Abstract
Few studies have assessed the associations between residential proximity to power plants and adverse birth outcomes including preterm delivery (PTD), very preterm delivery (VPTD), and term low birth weight (LBW). We geocoded 423,719 singleton Florida births born from 2004 to 2005 and all active power plants and determined residential proximity to the nearest power plant for each birth. Prenatal exposure to particulate matter less than 2.5 µm in diameter for women living near different types of power plants was also determined by using National Environmental Public Health Tracking Network data. Logistic regression models were used to test the hypothesized associations. Women who lived closer to coal and solid waste power plants were exposed to higher levels of particulate matter less than 2.5 µm in diameter compared with other types. We observed a 1.8% (95% confidence interval (CI): 1.3, 2.3) increased odds for PTD, 2.2% (95% CI: 1.0, 3.4) for VPTD, and 1.1% (95% CI: 0.2, 2.0) for term LBW for each 5 km closer to any power plant. When stratifying by different fuel type, we found that only solid waste had an association with term LBW, whereas oil, gas, and solid waste all had an association with PTD and VPTD. Results were consistent when exposure was categorized by number of power plants. Our study found evidence of increasing odds of adverse birth outcomes among infants born to pregnant women living closer to power plants. More research is warranted to better understand the causal relationship.
Keywords: birth outcomes, environment, low birth weight, pollution, power plants, preterm delivery
Air pollution has been extensively linked to many negative health outcomes ranging from cardiorespiratory diseases (1–3) to hospitalizations (4–6) and mortality (7, 8). A recent risk analysis estimated that particulate matter less than 2.5 µm in diameter (PM2.5) and ozone, estimated from the 2005 air quality level, were responsible for 130,000 and 4,700 excess deaths, respectively (9). These mortality estimates were significantly higher for the older population. In populous cities, the percentage of deaths attributable to PM2.5 and ozone ranges from 3.5% in San Jose to 10% in Los Angeles (both in California) (9). Exposure to various air pollutants has also been linked to adverse pregnancy-related and birth outcomes including gestational hypertension, premature delivery, and low birth weight (10–13).
The American Lung Association has estimated that pollution specifically from power plants is responsible for approximately 13,000 excess deaths annually in the United States (14). Because living near power plants may expose people to additional sources of air pollution, this proximity may increase their risk of having negative health endpoints. According to the US Environmental Protection Agency, power plants release toxic chemicals into the air including mercury, heavy metals, and acid gases, all of which are known to be deleterious to human health, especially that of the unborn fetus (15). Moreover, there is recent evidence suggesting that harmful emissions from power plants may be increasing (16).
Because of accumulating evidence of a negative relationship between power plants and human health, a few studies have assessed the association between proximity to nuclear power plants and adverse birth outcomes, but no association was found (17–19). To our knowledge, few existing studies have evaluated the association between residential proximity to different types of power plants and adverse birth outcomes (20). Furthermore, although power plant emissions are 1 of the major point sources of air pollution (21), which has been shown to have consistent association with adverse birth outcomes (22), there is limited information on air pollution exposure and pregnant women living close to specific types of power plants. Such information would be valuable in public health efforts to reduce adverse birth outcomes. Given that PM2.5 is a measurable air pollutant and a major component of power plant emissions (21), as well as its consistent associations with adverse birth outcomes (22, 23), PM2.5 can serve as a good indicator for air pollution from power plants. Florida has relatively high power plant emissions (16, 24) that provide a unique opportunity to investigate the potential association between power plant emissions and adverse birth outcomes. Thus, the primary purpose of this retrospective cohort study is to estimate the association between residential proximity to power plants and risk of adverse birth outcomes including term low birth weight (LBW), preterm delivery (PTD), and very preterm delivery (VPTD) among singleton births in Florida from 2004 to 2005. We further stratify these associations by fuel type. Second, we use PM2.5 as a surrogate for “pollution” from power plants to determine 1) the level of “pollution” exposure during pregnancy for women living close to power plants and 2) whether the amount of pollution depends on fuel type.
METHODS
Setting and participants
The source population was all livebirths recorded by the Florida Department of Health, Office of Vital Statistics (Florida Vital Records), from January 1, 2004, through December 31, 2005 (n = 445,028). After exclusion of births that had addresses outside Florida (n = 4,672); births that were missing address (n = 423), unable to geocode (e.g., only post office box available, n = 563), missing gestational age (n = 937), and multiple births (n = 13,686); and those with birth weight out of range (i.e., <500 and >5,000 g) (n = 903) and those with gestational age out of range (i.e., <140 days and >320 days) (n = 125), 423,719 births remained for analyses.
Exposure assessment
The exposure for this study was proximity to a nonrenewable-source power plant. All active power plants during the study period and eligible births were geocoded and mapped using ArcGIS V10.1 (ESRI, Redlands, California). Distance from the nearest power plants was measured in kilometers. The type of nearest power plant was also identified by fuel type. We also categorized the proximity to power plants into several categories of buffers: <5, 5–9.9, 10–19.9, and ≥20 km. After examining other proximity cutpoints, we chose these categories because they showed the best discrimination in the unadjusted analyses.
To describe prenatal exposures to PM2.5, we estimated average daily residential exposures to PM2.5 during pregnancy for each birth using data from the Centers for Disease Control and Prevention's National Environmental Public Health Tracking Network. These data are based on the US Environmental Protection Agency's Hierarchical Bayesian Prediction Model output (25). Briefly, this model uses hierarchical Bayesian methods to combine data from observed air quality data measured at air monitors, the National Emission Inventory, and meteorological and photochemical data to produce 12 × 12 km gridded estimates of daily average PM2.5 concentrations. We overlaid geocoded residential addresses over the 12 × 12 km grids. Prenatal exposure was assigned to each birth as the average daily PM2.5 concentration over the first trimester for the grid in which it falls. We chose first trimester because 1) first trimester exposure to PM2.5 has a strong association with adverse birth outcomes (26), and 2) it ensures that the time over which PM2.5 is averaged is similar for cases and controls. The first trimester was defined as the first 13 weeks of gestation. A detailed description of this method was reported elsewhere (23). In addition, we also overlaid geocoded active power plants and the 12 × 12 km grids to determine the daily PM2.5 concentrations near the power plants during the study period.
Outcome assessment
The main outcomes of interest in this study include the terms LBW, PTD, and VPTD, all of which were assessed through Florida Vital Records data. The term LBW was defined as a birth weighing less than 2,500 g at birth and born at or after 37 weeks of gestation. PTD was defined as a birth that occurred before 37 but at or after 32 weeks of gestation. VPTD was defined as a birth that occurred before 32 weeks of gestation. On Florida birth certificates, the gestational age in weeks is typically determined by ultrasound measurements. When ultrasound is not available, fundal height (determined by clinical examination) or menstrual history is used to estimate gestational age. For controls, we used eligible births that had none of the 3 outcomes we assessed.
Covariates
Covariates were chosen on the basis of a directed acyclic graph (Web Figure 1 available at http://aje.oxfordjournals.org/). They included mother's age (continuous), maternal education (less than high school, high school graduate and/or some college, college graduate, graduate school), maternal race (white, black, Hispanic, Asian/Pacific Islander, and others), and marital status (married, unmarried). We also used Census 2000 data to determine census block group income as a proxy for neighborhood socioeconomic status and urbanity.
Statistical analyses
To describe exposures to PM2.5 during pregnancy and throughout the study period according to nearest power plants, we used scatterplots with locally weighted scatterplot smoothing (LOESS) functions (smooth = 0.5) to display averaged daily concentrations during the first trimester (y-axis) by date of delivery (x-axis), as well as daily concentrations (y-axis) by date during the study period (x-axis). We stratified the plots by fuel type for comparison. To compare continuous and categorical characteristics for participants with and without the 3 adverse birth outcomes, we performed t tests and χ2 tests. We used logistic regression models to investigate the association between proximity to a power plant and the adverse birth outcomes. Two logistic regression models were used for each outcome: The first model was unadjusted, and the second model was a parsimonious model adjusted for potential confounders on the basis of our directed acyclic graph (Web Figure 1). We obtained the odds ratios and 95% confidence intervals for the increase in odds of having adverse birth outcomes for each 5 km closer to any power plant. We also compared the odds for different buffer sizes of <5, 5–9.9, 10–19.9, and ≥20 km from a power plant. We further stratified analyses for different types of plants. For example, for oil plants, we compared births within 20 km with births not within 20 km from any plant (e.g., excluding those who were closer to any other types of plant). We were unable to perform analyses for more refined proximity categories because of low sample size for certain cells. Data analyses were performed by using SAS, version 9.3, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Table 1 shows participants' characteristics by term LBW, PTD, and VPTD. The sample prevalence was 2.4% for term LBW, 8.2% for PTD, and 1.5% for VPTD. Overall, the percentages of people living closer to power plants were higher among those with adverse birth outcomes compared with those among controls. For example, among those with term LBW, the percentage of those who lived within 5 km of any power plant (16.1%) was higher than that of controls (14.9%). Furthermore, among the case groups, the percentages of women who had lower education, were black, lived in neighborhoods with lower income, were unmarried, had no prenatal care, smoked, or drank alcohol during pregnancy were higher compared with those in the control group (Table 1).
Table 1.
Characteristics of 423,719 Singleton Births in Florida From 2004 to 2005
| Characteristic | Term LBW |
PTD |
VPTD |
Controls |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | |
| Total participants | 9,320 | 2.4 | 33,402 | 8.2 | 5,680 | 1.5 | 375,317 | |||||
| Distance from plant, km | ||||||||||||
| Continuous | 14.5 (11.8) | 14.4 (11.6) | 14.1 (11.5) | 15.0 (12.1) | ||||||||
| Categorical | ||||||||||||
| <5 | 1,497 | 16.1 | 5,288 | 15.8 | 943 | 16.6 | 56,016 | 14.9 | ||||
| 5–9.9 | 2,441 | 26.2 | 8,900 | 26.7 | 1,531 | 27.0 | 97,167 | 25.9 | ||||
| 10–19.9 | 3,393 | 36.4 | 12,400 | 37.1 | 2,080 | 36.6 | 138,917 | 37.0 | ||||
| ≥20 | 1,989 | 21.3 | 6,814 | 20.4 | 1,126 | 19.8 | 83,217 | 22.2 | ||||
| Maternal age, years | 26.7 (7.1) | 27.6 (6.9) | 27.4 (7.3) | 27.5 (6.4) | ||||||||
| Gestational age, weeks | 38.0 (1.0) | 35.1 (1.2) | 27.9 (2.6) | 39.0 (1.1) | ||||||||
| Mother's education | ||||||||||||
| Less than high school | 2,560 | 27.8 | 7,730 | 23.4 | 1,397 | 25.2 | 77,011 | 20.7 | ||||
| High school/some college | 4,749 | 51.6 | 16,674 | 50.5 | 2,942 | 53.1 | 183,225 | 49.3 | ||||
| College degree | 1,504 | 16.4 | 6,896 | 20.9 | 984 | 17.8 | 88,270 | 23.7 | ||||
| Graduate school | 394 | 4.3 | 1,710 | 5.2 | 215 | 3.9 | 23,343 | 6.3 | ||||
| Maternal race | ||||||||||||
| White | 3,344 | 36.4 | 14,615 | 44.1 | 1,864 | 33.3 | 176,354 | 47.4 | ||||
| Black | 2,756 | 30.0 | 7,710 | 23.3 | 1,932 | 34.5 | 62,419 | 16.8 | ||||
| Hispanic | 1,881 | 20.5 | 6,451 | 19.5 | 1,155 | 20.6 | 83,470 | 22.4 | ||||
| Asian/Pacific Islander | 324 | 3.5 | 902 | 2.7 | 124 | 2.2 | 10,605 | 2.9 | ||||
| Others | 894 | 9.6 | 3,430 | 10.4 | 522 | 9.3 | 39,483 | 10.6 | ||||
| Census block group annual income, US dollars | ||||||||||||
| <29,643.00 (first quartile) | 3,026 | 32.5 | 9,174 | 27.5 | 1,892 | 33.3 | 92,338 | 24.6 | ||||
| 29,643.00 to <38,095.00 (second quartile) | 2,401 | 25.8 | 8,287 | 24.8 | 1,461 | 25.7 | 94,213 | 25.1 | ||||
| 38,095.00 to <49,457.00 (third quartile) | 2,152 | 23.1 | 8,288 | 24.8 | 1,320 | 23.2 | 94,303 | 25.1 | ||||
| ≥49,457.00 (fourth quartile) | 1,741 | 18.7 | 7,653 | 22.9 | 1,007 | 17.7 | 94,463 | 25.2 | ||||
| Urban neighborhood | 8,160 | 87.6 | 28,972 | 86.7 | 4,986 | 87.8 | 323,992 | 86.3 | ||||
| Infant's sex, female | 5,578 | 59.9 | 15,910 | 47.6 | 2,655 | 46.7 | 182,823 | 48.7 | ||||
| Marital status, married | 4,408 | 47.4 | 18,549 | 55.6 | 2,631 | 46.4 | 224,450 | 59.8 | ||||
| Prenatal care, yes | 9,055 | 97.2 | 32,214 | 96.4 | 5,224 | 92.0 | 370,271 | 98.7 | ||||
| Tobacco use | ||||||||||||
| Yes, <10/day | 1,519 | 16.3 | 4,960 | 14.9 | 1,031 | 18.2 | 51,941 | 13.8 | ||||
| Yes, ≥10/day | 1,128 | 12.1 | 2,586 | 7.5 | 472 | 8.3 | 22,611 | 6.0 | ||||
| Quit | 190 | 2.0 | 548 | 1.7 | 99 | 1.7 | 5,952 | 1.6 | ||||
| No | 6,483 | 69.6 | 25,308 | 75.8 | 4,078 | 71.8 | 294,813 | 78.6 | ||||
| Alcohol, yes | 69 | 0.7 | 160 | 0.5 | 32 | 0.6 | 1,180 | 0.3 | ||||
| Season of conception | ||||||||||||
| Warm (May–October) | 4,382 | 47.0 | 16,221 | 48.6 | 2,743 | 48.3 | 182,090 | 48.5 | ||||
| Cold (November–April) | 4,938 | 53.0 | 17,181 | 51.4 | 2,937 | 51.7 | 193,227 | 51.5 | ||||
| Year of conception | ||||||||||||
| 2003 | 3,243 | 34.8 | 10,772 | 32.3 | 1,448 | 25.5 | 135,717 | 36.2 | ||||
| 2004 | 4,664 | 50.0 | 16,924 | 50.7 | 2,866 | 50.5 | 189,748 | 50.6 | ||||
| 2005 | 1,413 | 15.2 | 5,706 | 17.1 | 1,366 | 24.1 | 49,852 | 13.3 | ||||
| Type of nearest power plant | ||||||||||||
| Coal | 634 | 6.8 | 2,555 | 7.7 | 403 | 7.1 | 27,160 | 7.2 | ||||
| Gas | 4,024 | 43.2 | 14,447 | 43.3 | 2,416 | 42.5 | 164,909 | 43.9 | ||||
| Nuclear | 63 | 0.7 | 222 | 0.7 | 37 | 0.7 | 2,913 | 0.8 | ||||
| Oil | 1,611 | 17.3 | 5,519 | 16.5 | 1,032 | 18.2 | 62,212 | 16.6 | ||||
| Solid waste | 2,818 | 30.2 | 10,148 | 30.4 | 1,718 | 30.3 | 112,135 | 29.9 | ||||
| Other | 170 | 1.8 | 511 | 1.5 | 74 | 1.3 | 5,988 | 1.6 | ||||
Abbreviations: LBW, low birth weight; PTD, preterm delivery; SD, standard deviation; VPTD, very preterm delivery.
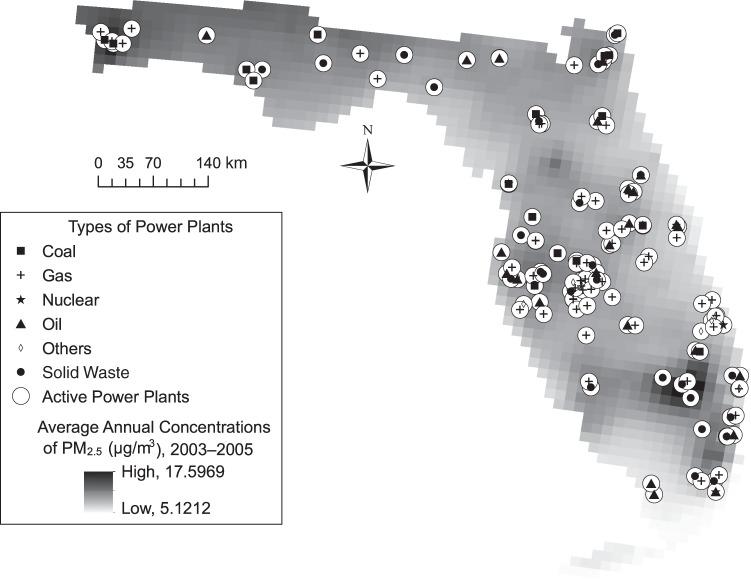
Figure 1 shows a map of the spatial distribution of active power plants and the average annual PM2.5 concentrations in Florida during 2003–2005. There were 150 active nonrenewable power plants with different fuel types including 17 coal plants, 66 gas plants, 3 nuclear plants, 28 oil plants, 29 solid waste plants, and 7 plants with other types of fuel (e.g., coke, etc.). In general, PM2.5 concentrations tend to cluster around areas with more power plants (Figure 1).
Figure 1.
Geographical distribution of power plants and average annual levels of particulate matter less than 2.5 µm in diameter (PM2.5) in Florida during the study period from 2003 to 2005.
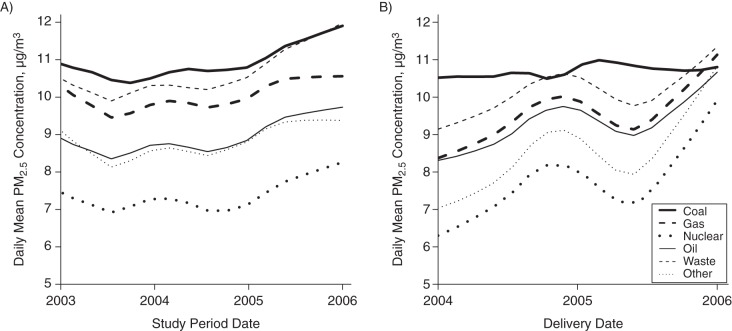
Figure 2A and 2B illustrate PM2.5 exposure by types of power plants. In general, the concentrations of PM2.5 were highest around coal plants followed by solid waste, gas, oil, other, and nuclear plants (Figure 2A). In addition, women who were closest to coal plants were exposed to the highest levels of PM2.5 (mean = 10.7 (standard deviation (SD), 2.7) μg/m3) during the first trimester, followed by those living close to solid waste plants (mean = 10.1 (SD, 1.8) μg/m3), gas plants (mean = 9.5 (SD, 2.1) μg/m3), and oil plants (mean = 9.3 (SD, 1.8) μg/m3). Those who were closest to nuclear plants (mean = 7.7 (SD, 1.9) μg/m3) and “other” plants (mean = 8.5 (SD, 2.0) μg/m3) were exposed to the lowest levels of PM2.5 (Figure 2B; Web Table 1).
Figure 2.
Exposures to levels of particulate matter less than 2.5 µm in diameter (PM2.5) stratified by power plant type in Florida from 2003 to 2005. A) Daily concentrations of PM2.5 during the study period 2003–2005 at power plants; B) mean daily PM2.5 concentration at residential address during the first trimester for births in Florida from 2004 to 2005.
Table 2 provides the unadjusted and adjusted odds ratios for the association between proximity to power plants and term LBW, PTD, and VPTD. Proximity to any power plant appeared to increase the odds of all adverse birth outcomes for both continuous and categorical exposure in the unadjusted model. Moreover, these associations showed an exposure-response relationship with closer residents having the higher odds. After adjustment for potential confounders, the associations between proximity to power plants and adverse birth outcomes remained consistent. Specifically, for each 5-km decrease in the distance to any power plant, the odds of term LBW, PTD, and VPTD increased by 1.1% (odds ratio (OR) = 1.011, 95% confidence interval (CI): 1.002, 1.020), 1.8% (OR = 1.018, 95% CI: 1.013, 1.023), and 2.2% (OR = 1.022, 95% CI: 1.010, 1.034), respectively. These associations remained consistent for PTD and VPTD when exposures were categorized (Table 2). For term LBW, only those living between 10 and 20 km away from any power plant had increased odds.
Table 2.
Association Between Proximity to Power Plants and Adverse Birth Outcomes in Florida From 2004 to 2005
| Distance From Power Plant, km | Term LBW |
PTD |
VPTD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | aORa | 95% CI | OR | 95% CI | aORa | 95% CI | OR | 95% CI | aORa | 95% CI | |
| Continuous (for each 5 km closer) | 1.018 | 1.009, 1.027 | 1.011 | 1.002, 1.020 | 1.020 | 1.015, 1.025 | 1.018 | 1.013, 1.023 | 1.031 | 1.019, 1.043 | 1.022 | 1.010, 1.034 |
| Categorical | ||||||||||||
| <5 | 1.118 | 1.045, 1.197 | 1.038 | 0.968, 1.113 | 1.153 | 1.110, 1.197 | 1.126 | 1.084, 1.170 | 1.244 | 1.140, 1.357 | 1.160 | 1.060, 1.270 |
| 5–9.9 | 1.051 | 0.990, 1.116 | 1.026 | 0.965, 1.092 | 1.119 | 1.082, 1.156 | 1.114 | 1.077, 1.152 | 1.164 | 1.078, 1.258 | 1.138 | 1.050, 1.233 |
| 10–19.9 | 1.022 | 0.966, 1.081 | 1.059 | 1.000, 1.121 | 1.090 | 1.057, 1.124 | 1.106 | 1.072, 1.141 | 1.107 | 1.029, 1.190 | 1.147 | 1.064, 1.236 |
| ≥20 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; LBW, low birth weight; OR, odds ratio; PTD, preterm delivery; VPTD, very preterm delivery.
a Adjusted for maternal age, maternal race, education, marital status, census block group income, and urban neighborhood.
Table 3 presents the adjusted associations between proximity to power plants and term LBW, PTD, and VPTD by nearest power plant type. Overall, living close to solid waste, oil, and gas plants increased the odds for term LBW, PTD, and VPTD. In the continuous exposure analyses, only solid waste plants (OR = 1.03, 95% CI: 1.01, 1.04) had an association with term LBW for each 5-km decrease in distance. All types of plants had a slightly elevated association with PTD, except nuclear plants, which had a negative association (OR = 0.90, 95% CI: 0.86, 0.95), and “others” and coal plants with no associations. For VPTD, only oil and solid waste plants had significant associations. The results remained consistent when exposure was categorized (Table 3).
Table 3.
Association Between Proximity to Power Plants, by Fuel Type, and Adverse Birth Outcomes in Florida From 2004 to 2005
| Type of Power Plants | Term LBW |
PTD |
VPTD |
|||
|---|---|---|---|---|---|---|
| aORa | 95% CI | aORa | 95% CI | aORa | 95% CI | |
| Continuous (for Each 5 km Closer in Distance) | ||||||
| Coal plant (n = 17) | 1.02 | 0.98, 1.06 | 1.01 | 0.99, 1.03 | 1.03 | 0.98, 1.09 |
| Gas plant (n = 66) | 1.00 | 0.99, 1.02 | 1.02 | 1.01, 1.03 | 1.01 | 0.99, 1.03 |
| Nuclear plant (n = 3) | 1.07 | 0.96, 1.20 | 0.90 | 0.86, 0.95 | 0.99 | 0.87, 1.14 |
| Oil plant (n = 28) | 1.00 | 0.99, 1.02 | 1.02 | 1.01, 1.03 | 1.03 | 1.01, 1.06 |
| Solid waste plant (n = 29) | 1.03 | 1.01, 1.04 | 1.02 | 1.02, 1.03 | 1.02 | 1.00, 1.05 |
| Other (n = 7) | 1.05 | 0.96, 1.15 | 1.01 | 0.96, 1.06 | 1.03 | 0.91, 1.18 |
| Categorical (≤20 km Away vs. >20 km Away From All Plants) | ||||||
| Coal plant (n = 16) | 1.19 | 0.98, 1.45 | 1.07 | 0.97, 1.18 | 1.19 | 0.93, 1.51 |
| Gas plant (n = 67) | 0.96 | 0.89, 1.04 | 1.09 | 1.04, 1.13 | 1.09 | 0.99, 1.20 |
| Nuclear plant (n = 3) | 1.37 | 0.81, 2.31 | 0.82 | 0.60, 1.11 | 0.88 | 0.39, 2.00 |
| Oil plant (n = 28) | 1.04 | 0.91, 1.19 | 1.14 | 1.06, 1.24 | 1.27 | 1.07, 1.52 |
| Solid waste plant (n =30) | 1.10 | 1.00, 1.22 | 1.16 | 1.09, 1.22 | 1.15 | 1.01, 1.32 |
| Other (n = 7) | 1.16 | 0.68, 1.98 | 1.23 | 0.89, 1.70 | 1.39 | 0.61, 3.17 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; LBW, low birth weight; PTD, preterm delivery; VPTD, very preterm delivery.
a Adjusted for maternal age, maternal race, marital status, census block group income, and urban neighborhood.
Because there was evidence of spatial clustering of power plants, distance from power plants may not be the best measure of exposure. We also created 20 km buffers around each birth and determined the total number of power plants within this buffer (Table 4). The association between adverse birth outcomes and total number of power plants within 20 km was determined. Compared with pregnant women who lived with no power plants within a 20 km radius, women living near ≥2 power plants had a 7% increased odds of term LBW (OR = 1.07, 95% CI: 1.01, 1.12), 12% increased odds of PTD (OR = 1.12, 95% CI: 1.09, 1.15), and 17% increased odds of VPTD (OR = 1.17, 95% CI: 1.09, 1.25). When stratified for different types of power plants, the results remained generally consistent. Coal was strongly associated with all adverse birth outcomes (Table 4).
Table 4.
Association Between Number of Plants Within 20 km, by Fuel Type, and Adverse Birth Outcomes in Florida From 2004 to 2005
| Type and No. of Power Plants Within 20 km | Term LBW |
PTD |
VPTD |
|||
|---|---|---|---|---|---|---|
| aORa | 95% CI | aORa | 95% CI | aORa | 95% CI | |
| All plants | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 0.93 | 0.86, 1.01 | 1.08 | 1.04, 1.13 | 1.06 | 0.95, 1.17 |
| ≥2 | 1.07 | 1.01, 1.12 | 1.12 | 1.09, 1.15 | 1.17 | 1.09, 1.25 |
| Coal plant | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 0.93 | 0.87, 1.00 | 1.09 | 1.05, 1.13 | 0.99 | 0.91, 1.08 |
| ≥2 | 1.12 | 1.03, 1.22 | 1.20 | 1.14, 1.25 | 1.23 | 1.10, 1.36 |
| Gas plant | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 1.07 | 1.02, 1.13 | 1.05 | 1.02, 1.08 | 1.13 | 1.06, 1.21 |
| ≥2 | 1.06 | 1.00, 1.12 | 1.13 | 1.10, 1.17 | 1.13 | 1.06, 1.21 |
| Oil plant | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 1.07 | 1.02, 1.12 | 1.10 | 1.07, 1.12 | 1.18 | 1.12, 1.25 |
| ≥2 | 0.97 | 0.89, 1.06 | 0.99 | 0.95, 1.04 | 0.83 | 0.74, 0.94 |
| Nuclear plantb | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| ≥1 | 1.03 | 0.93, 1.15 | 0.92 | 0.86, 0.98 | 0.88 | 0.75, 1.02 |
| Solid waste plant | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 1.08 | 1.04, 1.13 | 1.06 | 1.03, 1.09 | 1.13 | 1.06, 1.20 |
| ≥2 | 1.00 | 0.93, 1.07 | 1.09 | 1.06, 1.13 | 1.13 | 1.04, 1.22 |
| Other plant | ||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1 | 0.94 | 0.87, 1.02 | 1.03 | 0.99, 1.08 | 1.07 | 0.98, 1.19 |
| ≥2 | 0.95 | 0.80, 1.14 | 0.86 | 0.78, 0.96 | 0.77 | 0.60, 0.99 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; LBW, low birth weight; PTD, preterm delivery; VPTD, very preterm delivery.
a Adjusted for maternal age, maternal race, maternal education, marital status, census block group income, and urban neighborhood.
b Nuclear plants are categorized differently because there are no births with 2 or more nuclear plants within 20 km.
DISCUSSION
This study found that women with residential proximity to coal and solid waste plants were exposed to the highest concentrations of PM2.5 during the first trimester, and those closest to nuclear plants were exposed to the lowest concentrations. This pattern remained consistent when daily PM2.5 concentrations near different types of power plants were examined. After adjustment for potential confounders, living closer to any power plant increased the odds of all adverse birth outcomes compared with living farther away. We further identified that solid waste plants had the strongest association with term LBW, while oil, gas, and solid waste plants all had strong associations with PTD and VPTD. The study also found that women living near 1 or more power plants located within a 20 km radius from their residence had higher odds of adverse birth outcomes. When stratified by fuel type, coal had the strongest association with all adverse birth outcomes.
Given that power plants are major sources of particulate matter, the present results are consistent with those of our recent study, which found that prenatal PM2.5 exposure was positively associated with LBW, PTD, and VPTD (23). During the study period, power plants in the United States emitted an estimated annual average of 2,491,971 metric tons of carbon dioxide, 10,431 metric tons of sulfur dioxide, and 4,212 metric tons of nitric oxides (27). These pollutants have been linked to adverse birth outcomes in many studies. For example, higher exposure to sulfur dioxide has been linked to PTD among singleton births (28) and decreased term birth weight (29). In addition, nitric oxides have also been linked to term LBW and PTD (30, 31). Given the high emission of sulfur dioxide and nitric oxides from power plants and the positive association between these pollutants and adverse birth outcomes, it is plausible that proximity to power plants was associated with adverse birth outcomes. This finding has also been observed in Taiwan by Tsai et al. (20), who reported that the odds of PTD were 14 times higher among women who lived within 3 km of a thermal power plant compared with those who lived farther than 3 km.
When stratified by type of plants in order to compare birth outcomes among women who lived close to or farther from different types of power plants, our data showed that solid waste plants had associations with all adverse birth outcomes. In addition, when exposure was changed to number of plants within 20 km, coal plants had the highest association with all adverse birth outcomes. These findings are consistent with the fact that coal and solid waste power plants produce relatively larger amounts of particulate emissions compared with other types of power plants. Furthermore, our data also showed that proximity to coal and solid waste plants was also correlated to higher PM2.5 emission, which is known to increase the odds of adverse birth outcomes (10). With coal combustion accounting for approximately 45% of electricity produced in the United States, it may pose a serious public health issue. This is especially true because the by-products of coal plants also include toxic components including sulfur dioxide and nitric oxides, all of which are also associated with negative health outcomes (32). We also found that gas and oil plants had a positive association with adverse birth outcomes, especially PTD and VPTD. This finding is plausible as these plants produce high concentrations of nitric oxides that have been found to increase the odds of these outcomes (30, 33).
Our continuous exposure analysis showed that proximity to nuclear plants was protective against PTD, but not term LBW or VPTD. In categorical exposure analyses, no association was found. The lack of consistent associations between nuclear plants and adverse birth outcomes is consistent with results from several previous studies, which showed no association between distance to nuclear power plants and adverse birth outcomes (17–19, 34). The lack of a positive association could be explained by the fact that, compared with other types of plants, nuclear plants do not emit as many atmospheric pollutants in high concentrations (e.g., PM2.5, sulfur dioxide, and nitric oxides) (35). This association is also consistent with our data that showed, on average, that women close to nuclear plants and other plants were exposed to lower levels of PM2.5.
Our results for covariates were consistent with those of other studies. For example, the fact that our study showed an increased proportion of females among the term LBW group and a decreased proportion of females among the PTD and VPTD groups confirmed previous findings. On average, males weigh 150 and 200 g more than females (36) given term birth, and they have a 9%–24% increased risk for PTD (37). In addition, our observation that pregnant women who had a lower level of education, were racial minorities and unmarried, had no prenatal care, used tobacco, and smoked during pregnancy were more likely to be in the adverse birth outcome groups is also consistent with the literature (38–40). It is possible that socioeconomic status confounded the association that we observed in this study. However, in addition to adjustment for maternal education, which serves as a proxy for socioeconomic status, we also adjusted for census-tract-level median household income from the US Census 2000 to control for ecological (or population-level) socioeconomic status factors.
Like most retrospective studies, this study has several limitations. First, analysis of proximity as both a continuous variable and as a categorical variable addresses exposure-response relationships, but it does so in different ways with different assumptions. In the categorical exposure analyses, we made a fundamental assumption that each power plant has a uniform effect on participants within a certain buffer. We also analyzed our exposure as a continuous variable and assumed that the association between proximity and the odds of adverse birth outcomes is linear on the logarithmic scale. When the results for both analyses were compared, the conclusions were consistent, although those for categorical exposure are stronger. One of the reasons for the differences may be a result of the spatial clustering of power plants. To ensure that clustering was not influencing our results, we performed a sensitivity analysis using the number of power plants within 20 km as exposure. This analysis yielded results consistent with proximity to power plants as a measure of exposure.
Although we chose PM2.5 to “validate” our exposure, power plants also emit other gaseous pollutants including carbon oxides, sulfur dioxide, and nitric oxides (41), all of which are highly correlated (30). Because of the use of proximity and the unavailability of specific pollutant data from the US Environmental Protection Agency's Hierarchical Bayesian Prediction Model, we were unable to disentangle the specific pollutant. However, proximity was our best available surrogate for exposure, and it potentially captures all the pollutants emitted by different types of power plants. Additionally, we were not able to identify critical windows of exposure that underlie these associations. Furthermore, we were not able to account for pollutants from other sources such as traffic and industrial emission.
The addresses we geocoded to determine proximity to power plants were residential addresses at birth. This method assumes that pregnant women stayed at those same addresses throughout their pregnancy. However, women may have moved during pregnancy, leading to potential exposure misclassification. We unfortunately did not have residential mobility information to address this issue. However, studies on residential mobility and its effects on air pollution assessment suggest a very low degree of misclassification (42, 43).
We were also unable to adjust for daily activities patterns. Some women may spend most of their time outside their residential home; therefore, their exposure may be different from that estimated. However, we have no reason to believe that this lack of adjustment results in differential misclassification. Therefore, this discrepancy is likely to bias our results toward the null. Consequently, daily activity patterns cannot entirely explain the increased risk that we found.
One may argue that potential confounding may be caused by other variables that we did not adjust for. However, we have also used a fully adjusted model with all covariates in our directed acyclic graph. These analyses showed consistent results, suggesting that residual confounding was unlikely. Finally, the Hierarchical Bayesian Prediction Model data that we used to describe PM2.5 exposures were available at only 12 × 12 km resolution. Therefore, we were not able to account for local variation smaller than this spatial resolution.
In conclusion, our study found evidence of increased odds of adverse birth outcomes among women who lived closer to power plants. This association remained consistent in our analyses for total number of power plants within a 20 km radius and stratified analyses for different types of power plants. These results call for further investigation to confirm our results and to investigate the specific pollutants generated from power plants that are responsible for this association.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, College of Public Health and Health Professions and College of Medicine, University of Florida, Gainesville, Florida (Sandie Ha, Hui Hu, Xiaohui Xu); Department of Pediatrics, College of Medicine, University of Florida, Gainesville, Florida (Jeffrey Roth); and Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention (LAP3), School of Public Health, Fudan University, Shanghai, People's Republic of China (Haidong Kan).
This work was supported by grant K01ES019177 from the National Institute of Environmental Health Sciences (X.X.) and a University of Florida Graduate School fellowship (S.H.).
We wish to thank the Florida Department of Health for supplying the data.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funders or data provider.
Conflict of interests: none declared.
REFERENCES
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;12121:2331–2278. [DOI] [PubMed] [Google Scholar]
- 2.Peacock JL, Anderson HR, Bremner SA, et al. Outdoor air pollution and respiratory health in patients with COPD. Thorax. 2011;667:591–596. [DOI] [PubMed] [Google Scholar]
- 3.Teng TH, Williams TA, Bremner A, et al. A systematic review of air pollution and incidence of out-of-hospital cardiac arrest. J Epidemiol Community Health. 2014;681:37–43. [DOI] [PubMed] [Google Scholar]
- 4.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;29510:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halonen JI, Lanki T, Yli-Tuomi T, et al. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax. 2008;637:635–641. [DOI] [PubMed] [Google Scholar]
- 6.Neuberger M, Moshammer H, Rabczenko D. Acute and subacute effects of urban air pollution on cardiopulmonary emergencies and mortality: time series studies in Austrian cities. Int J Environ Res Public Health. 2013;1010:4728–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romieu I, Gouveia N, Cifuentes LA, et al. Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res Rep Health Eff Inst. 2012;171:5–86. [PubMed] [Google Scholar]
- 8.Simpson R, Williams G, Petroeschevsky A, et al. The short-term effects of air pollution on daily mortality in four Australian cities. Aust N Z J Public Health. 2005;293:205–212. [PubMed] [Google Scholar]
- 9.Fann N, Lamson AD, Anenberg SC, et al. Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Anal. 2012;321:81–95. [DOI] [PubMed] [Google Scholar]
- 10.Stieb DM, Chen L, Eshoul M, et al. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. [DOI] [PubMed] [Google Scholar]
- 11.Vrijheid M, Martinez D, Manzanares S, et al. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ Health Perspect. 2011;1195:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Hu H, Ha S, et al. Ambient air pollution and hypertensive disorder of pregnancy. J Epidemiol Community Health. 2014;681:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H, Ha S, Roth J, et al. Ambient air pollution and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Atmos Environ. 2014;97:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Lung Association. Toxic Air: The Case for Cleaning Up Coal-fired Power Plants. Washington, DC: American Lung Association; 2011. [Google Scholar]
- 15.US Environmental Protection Agency. Reducing air pollution from power plants. http://www.epa.gov/airquality/powerplants/ Published March 27, 2012. Updated March 27, 2012. Accessed December 6, 2014.
- 16.Environmental Integrity Project. US Power Plant Global Warming Emissions Rising in 2013 AfterYears of Decline. Washington, DC: Environmental Integrity Project; 2013. [Google Scholar]
- 17.Queisser-Luft A, Wiesel A, Stolz G, et al. Birth defects in the vicinity of nuclear power plants in Germany. Radiat Environ Biophys. 2011;502:313–323. [DOI] [PubMed] [Google Scholar]
- 18.Mangones T, Visintainer P, Brumberg HL. Congenital anomalies, prematurity, and low birth weight rates in relation to nuclear power plant proximity. J Perinat Med. 2013;414:429–435. [DOI] [PubMed] [Google Scholar]
- 19.Wang SI, Lee LT, Zou ML, et al. Pregnancy outcome of women in the vicinity of nuclear power plants in Taiwan. Radiat Environ Biophys. 2010;491:57–65. [DOI] [PubMed] [Google Scholar]
- 20.Tsai SS, Yu HS, Chang CC, et al. Increased risk of preterm delivery in women residing near thermal power plants in Taiwan. Arch Environ Health. 2004;599:478–483. [DOI] [PubMed] [Google Scholar]
- 21.Buonocore JJ, Dong X, Spengler JD, et al. Using the Community Multiscale Air Quality (CMAQ) Model to estimate public health impacts of PM2.5 from individual power plants. Environ Int. 2014;68:200–208. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Liu Y, Chen Y, et al. Maternal exposure to fine particulate matter (PM) and pregnancy outcomes: a meta-analysis Environ Sci Pollut Res Int. 2015;225:3383–3396. [DOI] [PubMed] [Google Scholar]
- 23.Ha S, Hu H, Roussos-Ross D, et al. The effects of air pollution on adverse birth outcomes. Environ Res. 2014;134:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodges A, Rahmani M. Fuel sources and carbon dioxide emissions by electric power plants in the United States. https://edis.ifas.ufl.edu/fe796 Published March 2009. Updated January 2012. Accessed March 8, 2014.
- 25.McMillan N, Holland DM, Morara M, et al. Combining different sources of particulate data using Bayesian space-time modeling. Environmetrics. 2009;211:48–65. [Google Scholar]
- 26.Lee PC, Roberts JM, Catov JM, et al. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Matern Child Health J. 2013;173:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Energy Information Administration. Electric power annual: emission from energy consumption at conventional power plants and combined-heat-and-power plants 2002–2012. http://www.eia.gov/electricity/annual/html/epa_09_01.html Published December 12, 2013. Accessed December 17, 2014.
- 28.Liu S, Krewski D, Shi Y, et al. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect. 2003;11114:1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geer LA, Weedon J, Bell ML. Ambient air pollution and term birth weight in Texas from 1998 to 2004. J Air Waste Manag Assoc. 2012;6211:1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilhelm M, Ghosh JK, Su J, et al. Traffic-related air toxics and term low birth weight in Los Angeles County, California. Environ Health Perspect. 2012;1201:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Ren C, Delfino RJ, et al. Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect. 2009;11711:1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Environmental Health & Engineering, Inc. Emissions of Hazardous Air Pollutants from Coal-fired Power Plants. Needham, MA: Environmental Health & Engineering, Inc; 2011. [Google Scholar]
- 33.Lee BE, Ha EH, Park HS, et al. Exposure to air pollution during different gestational phases contributes to risks of low birth weight. Hum Reprod. 2003;183:638–643. [DOI] [PubMed] [Google Scholar]
- 34.Siffel C, Otos M, Czeizel AE. Congenital abnormalities and indicators of germinal mutations in the vicinity of the Paks nuclear plant, Hungary. Mutagenesis. 1996;113:299–303. [DOI] [PubMed] [Google Scholar]
- 35.US Environmental Protection Agency. Clean energy: air emissions. http://www.epa.gov/cleanenergy/energy-and-you/affect/air-emissions.html. Updated May 22, 2014. Accessed December 17, 2014.
- 36.Valero De Bernabé J, Soriano T, Albaladejo R, et al. Risk factors for low birth weight: a review. Eur J Obstet Gynaecol Reprod Biol. 2004;1161:3–15. [DOI] [PubMed] [Google Scholar]
- 37.Zeitlin J, Saurel-Cubizolles MJ, De Mouzon J, et al. Fetal sex and preterm birth: Are males at greater risk? Hum Reprod. 2002;1710:2762–2768. [DOI] [PubMed] [Google Scholar]
- 38.Chang SC, O'Brien KO, Nathanson MS, et al. Characteristics and risk factors for adverse birth outcomes in pregnant black adolescents. J Pediatr. 2003;1432:250–257. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Birth outcome and risk factor analysis. http://www.cdc.gov/pednss/how_to/read_a_data_table/prevalence_tables/birth_outcome.htm. Updated March 4, 2010. Accessed November 8, 2013.
- 40.Graham J, Zhang L. Factors associated with negative birth outcomes: findings from a birth cohort study. http://msdh.ms.gov/msdhsite/_static/resources/2546.pdf. Updated January 15, 2015. Accessed January 24, 2015.
- 41.Vijayaraghavan K, Seigneur C, Bronson R, et al. A case study of the relative effects of power plant nitrogen oxides and sulfur dioxide emission reductions on atmospheric nitrogen deposition. J Air Waste Manag Assoc. 2010;603:287–293. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Bell EM, Caton AR, et al. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res. 2010;1102:162–168. [DOI] [PubMed] [Google Scholar]
- 43.Oudin A, Forsberg B, Strömgren M, et al. Impact of residential mobility on exposure assessment in longitudinal air pollution studies: a sensitivity analysis within the ESCAPE Project. Sci World J. 2012;2012:125818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.