shown) (McNaughton, 1980). We first compared the paired-pulse ratio (PPR), which provides a measure of the probability of transmitter release. Synapses with high probability of transmitter release often display low PPR, whereas synapses with low probability of transmitter release display high PPR (Zucker and Regehr, 2002). Neither CUS nor chronic JZL184 treatments had significant effects on the PPR (CUS: F1,28 = 0.228, p > 0.05; JZL184: F1,28 = 0.439, p > 0.05; CUS × JZL184: F1,28 = 0.148, p > 0.05; Fig. 3C). Thus, CUS and chronic JZL184 do not significantly alter the probability of transmitter release of the MPP-granule cell synapses.
Figure 3.
Effects of CUS and chronic JZL184 treatments on basal synaptic properties in the DG of the hippocampus. (A) Scheme of the arrangement of electrodes for stimulating (Stim) and recording (Rec). MPP, the medial perforant path; LPP, the lateral perforant path. (B) Sample recordings of fEPSPs evoked by stimulation of the MPP exhibited paired-pulse depression (B). (C) Summarized results showed that neither CUS nor chronic JZL184 treatments altered paired-pulse ratio (PPR) of fEPSPs (n = 7-9 slices/N = 3-4 mice; p > 0.05). (D) Representative fEPSPs evoked with increasing stimulus intensities were shown for vehicle- and JZL184-treated control and CUS-exposed mice. (E) CUS significantly decreased the slope of input-output (I/O) curves (n = 9-10 slices/N = 4-5 mice; *p < 0.05) and chronic JZL184 treatments partially recovered the effect of CUS on the I/O curves (n = 6-8 slices/N = 3-4 mice; *p < 0.05).
We next examined the input–output (I/O) relationships for fEPSPs by stimulating the MPP with incremental intensities. CUS and chronic JZL184 treatments significantly altered the slope of I/O curves (CUS: F1,29 = 32.894, p < 0.001; JZL184: F1,29 = 7.527, p = 0.01; CUS × JZL184: F1,29 = 7.417, p < 0.05; Fig. 3D,E). Post-hoc analysis indicates that CUS significantly decreased the slope of I/O curves (p < 0.05); JZL184 increased the slope of I/O curves in CUS-exposed mice (p < 0.05), but not in control mice (p > 0.05). These results indicate that CUS decreased basal synaptic strength in the DG, which was partially prevented by chronic JZL184 treatments.
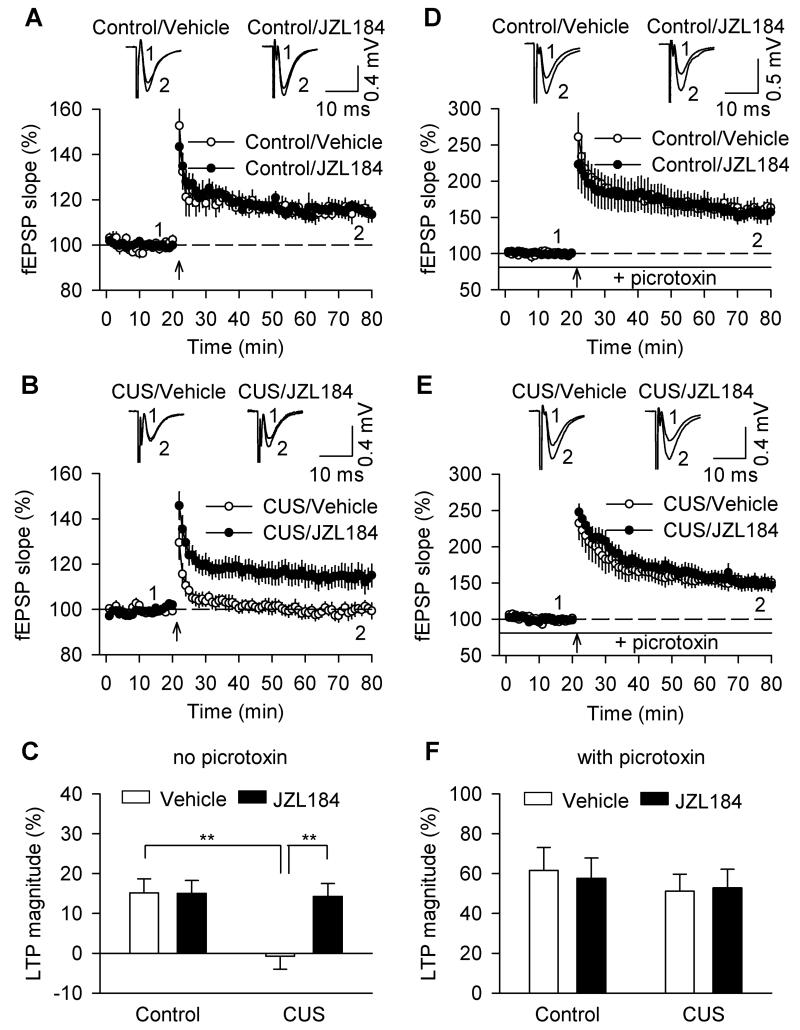
A modest LTP can be induced in the MPP-granule cell synapses in the absence of GABAA receptor antagonists, and this form of LTP is dependent on hippocampal neurogenesis since such LTP was blocked following X-irradiation of the hippocampus (Saxe et al., 2006; Snyder et al., 2001; Wang et al., 2008). We examined whether CUS- and chronic JZL184-induced alterations of hippocampal neurogenesis affected LTP induction. Tetanic stimulation of the MPP was applied to induce LTP and GABAA receptor blocker picrotoxin was omitted from the ACSF. CUS and chronic JZL184 treatments had significant effects on LTP induction (CUS: F1,29 = 32.894, p < 0.001; JZL184: F1,29 = 7.527, p = 0.01; CUS × JZL184: F1,29 = 7.417, p < 0.05; Fig. 4A,B,C). Post-hoc analysis indicates that LTP was impaired in slices prepared from CUS-exposed mice (p < 0.01), chronic JZL184 did not affect LTP in control mice (p > 0.05), but prevented CUS-induced impairment of LTP induction (p < 0.01).
Figure 4.
Effects of CUS and chronic JZL184 treatments on LTP in the DG. (A) In hippocampal slices from control mice, chronic JZL184 treatments did not significantly alter LTP compared with vehicle treatment (n = 7-8 slices/N = 4-5 mice). Sample fEPSPs are shown on the top. (B) In slices from CUS-exposed mice, chronic JZL184 treatments prevented CUS-induced impairment of LTP (n = 8-8 slices/N = 5-6 mice). (C) ANOVA performed on the last 10 min of LTP recording showed that CUS impaired LTP induction, and this effect was prevented by chronic JZL184 treatments (n = 7-8 slices/N = 4-5 mice; **p < 0.01). (D,E) In the presence of picrotoxin (50 μM), robust LTP was induced in slices from vehicle and JZL184-treated control (D) and CUS mice (E). (F) Summarized results showed that neither CUS nor JZL184 had significant effect on LTP induction (n = 7-8 slices/N = 4-5 mice; p > 0.05).
In the presence of picrotoxin that blocked GABAA receptors, stimulation of MPP induces robust LTP that is independent of hippocampal neurogenesis (Saxe et al., 2006; Snyder et al., 2001; Wang et al., 2008). In the continuous presence of picrotoxin (50 μM) in the ACSF, robust LTP was induced in slices from vehicle and JZL184-treated control and CUS mice (Fig. 4D,E). Neither CUS nor chronic JZL184 significantly altered the magnitude of LTP (CUS: F1,25 = 0.599, p >0.05; JZL184: F1,25 = 0.014, p >0.05; CUS × JZL184: F1,25 = 0.081, p > 0.05; Fig. 4F).
Discussion
In this study, we showed that CUS impaired hippocampal neurogenesis and LTP in the DG and induced depressive-like behaviors, while chronic in vivo administration of MAGL inhibitor JZL184 prevented CUS-induced cellular and behavioral deficits. Enhancement of hippocampal neurogenesis and synaptic plasticity might contribute to antidepressant-like behavioral effects of JZL184.
CUS exhibits high predictive, face and construct validity as an animal model of depression (Willner, 2005). Consistent with previous studies (Zhong et al., 2014), we have shown that chronic JZL184 treatments prevented the CUS-induced increase in the immobility time in the FST and the latency to feed in the novel environment in the NSF test but had no significant effects on these parameters in control mice. These results suggest that blocking 2-AG hydrolysis with MAGL inhibitor JZL184 prevented CUS-induced depressive-like behaviors. The present study investigated cellular mechanisms for antidepressant-like behavioral effects of chronic JZL184 treatments. Both pharmacological and non-pharmacological antidepressant treatments (e.g. electroconvulsive shock) increased adult hippocampal neurogenesis (David et al., 2009; Malberg et al., 2000; Santarelli et al., 2003). However, there are also studies showing that antidepressants at behaviorally active or clinically relevant doses did not affect hippocampal neurogenesis (Hanson et al., 2011) and X-ray irradiation that killed new born neurons did not block behavioral effects of antidepressants (David et al., 2009; Holick et al., 2008). Thus, antidepressant behavioral effects can be either dependent or independent of hippocampal neurogenesis. We showed that CUS decreased the number of BrdU+ and DCX+ cells in the DG, and these effects were prevented by chronic JZL184 treatments. Due to technical constraints, we did not test whether X-ray irradiation of hippocampus blocked antidepressant-like behavioral effects of chronic JZL184 treatments. Accumulating evidence indicates that CB1 receptors play an important role in adult hippocampal neurogenesis. First, the DG of the hippocampus expresses one of the highest levels of CB1 receptors in the brain (Katona et al., 2006; Monory et al., 2006). Second, hippocampal neurogenesis is impaired in CB1 knockout mice (Jin et al., 2004). Third, chronic administration of direct-acting CB1 agonist HU210 increased the number of BrdU+ cells in the DG in rats and produced antidepressant-like behavioral effects (Jiang et al., 2005). Fourth, cannabidiol, a non-psychotomimetic component of Cannabis sativa, increases hippocampal neurogenesis via CB1 receptors (Campos et al., 2013; Wolf et al., 2010). CB1 receptors mediate not only baseline hippocampal neurogenesis, but also activity- and enriched environment-induced increase in hippocampal neurogenesis (Hill et al., 2010; Wolf et al., 2010). Finally, kainate excitotoxicity induced hippocampal neurogenesis via the CB1 receptor (Aguado et al., 2007). It is thus likely that enhancement of hippocampal neurogenesis might contribute to antidepressant-like behavioral effects of chronic JZL184 treatments. Nevertheless, our data do not exclude the possibility that other mechanisms may contribute to behavioral effects of JZL184.
We have shown that CUS decreased 2-AG levels in the hippocampus (Zhong et al., 2014). Chronic JZL184 treatments prevented CUS-induced decrease in the number of BrdU+ and DCX+ cells in the DG of the hippocampus but did not significantly alter the number of BrdU+ and DCX+ cells in control mice. The reason for this differential modulation is not yet clear. We speculate that endogenous 2-AG levels are sufficient to support adult hippocampal neurogenesis in control mice, while CUS-induced decrease in 2-AG levels in the hippocampus (Zhong et al., 2014) would allow JZL184 to exert more significant effect on hippocampal neurogenesis.
We have shown that CB1 receptor-mediated activation of mTOR singling is important for the antidepressant-like behavioral effects of JZL184 (Zhong et al., 2014). mTOR is a serine/threonine protein kinase that regulates cell metabolism, growth, survival, protein synthesis-dependent synaptic plasticity (Jaworski and Sheng, 2006). mTOR-dependent protein synthesis and synaptogenesis might mediate the rapid antidepressant-like behavioral effects of NMDA receptor antagonists including ketamine (Li et al., 2010). The mTOR signaling pathway has been implicated in adult hippocampal neurogenesis (Corsini et al., 2009; Kim et al., 2009; Zhou et al., 2013). Future research is needed to address whether JZL184-induced activation of mTOR signaling pathway is responsible for the enhancement of hippocampal neurogenesis by JZL184.
Newborn neurons make synaptic connections with neighboring neurons and are functionally integrated into preexisting neuronal networks (Ming and Song, 2005; van Praag et al., 2002). Given that CUS and chronic JZL184 altered the number of DCX+ immature neurons, they may affect basic synaptic properties and LTP in the DG. In the DG, the medial and lateral perforant paths (MPP and LPP) form synapses on granule cell (GC) dendrites in the middle and outer one-thirds of the molecular layer, respectively (Burwell and Amaral, 1998). We chose to study basic synaptic properties and LTP in the MPP (Fig. 3A) for the following reasons. First, LTP of the MPP is neurogenesis-dependent as it was blocked by X-irradiation of the hippocampus (Saxe et al., 2006; Wang et al., 2008). Second, newborn GCs migrate from inner molecular layer to outer molecular layer of the DG as they gradually mature (Vivar and van Praag, 2013; Wang et al., 2000). Third, CB1 receptors are highly expressed in the inner molecular layer of the DG but are expressed at much lower levels in the outer molecular layer of the DG (Katona et al., 2006; Monory et al., 2006). We found that neither CUS nor chronic JZL184 had significant effect on the paired-pulse ratio, suggesting that the probability of presynaptic transmitter release was not altered by either treatment. On the other hand, CUS decreased I/O functions and impaired a form of LTP induced in the absence of GABAA receptor antagonists, both deficiencies were prevented by chronic JZL184 treatments. In many excitatory synapses, LTP is tightly controlled by GABAergic inhibition such that LTP cannot be induced unless GABAA receptor antagonists are present (Huang et al., 1999; Liu et al., 2005). However, a modest LTP can be induced in the DG induced in the absence of GABAA receptor antagonists, and this form of LTP is dependent on neurogenesis since it was blocked by X-irradiation (Saxe et al., 2006; Snyder et al., 2001; Wang et al., 2000). Immature neurons exhibit several unique properties that make them susceptible to LTP induction. First, GABAergic inputs to immature neurons in the DG are excitatory (Ge et al., 2006). Second, immature neurons express predominantly the NR2B subtype of NMDA receptor, which facilitates LTP induction (Ge et al., 2007). Third, immature neurons express T-type Ca2+ channels, which facilitate LTP by inducing Ca2+ spikes and enhancing fast Na+ action potentials (Schmidt-Hieber et al., 2004). The enhanced LTP occurs in a critical period between 1 and 1.5 months after cell birth (Ge et al., 2007), a stage at which the newborn neurons have ceased to express DCX (Brown et al., 2003; Kempermann et al., 2003). Thus, CUS- and JZL184-induced changes in the number of immature neurons may underlie the observed effects on I/O functions and LTP induction.
Both immature and mature neurons are recruited for LTP induction in the DG in the presence of GABAA receptor antagonist picrotoxin, and this form of LTP is not dependent on neurogenesis (Saxe et al., 2006; Snyder et al., 2001; Wang et al., 2000). We found that neither CUS nor chronic JZL184 had significant effects on LTP in picrotoxin. Although CUS and JZL184 change the number of DCX+ immature neurons, these neurons represent only a small percentage of the total granule cell population, which may explain why CUS and JZL184 did not affect LTP induced in the presence of picrotoxin. It is worth noting that FAAH inhibitor URB597 (Basavarajappa et al., 2014) and THC impair LTP in the CA1 region of the hippocampus (Fan et al., 2010; Hoffman et al., 2007), although this LTP is enhanced in MAGL knockout mice (Pan et al., 2011).
MAGL inhibition not only increases 2-AG levels but also reduces the metabolites of 2-AG, including arachidonic acid and prostaglandins (Nomura et al., 2011). Chronic JZL184 treatments produce CB1-independent neuroprotective effects through reduction of prostaglandin-induced neuroinflammation (Chen et al., 2012; Nomura et al., 2011; Piro et al., 2012). We have shown that the antidepressant-like behavioral effects of chronic JZL184 treatments were blocked by the CB1 receptor antagonist rimonabant (Zhong et al., 2014). However, it remained unknown whether the effects of chronic JZL184 treatments on hippocampal neurogenesis and LTP in the DG are mediated by 2-AG-induced activation of CB1 receptors or by the reduction of 2-AG metabolites. CB1 antagonists rimonabant and AM251 produced CB1-independent, unspecific effects on hippocampal neurogenesis (Jin et al., 2004), which would confound data interpretation. Nevertheless, future studies will be carried out in CB1 knockout mice to address whether the effects of JZL184 on hippocampal neurogenesis and LTP are mediated by CB1 receptors.
In summary, our studies indicate that MAGL inhibitor JZL184 produces antidepressant-like effects in a CUS model of depression and these effects are likely mediated through the enhancement of hippocampal neurogenesis. Chronic JZL184 treatments also produce neuroprotective effects against Alzheimer’s disease and Parkinson’s disease (Chen et al., 2012; Nomura et al., 2011; Piro et al., 2012) and reverse deficits in learning, memory and long-term synaptic plasticity in a mouse model of Alzheimer’s disease (Chen et al., 2012). Thus, MAGL inhibition may represent a useful strategy for pharmacotherapy of a variety of disease conditions including depression.
Acknowledgments
This work was supported by NIH Grants R21 MH095921 and R01 DA035217 and Extendicare Foundation. It was also partially funded through the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and National Institutes of Health grant UL1RR031973 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources and the National Center for Advancing Translational Sciences.
Footnotes
Conflict of Interest
The authors have no potential conflict of interest.
References
- Aguado T, Romero E, Monory K, Palazuelos J, Sendtner M, Marsicano G, Lutz B, Guzman M, Galve-Roperh I. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J Biol Chem. 2007;282(33):23892–8. doi: 10.1074/jbc.M700678200. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Gobbi G. The cannabinoid CB1 receptor and the endocannabinoid anandamide: possible antidepressant targets. Expert Opin Ther Targets. 2008;12(11):1347–66. doi: 10.1517/14728222.12.11.1347. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Nagre NN, Xie S, Subbanna S. Elevation of endogenous anandamide impairs LTP, learning, and memory through CB1 receptor signaling in mice. Hippocampus. 2014 doi: 10.1002/hipo.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163(1):111–7. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163(3):463–8. doi: 10.1083/jcb.200305129. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14(12):1347–56. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62(10):1103–10. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998;391(3):293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70(5):479–86. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Campos AC, Ortega Z, Palazuelos J, Fogaca MV, Aguiar DC, Diaz-Alonso J, Ortega-Gutierrez S, Vazquez-Villa H, Moreira FA, Guzman M. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int J Neuropsychopharmacol. 2013;16(6):1407–19. doi: 10.1017/S1461145712001502. others. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, Teng Z, Chen C. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep. 2012;2(5):1329–39. doi: 10.1016/j.celrep.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini NS, Sancho-Martinez I, Laudenklos S, Glagow D, Kumar S, Letellier E, Koch P, Teodorczyk M, Kleber S, Klussmann S. The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell. 2009;5(2):178–90. doi: 10.1016/j.stem.2009.05.004. others. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–7. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–93. doi: 10.1016/j.neuron.2009.04.017. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21(12):521–8. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97(13):7579–84. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci U S A. 2003;100(10):6227–32. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan N, Yang H, Zhang J, Chen C. Reduced expression of glutamate receptors and phosphorylation of CREB are responsible for in vivo Delta9-THC exposure-impaired hippocampal synaptic plasticity. J Neurochem. 2010;112(3):691–702. doi: 10.1111/j.1471-4159.2009.06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30(6):2017–24. doi: 10.1523/JNEUROSCI.5693-09.2010. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–66. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102(51):18620–5. doi: 10.1073/pnas.0509591102. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG, Jr., Brown ME. Do patients use marijuana as an antidepressant? Depression. 1996;4(2):77–80. doi: 10.1002/(SICI)1522-7162(1996)4:2<77::AID-DEPR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36(13):2589–602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, Bambico FR, Patel S, Gorzalka BB, Gobbi G. The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol Sci. 2009;30(9):484–93. doi: 10.1016/j.tips.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TT, Gil-Mohapel J, Gorzalka BB, Hillard CJ, Christie BR. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20(4):513–23. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Liu QS. Endocannabinoid signaling in the etiology and treatment of major depressive illness. Curr Pharm Des. 2013 Oct 28; doi: 10.2174/13816128113196660735. 2013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR. Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem. 2007;14(1-2):63–74. doi: 10.1101/lm.439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33(2):406–17. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–55. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34(3):205–19. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115(11):3104–16. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, Childs J, Greenberg DA. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol. 2004;66(2):204–8. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26(21):5628–37. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63(6):761–73. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 2011;98(1):21–7. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, Lichtman AH. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther. 2013;345(3):492–501. doi: 10.1124/jpet.112.201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105(2):751–6. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437(7061):1027–31. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009a;5(1):37–44. doi: 10.1038/nchembio.129. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009b;16(7):744–53. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL. Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res. 1980;199(1):1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51(4):455–66. doi: 10.1016/j.neuron.2006.07.006. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334(6057):809–13. doi: 10.1126/science.1209200. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008;28(6):1385–97. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31(38):13420–30. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318(1):304–11. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4(11):873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piro JR, Benjamin DI, Duerr JM, Pi Y, Gonzales C, Wood KM, Schwartz JW, Nomura DK, Samad TA. A dysregulated endocannabinoid-eicosanoid network supports pathogenesis in a mouse model of Alzheimer’s disease. Cell Rep. 2012;1(6):617–23. doi: 10.1016/j.celrep.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–5. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. others. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–6. doi: 10.1073/pnas.0607207103. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–7. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res. 2011;64(3):226–34. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85(6):2423–31. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Sumislawski JJ, Ramikie TS, Patel S. Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology. 2011;36(13):2750–61. doi: 10.1038/npp.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr. BUdR as an S-phase marker for quantitative studies of cytokinetic behaviour in the murine cerebral ventricular zone. J Neurocytol. 1992;21(3):185–97. doi: 10.1007/BF01194977. [DOI] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135(2):564–78. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, van Praag H. Functional circuits of new neurons in the dentate gyrus. Front Neural Circuits. 2013;7:15. doi: 10.3389/fncir.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28(6):1374–84. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42(2):248–57. [PubMed] [Google Scholar]
- Wang W, Sun D, Pan B, Roberts CJ, Sun X, Hillard CJ, Liu QS. Deficiency in Endocannabinoid Signaling in the Nucleus Accumbens Induced by Chronic Unpredictable Stress. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.99. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat Protoc. 2006;1(3):1399–405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Bick-Sander A, Fabel K, Leal-Galicia P, Tauber S, Ramirez-Rodriguez G, Muller A, Melnik A, Waltinger TP, Ullrich O. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun Signal. 2010;8:12. doi: 10.1186/1478-811X-8-12. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ, Zhang HT, Cravatt BF, Liu QS. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR Signaling. Neuropsychopharmacology. 2014;39(7):1763–76. doi: 10.1038/npp.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Li W, Huang S, Song J, Kim JY, Tian X, Kang E, Sano Y, Liu C, Balaji J. mTOR Inhibition ameliorates cognitive and affective deficits caused by Disc1 knockdown in adult-born dentate granule neurons. Neuron. 2013;77(4):647–54. doi: 10.1016/j.neuron.2012.12.033. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]