Abstract
Purpose
There is insufficient evidence to recommend mammography for women >75 years. Guidelines recommend that older women be informed of the uncertainty of benefit and potential for harm, especially for women with short life expectancy. However, few older women are informed of harms of screening and many with short life expectancy are screened. Therefore, we aim to test whether a mammography screening decision aid (DA) for women >75 years affects their use of mammography, particularly for women with <10 year life expectancy.
Methods/Design
The DA is a self-administered pamphlet that includes information on screening outcomes, tailored information on breast cancer risk, health, life expectancy, and competing mortality risks, and includes a values clarification exercise. We are conducting a large cluster randomized controlled trial (RCT) of the DA with the primary care provider (PCP) as the unit of randomization to evaluate its efficacy. We plan to recruit 550 women 75-89 years from 100 PCPs to receive either the mammography DA or a pamphlet on home safety for older adults (control arm) before a visit with their PCP, depending on their PCP's randomization assignment. The primary outcome is receipt of mammography screening assessed through chart abstraction. Secondary outcomes include effect of the DA on older women's screening intentions, knowledge, and decisional conflict, and on documented discussions about mammography by their PCPs. We will recruit women from 5 Boston-based primary care practices (3 community-based internal medicine practices and 2 academic practices), and 2 North Carolina-based academic primary care practices.
Discussion
It is essential that we test the DA in a large RCT to determine if it is efficacious and to substantiate the need for broad translation into clinical practice. Our DA has the potential to improve health care utilization and care in a manner dictated by patient preferences.
Keywords: Mammography screening, Decision aid, Older women, Randomized trial
Introduction
Women aged 75 and older are the fastest growing segment of the US population and are at the highest risk of breast cancer [1,2]. However, none of the randomized trials of mammography screening included women >75 years and it is not known if mammography helps these women live longer [3-6]. Among women 50-74 years, mammography is estimated to reduce breast cancer mortality by 15% to 25% [3-6] and screening is recommended every 1-2 years [7,8]. However, the reduced breast cancer mortality associated with mammography is likely smaller for older women due to shorter life expectancies, slower growing tumors, and competing illnesses [9,10]. Increasingly, data suggest that women need an approximate 10 year life expectancy to have a chance at a mortality benefit from being screened with mammography [11-13]. Meanwhile, there are immediate harms to screening older women including: pain, anxiety, complications from tests after a false positive mammogram (e.g., breast biopsy), and over diagnosis (finding cancers that otherwise would never have caused symptoms in one's lifetime) [10,14]. Over diagnosis is particularly concerning since some older women experience significant complications from breast cancer treatment [15-21].
Currently, there is insufficient evidence to recommend mammography screening for women >75 years. Guidelines encourage clinicians to discuss the uncertainty about the balance of benefits and harms with older women [7,8,22]. Yet, few older women are informed of potential harms of mammography before being screened, likely because explaining such uncertainty can be challenging and time consuming [3,23,24]. As a result 56% of women >75 years are screened, including 50% of women with <10 year life expectancy - an estimated 2.8 million US women [25].
To improve older women's understanding of the benefits and risks of mammography screening, we propose a large cluster randomized controlled trial (RCT) of a pamphlet-based decision aid (DA), using primary care provider (PCP) as the unit of randomization, to evaluate the DA's efficacy. We previously developed and pilot tested the DA. Our pilot pretest/posttest trial of 45 women >75 years found that the DA resulted in older women being more knowledgeable about the benefits and risks of mammography, clearer in their values, and fewer intended to be screened, especially those with <10 year life expectancy [26]. We aim to recruit 550 women 75-89 years from 100 PCPs who provide care at an academic primary care or geriatrics practice in Boston, three community practices in the Boston metro area, or at an academic internal medicine or family practice in North Carolina. Patient participants will either receive the DA (intervention arm) or an educational pamphlet on home safety for older adults (control arm) [27]. We chose to use PCPs as the unit of randomization rather than individual patients because we anticipate that some patients will share the DA with their PCPs. Once PCPs are exposed to the DA for one patient they could change their approach to screening. This could lead to contamination of the control group making it more difficult to show an effect of the DA if we chose to randomize at the patient level.
We have developed a promising DA to inform and improve older women's mammography screening decisions. It is essential that we now test this DA in a large RCT, because if efficacious, it will provide compelling data for busy primary care practices to implement the DA nationally.
Aims
We will examine the efficacy of the DA on several patient level outcomes:
Receipt of screening;
Intentions of being screened;
Knowledge of the pros and cons of being screened;
Decisional conflict around screening;
Preferred decision-making role around mammography (active vs. passive/shared with physician);
Documented discussions by PCPs of the risks and benefits of mammography screening in participants’ notes.
Methods and Analysis
Study design
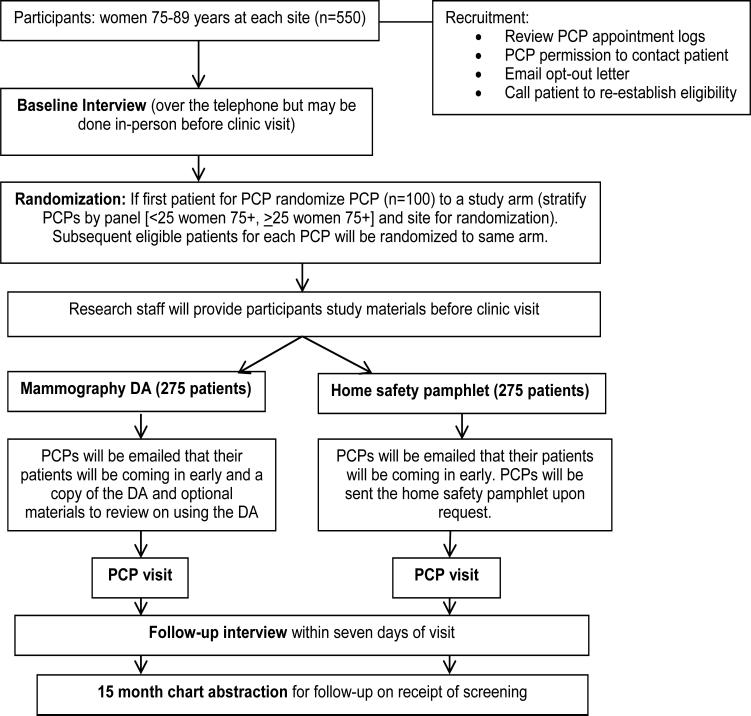
We propose a cluster randomized controlled trial (RCT) with primary care provider (PCP) as the unit of randomization to determine the effect of a mammography screening decision aid (DA) for women >75 years on receipt of mammography screening (Figure 1).
Figure 1.
Design of the cluster randomized controlled trial.
Setting
We will recruit patients from PCPs from multiple diverse sites including: Beth Israel Deaconess Medical Center's (BIDMC) primary care (HealthCare Associates [HCA]) and geriatrics’ (Senior Health) practices, at least three community practices affiliated with Harvard Vanguard Medical Associates (HVMA), a non-profit medical group with 17 practices in the greater Boston area, and an academic internal medicine practice and family medicine practice affiliated with the University of North Carolina (UNC). Our seven primary practices provide 4,650 patients from 166 eligible PCPs. We will recruit from two additional HVMA sites if needed to meet recruitment goals. Table 1 provides a description of each practice. All practice sites use robust electronic medical records that capture all clinician notes, labs, reports, and screening received.
Table 1.
Practice characteristics of recruitment sites.
| Practices | Description | PCP | Patient | Age | Race/Ethnicity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| #s | #s | 75-79 | 80-84 | 85-89 | Wa | Ba | Aa | Ha | ||
| BIDMC primary care-HCAa | Large diverse Boston-based academic internal medicine practice | 30b | 695 | 47% | 33% | 20% | 67% | 23% | 4% | 3% |
| BIDMC Senior Health | Boston-based academic geriatrics practice | 5b | 365 | 27% | 34% | 39% | 83% | 9% | 4% | 1% |
| HVMA-Kenmorec | Large diverse Boston-based community primary care practice | 19 | 814 | 47% | 31% | 22% | 55% | 33% | 2% | 3% |
| HVMA-West Roxbury | Semi-urban diverse community primary care practice outside Boston | 11 | 598 | 41% | 36% | 24% | 75% | 16% | 2% | 3% |
| HVMA-Wellesley | Suburban affluent community primary care practice outside Boston | 17 | 1031 | 44% | 36% | 21% | 87% | 2% | 3% | 1% |
| UNC general internal medicinec | Diverse academic internal medicine practice in Chapel Hill, NC | 49b | 741 | 48% | 33% | 19% | 62% | 26% | 4% | 8% |
| UNC family medicinec | Diverse academic family practice in Chapel Hill, NC | 35b | 406 | 44% | 32% | 24% | 60% | 30% | 4% | 6% |
| Total | 166 | 4,650 | ||||||||
| If needed: HVMA-Medford | Semi-urban diverse community primary care practice outside Boston | 15 | 922 | 40% | 36% | 25% | 93% | 3% | 1% | 0% |
| If needed: HVMA-Braintree | Suburban community primary care practice outside Boston | 14 | 882 | 49% | 34% | 17% | 90% | 4% | 1% | 0% |
Abbreviations: HCA=HealthCare Associates, HVMA= Harvard Vanguard Medical Associates, UNC= University of North Carolina, NA=Not Applicable, W=White, B=Black, A=Asian, H=Hispanic.
Excludes PCPs that participated in the pilot, the research team, and residents
HVMA data were pulled 8/2013; UNC and BIDMC data were pulled 9/2014
Participants
Inclusion criteria
We will recruit English-speaking women, aged 75 to 89 years, scheduled for a routine visit or physical with their PCP in the next 4-12 weeks. We chose this time frame since women scheduled for urgent issues would be unlikely to discuss mammography with their PCPs. Also, since our DA is designed to help women who have regularly undergone screening decide whether or not to continue screening, we will include women who have not had a mammogram in 9 months but have had one in 2.5 years. We chose 2.5 years as our upper threshold since data suggest that if older women continue to be screened that they be screened every 2 years [28]. Since not all women that plan to continue screening will have completed screening by 2 years exactly, we chose to include women who had not been screened in the past 2.5 years.
Exclusion criteria
We will exclude women who have it documented on their screening sheet that they have chosen to stop screening, since this is a major medical decision for older women while there is often no decision-making involved in continuing screening [29]. Also, while some older women with >10 year life expectancy may have chosen to stop being screened even though there is a chance of benefit, it is unlikely that the DA would change their preferences when years of public health messages and widespread enthusiasm for screening have not.
We will also exclude women with a history of Atypical Ductal Hyperplasia (ADH) or non-invasive or invasive breast cancer, since these women may be at higher risk of breast cancer and physicians may recommend screening regardless of patient preferences. We will exclude women with dementia (on problem list/reported by PCP). We will also exclude women who are scheduled for their first visit with their PCP since their medical records may be incomplete and because PCPs may feel uncomfortable discussing the risks and benefits of mammography in the context of patient life expectancy on a first visit with a patient. In addition, we will exclude women without capacity for informed consent. To determine capacity, we will ask women seven questions about their understanding of the study, the benefits and harms, and their role; women need to answer at least four questions correctly for inclusion. After enrollment, we plan to assess cognition with the Orientation-Memory-Concentration (OMC) test [30,31]. If a woman scores>19 on the OMC test (indicative of dementia), the research team will discuss the case with the site principal investigator to make sure that her enrollment is appropriate. We do not plan to exclude women with capacity for informed consent but mild cognitive impairment (MCI) since: 1) ~20% of US women ≥ 75 years have MCI [32-34]; 2) many are screened without discussion of risks of mammography [35]; 3) MCI increases with age and is associated with comorbidity and shorter life expectancy [36]; and 4) women with MCI successfully participated in our pilot. Since on average women ≥ 90 years have <5 year life expectancy [1] and dementia is common (36%), we will exclude women ≥ 90 years [37]. We will also exclude women that report <7th grade education (the reading level of study materials) and patients from the 25 PCPs at BIDMC that participated in the pilot. Finally, we will exclude women whose PCPs already had 15 patients participate in the study (the cap per PCP).
Participant recruitment
To identify potential participants, a research assistant (RA) will review PCP appointment logs to find women 75-89 years scheduled to see their PCP in the next 4 to 12 weeks (with approval from a HIPAA waiver). Once a woman aged 75-89 is identified, a research assistant (RA) will review the patient's medical records to see if the patient meets eligibility criteria. In order to contact patients about the study, the RA will also contact patients’ PCPs to obtain permission to send their patients information about the study. We will explain to PCPs that the study aims to evaluate educational materials on cancer screening or falls and home safety for older adults. If the PCP is willing to have his/her patient contacted, an RA will mail the patient an informational letter about the study. The letter will include a self-addressed post-card for the patient to return to opt-out of being contacted. After 10 days, an RA will call patients who have not opted-out to describe the study. For those interested in participating, the RA will re-establish eligibility and assess patients’ capacity to participate. The RA will then obtain verbal informed consent from eligible patients.
Intervention and control arms
We will compare responses and outcomes of women that receive the DA (intervention) to women that receive an educational pamphlet on home safety (control). RAs will provide the DA or the home safety pamphlet to patients before a routine visit with their PCP (depending on PCP randomization assignment). The RAs will be instructed not to explain or discuss the content of the educational pamphlets with participants. Instead, the RA will encourage participants to ask their PCPs any questions about the educational materials.
Intervention
Development and pilot testing of the DA has been described previously [26]. In brief, the DA is written at a 6th grade reading level and includes information on 1) breast cancer risk factors for women >75 years; 2) health/life expectancy; 3) likely outcomes if screened and not screened with mammography; 4) competing mortality risks; 5) breast cancer treatments; and 6) a values clarification exercise. The last page asks users their intentions of being screened on a 15-point validated scale and invites users to share this information with their clinician [23]. PCPs whose patients are randomized to receive the DA will be sent a copy of the DA via email and a link to an optional training on using the DA (5 informational slides and a 3-minute video).
Control
To reduce response bias and to compensate for the time and attention required by the intervention group to read the DA, patients in the control arm will be provided a two page pamphlet on home safety for older adults developed by the American Geriatrics Society (AGS) Foundation for Health in Aging [27]. PCPs whose patients are randomized to receive the home safety pamphlet, will be sent an email informing them that their patient will be coming in early to read health educational materials for older adults as part of a study. We otherwise do not plan any intervention for control group PCPs because we do not want to change their usual behavior. However, if PCPs in the control arm request a copy of the educational materials then we will email them a copy of the home safety pamphlet.
Pre-intervention measures
The baseline questionnaire will assess intentions to get a mammogram, concerns about breast cancer and perceived risk [38], subjective norms around mammography (e.g., how strongly women agree with the statement “my family thinks I should have a mammogram”) [39], family history of breast cancer and reproductive history [40]. To keep patients blinded to the intervention of interest it will also assess history of falls, and home safety. In addition, we will assess participants’ sociodemographic characteristics (race/ethnicity, education, insurance, marital status, socioeconomic status using the MacArthur scale) [41], life expectancy using the Schonberg Mortality Index and the Lee Mortality Index [42-44], cognition (OMC test) [30], numeracy [45], and medical literacy (REALM 7 [46], assessed in person on the day of the PCP visit).
Outcomes
Each outcome, when and how it is assessed is described in detail in Table 1.
Primary outcome
Receipt of mammography screening
We chose receipt of screening as our primary outcome since we anticipate our DA directly impacts mammography use, especially for women with short life expectancy. We will follow women for 15 months to guarantee at least two years of data since their last mammogram (the upper bound of the recommended screening interval) [7]. We will review primary care notes, radiology records, and screening sheets (mammograms performed outside the medical system are manually entered on screening sheets). We will contact patients by telephone if the medical records indicate a participant has moved or changed to a different medical system or has had no documented contact with the medical system in six months (and the last note does not indicate the participant has died), to assess when a participant received her last mammogram and what follow-up was received. If we cannot reach the participant we will try her proxy.
Secondary outcomes
For secondary outcomes, we will assess women's intentions to be screened in the next year [47], knowledge of the pros and cons of mammography screening [26], decisional conflict around mammography screening [48], preferred decision-making role in deciding on mammography screening [49], and whether or not they discussed mammography screening with their PCP after participants have read the DA and met with their PCP. We will also ask women in the intervention arm (DA group) a validated 10 item index to see if the DA prepared them to communicate with their clinician about decision-making around mammography screening [50,51]. For the control arm, we will modify this index to ask participants how the pamphlet affected their thoughts and plans for making their home safer. To assess whether the educational materials provoke anxiety we will ask participants the Spielberger State-Trait Anxiety Inventory short-form [52]. In addition, we will ask participants about the length, clarity, and whether women found the educational materials anxiety invoking and/or whether they would recommend them to a friend [53]. For the DA only, we will ask participants if they feel that the material in the DA is balanced.
To keep participants blinded to whether they were randomized to the intervention of interest, we will ask both groups their intentions to perform several home safety measures (e.g., check hot water setting) and whether they discussed home safety with their PCP during the visit.
In addition to asking in follow-up if women discussed mammography/home safety with their PCP, we will review PCP notes up to 6 months after participation (in case patients choose to bring up screening at the next visit rather than the index visit) to see if PCPs documented a discussion on mammography or home safety (e.g., fall prevention tips). We plan to assess both screening and home safety to keep chart abstractors blinded to the outcome of interest. We will categorize a woman as having received a balanced screening discussion if a note includes documentation beyond the typical notation endorsing mammography (e.g., “mammogram recommended”). The note must either include: a) discussion of a limitation of screening or b) that mammography was discussed AND whether or not the patient chose to continue screening. To ensure the validity of coding of whether screening/home safety discussions occurred, at least 3 investigators will read the de-identified paragraphs of participants’ records and code whether they think a balanced discussion occurred. Discrepancies will be adjudicated by consensus between investigators.
Randomization
We will randomize PCPs to the DA vs. home safety pamphlet after their first eligible patient agrees to participate. Subsequent patients of each PCP (up to 15) that participate will receive the same study materials as the first patient eligible for that PCP. To ensure that PCPs who see a large number of women >75 years are not all randomized to one arm, we will stratify randomization by PCP panel size (<25 women 75+ in panel vs. >25). We will also stratify by site in case of institutional differences in the approach to screening. Randomization assignment will be determined using a permuted block randomization scheme with randomly-varying block sizes. We will place assignments in sealed, opaque, sequentially numbered envelopes.
Blinding
Although RAs will be blinded to allocation status when identifying the first patient eligible for each PCP, they will not be blinded to allocation status for subsequent patients. However, RAs will be trained to review patient charts sequentially by their appointment date. If there are questions as to whether a patient is eligible the case will be reviewed by an investigator blinded to assignment. Recruitment materials informing patients of the study and verbal informed consent scripts will be the same regardless of randomization assignment. While RAs that administer follow-up interviews will not be blinded to participant intervention status, they will be trained to read all questionnaires verbatim and not to add commentary. We have learned from prior work that having older women complete questionnaires for themselves leads to more missing data. Chart abstractors that assess receipt of mammography screening (our primary outcome) will be blinded to participant randomization assignment.
Interim analyses
We do not plan interim analyses since this is a minimal risk trial of an educational pamphlet.
Sample size
In our pilot, 84% of women were screened in the two years before reading the DA and 63% in the 15 months after (a 21% decline) [26]. Since our pilot was small and used a quasi-experimental design, we aim to detect a smaller difference (15%) between study arms in the RCT. Although there is little prior data on which to base the intra-class correlation coefficient (ICC) to account for clustering of patients by PCP, several primary-care based trials have used an ICC of 0.05 [54-59]. We conservatively chose to base our sample size estimate on an ICC of 0.1. Since we anticipate recruiting on average 5 patients from 100 PCPs, with an alpha of 0.05, we will need to recruit 516 women (258 per arm) to have 0.90 power to detect a 15% difference in receipt of screening (80% in the control arm vs. 65% in the DA arm); even if the ICC is 0.2 (unlikely) we will have 0.81 power. In our pilot we excluded 2 women (4%) from follow-up analyses whose first mammogram was done for diagnostic reasons; there was no other loss of follow-up and no one had missing data on intentions to be screened. To provide an extra margin in case of greater loss of follow-up or missing data in the RCT, we plan to recruit 7% more women (n=34) to our RCT for a total of 550 women. In addition, based on our pilot, we anticipate that at least 50% of participants will have <10 year life expectancy. Thus, we anticipate having at least 258 women with <10-year life expectancy for these subset analyses. With an ICC of 0.1 and 3 patients on average for 90 PCPs (assuming a few PCPs will not have patients with <10-year life expectancy included), we will have 0.90 power to detect a 20% difference in receipt of screening (we found a 28% difference in the pilot for women with <10-year life expectancy) between intervention arms [26].
Statistical Methods
Our primary outcome is receipt of screening within 15 months (yes/no; reflecting screening within 24 months, given inclusion requires that patients did not have mammogram during 9 months before study entry). Our secondary outcomes are 1) intentions to be screened (yes vs. no/unsure), 2) knowledge (mean of correct responses on the 10 item test), 3) decisional conflict (mean decisional conflict scale [DCS] score, ranges from 0 [none] to 100 [extremely high decisional conflict]), 4) preferred decision-making role around mammography (active vs. passive/shared with physician); 5) anxiety; and 6) documentation by PCPs of a screening discussion within 6 months (yes/no).
To examine the DA's effect on our outcomes of interest, we will use marginal linear and logistic regression models using Generalized Estimating Equations (GEE) with sandwich estimates of standard error to allow for clustering by PCP. We will fit each model with three independent variables: intervention group (DA vs. home safety pamphlet), PCP panel size (<25, 25+), and site (BIDMC, Atrius, UNC). In secondary analyses, we will consider the following potential patient-level cofounders: baseline intentions to be screened, concerns about breast cancer and perceived risk, family history of breast cancer, educational attainment, patient sociodemographics, subjective norms, life expectancy, and literacy. In subset analyses, we will examine receipt of screening, intentions to be screened, and documentation of a physician discussion around screening limiting the sample to women with <10 year life expectancy [42-44]. Since we expect few missing data for our primary outcomes we will perform complete case analyses for our primary analyses. In sensitivity analyses we will use multiple imputations to account for missing data. In addition, we will use chi-square statistics to explore cultural, racial, ethnic, and socioeconomic differences in effects of the DA.
We will use descriptive statistics to report whether the intervention group found the DA acceptable and/or prepared them for decision-making. We will also use descriptive statistics to report the number of PCPs in the intervention arm that viewed the training materials. Within the intervention group, we will use McNemar's test to examine if screening intentions changed between reading the DA and meeting with their PCP. All analyses will be completed on an intention-to-treat basis (Table 2).
Table 2.
Data Collection.
| Outcomes | Outcome Measure | Description | Scoring | When | DA group |
Control group |
From |
|---|---|---|---|---|---|---|---|
| Primary Outcome | Receipt of screening | Review primary care notes, radiology records, and screening sheets; will contact patients or their proxy if follow-up is not complete in the medical records. | Yes vs. no | 15 months | X | X | Medical records |
| Secondary Outcomes | Intentions to be screened | Predisposition/choice: a validated 15-point scale to assess one's propensity to being screened. We will categorize scores as 1-5 (yes), 6-10 (unsure), or 11-15 (no) [23]. | Yes vs. those who are unsure or plan not to be screened | Baseline, Follow-upa,b | X | X | Participant reported |
| Knowledge | 10 questions (2 multiple choice and 8 true/false); 7 were adapted from other studies [75-78] and 3 were developed based on the material in the DA. | Sum of correct answers | Folow-up | X | X | Participant reported | |
| Decisional Conflict Scale (DCS) | A validated 16 item scale (each item is scored on a 5-point Likert scale) to measure uncertainty around a decision, whether one feels informed, clear about their personal values, and supported in their decision-making (Cronbach's alpha=0.78 to 0.92) [15,79,80]. | Scores range 0-100; lower scores indicate less conflict | Folow-up | X | X | Participant reported | |
| Preferred decision-making role | The Control Preferences Scale (CPS) assesses whether patients prefer to make medical decisions on their own or share responsibility with their doctor or have their doctor make their decision [49,80]. | Active vs. passive/shared with doctor (since aim of decision aids is to help patients be more active in decision-making) | Folow-up | X | X | Participant reported | |
| Preparation for decision-makingc | A validated 10 item index (each item scored on a 5 point Likert scale) to see if the DA prepares patients to communicate with clinicians about mammography (Cronbach's alpha=0.92-0.96) [50,51]. For the control arm, we will modify this index to ask participants how the pamphlet affects their thoughts for making their home safer. | Scores range 0-100; higher scores indicate greater preparation | Folow-up | X | X | Participant reported | |
| Acceptability of the materials | Will assess participant perceptions about length, clarity, and whether they found the materials helpful or would recommend them to a friend [81]. For the DA only, we will ask whether the information is slanted towards or against getting a mammogram or whether the information is balanced. | Descriptive | Folow-up | X | X | Participant reported | |
| Anxiety | We will examine whether the educational materials provoke anxiety using the Spielberger State-Trait Anxiety Inventory short-form (6 items on a 4 point Likert scale) [52]. | Scores range from 6 -24 with lower scores indicating less anxiety | Folow-up | X | X | Participant reported | |
| Balanced mammography discussion | Per patient report and per review of primary care notes up to 6 months after participation (in case patients choose to bring up screening at the next visit rather than the index visit) we plan to assess whether discussions on the benefits and harms of mammography screening and/or home safety discussions occurred. | Yes vs. no | 15 months | X | X | Medical records |
Since screening intentions are assessed at the end of the DA, these data will also be recorded from the intervention group then.
An RA will attempt to administer the follow-up interview immediately after a participant has read the DA and has met with her PCP (but no longer than seven days after the PCP visit).
Since the home safety pamphlet is not a decision aid, the questions in the preparation for decision-making index will be modified in the control arm so that the questions ask participants how the pamphlet affects their thoughts around home safety.
Ethical approval and trial registration
The Committee of Clinical Investigations at BIDMC approved this study (protocol number: 2014-P-000108; BIDMC). We will publish our findings regardless of the results and will follow the CONSORT guidelines for reporting RCTs [60]. The trial is registered at clinicaltrials.gov (NCT02198690).
Discussion
The population of women over age 75 is rising rapidly and increasingly these women are being screened with mammography without a balanced discussion of the potential benefits and harms [1-3,24,29]. Some experts consider it a medical error if a patient undergoes a test that they would not have chosen if they had a better understanding of the likely outcomes. Furthermore, screening older women with short life expectancies (which occurs commonly) may only cause harm without the chance for a survival benefit. If effective, our DA will lead to more informed mammography screening decisions. It will also lead to decreased screening, particularly among older women with short life expectancies, thereby reducing harms from screening. However, since implementing any DA takes time and resources, our DA should not be broadly translated into clinical practice without compelling data from a large RCT.
Strengths and limitations
We chose to test the DA under ideal (explanatory) rather than usual (pragmatic) circumstances at this stage because we feel we need compelling data that the DA helps patients and does not cause any harms before we work on a larger dissemination of the tool [61,62]. If we went straight to a pragmatic trial we would not know which patients, if any, received the DA. Furthermore, if the DA was found to be ineffective, it would be difficult to determine if its lack of effectiveness was due to the content of the DA itself or because patients did not receive it. However, we designed our trial to reflect how we anticipate that many practices will implement the DA, especially those that are part of a patient-centered medical home.
There are logistical challenges to using DAs in practice including: limited clinic time with competing agendas, lack of incentives, and difficulty identifying appropriate patients for use [63]. Since prior research has demonstrated that relying on PCPs to give patients DAs results in inconsistent delivery [63,64], in the RCT our research assistants (RAs) will function as panel managers and identify patients and give them the DA [65]. Providing a DA before a visit has been shown to improve communication during a visit [63,66], therefore, we plan for patients to come early to read the DA. Our study design ensures that patients will have opportunity to discuss the DA with their PCP before finalizing their decision.
While the study aims to include a diverse group of women >75 years from multiple geographical and clinical settings and from varied socioeconomic and educational backgrounds, we will exclude women that do not speak English and those with dementia. The DA does not include data on impact of dementia on life expectancy and was not developed for proxy use. If efficacious, we will modify the DA for proxy use and will have it translated to Spanish. While we will have limited power to examine the effects of the DA in subgroup analyses, we will explore if there are racial/ethnic and/or socioeconomic differences in perceptions of the DA.
We also plan to develop an interactive web-based version of the DA if it is effective. We chose a pamphlet format for the DA for this RCT since 1) 98% of pilot study participants reported preference for paper-based educational materials; 2) the current cohort of women >75 years tends to have low computer and internet literacy and may require computer training which may not be feasible in many practices [67,68]; 3) paper-based DAs have been shown to have equivalent effects on cancer screening behavior and tend to be associated with greater satisfaction and use [69,70]; and 4) physicians tend to prefer to give pamphlets to patients [71,72].
We also considered asking study participants about any anticipated regret if they choose not to be screened but are subsequently diagnosed with breast cancer. However, the mere measurement of anticipated regret can lead to behavioral change and we did not want our follow-up questionnaire to influence participants’ screening decisions [73,74].
We chose to randomize PCPs to a study arm rather than practices since patients seen at each practice may vary significantly. We found little cross-practice contamination in the pilot.
Conclusion
We aim to test our novel mammography screening DA for women >75 years in a large RCT to determine if it is efficacious and to substantiate the need for broad translation into clinical practice. Our DA has the potential to improve health care utilization and care in a manner dictated by patient preferences.
Acknowledgments
This research is supported by the National Cancer Institute (R01 CA181357).
Footnotes
Citation: Schonberg MA, Kistler CE, Nekhlyudov L, Fagerlin A, Davis RB, et al. (2014) Evaluation of a Mammography Screening Decision Aid for Women Aged 75 and Older: Protocol for a Cluster-randomized Controlled Trial. J Clin Trials 4: 191. doi:10.4172/2167-0870.1000191
Trial registration: NCT02198690.
References
- 1.Arias E. United States life tables, 2008. Natl Vital Stat Rep. 2012;61:1–63. [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2009:CD001877. doi: 10.1002/14651858.CD001877.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Independent UK Panel on Breast Cancer Screening The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–1786. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 5.Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;6:CD001877. doi: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–737. 237–242. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Preventive Services Task Force1 Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. See comment in PubMed Commons below Ann Intern Med. 2009;151:716–726. W-236. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society Guidelines for the Early Detection of Cancer. 2014 [Google Scholar]
- 9.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92:550–556. doi: 10.1093/jnci/92.7.550. [DOI] [PubMed] [Google Scholar]
- 10.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med. 2003;348:1672–1680. doi: 10.1056/NEJMcp021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warner E. Clinical practice. Breast-cancer screening. N Engl J Med. 2011;365:1025–1032. doi: 10.1056/NEJMcp1101540. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O'Brien S, et al. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441. doi: 10.1136/bmj.e8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonberg MA, Silliman RA, Marcantonio ER. Weighing the benefits and burdens of mammography screening among women age 80 years or older. J Clin Oncol. 2009;27:1774–1780. doi: 10.1200/JCO.2008.19.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Audisio RA. The surgical risk of elderly patients with cancer. Surg Oncol. 2004;13:169–173. doi: 10.1016/j.suronc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. N Engl J Med. 2009;360:63–70. doi: 10.1056/NEJMct0803525. [DOI] [PubMed] [Google Scholar]
- 17.Lind PA, Marks LB, Hardenbergh PH, Clough R, Fan M, et al. Technical factors associated with radiation pneumonitis after local +/− regional radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2002;52:137–143. doi: 10.1016/s0360-3016(01)01715-1. [DOI] [PubMed] [Google Scholar]
- 18.Mandelblatt JS, Edge SB, Meropol NJ, Senie R, Tsangaris T, et al. Sequelae of axillary lymph node dissection in older women with stage 1 and 2 breast carcinoma. Cancer. 2002;95:2445–2454. doi: 10.1002/cncr.10983. [DOI] [PubMed] [Google Scholar]
- 19.Svastics E, Sulyok Z, Besznyak I. Treatment of breast cancer in women older than 70 years. J Surg Oncol. 1989;41:19–21. doi: 10.1002/jso.2930410108. [DOI] [PubMed] [Google Scholar]
- 20.Welch HG, Albertsen PC, Nease RF, Bubolz TA, Wasson JH. Estimating treatment benefits for the elderly: the effect of competing risks. Ann Intern Med. 1996;124:577–584. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 21.Wildiers H, Kunkler I, Biganzoli L, Fracheboud J, Vlastos G, et al. Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8:1101–1115. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 22.Breast cancer screening in older women. American Geriatrics Society Clinical Practice Committee. J Am Geriatr Soc. 2000;48:842–844. [PubMed] [Google Scholar]
- 23.Hoffman RM, Lewis CL, Pignone MP, Couper MP, Barry MJ, et al. Decision-making processes for breast, colorectal, and prostate cancer screening: the DECISIONS survey. Med Decis Making. 2010;30:53S–64S. doi: 10.1177/0272989X10378701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schonberg MA, Ramanan RA, McCarthy EP, Marcantonio ER. Decision making and counseling around mammography screening for women aged 80 or older. J Gen Intern Med. 2006;21:979–985. doi: 10.1111/j.1525-1497.2006.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schonberg MA, Breslau ES, McCarthy EP. Targeting of mammography screening according to life expectancy in women aged 75 and older. J Am Geriatr Soc. 2013;61:388–395. doi: 10.1111/jgs.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schonberg MA, Hamel MB, Davis RB, Griggs MC, Wee CC, et al. Development and evaluation of a decision aid on mammography screening for women 75 years and older. JAMA Intern Med. 2014;174:417–424. doi: 10.1001/jamainternmed.2013.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Home Safety Tips for Older Adults. 2014 [Google Scholar]
- 28.Braithwaite D, Zhu W, Hubbard RA, O'Meara ES, Miglioretti DL, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst. 2013;105:334–341. doi: 10.1093/jnci/djs645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torke AM, Schwartz PH, Holtz LR, Montz K, Sachs GA. Older adults and forgoing cancer screening: “I think it would be strange”. JAMA Intern Med. 2013;173:526–531. doi: 10.1001/jamainternmed.2013.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzman R, Brown T, Fuld P, Peck A, Schechter R, et al. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 31.Queally VR, Evans JJ, McMillan TM. Accuracy in scoring vignettes using the mini mental state examination and the short orientation memory concentration test. J Geriatr Psychiatry Neurol. 2010;23:160–164. doi: 10.1177/0891988710363712. [DOI] [PubMed] [Google Scholar]
- 32.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peltz CB, Corrada MM, Berlau DJ, Kawas CH. Cognitive impairment in nondemented oldest-old: prevalence and relationship to cardiovascular risk factors. Alzheimers Dement. 2012;8:87–94. doi: 10.1016/j.jalz.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta KM, Fung KZ, Kistler CE, Chang A, Walter LC. Impact of cognitive impairment on screening mammography use in older US women. Am J Public Health. 2010;100:1917–1923. doi: 10.2105/AJPH.2008.158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wray LO, Wade M, Beehler GP, Hershey LA, Vair CL. A program to improve detection of undiagnosed dementia in primary care and its association with healthcare utilization. Am J Geriatr Psychiatry. 2014;22:1282–1291. doi: 10.1016/j.jagp.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipkus IM, Iden D, Terrenoire J, Feaganes JR. Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:533–539. [PubMed] [Google Scholar]
- 39.Tiro JA, Diamond PM, Perz CA, Fernandez M, Rakowski W, et al. Validation of scales measuring attitudes and norms related to mammography screening in women veterans. Health Psychol. 2005;24:555–566. doi: 10.1037/0278-6133.24.6.555. [DOI] [PubMed] [Google Scholar]
- 40.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 41.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 42.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 43.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med. 2009;24:1115–1122. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. External validation of an index to predict up to 9-year mortality of community-dwelling adults aged 65 and older. J Am Geriatr Soc. 2011;59:1444–1451. doi: 10.1111/j.1532-5415.2011.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, et al. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making. 2007;27:672–680. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 46.Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25:391–395. [PubMed] [Google Scholar]
- 47.O'Connor AM. User Manual- Measures of Decision/Choice Predisposition. Ottawa Hospital Research Institute; Ottawa: 2013. 1996. [Google Scholar]
- 48.O'Connor AM. User Manual- Decisional Conflict Scale (16 item statement format) Ottawa Hospital Resarch Institute; Ottawa: 2014. 1993. [Google Scholar]
- 49.Degner LF, Kristjanson LJ, Bowman D, Sloan JA, Carriere KC, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277:1485–1492. [PubMed] [Google Scholar]
- 50.Rainey LC. Effects of preparatory patient education for radiation oncology patients. Cancer. 1985;56:1056–1061. doi: 10.1002/1097-0142(19850901)56:5<1056::aid-cncr2820560516>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 51.Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, et al. Validation of a preparation for decision making scale. Patient Educ Couns. 2010;78:130–133. doi: 10.1016/j.pec.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31:301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 53.O'Connor AM. User Manual-Acceptability [document on the Internet] Ottawa Hospital Research Institute; Ottawa: 1996. [Google Scholar]
- 54.Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169:1560–1568. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 55.Nagle C, Gunn J, Bell R, Lewis S, Meiser B, et al. Use of a decision aid for prenatal testing of fetal abnormalities to improve women's informed decision making: a cluster randomised controlled trial [ISRCTN22532458]. BJOG. 2008;115:339–347. doi: 10.1111/j.1471-0528.2007.01576.x. [DOI] [PubMed] [Google Scholar]
- 56.Kerry SM, Bland JM. The intracluster correlation coefficient in cluster randomisation. BMJ. 1998;316:1455. doi: 10.1136/bmj.316.7142.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legare F, Labrecque M, Godin G, LeBlanc A, Laurier C, et al. Training family physicians and residents in family medicine in shared decision making to improve clinical decisions regarding the use of antibiotics for acute respiratory infections: protocol for a clustered randomized controlled trial. BMC Fam Pract. 2011;12:3. doi: 10.1186/1471-2296-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Junius-Walker U, Wrede J, Voigt I, Hofmann W, Wiese B, et al. Impact of a priority-setting consultation on doctor-patient agreement after a geriatric assessment: cluster randomised controlled trial in German general practices. Qual Prim Care. 2012;20:321–334. [PubMed] [Google Scholar]
- 59.Heselmans A, Van de Velde S, Ramaekers D, Vander Stichele R, Aertgeerts B. Feasibility and impact of an evidence-based electronic decision support system for diabetes care in family medicine: protocol for a cluster randomized controlled trial. Implement Sci. 2013;8:83. doi: 10.1186/1748-5908-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis. 1967;20:637–648. doi: 10.1016/0021-9681(67)90041-0. [DOI] [PubMed] [Google Scholar]
- 62.Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62:464–475. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 63.Brackett C, Kearing S, Cochran N, Tosteson AN, Blair Brooks W. Strategies for distributing cancer screening decision aids in primary care. See comment in PubMed Commons below Patient Educ Couns. 2010;78:166–168. doi: 10.1016/j.pec.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Legare F, Ratte S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526–535. doi: 10.1016/j.pec.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 65.Defining the Patient Centered Medical Home. Agency for Healthcare Research and Quality [Google Scholar]
- 66.Hsu C, Liss DT, Westbrook EO, Arterburn D. Incorporating patient decision aids into standard clinical practice in an integrated delivery system. Med Decis Making. 2013;33:85–97. doi: 10.1177/0272989X12468615. [DOI] [PubMed] [Google Scholar]
- 67.Xie B, Jaeger PT. Older adults and political participation on the Internet: a cross-cultural comparison of the USA and China. J Cross Cult Gerontol. 2008;23:1–15. doi: 10.1007/s10823-007-9050-6. [DOI] [PubMed] [Google Scholar]
- 68.Rideout V, Neuman T, Kichman M, Brodie M, Kaiser Family Foundation website e-Health and the Elderly: How Seniors Use the Internet for Health Information. 2005 [Google Scholar]
- 69.Weinberg DS, Keenan E, Ruth K, Devarajan K, Rodoletz M, et al. A randomized comparison of print and web communication on colorectal cancer screening. JAMA Intern Med. 2013;173:122–129. doi: 10.1001/2013.jamainternmed.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor KL, Williams RM, Davis K, Luta G, Penek S, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med. 2013;173:1704–1712. doi: 10.1001/jamainternmed.2013.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.King VJ, Davis MM, Gorman PN, Rugge JB, Fagnan LJ. Perceptions of shared decision making and decision aids among rural primary care clinicians. Med Decis Making. 2012;32:636–644. doi: 10.1177/0272989X11431961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foundation for Informed Medical Decision Making, (FIMDM) Informing and Involving Patients in Medical Decisions: The Primary Care Physicians’ Perspective. 2014 [Google Scholar]
- 73.Sandberg T, Conner M. A mere measurement effect for anticipated regret: impacts on cervical screening attendance. Br J Soc Psychol. 2009;48:221–236. doi: 10.1348/014466608X347001. [DOI] [PubMed] [Google Scholar]
- 74.Ziarnowski KL, Brewer NT, Weber B. Present choices, future outcomes: anticipated regret and HPV vaccination. below Prev Med. 2009;48:411–414. doi: 10.1016/j.ypmed.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Mathieu E, Barratt A, Davey HM, McGeechan K, Howard K, et al. Informed choice in mammography screening: a randomized trial of a decision aid for 70-year-old women. Arch Intern Med. 2007;167:2039–2046. doi: 10.1001/archinte.167.19.2039. [DOI] [PubMed] [Google Scholar]
- 76.Stager JL. The comprehensive Breast Cancer Knowledge Test: validity and reliability. J Adv Nurs. 1993;18:1133–1140. doi: 10.1046/j.1365-2648.1993.18071133.x. [DOI] [PubMed] [Google Scholar]
- 77.van Agt H, Fracheboud J, van der Steen A, de Koning H. Do women make an informed choice about participating in breast cancer screening? A survey among women invited for a first mammography screening examination. Patient Educ Couns. 2012;89:353–359. doi: 10.1016/j.pec.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Wilcox S, Stefanick ML. Knowledge and perceived risk of major diseases in middle-aged and older women. Health Psychol. 1999;18:346–353. doi: 10.1037//0278-6133.18.4.346. [DOI] [PubMed] [Google Scholar]
- 79.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 80.Kryworuchko J, Stacey D, Bennett C, Graham ID. Appraisal of primary outcome measures used in trials of patient decision support. Patient Educ Couns. 2008;73:497–503. doi: 10.1016/j.pec.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 81.Pinquart M, Duberstein PR. Information needs and decision-making processes in older cancer patients. Crit Rev Oncol Hematol. 2004;51:69–80. doi: 10.1016/j.critrevonc.2004.04.002. [DOI] [PubMed] [Google Scholar]