Abstract
We used electronic health record data from 162 patients enrolled in the NUgene Project (2002–2013) to determine demographic factors associated with long-term (from 1 to up to 9.5 (mean = 5.6) years) weight loss following Roux-en-Y gastric bypass surgery. Ninety-nine (61.1%) patients self-reported white, and 63 (38.9%) self-reported black, mixed, or missing race. The average percent weight loss was −33.4% (standard deviation, 9.3) at 1 year after surgery and −30.7% (standard deviation, 12.5) at the last follow-up point. We used linear mixed and semiparametric trajectory models to test the association of surgical and demographic factors (height, surgery age, surgery weight, surgery body mass index, marital status, sex, educational level, site, International Classification of Diseases code, Current Procedural Terminology code, Hispanic ethnicity, and self-reported race) with long-term percent weight loss and pattern of weight loss. We found that black, mixed, and missing races (combined) in comparison with white race were associated with a decreased percent weight loss of −4.31% (95% confidence interval: −7.30, −1.32) and were less likely to have higher and sustained percent weight loss (P = 0.04). We also found that less obese patients were less likely to have higher and sustained percent weight loss (P = 0.01). These findings may be helpful to patients in setting expectations after weight loss surgery.
Keywords: bariatric surgery, long-term weight loss, predictors, race, repeated measures, Roux-en-Y gastric bypass
Few previous studies have examined long-term (greater than 3 years after surgery) predictors of weight loss following bariatric surgery (1). Long-term follow-up of bariatric surgery patients may be hampered by the collection of data solely through follow-up visits with the patient's bariatric surgeon, and the associated loss of follow-up among patients with treatment failure is a potential source of unmeasured bias in the analysis of long-term studies (2, 3). Additionally, very few studies have applied statistical methods for analysis of repeated measures. Recent studies incorporating long-term follow-up focus primarily on clinical outcomes and comorbid conditions rather than predictors of long-term success, defined as high or sustained weight loss (4–6).
Our aim was to assess surgical and demographic predictors of long-term (up to 9.5 (mean = 5.6) years) weight loss among Roux-en-Y gastric bypass (RYGB) surgery patients. To increase the length of follow-up and decrease potential attrition, we used electronic health records (EHRs) from clinic visits across 2 health systems, avoiding the limitation of data collection occurring only with the patient's bariatric surgeon. We applied 2 methods for analysis of repeated measures and identification of statistically significant predictors, both linear mixed model and semiparametric trajectory models (a mixture model). To our knowledge, no study has previously examined the consistency of predictors across statistical methods or applied group-based trajectory modeling to assess differences in demographic characteristics between groups (7).
METHODS
Data collection
We identified individuals to include in this analysis from the NUgene biobank. The NUgene Project, run by Northwestern University, is a growing collection of DNA samples with associated health information collected from both a questionnaire and EHRs. Participants give a broad consent allowing for mining of the EHRs for phenotypes (https://www.nugene.org/). NUgene currently houses EHR data from 10,933 enrolled participants. Individuals were recruited into NUgene between 2002 and 2013 at both Northwestern Medicine Hospitals and Clinics and the NorthShore University HealthSystem (formerly Evanston Northwestern Hospital). Institutional review boards at Northwestern University and the NorthShore University HealthSystem (NorthShore) have approved both the NUgene study and this project. EHR data from Northwestern were extracted from the Enterprise Data Warehouse (edw.northwestern.org) (which comprises EHR data from the Cerner and Epic systems used at Northwestern Medicine for inpatient and outpatient care). NorthShore data were extracted from NorthShore's Enterprise Data Warehouse (which includes EHR data from Epic and many other ancillary clinical and financial systems for both inpatient and outpatient care, quality improvement, and research, at NorthShore).
Among NUgene patients recruited at Northwestern Medicine, NUgene staff initially identified eligible patients by International Classification of Diseases, Ninth Revision (ICD-9), procedure code or Current Procedural Terminology (CPT) surgical history code (Appendix Table 1). At NorthShore, eligible patients were identified by CPT surgical history code or having one of several specific surgical descriptions documented in the EHRs (i.e., LAP GAS BYPASS/ROUX-EN-Y). A total of 228 eligible NUgene participants were identified at Northwestern, and 83 were identified at NorthShore.
Sample selection
Figure 1 summarizes the exclusion criteria used to generate the analytical sample. Fifty-one patients were excluded because they did not have ICD-9 codes for the most common types (bypass and banding) of bariatric surgery performed during this time frame (2003–2011) or were missing certain age or weight data from the EHRs. All remaining patient records from Northwestern Medicine (195 patients) and NorthShore Hospital (65 patients) were merged. Weight observations during or shortly after pregnancy were excluded from the analysis data set. Three patients were excluded because a large number of follow-up weight observations occurred during a reported pregnancy. Because of known differences in the pattern of weight loss following different types of bariatric surgeries (with banding resulting in lower sustained weight loss than RYGB) (8, 9) and the small sample size of banding patients, we excluded all patients who underwent laparoscopic banding (38 patients) from the analyses presented in this paper, choosing to focus only on RYGB procedures. As many of our analyses focused only on weight loss at least 1 year after surgery, we excluded 35 patients from this analysis who had only postsurgical weight loss measurements in the first year after surgery. An additional 22 patients who had only 1 weight observation at least 1 year after surgery were excluded because multiple observations were necessary for inclusion in our chosen statistical models. We identified all patients for whom the weight at time of surgery (surgery weight) was not recorded. Within this subset of patients, we carried forward the last recorded presurgery weight as the surgery weight. Our final sample included 162 patients having a total of 3,071 postsurgical weight observations.
Figure 1.
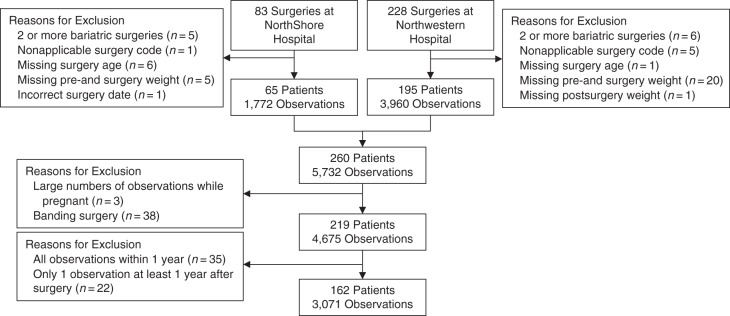
The analytical sample used in this analysis was drawn from patients enrolled in the NUgene Project (2002–2013) at either Northwestern Medicine Hospital or NorthShore University HealthSystem. Of all the patients who underwent bariatric surgery, patients were initially excluded if they had 2 or more surgeries, if they had a nonapplicable surgery, if their health records were missing critical information (surgery age and relevant weights), or if the surgery date was incorrect. Patients were further excluded if a large proportion of observations were taken while pregnant or if the surgery was not a bypass. Specific observations were excluded if they occurred within 1 year after surgery, and any patients were excluded if all of their observations were within 1 year after surgery.
Of the 162 patients included in the analysis data set, 99 (61.1%) self-reported white, 52 (32.1%) self-reported black, and 11 (6.8%) self-reported mixed or missing race. Ten (6.2%) patients separately reported Hispanic ethnicity, of which 8 (4.9%) also reported mixed or missing race, and 2 (1.2%) reported white race. Hispanic ethnicity was not statistically significantly associated with percent weight loss, and adjustment for ethnicity did not meaningfully change other estimates, so Hispanic ethnicity was not included in further analyses. We dichotomized race as either white or black (including mixed and missing). Sensitivity analyses that included only individuals self-reporting black or white race showed results similar to the model also including those reporting mixed and missing race.
Outcome and predictor variables
To protect patient privacy, we did not include specific dates in the analytical data set, so information was transformed into an “age at” form by using participant birthdate information in the EHRs. Variables extracted from the EHRs (in addition to surgery type from CPT, ICD-9, or surgical description) included (decimal) age at surgery, all weight measures in the EHRs with associated “age at weight measurement” values, and surgery year. Variables extracted from the NUgene questionnaire (administered at the time of NUgene enrollment) included sex, race, enrollment year (2003–2011), marital status, educational level, and height. We examined all relevant demographic and surgical predictors (based on previous studies and biological plausibility) that we could extract from the EHRs or the NUgene entrance questionnaire (height, surgery age, surgery weight, surgery body mass index, marital status, sex, educational level, site, ICD-9 code, CPT code, Hispanic ethnicity, and self-reported race).
Our primary outcome was percent weight loss, which was calculated by subtracting postsurgical weight values from the surgery weight, and then dividing by the surgery weight. Percent weight loss was calculated at each weight observation after surgery and used as a repeated outcome variable in our models. We also ran supplementary models using repeated measures of postsurgical body mass index and weight; we selected percent weight loss for our final models, rather than weight or body mass index, as these results are easier to interpret by accounting for varying frame sizes of the participants in this study. A previous study indicated that, compared with both change in body mass index and percent excess body weight loss, percent weight loss is robust to changes in preoperative body mass index and, therefore, is the most sensitive outcome for identification of significant weight loss predictors (10).
Previous studies have demonstrated that RYGB patients typically rapidly lose weight initially after surgery, reach a weight nadir 12–18 months after surgery, and then experience slow or no weight regain thereafter (6, 11, 12). Given our interest in long-term weight loss and regain, as well as the complications of interpreting coefficients in nonlinear models, we focused on linear mixed models including only weight measurements obtained at least 1 year after surgery. However, we conducted sensitivity analyses by using trajectory models with a semiparametric, group-based mixture model (Proc Traj; SAS Institute, Inc., Cary, North Carolina) including all weight measurements obtained anytime 30 days after surgery.
Statistical analysis
We selected 2 different modeling methods to determine the demographic predictors of long-term weight loss (beginning 1 year after surgery). First, linear mixed models were used to determine the statistical significance of demographic predictors with repeated outcome measures. Second, a semiparametric, group-based mixture model was used to distinguish unique long-term weight regain patterns. We determined the statistical significance of demographic predictors across groups defined by these patterns. Results of these models were compared to identify the robust demographic predictors of long-term weight loss after bariatric surgery.
We used SAS Proc Mixed to create linear mixed models including repeated measures with a random intercept and unstructured covariance matrix to determine which predictors were associated with long-term percent weight loss and regain among our sample. Each demographic predictor was tested univariably in an unadjusted linear mixed model with and without an additional test for interaction with time. Any demographic predictors that were individually statistically significantly (P < 0.05) associated with percent weight loss or associated with percent weight loss over time (interactive effect) were further assessed in a multivariable model. The final model included all variables significant in the multivariable model as well as some (sex, site) that we chose to include to be consistent with previously published papers examining demographic predictors of weight loss.
We used SAS Proc Traj to create trajectory models of percent weight loss from 1 year after surgery. Based on a priori knowledge of weight loss patterns after bariatric surgery among RYGB patients, we modeled between 2 and 6 groups with quadratic trajectories (11, 13). We eliminated any models where any group membership was below 5%. After selecting the number of groups based on the Bayesian Information Criterion, we tested additional trajectory patterns based on the statistical significance of the parameter estimates, total Bayesian Information Criterion, and visual fit. After selecting a final model, we tested the statistical significance of demographic predictors against trajectory model group membership (as a categorical variable) using Pearson's χ2 or 1-way analysis of variance (ANOVA) test.
RESULTS
Demographics
Demographic characteristics and surgical factors (height, surgery age, surgery weight, surgery body mass index, marital status, sex, educational level, site, ICD-9 code, CPT code, Hispanic ethnicity, and self-reported race) among our final sample of 162 patients are presented in Table 1. The median number of weight observations 1 year or more after surgery per participant was 10 (interquartile range, 4–25), and the average length of follow-up after surgery was 5.6 (standard deviation (SD), 2.2) years. Length of follow-up ranged from 1.2 to 9.6 years. The average body mass index expressed as weight (kg)/height (m)2) was 50.1 (SD, 8.9) at the time of surgery, 33.1 (SD, 6.6) at 1 year after surgery, and 34.3 (SD, 7.1) at the final observation. The average percent weight loss among all participants was 33.4% (SD, 9.3) at 1 year after surgery and 30.7% (SD, 12.5) at the last point of follow-up.
Table 1.
Demographic Features of 162 Patients Who Underwent Roux-en-Y Gastric Bypass for Weight Loss at Northwestern Medicine Hospital and NorthShore University HealthSystem and Were Enrolled in the NUgene Project, 2002–2013
| Characteristic | All Patients (n = 162) |
||
|---|---|---|---|
| Mean (SD) | No. of Patients | % | |
| Height, inchesa | 65.8 (3.4) | ||
| Age at surgery, years | 46.7 (10.8) | ||
| Weight at surgery, kg | 140.1 (28.4) | ||
| Body mass index at surgeryb | 50.1 (8.9) | ||
| Marital status | |||
| Married | 68 | 42.0 | |
| Unmarried | 94 | 58.0 | |
| Sex | |||
| Male | 25 | 15.4 | |
| Female | 137 | 84.6 | |
| Educational level | |||
| Postsecondary or higher | 144 | 88.9 | |
| Less than postsecondary | 18 | 11.1 | |
| Site | |||
| Northwestern Medicine Hospital | 119 | 73.5 | |
| NorthShore HealthSystem | 43 | 26.5 | |
| ICD-9 codec | |||
| 4431 | 34 | 21.0 | |
| 4438 | 39 | 24.1 | |
| 4439 | 45 | 27.8 | |
| Missing | 44 | 27.2 | |
| CPT codec | |||
| 43644 | 74 | 45.7 | |
| 43846 | 32 | 19.8 | |
| Missing | 56 | 34.6 | |
| Hispanic ethnicity | |||
| Hispanic | 10 | 6.2 | |
| Non-Hispanic or not reported | 152 | 93.8 | |
| Racec | |||
| White | 99 | 61.1 | |
| Black/mixed/missing ethnicity | 63 | 38.9 | |
Abbreviations: CPT, Current Procedural Terminology; ICD-9, International Classification of Diseases, Ninth Revision; SD, standard deviation.
a One inch = 2.54 cm.
b Expressed as weight (kg)/height (m)2.
c Each participant had a CPT, ICD-9, or both codes reported.
Predictors of long-term weight loss in linear mixed models
When modeled univariably, race, surgery body mass index, and surgery weight were statistically significant predictors of percent weight loss (Table 1). Surgery age was an independently statistically significant predictor of percent weight loss when modeled as an interaction effect with time. Interaction terms for other variables were not statistically significant and did not have a large magnitude of association (data available upon request from the authors). Race, surgery weight, and surgery age remained statistically significant in a multivariable model, with additional adjustment for sex, height, site, and time (Table 2). We found no difference in the statistical significance of these covariates when modeled with the alternate outcomes body mass index and weight or when modeled with a squared time term to account for nonlinearity (data not shown).
Table 2.
Linear Mixed Model of Percent Weight Loss From 1 to Over 9.5 Years of Follow-up After Roux-en-Y Gastric Bypass Surgery at Northwestern Medicine Hospital and NorthShore University HealthSystem Among 162 Patients Enrolled in the NUgene Project, 2002–2013
| Covariate | Percent Weight Loss |
||
|---|---|---|---|
| βa | 95% CI | P Value | |
| Race | |||
| White | 0.00 | Referent | <0.01 |
| Black/mixed/missing | −4.31 | −7.30, −1.32 | |
| Sex | |||
| Female | 0.00 | Referent | 0.24 |
| Male | 3.15 | −2.12, 8.43 | |
| Site | |||
| NorthShore | 0 | Referent | 0.44 |
| Northwestern | −1.29 | −4.57, 1.98 | |
| Surgery ageb | |||
| Age, years | −0.22 | −0.40, −0.03 | 0.02 |
| Surgery age × time, years | 0.05 | 0.00, 0.09 | 0.03 |
| Time, years | −3.07 | −5.22, −0.93 | <0.01 |
| Surgery weight, kg | 0.13 | 0.08, 0.19 | <0.01 |
| Height, inchesc | −1.06 | −1.65, −0.47 | <0.01 |
Abbreviation: CI, confidence interval.
a Reported β coefficients are for a multivariable linear mixed model of percent weight lost from 1 to over 9.5 years of follow-up in 162 patients. Negative β coefficients indicate lower long-term percent weight loss.
b Time and surgery age terms should not be interpreted individually, given the presence of the interaction term. A positive value for this interaction term indicates that the rate of weight regain (reduction in weight loss) is slower in older individuals.
c One inch = 2.54 inches.
In our final model, we found that black, mixed, and missing races (combined) in comparison with white race were associated with a decreased percent weight loss of −4.31% (95% confidence interval: −7.30, −1.32) over 1–9.5 years of follow-up. Taller stature (βinch = −1.06 (95% confidence interval: −1.65, −0.47)) was also associated with a decreased percent weight loss, with 1 inch = 2.54 cm; every additional inch in height was associated with a −1.06% lower percent weight loss over follow-up. Higher surgery weight was associated with an increased (βkg = 0.13 (95% confidence interval: 0.08, 0.19)) percent weight loss; every additional kilogram of weight at surgery was associated with 0.13% higher percent weight loss over follow-up.
Predictors of long-term weight regain in linear mixed models
We found that surgery age was a statistically significant predictor of long-term weight regain through an interaction effect with time (βyears = −0.22 (95% confidence interval: −0.40, −0.03); βyears × time = 0.05 (95% confidence interval: 0.00, 0.09); βtime = −3.07 (95% confidence interval: −5.22, −0.93)). The model suggests that the association with time is different by age, and older persons were found to regain a smaller percent weight over time despite having a lower initial percent weight loss. For example, our model found that a white female aged 40 years weighing 130 kg and being 70 inches (177.8 cm) tall at the time of surgery would be predicted to have 31.1% weight loss at 1 year, 26.8% weight loss at 5 years, and 22.0% weight loss at 9.5 years. A white female aged 50 years weighing 130 kg and being 70 inches tall at time of surgery would be predicted to have 29.4%, 27.1%, and 24.5% weight loss at respective time points. A white female aged 60 years weighing 130 kg and being 70 inches tall at time of surgery would be predicted to have a relatively stable weight loss of approximately 27% over 9.5 years of follow-up.
Predictors of weight loss patterns in trajectory models
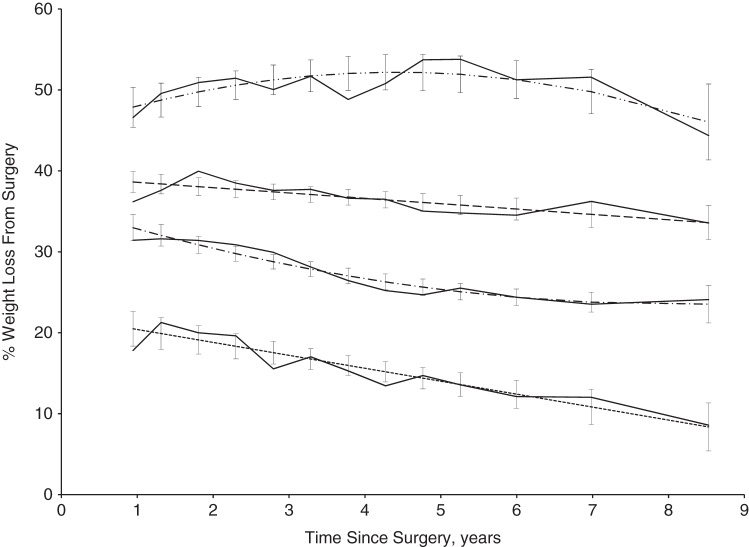
With quadratic trajectories specified for all groups, the Bayesian Information Criterion was minimized in a 4-group model (13). Through comparison of the Bayesian Information Criterion and visual fit, these 4 groups were best modeled with 2 linear and 2 quadratic trajectories. Within our final model, depicted in Figure 2, the average posterior probability of group membership was 0.92 (SD, 0.12). We also compared trajectory models using data from 30 days after surgery. We found that addition of data within 1 year of surgery did not alter the overall long-term patterns of weight loss by group.
Figure 2.
Trajectory-based model and average observed percent weight loss by group among 162 bypass patients enrolled in the NUgene Project (2002–2013) from 1 to over 9.5 years of follow-up after Roux-en-Y gastric bypass surgery at Northwestern Medicine Hospital and NorthShore University HealthSystem. The dash-dot-dot-dash line represents the trajectory plot of group 4, the large dashed line represents the trajectory plot of group 3, the dot-dashed-dot line represents the trajectory plot of group 2, and the small dashed line represents the trajectory plot of group 1. For each group, confidence intervals are depicted for the trajectory plots, and the average observed percent weight loss among each group is shown as a solid line.
Each of the 4 groups shows predicted weight regain (reduction in percent weight lost) over the 1–9.5 years after surgery; however, we found differences in both 1 year postsurgical weight loss by group and pattern of weight regain. Groups 1 (n = 19; 11.7%) and 3 (n = 54; 33.3%) patients were fit best by linear trajectories, indicating consistent and persistent weight regain. In comparison with group 1, group 3 patients had a higher percent weight loss at 1 year after surgery and regained less weight per year through follow-up. Groups 2 (n =69; 42.6%) and 4 (n = 20; 12.4%) were fit best by quadratic trajectories, indicating changes in weight regain over time. Patients in group 2 had moderate weight loss at 1 year after surgery and displayed a group tendency to regain weight up to 7 years after surgery with apparent stasis thereafter. Patients in group 4 had a very high percent weight loss at 1 year after surgery and continued to lose weight until 4.5 years after surgery, at which point they slowly regained weight over time.
We tested the distribution of demographic predictors against group membership to determine if the same predictors from our linear mixed models were also significantly associated with trajectory patterns. Table 3 includes all the demographic predictors that were included in our final linear mixed model; no other demographic predictors were statistically significantly different by group. We found that, similar to our linear mixed models of independent predictors, race (P = 0.04), surgery weight (P = 0.01), and surgery body mass index (P < 0.01) were statistically significantly associated with group membership as determined by SAS Proc Traj. Participants who self-reported race as either black, mixed, or missing were less likely to have higher and sustained percent weight loss (groups 3 and 4). Likewise, less obese patients were also less likely to have higher and sustained percent weight loss. We found no associations between distribution of height (P =0.61), surgery age (P = 0.66), marital status (P = 0.09), sex (P = 0.93), educational level (P = 0.38), hospital (P = 0.94), ICD-9 code (P = 0.13), CPT code (P = 0.39), or self-reported Hispanic ethnicity (P = 0.71) with trajectory group using SAS Proc Traj.
Table 3.
Association of Demographic Predictors of Weight Loss by Group Membership as Assigned by SAS Proc Traja Among 162 Roux-en-Y Gastric Bypass Surgery Patients at Northwestern Medicine Hospital and NorthShore University HealthSystem Who Were Enrolled in the NUgene Project, 2002–2013
| Characteristic | Group 1 (n = 19) |
Group 2 (n = 69) |
Group 3 (n = 54) |
Group 4 (n = 20) |
P Valueb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | Mean (SD) | No. of Patients | % | Mean (SD) | No. of Patients | % | Mean (SD) | No. of Patients | % | Mean (SD) | ||
| Race | 0.0385 | ||||||||||||
| White | 10 | 52.6 | 36 | 52.2 | 36 | 66.7 | 17 | 85.0 | |||||
| Black/mixed/missing ethnicity | 9 | 47.4 | 33 | 47.8 | 18 | 33.3 | 3 | 15.0 | |||||
| Sex | 0.9340 | ||||||||||||
| Male | 2 | 10.5 | 11 | 15.9 | 9 | 16.7 | 3 | 15.0 | |||||
| Female | 17 | 89.5 | 58 | 84.1 | 45 | 83.3 | 17 | 85.0 | |||||
| Site | 0.9412 | ||||||||||||
| Northwestern Medicine Hospital | 15 | 78.9 | 50 | 72.5 | 39 | 72.2 | 15 | 75.0 | |||||
| NorthShore HealthSystem | 4 | 21.1 | 19 | 27.5 | 15 | 27.8 | 5 | 25.0 | |||||
| Age at surgery, years | 49.6 (9.7) | 46.3 (10.5) | 46.3 (12.3) | 46.9 (8.4) | 0.6639 | ||||||||
| Weight at surgery, kg | 127.8 (22.5) | 136 (26.2) | 144.9 (29.0) | 153.1 (33.3) | 0.0125 | ||||||||
| Height, inchesc | 65.8 (2.6) | 65.9 (3.8) | 66.1 (3.1) | 64.9 (3.8) | 0.6076 | ||||||||
Abbreviations: ANOVA, analysis of variance; SD, standard deviation.
a SAS Proc Traj (SAS Institute, Inc., Cary, North Carolina).
b Reported P values are based on χ2 or ANOVA tests as appropriate. These tests examine if means or percentages of the demographic characteristics vary significantly across the 4 population groups assigned by SAS Proc Traj.
c One inch = 2.54 cm.
DISCUSSION
In this study of long-term weight loss follow-up after bariatric surgery, we found that race (black, mixed, and missing combined in comparison with white) and taller stature were associated with decreased percent weight loss and that higher surgery weight was associated with increased percent weight loss from 1 year to over 9.5 years of follow-up. We also found that older patients have decreased overall percent weight loss but slower weight regain over the course of follow-up. Participants with higher and longer sustained weight loss were more likely to be white and were more likely to be heavier at the time of surgery.
Comparison of results with those of other studies
Traditional approaches to assessing differences in outcomes and differential associations with demographic predictors have relied on single time-point analysis, either through bivariate models (i.e., t tests or χ2 tests) or through regression models. Several of these analyses have shown statistically significant associations with race: Generally whites have been found to have increased weight loss or excess weight loss in comparison with black or minority patients (14–17). In 1 recent meta-analysis including 3,801 individuals 2 years after surgery, blacks had, on average, 8.36% less excess weight loss than whites (14). Other studies have not reported statistically significant differences by race, although the durations of these studies have been 2 years or less and they may have been underpowered for such a comparison (3, 18–21). Age has previously been assessed as a demographic predictor of safety and efficacy of treatment by RYGB in older (greater than 60 years of age) patients. Although the reported results correlate with our finding that older patients experience reduced overall weight loss, relevant studies are limited by small sample sizes and have not formally reported effect sizes for long-term weight loss (22, 23).
Linear mixed models have been shown to be advantageous over traditional bivariate models through increased efficiency of incorporating observations at all available time points and by additional consideration of within-patient variation over time (7). However, repeated measures models assessing bariatric surgery outcomes and predictors have been used in very few studies. Repeated measures regression was recently used in a study of 2,365 RYGB surgery patients at the Geisinger Clinic to estimate percent excess body weight loss and independently associated preoperative variables in 3 postsurgical phases (6-month weight loss, weight loss nadir, and long-term (>36-months) weight loss). Race was not a significant predictor of weight loss outcomes in this study, but there was little power for comparison, as the population was 97% Caucasian (24). The Longitudinal Assessment of Bariatric Surgery (24) study is the only other study we know of that has used trajectory or growth mixture–based methods to model weight loss trajectories. In this analysis, the investigators applied growth mixture methods to estimate differential percent weight change and outcomes between groups with different trajectory patterns over 3 years of follow-up. Although application of the growth mixture–based method in this study is an improvement over previous studies, the primary aim of their assessment was to determine differences in major clinical outcomes, and predictors of long-term weight loss were not studied (11). In our study, we applied the growth mixture–based method to examine predictors of long-term weight loss, an outcome of critical importance in the field. Trajectory-based methods are well-suited to the analysis of long-term weight loss after surgery and should be applied more widely to identify predictors of differences in bariatric surgery outcomes.
The identification of race and age as potential predictors of long-term weight loss after RYGB (an outcome arguably more important than maximal weight loss or weight loss less than 5 years after weight loss surgery) may have implications for future research and policy in the area of bariatric surgery. If these findings can be replicated, research should be undertaken to understand the mechanisms behind the racial disparity in weight loss after surgery, and treatment adjustments including offering more intensive lifestyle interventions on postsurgical patients at higher risk of regain should be considered. Associations of reduced overall weight loss but particularly also reduced weight gain with aging should be examined in the context of sarcopenic obesity, that is, the loss of muscle mass with an increase in fat mass that often occurs with aging (25).
Strengths and limitations
First, we included only bypass surgery, and our results would not be expected to generalize to other types of bariatric surgery (5, 6, 11). We felt it was important to restrict to bypass surgery given the documented different patterns of weight loss and the amount of total long-term weight loss observed in studies of bypass and banding surgery. Second, we included 2 hospitals within the Chicago area, limiting the generalizability to other populations. Although this may limit our results regionally, we included in this analysis both whites and minority groups, an improvement over many previous studies that have been limited to white populations. We excluded patients who did not have weights recorded at surgery and for whom no presurgery weights were available. However, by extracting data from EHRs, we were able to include weight observations objectively measured at all clinic visits across the 2 health systems. This is an improvement over previous studies that may be limited to observations solely from patient follow-up visits with bariatric surgeons, given that a previous study has shown higher frequency of treatment failure in patients who cease follow-up with their bariatric surgeon (26). Our inclusion of up to 9.5 years of follow-up is a significant improvement over other studies that have not been able to make a long-term assessment of bariatric surgery outcomes or demographic predictors of long-term weight loss.
Conclusion
We used 2 different methods for analysis of longitudinal repeated measures data, including novel use of a semiparametric trajectory model to assess demographic differences in bariatric surgery patients, and obtained concordant results about association of long-term weight loss with age and race using data gathered from an EHR database. The findings may be useful in planning future studies of weight loss and weight regain after bariatric surgery.
ACKNOWLEDGMENTS
Author affiliations: Department of Preventive Medicine (Abigail S. Baldridge, Kwang-Youn A. Kim, Norrina B. Allen, Philip Greenland, Laura J. Rasmussen-Torvik), Center for Genetic Medicine (Jennifer A. Pacheco, Sharon A. Aufox, Maureen E. Smith), and Department of Surgery (E. Hungness), Feinberg School of Medicine, Northwestern University, Chicago, Illinois; and Center for Biomedical Research Informatics (J. C. Silverstein) and Department of Surgery (J. C. Silverstein, W. Denham), NorthShore University HealthSystem, Evanston, Illinois.
The project described was supported by the National Center for Research and the National Center for Advancing Translational Sciences, National Institutes of Health, through grants KL2 RR025740 and KL2 TR000107.
An early version of this paper was presented at the American Heart Association Cardiovascular Disease, Epidemiology, and Prevention/Nutrition, Physical Activity, and Metabolism 2014 Scientific Sessions, March 18–21, 2014, San Francisco, California.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
Appendix Table 1.
ICD-9 Procedure Codes and CPT History Codes Used to Determine Patients Eligible for Data Abstraction From Electronic Health Records, NUgene Project, 2002–2013
| Code | Description | Included in Analysis |
|---|---|---|
| ICD-9 Procedure Codes | ||
| 44.38 | Laparoscopic gastroenterostomy. Bypass: gastroduodenostomy, gastroenterostomy, gastrogastrostomy. Laparoscopic gastrojejunostomy without gastrectomy not elsewhere classified. Excludes gastroenterostomy, open approach (code 44.39). | Yes |
| 44.39 | Other gastroenterostomy. Bypass: gastroduodenostomy, gastroenterostomy, gastrogastrostomy. Gastrojejunostomy without gastrectomy not otherwise specified. | Yes |
| 44.31 | High gastric bypass. Printen and Mason gastric bypass. | Yes |
| 44.95 | Laparoscopic gastric restrictive procedure. Adjustable gastric band and port insertion. Excludes laparoscopic gastroplasty (code 44.68), other repair of stomach (code 44.69). | No |
| 43.89 | Other: Partial gastrectomy with bypass gastrogastrostomy. Sleeve resection of stomach. | No |
| 45.91 | Small-to-small intestinal anastomosis. | No |
| 44.68 | Laparoscopic gastroplasty. Banding. Silastic vertical banding. Vertical banded gastroplasty. Code also any synchronous laparoscopic gastroenterostomy (code 44.38). Excludes insertion, laparoscopic adjustable gastric band (restrictive procedure) (code 44.95); other repair of stomach, open approach (codes 44.61–44.65, 44.69). | No |
| CPT History Codes | ||
| 43644 | Laparoscopy, surgical, gastric restrictive procedure; with gastric bypass and Roux-en-Y gastroenterostomy (Roux limb 150 cm or less). | Yes |
| 43846 | Gastric restrictive procedure, with gastric bypass for morbid obesity; with short limb (150 cm or less) Roux-en-Y gastroenterostomy. | Yes |
| 43770 | Laparoscopy, surgical, gastric restrictive procedure; placement of adjustable gastric restrictive device (e.g., gastric band and subcutaneous port components). | No |
| 43645 | Laparoscopy, surgical, gastric restrictive procedure; with gastric bypass and small intestine reconstruction to limit absorption. | No |
| 43847 | Gastric restrictive procedure, with gastric bypass for morbid obesity; with small intestine reconstruction to limit absorption. | No |
| 43842 | Gastric restrictive procedure, without gastric bypass, for morbid obesity; vertical-banded gastroplasty. | No |
| 43843 | Gastric restrictive procedure, without gastric bypass, for morbid obesity; other than vertical-banded gastroplasty. | No |
| 43845 | Gastric restrictive procedure with partial gastrectomy, pylorus-preserving duodenoileostomy and ileoileostomy (50–100 cm common channel) to limit absorption (biliopancreatic diversion with duodenal switch). | No |
Abbreviations: CPT, Current Procedural Terminology; ICD-9, International Classification of Diseases, Ninth Revision.
REFERENCES
- 1.Puzziferri N, Roshek TB, 3rd, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;3129:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Y, Pagoto SL, Olendzki BC, et al. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. 2006;169:1227–1231. [DOI] [PubMed] [Google Scholar]
- 3.Snyder B, Nguyen A, Scarbourough T, et al. Comparison of those who succeed in losing significant excessive weight after bariatric surgery and those who fail. Surg Endosc. 2009;2310:2302–2306. [DOI] [PubMed] [Google Scholar]
- 4.Higa K, Ho T, Tercero F, et al. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011;74:516–525. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen NQ, Game P, Bessell J, et al. Outcomes of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. World J Gastroenterol. 2013;1936:6035–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;3578:741–752. [DOI] [PubMed] [Google Scholar]
- 7.Dallal RM, Quebbemann BB, Hunt LH, et al. Analysis of weight loss after bariatric surgery using mixed-effects linear modeling. Obes Surg. 2009;196:732–737. [DOI] [PubMed] [Google Scholar]
- 8.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;1223:248–256.e5. [DOI] [PubMed] [Google Scholar]
- 9.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;3071:56–65. [DOI] [PubMed] [Google Scholar]
- 10.Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity. 2013;218:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;31022:2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) Trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;2733:219–234. [DOI] [PubMed] [Google Scholar]
- 13.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 14.Admiraal WM, Celik F, Gerdes VE, et al. Ethnic differences in weight loss and diabetes remission after bariatric surgery: a meta-analysis. Diabetes Care. 2012;359:1951–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung LK, Lal LS, Chow DS, et al. Racial disparity in short-term outcomes after gastric bypass surgery. Obes Surg. 2013;2312:2096–2103. [DOI] [PubMed] [Google Scholar]
- 16.Limbach KE, Ashton K, Merrell J, et al. Relative contribution of modifiable versus non-modifiable factors as predictors of racial variance in Roux-en-Y gastric bypass weight loss outcomes. Obes Surg. 2014;248:1379–1385. [DOI] [PubMed] [Google Scholar]
- 17.Sudan R, Winegar D, Thomas S, et al. Influence of ethnicity on the efficacy and utilization of bariatric surgery in the USA. J Gastrointest Surg. 2014;181:130–136. [DOI] [PubMed] [Google Scholar]
- 18.Benoit SC, Hunter TD, Francis DM, et al. Use of bariatric outcomes longitudinal database (BOLD) to study variability in patient success after bariatric surgery. Obes Surg. 2014;246:936–943. [DOI] [PubMed] [Google Scholar]
- 19.Lutfi R, Torquati A, Sekhar N, et al. Predictors of success after laparoscopic gastric bypass: a multivariate analysis of socioeconomic factors. Surg Endosc. 2006;206:864–867. [DOI] [PubMed] [Google Scholar]
- 20.Welch G, Wesolowski C, Piepul B, et al. Physical activity predicts weight loss following gastric bypass surgery: findings from a support group survey. Obes Surg. 2008;185:517–524. [DOI] [PubMed] [Google Scholar]
- 21.Coleman KJ, Brookey J. Gender and racial/ethnic background predict weight loss after Roux-en-Y gastric bypass independent of health and lifestyle behaviors. Obes Surg. 2014;2410:1729–1736. [DOI] [PubMed] [Google Scholar]
- 22.Sosa JL, Pombo H, Pallavicini H, et al. Laparoscopic gastric bypass beyond age 60. Obes Surg. 2004;1410:1398–1401. [DOI] [PubMed] [Google Scholar]
- 23.St Peter SD, Craft RO, Tiede JL, et al. Impact of advanced age on weight loss and health benefits after laparoscopic gastric bypass. Arch Surg. 2005;1402:165–168. [DOI] [PubMed] [Google Scholar]
- 24.Still CD, Wood GC, Chu X, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring). 2014;223:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenholm S, Harris TB, Rantanen T, et al. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;116:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McVay MA, Friedman KE, Applegate KL, et al. Patient predictors of follow-up care attendance in Roux-en-Y gastric bypass patients. Surg Obes Relat Dis. 2013;96:956–962. [DOI] [PubMed] [Google Scholar]