Abstract
The prevalence of obesity has reached epidemic proportion with enormous costs in both human lives and healthcare dollars spent. Obesity-related metabolic disorders are much lower in premenopausal women than men; however, there is a dramatic increase following menopause in women. The health risks associated with obesity vary depending on the location of adipose tissue. Adipose tissue distributed in the abdominal visceral carry a much greater risk for metabolic disorders than does adipose tissue distributed subcutaneously. There are distinct sex-dependent differences in the regional fat distribution, women carry more fat subcutaneously whereas men carry more fat viscerally. Males and females differ with respect to their regulation of energy homeostasis. Peripheral adiposity hormones such as leptin and insulin as well as sex hormones directly influence energy balance. Sexual dimorphisms in energy balance, body fat distribution, and the role sex hormones have in mediating these differences are the focus of this review.
Keywords: Fat distribution, Gonadal steroids, Subcutaneous adipose tissue, Intra-abdominal adipose tissue, Leptin, Insulin, Estrogens, Estrogen receptor
1. Introduction
1.1. Incidence of obesity and its related metabolic disorders
1.1.1. Male vs. female
The increasing prevalence of obesity throughout the world [51], [122] and [127] is associated with an escalating incidence of obesity-related disorders and health costs [5]. Obesity is a leading cause for the development of adverse metabolic effects, including non-insulin dependent diabetes mellitus, dyslipidemia, and cardiovascular disease [34] and [45]. It has been estimated that 47 million individuals in the United States have obesity-related metabolic diseases [53]. There are important sex differences in the prevalence of these metabolic diseases. Women under the age of 50 have much less obesity-related metabolic disorders; however, the prevalence of these metabolic disorders increases dramatically in women after menopause [52]. Children who have metabolic diseases have a higher risk of developing adverse events later in life [55] and [116]. In today’s society there is an increase in the prevalence of obesity and its related metabolic diseases in adolescents and it is significantly higher among males than females aged 12 to 18 years [54], [70] and [154]. Data suggest that ovarian hormones may be protective against the metabolic syndrome because prior to menopause, the prevalence of the metabolic disorders is higher among males than females; however, after menopause, women are more likely to suffer from metabolic disorders.
1.1.2. Visceral fat vs. subcutaneous fat
The increased health risks due to obesity vary depending on the location / accrual of adipose tissue [15], [16], [17], [19] and [123]. Specifically, adipose tissue distributed in the abdominal or visceral region carries a much greater risk for metabolic disorders, than does adipose tissue distributed subcutaneously [19], [20] and [21]. Differences in distribution of adipose tissue and the relative risk for diseases suggest that not all adipose tissue is created equally. Rather, different adipose depots have different properties that can have important consequences on health outcomes.
There are distinct sex-dependent differences in the regional fat distribution. If age and body mass index (BMI) are matched, women have lower waist-to-hip ratio, indicating a greater amount of subcutaneous adipose tissue than men do [95] and [117]. Excess adiposity in the central visceral region of the body (‘android’ or male-pattern obesity [167]) is correlated with increased risk and mortality from disorders including diabetes, hyperlipidemia, hypertension, and atherosclerosis [11], [56] and [73]. In contrast, excess adiposity in the gluteal / femoral subcutaneous region (‘gynoid’ or female-pattern) is poorly correlated with risk for these metabolic disorders [18], [39], [40], [88] and [123]. Hence, there are sex-based differences with regard to obesity-associated health risks with obese men being more likely to develop secondary metabolic complications and cardiovascular diseases than obese women [36], [83], [87], [92] and [169]. Therefore, the distribution of fat is more directly associated with the metabolic syndrome than total body fat.
There are two important implications that follow from these observations. The first is that males and females may differ in their susceptibility to the metabolic syndrome based on where they deposit adipose tissue. The second is that whereas we know the health consequences associated with visceral fat deposition, very little is known about how excess nutrients are partitioned / stored into the different adipose tissue depots. The goal of this review is to explore what we know about these sex differences in energy balance which are associated with adipose tissue accrual and deposition, as well as the role that sex hormones play in these differences.
2. Sex differences in body fat distribution
2.1. Sex steroids regulate fat distributions
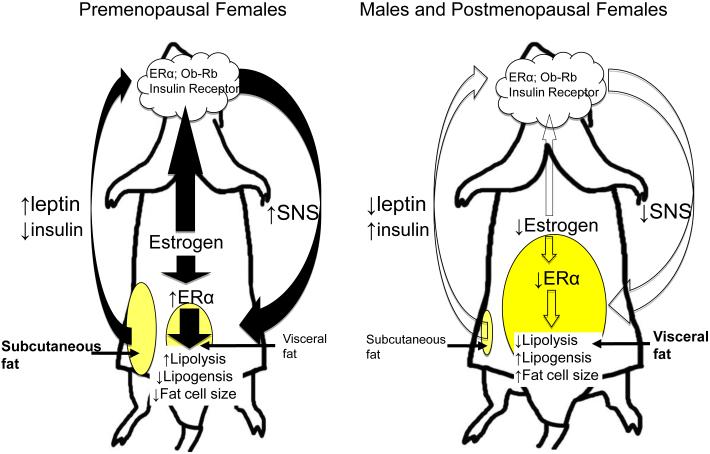
As previously mentioned, on average, women carry more fat subcutaneously [42], [66],[80], [89] and [90]; whereas men carry more fat viscerally [167]. Gonadal / sex steroids have been proposed as regulators of fat distribution [47] and [48]. Men have lower estrogen, and on average, men also have less total fat and a more central or intra-abdominal distribution; whereas premenopausal women have more total fat and a more gluteal / femoral subcutaneous fat distribution (Fig. 1). Intra-abdominal fat varies inversely with estrogen levels [12], [13], [15], [25] and [49]. After menopause and the decline of estrogen, women develop increased intra-abdominal adiposity, but those who receive estrogen replacement therapy do not [59], [63] and [64], suggesting a specific role of estrogen in limiting intra-abdominal fat mass [10] and [31]. Androgens favor abdominal fat deposition. Most women with polycystic ovary syndrome (PCOS), a hyperandrogenic disorder, have increased abdominal fat [37]. Exaggerated androgen synthesis and secretion by the ovaries and the adrenal glands are associated with insulin resistance and impaired glucose tolerance [37]. Consequently women with PCOS have increased risk for the metabolic syndrome. Given these differences in body-fat distribution and co-morbidities, it is likely that the mechanisms that regulate body fat distribution differ in males and females.
Fig. 1.
Potential model depicting how sex hormones and adiposity signals may interact to regulate body fat distribution.
We propose that female sex steroid estrogen regulates body fat distribution. Females carry more fat subcutaneously whereas males with lower estrogen carry more fat viscerally. Reductions in estrogen, as occurs in menopause, is associated with an increase in visceral adiposity.
Estrogen receptors (ER) are expressed in adipose tissues and hypothalamus. Estrogen regulates energy balance and body fat distribution by either directly interacting with the leptin signaling pathway or through activation of estrogen receptors. Specifically, estrogen may directly act on estrogen receptor alpha (ERα) in visceral adipose tissues to regulate lipid metabolism. Estrogen may influence adiposity by interacting with leptin, and potentially enhancing leptin-induced activation of the sympathetic nervous system which innervates visceral adipose tissue, thereby reducing fat accrual in the visceral depot. Additionally, subcutaneous adipose tissue, which accounts for a higher percentage of adipose tissue in females, secretes leptin, and the secreted leptin may activate CNS leptin receptors, and this may directly influence leptin-induced activation of the sympathetic nervous system.
2.2. Differences in subcutaneous vs. visceral fat
2.2.1. Lipid mobilization (lipolysis) vs. lipid accumulation (lipogenesis)
In all mammalian species, energy is primarily stored in the form of lipid in white adipose tissue. The amount of fat stored in adipose tissue is the net difference between the rates of lipogenesis and lipolysis. In situations where metabolic fuels are not sufficient to meet energy needs, a lipolytic cascade is initiated that results in the breakdown of energy stored in the form of triglycerides into free fatty acids and glycerol via hormone-sensitive lipase, the enzyme that turns on lipolysis. Catecholamines trigger lipolysis via membrane-bound α- and β-adrenoceptors [27] and [85]. Specifically catecholamines stimulate lipolysis via β1-, β2- and β3-adrenoceptors and inhibit lipolysis via α2-adrenoceptors [27] and [85]. Lipolysis correlates positively with activation of the sympathetic nervous system [4] which may further enhance free fatty acid release into portal circulation [95]. In situations where there is a prolonged positive energy balance, adipocytes take up circulating fatty acids and leads to increases in both adipocyte size and number, which is manifested more generally as an increased body fat mass [50]. The major pathway of free fatty acid uptake is mediated by lipoprotein lipase, an enzyme that hydrolyses meal-derived triglycerides in chylomicrons and very low density lipoprotein triglyceride at the capillary endothelium. In addition, circulating free fatty acids are directly taken up and stored via a lipoprotein lipase-independent pathway [9], [32] and [142].
There are sex differences in the lipolytic response. Female rats have higher lipolytic capacities and a lower α2/β3- adrenoceptor ratio in intra-abdominal retroperitoneal adipose tissue than male rats [94]. High-fat diet feeding changes α2- and β3- adrenoceptors differentially in males and females, specifically, in males there is an increase in antilipolytic α2- adrenoceptor and reductions in lipolytic β3- adrenoceptor in female rats. In addition, the decrease of α2/β3-adrenoceptor ratio is greater in males than females, which leads to a greater amount of fat accumulation in males fed with a high-fat diet [94]. In humans, lipolytic response of abdominal subcutaneous adipose tissue to norepinephrine, an adrenergic agonist, is greater in obese women, whereas obese men have greater abdominal α2 receptor antilipolytic function than women [91]. Thus, abdominal subcutaneous adipose tissues of obese females are more easily mobilized and utilized than those of obese males due to its greater lipolytic activity.
There are sex differences in lipid storage. Visceral adipose tissue uptake of triglyceride fatty acids is greater in men than in women [119]. Lipoprotein lipase is an enzyme that facilitates free fatty acid uptake. Premenopausal women have lower activity of lipoprotein lipase in their intra-abdominal adipose tissue than men [132] and [133]. The net result of these differences is that women store less lipid in intra-abdominal adipose tissue than men do.
2.2.2. Intra-abdominal adipose tissue
Intra-abdominal adipose tissue is metabolically and functionally different from subcutaneous adipose tissue. Intra-abdominal adipose tissue has adipogenic, metabolic, pro-atherogenic, and pro-thrombotic characteristics [86], [112] and [158]. Intra-abdominal fat has relatively more capillaries and efferent sympathetic axons per unit volume than subcutaneous adipose issue.
Weight loss is characterized by an initial reduction in intra-abdominal rather than subcutaneous adipose tissue in part because intra-abdominal adipocytes are more metabolically active [12], [14], [16], [103], [110] and [166]. Reduction of equal amounts of visceral and subcutaneous fat does not have the same net effect on glucose homeostasis. Surgical removal of intra-abdominal adipose tissue results in decreased insulin and glucose levels in humans [156]; improves glucose tolerance in male and female mice [150]; and prevents the onset of age-dependent insulin resistance and glucose intolerance in male rats [58]. Thus, men’s visceral adipose tissue has greater lipolytic activity than women’s, which contributes to sex-specific differences in metabolic and cardiovascular diseases accompanied by obesity.
2.2.3. Subcutaneous fat
Subcutaneous fat is dispersed within a broad area under the skin, is relatively poorly innervated and vascularized, and has a larger average cell diameter than intra-abdominal adipocytes [167]. Lipid deposition is an evolutionary advantageous process that allows efficient storage of maximal calories per unit volume of tissue. The adipose tissue intended for fatty acid uptake and storage of excess calories is the subcutaneous fat depot in both men and women [81], [105], [147] and [161]. The capacity to store lipids within the subcutaneous depot is the key to facing famine and limited caloric supply, especially in females. Females mobilize adipose tissue stored in this depot to augment the caloric demands placed on the body during lactation and breast feeding. From this perspective, we proposed that deposition of adipose tissue in the subcutaneous depot of females would be evolutionarily conserved.
Sex differences exist in fatty acid release and uptake in subcutaneous adipose tissue. Women have a greater number of antilipolytic α2-adrenoceptors in the gluteal / femoral subcutaneous region [136]. In contrast to what was previously reported for intra-abdominal adipose tissue, catecholamine-mediated lipolytic activity and free fatty acid release from subcutaneous adipose tissue is lower in women than in men [71]. In addition, free fatty acid uptake by subcutaneous adipose tissue is much greater in premenopausal women than men [147]. Using fatty acid tracers in food and adipose tissue biopsies, Jensen and colleagues found a higher proportion of dietary fat is stored in the lower body gluteal / femoral subcutaneous adipose tissue in women than men [139]. Therefore, subcutaneous adipose tissues of women release less and take up more free fatty acids than those of men, leading to greater fat storage at subcutaneous region in women and contributes to regional differences in fat distribution between men and women.
In accordance with these in vivo findings, in vitro subcutaneous adipose tissue from women esterifies greater amounts of extracellular free fatty acids (i.e., free fatty acid uptake) than comparable fat tissue from men [46]; in vitro catecholamine-induced lipolysis is significantly lower in subcutaneous gluteal adipocytes than in abdominal adipocytes from women, but are comparable between these adipocytes from men [136]. These in vitro findings imply there is a greater amount of lipid storage in subcutaneous adipose tissue from women than that from men.
It’s noteworthy that men also accumulate fat in their subcutaneous depots. Upper body abdominal subcutaneous adipose tissue takes up free fatty acid and stores lipid more avidly than lower body gluteal / femoral subcutaneous adipose tissue in men [147]. In addition, gene expression of fatty acid transporters is greater in abdominal than gluteal / femoral subcutaneous fat in men [147]. In contrast, in obese women the efficiency of free fatty acid storage is greater in lower body gluteal / femoral subcutaneous than upper body abdominal subcutaneous fat [81] and [147]. To summarize, obese men tend to store fat in intra-abdominal adipose tissue depots and upper body abdominal subcutaneous adipose tissues, whereas obese women store more fat in lower body gluteal / femoral subcutaneous adipose tissue depots. The differences in regional efficiency of free fatty acid uptake are in concordance of sex-specific body fat distribution that women tend to store more fat in the lower body and men in the upper body.
In contrast to what occurs in intra-abdominal adipose tissue, removal of subcutaneous adipose tissue does not result in improvement of any aspect of the metabolic syndrome in humans [79] or in rodents [58] and [150]. In a recent paper by Tran et al. [157], they found transplantation of subcutaneous adipose tissue into intra-abdominal adipose tissue improved metabolic parameters. When obese ob/ob mice are engineered to overexpress adiponectin in adipose tissue, there is a massive increase in subcutaneous fat, and this is associated with improved insulin sensitivity and decreased glucose and insulin levels, increased lipid clearance, improved diacylglycerol levels and fully functional healthy pancreatic β-cells [78]. Therefore subcutaneous fat could be insulin sensitive tissue, through an elevated lipoprotein lipase activity that favors the clearance of circulating triglycerides of dietary origin and facilitates free fatty acid storage in adipocytes [81], [105], [147] and [161].
2.3. Teleological explanation for differences in fat distribution
2.3.1. Why do males store visceral fat?
A key question to guide a better understanding of these key biological differences is the underlying reasons that males and females store excess calories in different places. These differences are presumably due to differential evolutionary and sexual selection pressures [68]. Visceral fat can be mobilized more rapidly to respond to shorter-term energetic challenges. Consequently one reason to store fat in the visceral depot is to make it more accessible for specific intermittent activities. If males are more responsible for hunting, gathering or immediate protection, it would make sense to put stored calories in fat with greater lipolytic activity where it can be mobilized over the shorter time frame required for these activities.
2.3.2. Why do females store subcutaneous fat?
Given the lower lipolytic rates in subcutaneous adipose tissue, it is much better suited to respond to chronic metabolic challenges such as occur during gestation and lactation in females. Consistent with this hypothesis, female rats gain weight during the early part of gestation and that weight gain is disproportionately in subcutaneous adipose tissue. Such a build-up of subcutaneous fat would facilitate female’s ability to counteract the enormous and chronic metabolic challenge associated with gestation and lactation. This is a period of high energetic demand with a relatively low level of ability for the organism to effectively hunt and/or gather calories from its environment. In rats this means that subcutaneous fat increases till day 12 of gestation, and declines progressively thereafter following gestation and lactation as it is utilized to provide energy; whereas visceral fat depots including lumbar and mesenteric adipose tissues progressively increase till the 19th day of gestation [96]. This indicates that subcutaneous but not visceral adipose tissue becomes the preferred energy source and being utilized during the last stage of gestation in female rats. In women, subcutaneous fat depots become more lipolytically active during lactation than visceral fat depots; thus subcutaneous adipose tissue is utilized as an important source of energy supply during lactation [133]. In contrast to what occurs in non-pregnant woman, lipolysis in the gluteal / femoral subcutaneous adipose tissue is significantly less, thereby supporting lipogenesis until early pregnancy [133].
3. Energy Balance Regulation
Obesity is a disorder of energy homeostasis. However, most animals match caloric intake with caloric expenditure quite precisely, resulting in relatively stable fat stores [74] and [77]. An organism’s ability to regulate energy homeostasis requires that there is an ability to sense changes in energy flux and the CNS must be a key player in both the sensing and responding to changes in energy flux. Key to the ability to sense this change in energy flux are signals indicating the amount and distribution of stored fat. Such ‘adiposity signals’ are proportional to body fat content and therefore monitor adiposity levels to inform the brain of changes in stored fuel levels.
Body weight regulation is thought to occur through negative feedback mechanisms which characterize most homeostatic systems [43], [131], [144], [146] and [172] involving adiposity signals. These signals act in the brain to regulate food intake, and ultimately the amount of calories stored in adipose tissue and thereby work to keep overall adiposity levels relatively constant. In addition to paying attention to total body fat, the brain should also pay attention to where the fat is distributed. Hence, signals that provide information, not just on overall adipose tissue levels but also where such adipose tissue is located, should provide feedback to the CNS. While there is a potential for a wide variety of signals to play a role in communicating with the CNS both overall body fat and body fat distribution, the best case can be made for three signals: leptin, insulin and estrogen.
3.1. Leptin
Leptin is secreted in direct proportion to body fat, entering the brain from the blood, and interacting with specific receptors on neurons in the hypothalamus and other areas [2], [145] and [171]. Increased activity of leptin locally in the vicinity of the ventral hypothalamus causes an overall catabolic response (i.e., reduced food intake, increased energy expenditure, increased sympathetic activation, and loss of body weight) whereas decreased leptin causes an overall anabolic response (i.e., increased food intake, decreased energy expenditure, and increased body weight) [145] and [170].
In addition to providing information about overall adipose mass, leptin also provides information about body fat distribution. Leptin is secreted at a higher rate from subcutaneous fat than from visceral fat [28] and [113], thus circulating leptin correlates better with total subcutaneous fat than with total body fat [30], [35], [42], [57], [76], [106],[126], [140] and [141].
Because females have more subcutaneous fat than males, an important implication is that the “adiposity” message conveyed to the brain differs in males and females, and is correlated with fat distribution [29] and [30]. Leptin levels are higher in females, even before puberty, compared with males [35]. After puberty, estrogen and testosterone modulate leptin synthesis and secretion via sex steroid receptor-dependent transcriptional mechanisms [99]. Leptin levels are inversely correlated with testosterone [69], [82], [98], [160] and [162] and exposure of human fat cells to testosterone or dihydrotestosterone inhibits leptin expression [162]. In aging and obese men, there is increased aromatase activity and conversion of androgens to estrogen and this is associated with increased plasma leptin [72], [115] and [175]. Testosterone replacement normalizes elevated serum leptin levels in hypogonadal men and in castrated male rats. In women, leptin fluctuations during the menstrual cycle directly correlate with estrogen, but not with progesterone [102], [129] and [130]. In male rats, dihydrotestosterone decreases adipose tissue leptin mRNA, whereas in female rats, 17-β estradiol increases adipose tissue leptin mRNA levels [82]. Finally, peripheral or central estradiol administered either to ovariectomized females or intact males increases hypothalamic sensitivity to leptin and favors body fat accrual in the subcutaneous over visceral adipose depot [29]. These studies suggest that estrogen regulates energy balance and body fat distribution by interacting with leptin signaling pathways (Fig. 1). Consistent with this hypothesis estrogen deficiency impairs central leptin sensitivity [3], [29] and [30].
3.2. Insulin
Similar to leptin, insulin is also considered as an adiposity signal despite several important differences with leptin. First, leptin is secreted directly from adipocytes in proportion to their metabolic activity, whereas insulin is secreted from pancreatic β cells in response to increases of circulating glucose. The metabolic activity of adipocytes is more stable than circulating glucose levels that change with feeding, exercise, and stress. In addition, the half-life of the plasma leptin is approximately 45 minutes, much longer than that of insulin with a half-life of approximately 2 - 3 min. Thus, although both the circulating levels of leptin and insulin are directly proportional to the amount of total adiposity, leptin is a more stable signal to indicate adiposity. Consequently, insulin’s ability to predict adipose tissue levels is a result of the integrated signal of insulin over time rather than at any particular moment in time.
The levels of leptin and insulin differ in regard to which fat depots they better reflect. Serum leptin is more tightly correlated with subcutaneous fat, whereas insulin secretion is better correlated with visceral fat, thus its levels better reflect visceral rather than total body adiposity. As previously discussed, visceral but not subcutaneous fat provides the risk factor for the metabolic syndrome as adiposity increases [19]. The result of differences in circulating levels of adiposity signals is that they differ between males and females and are correlated with differences in body fat distribution. Intra-abdominal fat is relatively insensitive to insulin [23] and [104], and insulin action is markedly impaired in individuals with visceral obesity [26] and [121]. Thus, men have greater risk to develop the metabolic syndrome as adiposity increases than premenopausal obese women do [36], [52] and [87]. It is noteworthy that although obese women with increased subcutaneous fat are protected from metabolic syndrome, the degree of protection would be lost if visceral fat is accrued.
While both leptin and insulin cross into the brain via dedicated transport processes to act on specific receptors to regulate energy balance and elicit net catabolic responses, there are sexually dimorphic responses to their actions [29], [30] and [65]. Male rats are relatively more sensitive to the catabolic action of insulin delivered into the CNS, whereas female rats are relatively more sensitive to the catabolic action of leptin delivered into the CNS [29] and [30]. Comparable phenomenon has been reported in a recent human study that men, but not women, lose body weight, body fat and waist circumference following intranasal insulin administration [65], an approach that increases insulin concentration of the cerebrospinal fluid and thereby alters brain functions [24]. Therefore, sexually differential sensitivity to catabolic effects of insulin exists in rodents and humans.
3.3. Estrogen
3.3.1. Estrogen regulates adiposity
Visceral fat varies inversely with estrogen levels [12], [13], [15], [25] and [49]. Visceral fat accumulation occurs in females when estrogen levels become sufficiently low, possibly due to direct effects of estrogen on adipose tissue. Estrogen (ER), progesterone (PR), and androgen receptors (AR) are expressed in adipose tissues [33], [111] and [128]. Subcutaneous adipose tissue has higher concentrations of ER and PR; however, visceral adipose tissue has higher concentrations of AR [97]. In accordance with the negative regulation between estrogen and AR in the adipose tissue, adipose tissue-specific AR knockout mice have increased intra-adipose estradiol levels, which further leads to hyperleptinemia with enhanced leptin sensitivity [174].
Reductions in estrogen, as occurs in menopause, is associated with an increase in visceral adiposity and a shift toward upper body fat distribution in humans [59], [63] and [64] and in rats [29]. Importantly, fat distribution changes are due primarily to reductions in circulating estrogen levels rather than aging [62] and [114]. A recent study reported that total body fat percentage of age-matched women is significantly higher in the perimenopausal and postmenopausal groups with decreased estrogen levels than in the premenopausal group [62] and [114]. Therefore, reduced estrogen levels during the menopausal transition, rather than the aging process, cause total body fat increase and fat accumulation in the visceral region. [62] and [114].
In rodents, ovariectomized female rats gain fat, preferentially gain visceral fat [29]. Peripheral or central administration of 17 β-estradiol to ovariectomized females restores their central leptin sensitivity and changes their body fat distribution to mirror that of intact females; additionally, altering the sex hormone milieu in males with 17 β-estradiol administration increases sensitivity to central leptin and increases subcutaneous fat deposition [29]. An important implication from these findings is that estrogen regulates body fat distribution, interacts with the integrated adiposity message conveyed to the brain by leptin, and enhances leptin’s action in the visceral fat, which facilitates fat mobilization in the visceral depot and fat deposition in the subcutaneous depot (Fig. 1).
3.3.2. Estrogen regulates adiposity through ER
Estrogen regulates body adiposity and fat distribution potentially through its receptors in the brain [33], [111] and [128]. The “classical” nuclear ER was cloned in 1985 [107] and renamed ERα when a second nuclear ER, ERβ, was discovered ten years later [84]. However, only ERα has been reported to have a major influence on energy homeostasis [67]. ERs are members of the nuclear receptor superfamily. Nuclear receptors are ligand-activated transcription factors, and they regulate the expression of target genes by binding to specific estrogen response elements on DNA. In the brain, estrogen modulates neuronal activity through ERs. ERα is necessary for estradiol’s genomic actions with respect to body weight regulation [118], whereas ERβ functions more as a modulator of estrogen actions [143]. Rapid, non-genomic actions of estradiol also have been described and some of them appear to involve ERα [1] and [108].
Heine et al. [67] reported that male and female mice with a targeted deletion in the ERα subunit (αERKO) have increased adiposity in both male and female mice, consistent with other evidence linking estrogen with body weight regulation and adipocyte function. Recently, site-specific deletion of ERα in the ventromedial hypothalamus, a brain region critical for body weight regulation, demonstrates the role of estrogen signaling through ERα in the regulation of body weight homeostasis [118]. Lack of estrogen signaling through ERα results in obesity due to an anabolic process, with changes in energy expenditure primarily mediating the weight gain [118]. These data are consistent with previous finding in the ERα total body knockout animals where it has been demonstrated that the obesity is primarily due to changes in energy expenditure rather than changes in food intake [67] and [124] and those mice are viscerally fat (unpublished data) . These findings suggest that estrogen signaling within critical hypothalamic nuclei is responsible for the regulation of body weight via modulating energy expenditure.
Further supporting a role for estrogen signaling through ERα in the regulation of body weight are the findings that abnormal adiposity has been associated with the XbaI polymorphism of the human ERα gene, in which guanidine is substituted for adenine in exon one of the [125], [152] and [173]. In a cross-sectional epidemiological sample of over two thousand middle-aged Japanese, pre-menopausal women that have the polymorphism, there is increased fat mass and increased waist-hip ratios, an index of visceral adiposity, compared to pre-menopausal women with the normal genotype [125] and [173]. The polymorphism does not affect adiposity in postmenopausal women or in men. Thus, polymorphisms of the human ERα gene may impair estrogen signaling and lead to increased visceral adiposity and its attendant health risks.
3.3.3. Estrogen regulates food intake
The preovulatory rise in estradiol secretion is associated with a decrease in food intake during estrus in ovarian-intact, cycling rats [44] and [155]. Estradiol exerts an inhibitory effect on energy intake by decreasing meal size but not meal number [7]. Estradiol interacts with many circulating signals, including insulin and leptin, to mediate the estrogenic inhibition of feeding behavior. This has been reviewed by Asarian and Geary [7]. Consistent with numerous previous reports [22], [41], [75], [93], [163], [164],[165] and [168], OVX results in a rapid increase in food intake and body weight relative to sham operated females, and this behavioral response is abolished by estradiol treatment alone [6]. Once a new body weight has been established, food intake returns to the level of the sham-operated females, yet the OVX defend this higher body weight. Addition of estrogen with administrations of exogenous estrogen at physiological doses every four days mimics estrus cycles in OVX rats and returns adiposity to the level of the shams [93], [164] and [165]. Therefore, estrogen appears to regulate body weight by recruiting initial changes of food intake, body adiposity, and/or energy expenditure.
3.3.4. Estrogen, a possible adiposity signal, closely interacts with leptin signaling
As previously indicated, adiposity signals transduce hormonal input into neurobiological responses to make compensatory adjustments by regulating food intake and energy expenditure, and consequently regulating body fat distribution [145] and [172]. Estrogen also fulfills these criteria and thus can be considered another potential adiposity signal. Specifically, it is released from the ovaries, crosses the blood brain barrier, binds to estrogen receptors located in key hypothalamic nuclei, and reduces food intake and body weight. Additionally, when delivered directly into the central ventricular system, it decreases food intake possibly through its actions on the same neurons that are responsible for leptin’s anorectic responses [60].
Estrogen and leptin have overlapping targeted nuclei. Hypothalamic cells that are immunoreactive for estrogen receptors also express leptin receptors [38]. The extensive hypothalamic co-localization of the long form of the leptin and estrogen receptors, Ob-Rb and ERα in the critical brain regions that modulate energy homeostasis, including arcuate nucleus (ARC), ventromedial hypothalamic nucleus (VMN) and parvicellular portion of the paraventricular nucleus (PVN), suggests a closely coupled interaction between these peripheral signals in the regulation of behavioral and neuroendocrine mechanisms of energy homeostasis at a central level [38]. In addition to anatomic overlapping of their receptors, estrogen influences leptin receptor expression. Estrogen levels appear to regulate the expression of the leptin receptors during the estrous cycle. Leptin receptor expression levels are lowest in proestrus, the point of the estrus cycle with the highest levels of estradiol, in the choroid plexus, and these changes correspond inversely with levels of circulating estradiol over the 4-day estrous cycle in the rat [8]. Estradiol might regulate leptin receptor expression independent of leptin levels. Although circulating leptin does not change during the estrous cycle, ARC Ob-Rb expression is highest during estrous and metestrous [8], providing a potential mechanism for cyclic variations in energy intake and activity seen in females. During normal estrous cycle, high estrogen level is associated with low expression of the leptin receptors. Ovariectomy has been reported to cause a marked reduction in expression of long form of the leptin receptor in the hypothalamus and estradiol replacement restores its expression [109], suggesting development of leptin resistance when estrogen is experimentally removed. However, it is counterintuitive since there is an estrogen response element in the leptin receptor gene. More research in this area is warranted.
Estrogen and leptin have similar molecular effects. STAT3 is a downstream target of leptin signaling and is activated by leptin. Similarly, intraperitoneal estrogen administration induces tyrosine phosphorylation of STAT3 in the hypothalamus in less than 30 min [61]. These findings provide molecular support for the interaction between estrogen and leptin in the regulation of energy homeostasis.
Estrogen alters sensitivity to centrally administered leptin. Peripheral or central administration of 17 β-estradiol to ovariectomised females restores the central leptin sensitivity [29]. In addition, administration of 17 β-estradiol increases sensitivity to central leptin, decreases sensitivity to central insulin in males [29]. These findings suggest that gonadal steroids interact with the adiposity message conveyed to the brain by leptin and insulin, resulting in differential sensitivity to these signals in males and females [29]. Central leptin signaling might affect hypothalamic ERα gene expression. Female mice that lack leptin receptors specifically in the proopiomelanocortin neurons (POMC Lepr-KO), one critical population of leptin receptors, have normal circulating estradiol but reduced hypothalamic ERα mRNA level [148]. Additionally, POMC Lepr-KO females accumulate greater percentage of visceral adipose tissue than male POMC Lepr-KO mice do [149]. Therefore, the combination of estrogen and functional leptin signaling is required for sex-specific fat distribution. Lack of either estrogen or functional leptin signaling leads to visceral obesity (Fig. 1).
4. Sexual dimorphism in regulation of energy balance
The phenomenon of maintaining typical sex-specific fat distributions in males and females suggests sex-specific mechanisms that regulate energy balance and adiposity. Male and female rodents use different behavioral and metabolic strategies to regulate energy balance. When overfed voluntarily with a palatable high fat diet chronically [137] or acutely [138], female rats gain more body weight than males due to greater conservation of energy expenditure with lower activation of thermogenesis in brown adipose tissue combined with lower energy intake. Male and female rodents respond differentially to two distinct approaches to reduce fat mass: caloric restriction (CR) and surgical removal of adipose tissue (i.e., lipectomy). Specifically, females but not males decrease oxygen consumption and thermogenesis during [151] and [159] or after lipectomy [151], whereas males respond by eating more food when food is returned following CR or lipectomy [151].
The phenomenon of using different behavioral strategies to gain or lose fat between males and females is also seen in some genetic mouse models. Syndecan-3 is a cell surface molecule found in the hypothalamus which facilitates the blockade of melanocortin receptors MC3/4R by the endogenous melanocortin antagonist agouti-related protein [135]. As a result, syndecan-3-deficient mice are more sensitive to the anorectic actions of melanocortin receptor agonists and are resistant to the weight gain that occurs when exposed to a high-fat diet. However, male and female syndean-3-deficient mice accomplish this reduced weight gain by different strategies. Males maintain their leanness as a primary result of consuming less high-fat diet while females maintain their leanness by expending more energy [153]. Lack of leptin receptors in POMC neurons increases body fat accumulation in both males and females, and leads to conservation of energy expenditure in females but not males [149]. In a separate model, both male and female mice with targeted disruption of the gene encoding granulocyte macrophage-colony stimulating factor show greater fat mass, and the mechanism is again sexually dimorphic with the males showing pronounced increase in intake while females have greater decreases in expenditure [134].
Collectively these examples point to unique strategies for body weight regulation in males as opposed to females, i.e., males primarily adjust energy intake whereas females primarily alter expenditure to regulate energy homeostasis. The primary strategy of males is to gain fat by increasing energy intake whereas that of females is to gain fat by decreasing energy expenditure.
5. Summary
Sex specific distribution of body fat has important implications for how obesity influences a wide variety of co-morbid conditions. A wide range of evidence links these differences in body fat distribution to gonadal steroids that also have important effects on the regulation of energy balance. As a result, males and females also appear to have important differences in the systems that regulate energy balance and body weight. Specifically, females store energy in the subcutaneous depot when energy is surfeit and utilize subcutaneous fat under energy-challenged conditions in which less energy is taken in than is expended in metabolism. Females tend to adjust the energy expenditure whereas males adjust the energy intake side of the energy balance equation. Males and females respond differently to the adiposity signals with females being more sensitive to leptin and males being more sensitive to insulin. There appears to have commonality among the intracellular signaling pathways activated by leptin, insulin, and estrogen. Leptin and insulin signaling converges on the phosphoinositide 3-kinase (PI3K) pathway and their actions depend on PI3K activation [120]. Estrogen also activates the PI3K signaling cascade [100] and [101]. More research is needed to better understand the cross-talk between insulin, leptin, and estrogen signaling at a molecular level.
The large differences between males and females in the regulation of energy homeostasis suggest the need for potentially different strategies for males and females to produce therapeutic weight loss. Unfortunately very little work actually addresses these potentially important differences between males and females. It is our contention that much more research must be done to understand how males and females differ and how approaches to weight loss can be tailored to each sex.
Acknowledgments
This work is supported by NIH grants National Research Service Award DK75255 (HS) and DK73689 (DJC).
References
- [1].Abraham IM, Han S-K, Todman MG, Korach KS, Harrison AE. Estrogen receptor {beta} mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J. Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ahima RS, Kelly J, Elmquist JK, Flier JS. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology. 1999;140:4923–4931. doi: 10.1210/endo.140.11.7105. [DOI] [PubMed] [Google Scholar]
- [3].Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int. J. Obes. Relat. Metab. Disord. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- [4].Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- [5].Andreyeva T, Sturm R, Ringel JS. Moderate and severe obesity have large differences in health care costs. Obes. Res. 2004;12:1936–1943. doi: 10.1038/oby.2004.243. [DOI] [PubMed] [Google Scholar]
- [6].Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- [7].Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bennett PA, Lindell K, Wilson C, Carlsson LM, Carlsson B, Robinson IC. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology. 1999;69:417–423. doi: 10.1159/000054444. [DOI] [PubMed] [Google Scholar]
- [9].Bickerton AST, Roberts R, Fielding BA, Hodson L, Blaak EE, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 2007;56:168–176. doi: 10.2337/db06-0822. [DOI] [PubMed] [Google Scholar]
- [10].Bjorkelund C, Lissner L, Andersson S, Lapidus L, Bengtsson C. Reproductive history in relation to relative weight and fat distribution. Int. J. Obes. Relat. Metab. Disord. 1996;20:213–219. [PubMed] [Google Scholar]
- [11].Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- [12].Bjorntorp P. Abdominal fat distribution and disease: an overview of epidemiological data. Ann. Med. 1992;24:15–18. doi: 10.3109/07853899209164140. [DOI] [PubMed] [Google Scholar]
- [13].Bjorntorp P. Abdominal fat distribution and the metabolic syndrome. J. Cardiovasc. Pharmacol. 1992;20(Suppl. 8):S26–S28. [PubMed] [Google Scholar]
- [14].Bjorntorp P. Abdominal obesity and the metabolic syndrome. Ann. Med. 1992;24:465–468. doi: 10.3109/07853899209166997. [DOI] [PubMed] [Google Scholar]
- [15].Bjorntorp P. Hormonal effects on fat distribution and its relationship to health risk factors. Acta Paediatr. 1992;(Suppl. 383):59–60. discussion 1. [PubMed] [Google Scholar]
- [16].Bjorntorp P. Metabolic abnormalities in visceral obesity. Ann. Med. 1992;24:3–5. doi: 10.3109/07853899209164137. [DOI] [PubMed] [Google Scholar]
- [17].Bjorntorp P. Regional fat distribution – implications for type II diabetes. Int. J. Obes. Relat. Metab. Disord. 1992;16(Suppl. 4):S19–S27. [PubMed] [Google Scholar]
- [18].Bjorntorp P. The android woman – a risky condition. J. Intern. Med. 1996;239:105–110. doi: 10.1046/j.1365-2796.1996.364690000.x. [DOI] [PubMed] [Google Scholar]
- [19].Bjorntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13:795–803. doi: 10.1016/s0899-9007(97)00191-3. [DOI] [PubMed] [Google Scholar]
- [20].Bjorntorp P. Hormonal control of regional fat distribution. Hum. Reprod. 1997;12(Suppl. 1):21–25. doi: 10.1093/humrep/12.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- [21].Bjorntorp P. Obesity. Lancet. 1997;350:423–426. doi: 10.1016/S0140-6736(97)04503-0. [DOI] [PubMed] [Google Scholar]
- [22].Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol. Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- [23].Bolinder J, Engfeldt P, Ostman J, Arner P. Site differences in insulin receptor binding and insulin action in subcutaneous fat of obese females. J. Clin. Endocrinol. Metab. 1983;57:455–461. doi: 10.1210/jcem-57-3-455. [DOI] [PubMed] [Google Scholar]
- [24].Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- [25].Bouchard C, Despres JP, Mauriege P. Genetic and nongenetic determinants of regional fat distribution. Endocr. Rev. 1993;14:72–93. doi: 10.1210/edrv-14-1-72. [DOI] [PubMed] [Google Scholar]
- [26].Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM Diabetes. 1996;45:633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- [27].Carpéné C, Bousquet-MÉLou A, Galitzky J, Berlan M, Lafontan M. Lipolytic effects of beta 1-, beta 2-, and beta 3-adrenergic agonists in white adipose tissue of mammals. Ann. NY Acad. Sci. 1998;839:186–189. doi: 10.1111/j.1749-6632.1998.tb10756.x. [DOI] [PubMed] [Google Scholar]
- [28].Casabiell X, Pineiro V, Peino R, Lage M, Camina J, et al. Gender differences in both spontaneous and stimulated leptin secretion by human omental adipose tissue in vitro: dexamethasone and estradiol stimulate leptin release in women, but not in men. J. Clin. Endocrinol. Metab. 1998;83:2149–2155. doi: 10.1210/jcem.83.6.4849. [DOI] [PubMed] [Google Scholar]
- [29].Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- [30].Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- [31].Colombel A, Charbonnel B. Weight gain and cardiovascular risk factors in the post-menopausal women. Hum. Reprod. 1997;12:134–145. doi: 10.1093/humrep/12.suppl_1.134. [DOI] [PubMed] [Google Scholar]
- [32].Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am. J. Physiol. 1999;276:E233–E240. doi: 10.1152/ajpendo.1999.276.2.E233. [DOI] [PubMed] [Google Scholar]
- [33].Crandall DL, Busler DE, Novak TJ, Weber RV, Kral JG. Identification of estrogen receptor beta RNA in human breast and abdominal subcutaneous adipose tissue. Biochem. Biophys. Res. Commun. 1998;248:523. doi: 10.1006/bbrc.1998.8997. [DOI] [PubMed] [Google Scholar]
- [34].Cummings DE, Schwartz MW. Genetics and pathophysiology of human obesity. Ann. Rev. Med. 2003;54:453–471. doi: 10.1146/annurev.med.54.101601.152403. [DOI] [PubMed] [Google Scholar]
- [35].Demerath EW, Towne B, Wisemandle W, Blangero J, Chumlea WC, Siervogel RM. Serum leptin concentration, body composition, and gonadal hormones during puberty. Int. J. Obes. Relat. Metab. Disord. 1999;23:678–685. doi: 10.1038/sj.ijo.0800902. [DOI] [PubMed] [Google Scholar]
- [36].Despres JP. The insulin-resistance-dyslipidemic syndrome of visceral obesity: effect on patients’ risk. Obes. Res. 1998;6(Suppl. 1):8S–17S. doi: 10.1002/j.1550-8528.1998.tb00683.x. [DOI] [PubMed] [Google Scholar]
- [37].Diamanti-Kandarakis E. Role of obesity and adiposity in polycystic ovary syndrome. Int. J. Obes. 2007;31:S8–S13. doi: 10.1038/sj.ijo.0803730. [DOI] [PubMed] [Google Scholar]
- [38].Diano S, Kalra SP, Sakamoto H, Horvath TL. Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain Res. 1998;812:256–259. doi: 10.1016/s0006-8993(98)00936-6. [DOI] [PubMed] [Google Scholar]
- [39].Donahue RP, Abbott RD. Central obesity and coronary heart disease in men. Lancet. 1987;2:1215. doi: 10.1016/s0140-6736(87)91357-2. [DOI] [PubMed] [Google Scholar]
- [40].Donahue RP, Orchard TJ, Becker DJ, Kuller LH, Drash AL. Sex differences in the coronary heart disease risk profile: a possible role for insulin. The Beaver County study. Am. J. Epidemiol. 1987;125:650–657. doi: 10.1093/oxfordjournals.aje.a114578. [DOI] [PubMed] [Google Scholar]
- [41].Drewett RF. Sexual behaviour and sexual motivation in the female rat. Nature. 1973;242:476–477. doi: 10.1038/242476a0. [DOI] [PubMed] [Google Scholar]
- [42].Dua A, Hennes MI, Hoffman RG, Maas DL, Krakower GR, et al. Leptin: a significant indicator of total body fat but not of visceral fat and insulin insensitivity in African–American women. Diabetes. 1996;45:1635–1637. doi: 10.2337/diab.45.11.1635. [DOI] [PubMed] [Google Scholar]
- [43].Dworkin BR. Learning and physiological regulation. University of Chicago Press; Chicago: 1993. [Google Scholar]
- [44].Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol. Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- [45].Eckel RH, Barouch WW, Ershow AG. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the Pathophysiology of Obesity-Associated Cardiovascular Disease. Circulation. 2002;105:2923–2928. doi: 10.1161/01.cir.0000017823.53114.4c. [DOI] [PubMed] [Google Scholar]
- [46].Edens NK, Fried SK, Kral JG, Hirsch J, Leibel RL. In vitro lipid synthesis in human adipose tissue from three abdominal sites. Am. J. Physiol. 1993;265:E374–E379. doi: 10.1152/ajpendo.1993.265.3.E374. [DOI] [PubMed] [Google Scholar]
- [47].Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am. J. Physiol. 1999;276:E317–E325. doi: 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]
- [48].Elbers JM, de Roo GW, Popp-Snijders C, Nicolaas-Merkus A, Westerveen E, et al. Effects of administration of 17beta-oestradiol on serum leptin levels in healthy postmenopausal women. Clin. Endocrinol. (Oxf.) 1999;51:449–454. doi: 10.1046/j.1365-2265.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- [49].Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am. J. Clin. Nutr. 1986;44:739–746. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- [50].Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am. J. Physiol. 1978;235:E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- [51].Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. Jama. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- [52].Ford ES. Prevalence of the metabolic syndrome defined by the international diabetes federation among adults in the US. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- [53].Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third national health and nutrition examination survey. Jama. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- [54].Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Prevalence of the metabolic syndrome among US adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008;31:587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- [55].Franks PW, Hanson RL, Knowler WC, Moffett C, Enos G, et al. Childhood predictors of young-onset type 2 diabetes. Diabetes. 2007;56:2964–2972. doi: 10.2337/db06-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Frayn KN, Coppack SW. Insulin resistance, adipose tissue and coronary heart disease. Clin. Sci. 1992;82:1–8. doi: 10.1042/cs0820001. [DOI] [PubMed] [Google Scholar]
- [57].Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- [58].Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- [59].Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, et al. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J. Clin. Endocrinol. Metab. 1997;82:414–417. doi: 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- [60].Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am. J. Physiol. 2008;294:E817–E826. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]
- [61].Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat. Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- [62].Genazzani AR, Gambacciani M. Effect of climacteric transition and hormone replacement therapy on body weight and body fat distribution. Gynecol. Endocrinol. 2006;22:145–150. doi: 10.1080/09513590600629092. [DOI] [PubMed] [Google Scholar]
- [63].Haarbo J, Hansen BF, Christiansen C. Hormone replacement therapy prevents coronary artery disease in ovariectomized cholesterol-fed rabbits. Apmis. 1991;99:721–727. doi: 10.1111/j.1699-0463.1991.tb01250.x. [DOI] [PubMed] [Google Scholar]
- [64].Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism. 1991;40:1323–1326. doi: 10.1016/0026-0495(91)90037-w. [DOI] [PubMed] [Google Scholar]
- [65].Hallschmid M, Benedict C, Schultes B, Fehm H-L, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004;53:3024–3029. doi: 10.2337/diabetes.53.11.3024. [DOI] [PubMed] [Google Scholar]
- [66].Havel PJ, Kasim-Karakas S, Dubuc GR, Mueller W, Phinney SD. Gender differences in plasma leptin concentrations. Nat. Med. 1996;2:949–950. doi: 10.1038/nm0996-949b. [DOI] [PubMed] [Google Scholar]
- [67].Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hoyenga KB, Hoyenga KT. Gender and energy balance: sex differences in adaptations for feast and famine. Physiol. Behav. 1982;28:545. doi: 10.1016/0031-9384(82)90153-6. [DOI] [PubMed] [Google Scholar]
- [69].Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J. Clin. Endocrinol. Metab. 1999;84:3673–3680. doi: 10.1210/jcem.84.10.6082. [DOI] [PubMed] [Google Scholar]
- [70].Jennifer AOD. Gender, ethnicity, culture and social class influences on childhood obesity among Australian schoolchildren: implications for treatment, prevention and community education. Health Soc. Care Community. 2008;16:282–290. doi: 10.1111/j.1365-2524.2008.00768.x. [DOI] [PubMed] [Google Scholar]
- [71].Jensen MD, Cryer PE, Johnson CM, Murray MJ. Effects of epinephrine on regional free fatty acid and energy metabolism in men and women. Am. J. Physiol. 1996;270:E259–E264. doi: 10.1152/ajpendo.1996.270.2.E259. [DOI] [PubMed] [Google Scholar]
- [72].Jockenhovel F, Blum WF, Vogel E, Englaro P, Muller-Wieland D, et al. Testosterone substitution normalizes elevated serum leptin levels in hypogonadal men. J. Clin. Endocrinol. Metab. 1997;82:2510–2513. doi: 10.1210/jcem.82.8.4174. [DOI] [PubMed] [Google Scholar]
- [73].Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch. Intern. Med. 1989;149:1514–1520. doi: 10.1001/archinte.149.7.1514. [DOI] [PubMed] [Google Scholar]
- [74].Keesey RE, Powley TL. The regulation of body weight. Ann. Rev. Psychol. 1986;37:109–133. doi: 10.1146/annurev.ps.37.020186.000545. [DOI] [PubMed] [Google Scholar]
- [75].Kemnitz JW, Gibber JR, Lindsay KA, Eisele SG. Effects of ovarian hormones on eating behaviors, body weight, and glucoregulation in rhesus monkeys. Horm. Behav. 1989;23:235–250. doi: 10.1016/0018-506x(89)90064-0. [DOI] [PubMed] [Google Scholar]
- [76].Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J. Clin. Endocrinol. Metab. 1997;82:1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- [77].Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. (Biol.) 1953;140:579–592. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- [78].Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Klein S, Fontana L, Young VL, Coggan AR, Kilo C, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. New Engl. J. Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- [80].Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int. J. Obes. Relat. Metab. Disord. 1994;18:2–207. [PubMed] [Google Scholar]
- [81].Koutsari C, Dumesic DA, Patterson BW, Votruba SB, Jensen MD. Plasma free fatty acid storage in subcutaneous and visceral adipose tissue in postabsorptive women. Diabetes. 2008;57:1186–1194. doi: 10.2337/db07-0664. [DOI] [PubMed] [Google Scholar]
- [82].Kristensen K, Pedersen SB, Richelsen B. Regulation of leptin by steroid hormones in rat adipose tissue. Biochem. Biophys. Res. Commun. 1999;259:624–630. doi: 10.1006/bbrc.1999.0842. [DOI] [PubMed] [Google Scholar]
- [83].Krotkiewski M, Björntorp P, Sjöström L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Invest. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-A. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lafontan M, Berlan M. Fat cell alpha 2-adrenoceptors: the regulation of fat cell function and lipolysis. Endocr. Rev. 1995;16:716–738. doi: 10.1210/edrv-16-6-716. [DOI] [PubMed] [Google Scholar]
- [86].Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol. Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- [87].Lamarche B. Abdominal obesity and its metabolic complications: implications for the risk of ischaemic heart disease. Coron. Artery Dis. 1998;9:473–481. doi: 10.1097/00019501-199809080-00002. [DOI] [PubMed] [Google Scholar]
- [88].Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Brit. Med. J. 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Legato MJ. Gender-specific aspects of obesity. Int. J. Fertil. Womens Med. 1997;42:184–197. [PubMed] [Google Scholar]
- [90].Legato MJ. Gender-specific physiology: how real is it? How important is it? Int. J. Fertil. Womens Med. 1997;42:19–29. [PubMed] [Google Scholar]
- [91].Leibel RL, Hirsch J. Site- and sex-related differences in adrenoreceptor status of human adipose tissue. J. Clin. Endocrinol. Metab. 1987;64:1205–1210. doi: 10.1210/jcem-64-6-1205. [DOI] [PubMed] [Google Scholar]
- [92].Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am. Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- [93].Leshner AI, Collier G. The effects of gonadectomy on the sex differences in dietary self-selection patterns and carcass compositions of rats. Physiol. Behav. 1973;11:671–676. doi: 10.1016/0031-9384(73)90253-9. [DOI] [PubMed] [Google Scholar]
- [94].Llado I, Rodriguez-Cuenca S, Pujol E, Monjo M, Estrany ME, et al. Gender effects on adrenergic receptor expression and lipolysis in white adipose tissue of rats. Obes. Res. 2002;10:296–305. doi: 10.1038/oby.2002.41. [DOI] [PubMed] [Google Scholar]
- [95].Lonnqvist F, Thorne A, Large V, Arner P. Sex differences in visceral fat lipolysis and metabolic complications of obesity. Arterioscler. Thromb. Vasc. Biol. 1997;17:1472–1480. doi: 10.1161/01.atv.17.7.1472. [DOI] [PubMed] [Google Scholar]
- [96].López-Luna P, Maier I, Herrera E. Carcass and tissue fat content in the pregnant rat. Biol. Neonate. 1991;60:29–38. doi: 10.1159/000243385. [DOI] [PubMed] [Google Scholar]
- [97].Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- [98].Luukkaa V, Pesonen U, Huhtaniemi I, Lehtonen A, Tilvis R, et al. Inverse correlation between serum testosterone and leptin in men. J Clin. Endocrinol. Metab. 1998;83:3243–3246. doi: 10.1210/jcem.83.9.5134. [DOI] [PubMed] [Google Scholar]
- [99].Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140:1567–1574. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- [100].Malyala A, Kelly MJ, Rønnekleiv OK. Estrogen modulation of hypothalamic neurons: activation of multiple signaling pathways and gene expression changes. Steroids. 2005;70:397. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [101].Malyala A, Zhang C, Bryant DN, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J. Comp. Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- [102].Mannucci E, Ognibene A, Becorpi A, Cremasco F, Pellegrini S, et al. Relationship between leptin and oestrogens in healthy women. Eur. J. Endocrinol. 1998;139:198–201. doi: 10.1530/eje.0.1390198. [DOI] [PubMed] [Google Scholar]
- [103].Marin BV, Tschann JM, Gomez CA, Kegeles SM. Acculturation and gender differences in sexual attitudes and behaviors: hispanic vs non-hispanic white unmarried adults. Am. J. Public Health. 1993;83:1759–1761. doi: 10.2105/ajph.83.12.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Marin P, Andersson B, Ottosson M, Olbe L, Chowdhury B, et al. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism. 1992;41:1242–1248. doi: 10.1016/0026-0495(92)90016-4. [DOI] [PubMed] [Google Scholar]
- [105].Marin P, Lonn L, Andersson B, Oden B, Olbe L, et al. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J. Clin. Endocrinol. Metab. 1996;81:1018–1022. doi: 10.1210/jcem.81.3.8772568. [DOI] [PubMed] [Google Scholar]
- [106].Masuzaki H, Ogawa Y, Isse N, Satoh N, Okazaki T, et al. Human obese gene expression: adipocyte-specific expression and regional differences in the adipose tissue. Diabetes. 1995;44:855–858. doi: 10.2337/diab.44.7.855. [DOI] [PubMed] [Google Scholar]
- [107].Matelski H, Greene R, Huberman M, Lokich J, Zipoli T. Randomized trial of estrogen vs. tamoxifen therapy for advanced breast cancer. Am. J. Clin. Oncol. 1985;8:128–133. doi: 10.1097/00000421-198504000-00004. [DOI] [PubMed] [Google Scholar]
- [108].McEwen BS. The molecular and neuroanatomical basis for estrogen effects in the central nervous system. J. Clin. Endocrinol. Metab. 1999;84:1790–1797. doi: 10.1210/jcem.84.6.5761. [DOI] [PubMed] [Google Scholar]
- [109].Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145:3115–3121. doi: 10.1210/en.2004-0129. [DOI] [PubMed] [Google Scholar]
- [110].Micheli H, Carlson LA, Hallberg D. Comparison of lipolysis in human subcutaneous and omental adipose tissue with regard to effects of noradrenaline, theophylline, prostaglandin E1 and age. Acta Chir. Scand. 1969;135:663–670. [PubMed] [Google Scholar]
- [111].Mizutani T, Nishikawa Y, Adachi H, Enomoto T, Ikegami H, et al. Identification of estrogen receptor in human adipose tissue and adipocytes. J. Clin. Endocrinol. Metab. 1994;78:950–954. doi: 10.1210/jcem.78.4.8157726. [DOI] [PubMed] [Google Scholar]
- [112].Montague CT, Prins JBS, Sanders LZ, Sewter CPD, Byrne CD, O’Rahilly S. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes. 1998;47:1384–1391. doi: 10.2337/diabetes.47.9.1384. [DOI] [PubMed] [Google Scholar]
- [113].Montague CT, Prins JBS, Digby JE, O’Rahilly S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46:342–347. doi: 10.2337/diab.46.3.342. [DOI] [PubMed] [Google Scholar]
- [114].Morita Y, Iwamoto I, Mizuma N, Kuwahata T, Matsuo T, et al. Precedence of the shift of body-fat distribution over the change in body composition after menopause. J. Obstet. Gynaecol. Res. 2006;32:513–516. doi: 10.1111/j.1447-0756.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- [115].Morley JE, Perry HM. Androgen deficiency in aging men: role of testosterone replacement therapy. J. Lab. Clin. Med. 2000;135:370–378. doi: 10.1067/mlc.2000.106455. 3rd. [DOI] [PubMed] [Google Scholar]
- [116].Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics follow-up study. Pediatrics. 2007;120:340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- [117].Mujica V, Leiva E, Icaza G, Diaz N, Arredondo M, et al. Evaluation of metabolic syndrome in adults of Talca city, Chile. Nutr. J. 2008;7:14. doi: 10.1186/1475-2891-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, et al. Silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Nguyen TT, Mijares AH, Johnson CM, Jensen MD. Postprandial leg and splanchnic fatty acid metabolism in nonobese men and women. Am. J. Physiol. 1996;271:E965–E972. doi: 10.1152/ajpendo.1996.271.6.E965. [DOI] [PubMed] [Google Scholar]
- [120].Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front. Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- [121].O’Shaughnessy IM, Myers TJ, Stepniakowski K, Nazzaro P, Kelly TM, et al. Glucose metabolism in abdominally obese hypertensive and normotensive subjects. Hypertension. 1995;26:186–192. doi: 10.1161/01.hyp.26.1.186. [DOI] [PubMed] [Google Scholar]
- [122].Ogden CL, Carroll MD, Flegal KM. Epidemiologic trends in overweight and obesity. Endocrinol. Metab. Clin. N. Am. 2003;32:741–760. doi: 10.1016/s0889-8529(03)00074-4. [DOI] [PubMed] [Google Scholar]
- [123].Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- [124].Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly M, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha- deficient male mice. Biochem. Biophys. Res. Commun. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- [125].Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int. J. Obes. Relat. Metab. Disord. 2003;27:1020–1027. doi: 10.1038/sj.ijo.0802378. [DOI] [PubMed] [Google Scholar]
- [126].Ostlund RE, Jr., Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J. Clin. Endocrinol. Metab. 1996;81:3909–3913. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- [127].Popkin BM, Doak C. The obesity epidemic is a worldwide phenomenon. Nutr. Rev. 1998;56:106–114. doi: 10.1111/j.1753-4887.1998.tb01722.x. [DOI] [PubMed] [Google Scholar]
- [128].Price TM, O’Brien SN. Determination of estrogen receptor messenger ribonucleic acid (mRNA) and cytochrome P450 aromatase mRNA levels in adipocytes and adipose stromal cells by competitive polymerase chain reaction amplification. J. Clin. Endocrinol. Metab. 1993;77:1041–1045. doi: 10.1210/jcem.77.4.8408452. [DOI] [PubMed] [Google Scholar]
- [129].Quinton ND, Laird SM, Okon MA, Li TC, Smith RF, et al. Serum leptin levels during the menstrual cycle of healthy fertile women. Brit. J. Biomed. Sci. 1999;56:16–19. [PubMed] [Google Scholar]
- [130].Quinton ND, Smith RF, Clayton PE, Gill MS, Shalet S, et al. Leptin binding activity changes with age: the link between leptin and puberty. J. Clin. Endocrinol. Metab. 1999;84:2336–2341. doi: 10.1210/jcem.84.7.5834. [DOI] [PubMed] [Google Scholar]
- [131].Ramsay DS, Seeley RJ, Bolles RC, Woods SC. Capaldi ED, editor. Ingestive homeostasis: the primacy of learning. Why We Eat What We Eat. 1996:11–27. [Google Scholar]
- [132].Rebuffé-Scrive M, Andersson B, Olbe L, Björntorp P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism. 1989;38:453–458. doi: 10.1016/0026-0495(89)90198-4. [DOI] [PubMed] [Google Scholar]
- [133].Rebuffé-Scrive M, Enk L, Crona N, Lönnroth P, Abrahamsson L, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J. Clin. Invest. 1985;75:1973–1976. doi: 10.1172/JCI111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Reed JA, Clegg DJ, Smith KB, Tolod-Richer EG, Matter EK, et al. GM-CSF action in the CNS decreases food intake and body weight. J. Clin. Invest. 2005;115:3035–3044. doi: 10.1172/JCI25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Reizes O, Lincecum J, Wang Z, Goldberger O, Huang L, et al. Transgenic expression of syndecan-1 uncovers a physiological control of feeding behavior by syndecan-3. Cell. 2001;106:105–116. doi: 10.1016/s0092-8674(01)00415-9. [DOI] [PubMed] [Google Scholar]
- [136].Richelsen B. Increased alpha 2- but similar beta-adrenergic receptor activities in subcutaneous gluteal adipocytes from females compared with males. Eur. J. Clin. Invest. 1986;16:302–309. doi: 10.1111/j.1365-2362.1986.tb01346.x. [DOI] [PubMed] [Google Scholar]
- [137].Roca P, Rodriguez AM, Oliver P, Bonet ML, Quevedo S, et al. Brown adipose tissue response to cafeteria diet-feeding involves induction of the UCP2 gene and is impaired in female rats as compared to males. Pflugers Arch. 1999;438:628–634. doi: 10.1007/s004249900107. [DOI] [PubMed] [Google Scholar]
- [138].Rodriguez E, Monjo M, Rodriguez-Cuenca S, Pujol E, Amengual B, et al. Sexual dimorphism in the adrenergic control of rat brown adipose tissue response to overfeeding. Pflugers Arch. 2001;442:396–403. doi: 10.1007/s004240100556. [DOI] [PubMed] [Google Scholar]
- [139].Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am. J. Physiol. 2000;279:E455–E462. doi: 10.1152/ajpendo.2000.279.2.E455. [DOI] [PubMed] [Google Scholar]
- [140].Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J. Clin. Endocrinol. Metab. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- [141].Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, et al. Sexual dimorphism in plasma leptin concentration. J. Clin. Endocrinol. Metab. 1997;82:579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- [142].Santosa S, Jensen MD. Why are we shaped differently and why does it matter? Am. J. Physiol. 2008;295:E531–E535. doi: 10.1152/ajpendo.90357.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, et al. Targeted disruption of the estrogen receptor-alpha gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology. 1999;140:2733–2744. doi: 10.1210/endo.140.6.6823. [DOI] [PubMed] [Google Scholar]
- [144].Schwartz MW, Seeley RJ. The new biology of body weight regulation. J. Am. Diet. Assoc. 1997;97:54–58. doi: 10.1016/S0002-8223(97)00018-7. [DOI] [PubMed] [Google Scholar]
- [145].Schwartz MW, Woods SC, Porte DJ, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- [146].Seeley RJ, Ramsay DS, Woods SC. Bouton ME, Fanselow MS, editors. Regulation of food intake: interactions between learning and physiology. Learning, Motivation, and Cognition: The Functional Behaviorism of Robert C. Bolles. 1997:99–115. [Google Scholar]
- [147].Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007;56:1369–1375. doi: 10.2337/db06-1680. [DOI] [PubMed] [Google Scholar]
- [148].Shi H, Sorrell JE, Clegg DJ, Woods SC, Seeley RJ. Leptin receptors in proopiomelanocortin (POMC) neurons and estrogen action. Endocr. Soc. Abst. 2008:OR31–OR35. [Google Scholar]
- [149].Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am. J. Physiol. 2008;294:E630–E639. doi: 10.1152/ajpendo.00704.2007. [DOI] [PubMed] [Google Scholar]
- [150].Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am. J. Physiol. 2007;293:E1012–E1020. doi: 10.1152/ajpendo.00649.2006. [DOI] [PubMed] [Google Scholar]
- [151].Shi H, Strader AD, Woods SC, Seeley RJ. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am. J. Physiol. 2007;293:E316–E326. doi: 10.1152/ajpendo.00710.2006. [DOI] [PubMed] [Google Scholar]
- [152].Speer G, Cseh K, Winkler G, Vargha P, Braun E, et al. Vitamin D and estrogen receptor gene polymorphisms in type 2 diabetes mellitus and in android type obesity. Eur. J. Endocrinol. 2001;144:385–389. doi: 10.1530/eje.0.1440385. [DOI] [PubMed] [Google Scholar]
- [153].Strader AD, Reizes O, Woods SC, Benoit SC, Seeley RJ. Mice lacking the syndecan-3 gene are resistant to diet-induced obesity. J. Clin. Invest. 2004;114:1354–1360. doi: 10.1172/JCI20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Syme C, Abrahamowicz M, Leonard GT, Perron M, Pitiot A, et al. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence: sex differences and underlying mechanisms. Arch. Pediatr. Adolesc. Med. 2008;162:453–461. doi: 10.1001/archpedi.162.5.453. [DOI] [PubMed] [Google Scholar]
- [155].Tarttelin MF, Gorski RA. Variations in food and water intake in the normal and acyclic female rat. Physiol. Behav. 1971;7:847–852. doi: 10.1016/0031-9384(71)90050-3. [DOI] [PubMed] [Google Scholar]
- [156].Thörne A, Lönnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int. J. Obes. Relat. Metab. Disord. 2002;26:193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- [157].Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol. Scand. 2005;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- [159].Valle A, Catala-Niell A, Colom B, Garcia-Palmer FJ, Oliver J, Roca P. Sex-related differences in energy balance in response to caloric restriction. Am. J. Physiol. 2005;289:E15–E22. doi: 10.1152/ajpendo.00553.2004. [DOI] [PubMed] [Google Scholar]
- [160].Vettor R, De Pergola G, Pagano C, Englaro P, Laudadio E, et al. Gender differences in serum leptin in obese people: relationships with testosterone, body fat distribution and insulin sensitivity. Eur. J. Clin. Invest. 1997;27:1016–1024. doi: 10.1046/j.1365-2362.1997.2270773.x. [DOI] [PubMed] [Google Scholar]
- [161].Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD. Meal fatty acid uptake in visceral fat in women. Diabetes. 2007;56:2589–2597. doi: 10.2337/db07-0439. [DOI] [PubMed] [Google Scholar]
- [162].Wabitsch M, Blum WF, Muche R, Braun M, Hube F, et al. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J. Clin. Invest. 1997;100:808–813. doi: 10.1172/JCI119595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol. Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- [164].Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol. Behav. 1979;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- [165].Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. Int. J. Obes. 1985;9(Suppl. 1):83–92. [PubMed] [Google Scholar]
- [166].Wahrenberg H, Lönnqvist F, Arner P. Mechanisms underlying regional differences in lipolysis in human adipose tissue. J. Clin. Invest. 1989;84:458–467. doi: 10.1172/JCI114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- [168].Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J. Nutr. 2001;131:2351–2357. doi: 10.1093/jn/131.9.2351. [DOI] [PubMed] [Google Scholar]
- [169].Wingard DL, Suarez L, Barrett-Connor E. The sex differential in mortality from all causes and ischemic heart disease. Am. J. Epidemiol. 1983;117:165–172. doi: 10.1093/oxfordjournals.aje.a113527. [DOI] [PubMed] [Google Scholar]
- [170].Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Ann. Rev. Psychol. 2000;51:255–277. doi: 10.1146/annurev.psych.51.1.255. [DOI] [PubMed] [Google Scholar]
- [171].Woods SC, Seeley RJ. Insulin as an adiposity signal. Int. J. Obes. Relat. Metab. Disord. 2001;25:S35–S38. doi: 10.1038/sj.ijo.0801909. [DOI] [PubMed] [Google Scholar]
- [172].Woods SC, Seeley RJ, Porte DJ, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- [173].Yamada Y, Ando F, Niino N, Ohta S, himokata H. Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density of the femoral neck in elderly Japanese women. J. Mol. Med. 2002;80:452–460. doi: 10.1007/s00109-002-0348-0. [DOI] [PubMed] [Google Scholar]
- [174].Yu IC, Lin H-Y, Liu N-C, Wang R-S, Sparks JD, et al. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology. 2008;149:2361–2368. doi: 10.1210/en.2007-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Zumoff B, Strain GW, Miller LK, Rosner W, Senie R, et al. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J. Clin. Endocrinol. Metab. 1990;71:929–931. doi: 10.1210/jcem-71-4-929. [DOI] [PubMed] [Google Scholar]