Abstract
Coastal degradation and habitat disruption are severely compromising sessile marine species. The fan shell Pinna nobilis is an endemic, vulnerable species and the largest bivalve in the Mediterranean basin. In spite of species legal protection, fan shell populations are declining. Models analyzed the contributions of environmental (mean depth, wave height, maximum wave height, period of waves with high energy and mean direction of wave source) versus human-derived stressors (anchoring, protection status, sewage effluents, fishing activity and diving) as explanatory variables depicting Pinna nobilis populations at a mesoscale level. Human stressors were explaining most of the variability in density spatial distribution of fan shell, significantly disturbing benthic communities. Habitat protection affected P. nobilis structure and physical aggression by anchoring reveals a high impact on densities. Environmental variables instead played a secondary role, indicating that global change processes are not so relevant in coastal benthic communities as human-derived impacts.
Introduction
The family Pinnidae Leach 1819 (order Pterioida) is a taxonomic family of large saltwater clams commonly known as pen- or fan-shells. These are filter-feeder marine bivalve molluscs with a long triangular shape and fragile shells with the pointed end anchored in the substrate using the byssus threads. The shells reach 15–35 cm length, exceptionally up to 120 cm [1]. This family includes two genera (Pinna and Atrina, [2]) with 61 species described worldwide [3]. Most of the species are geographically located in the Indo-Pacific area, with some inhabiting the Mediterranean Sea, Caribbean Sea, North East Atlantic, West Africa, and West America.
In the Mediterranean Sea, Pinna nobilis Linnaeus 1758 is the largest endemic bivalve [4] and lives at depths ranging from 0.5 to 60 m [5]. In the 20th century, P. nobilis populations have greatly declined due to anthropogenic activities, including recreational and commercial fishing, ornamental harvesting, and accidental killing by anchoring, bottom nets and trawlers [6,7]. Nowadays, this species is legally protected under both Annex II of the Barcelona Convention [8] and Annex IV of the EU Habitats Directive [9]. Consequently, scientific studies to assess the current populations status in the view of future management and to set up conservation strategies are a priority. This knowledge might be extrapolated elsewhere to other species of the Pinnidae family. However, in spite of protection, P. nobilis populations are severely affected by several impacts [10–13].
The way environmental conditions and population processes determine the abundance and distribution of species is a central problem of ecology and biogeography [14]. Usually, variation in population density is a combination of several factors, such as spatial variables that affects distribution of organisms. Human-driven impacts have adverse effects on marine biota and habitats and can act determining structural and functional changes in habitats and species [15].
In this regard, human stressors might guide and modify population traits in benthic species. Previous studies already pointed out responses of P. nobilis densities to fishing mortality was much higher than natural mortality acting as important driver of the spatial distribution of this species and population viability [10]; and that the species was also served as seafood in restaurants [16]. A recent study suggested that boat anchoring in shallow waters seemed to be one of the main factors influencing P. nobilis population densities [11]. Vázquez-Luis et al.[13] experimentally demonstrated the direct impact of anchoring on P. nobilis mimic units; and other research stressed impacts of boat propellers in shallow populations [12].
The second set of environmental stressors influencing P. nobilis population includes hydrodynamic processes and storms. García-March et al. [17] noted that selective pressures regulate P. nobilis population parameters, producing a trade-off between hydrodynamics and shell size and orientation, for different shore types and water depth; and in shallow population living at 6 m depth hydrodynamic forces killed large individuals. Hendriks et al. [18] demonstrated that P. oceanica meadows provide shelter from hydrodynamic forces to P. nobilis, most appreciable for seagrass meadows located in shallow waters where the smaller animals remain within the canopy. However, in the Gulf of Oristano, wave action was not a significant factor influencing the orientation of the P. nobilis shell due to the low hydrodynamic characteristics of the area [19]. These authors demonstrated that bottom current direction and speed have a greater influence on the spatial density pattern of the bivalve compared to exposure to waves [19].
Most of the studies conducted on Pinna nobilis focused on density and spatial distribution along the Mediterranean Sea in a regional context, with the majority addressing coastal lagoons. Some studies have evaluated biological aspects such as the shell epibiontic community, feeding ecology, growth, recruitment, genetic structure and contaminants, among others [20]. Nevertheless, few studies had been performed including a mesoscale spatial range, and up to our knowledge, no studies had been done addressing environmental and human stressors within Pinna nobilis populations.
This work aims to understand the role that both environmental and human stressors play in the distribution and abundance of fan mussel Pinna nobilis, a sessile benthic, vulnerable and long-living species. Cumulative impacts at coastal areas are increasing and responses of key species might provide clues for management actions fostering environmental protection. This is the first study that combines and compares partial contributions of environmental and human stressors to model the effect that these variables exert on the P. nobilis population. Knowledge of key process and factors shaping endangered species and linked marine ecosystems are essential to provide appropriate management strategies for coastal species conservation.
Methods
Parque Nacional Maritimo Terrestre del Archipielago de Cabrera provided permission for underwater surveys within the National Park. Conselleria de Medi Ambient del Govern Balear provided permission for surveying fan mussels in locations other than the National Park. Fan mussel Pinna nobilis is a protected species, but no extraction or manipulation was done on any of the surveyed individuals. Only scuba diving visual census for density counts and length measures estimations were carried out, not interfering with the species itself.
Study area
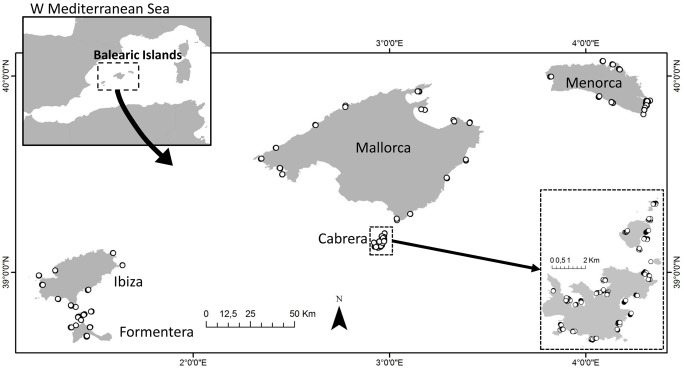
We conducted the studies in the Balearic Islands, western Mediterranean Sea at five main islands: Mallorca, Menorca, Ibiza, Formentera and Cabrera and several islets (Fig 1). Seagrass meadows (Posidonia oceanica (Linnaeus) Delile, 1813) are one of the most significant elements of the benthic environment of Balearic coasts, growing on carbonate sediments of biogenic origin [21]. Extensive and dense meadows up to depths of 40 m inhabit the archipelago, but many been degraded as a result of anchoring and pollution from recreational boating, which is largely unregulated around the islands [22]. This mesoscale study integrates variability among hundreds of kilometers covering an area of around 150,000 km2 [23].
Fig 1. Study area.
Study area in the Balearic Islands showing sampling localities (white dots) among islands.
Data Collection
Pinna nobilis surveys
We conducted a total of 661 visual censuses by scuba diving to survey fan mussels density in different islands of the Balearic Archipelago (Cabrera, Formentera, Ibiza, Mallorca and Menorca) at two different depths (10 and 20 m) in Posidonia oceanica seagrass meadows. We selected different localities at each island and depth sampling a total of 23 localities in Cabrera, 9 in Formentera, 8 in Ibiza, and 14 in Mallorca and Menorca (Fig 1). We carried out the field surveys from July 2011 to September 2013. We conducted the underwater visual censuses along strip transects of 30 m length and 2.5 m width (total area per transect of 75 m2 after Vazquez-Luis et al. [24]) in order to survey the fan mussel P. nobilis population density and shell size (measured as maximum shell width). We performed a total of 234 strip transects in Cabrera, 69 in Formentera, 80 in Ibiza, 140 in Mallorca and 138 in Menorca. The total surveyed surface accounting for all islands was 49,575 m2. We expressed P. nobilis densities as individuals per 100 m2.
Environmental and human variables
In the present study we choose 10 variables, considered as possible stressors for P. nobilis population structure (density, shell size and spatial distribution). We divided the variables in two groups (major variables): a) environmental variables which included: mean depth, Hs mean, Hs maximum, mean Tp and mean direction; and b) human variables which included: anchoring, protection status, sewage effluents, fishing activity and diving. Hs mean was the significant wave height, Hs maximum was the maximum wave height, Mean Tp was the period of the waves with higher energy and Mean direction as direction of wave source [25]. We obtained the wave data from the closest WANA node to each locality for the period 2000–2014 [25]. We divided Anchoring in three levels: 1-No anchoring pressure, 2-Low anchoring intensity 3-Medium anchoring intensity (with data obtained from local knowledge and historical Google images) [26]. Protection status comprised 5 categories from the highest to the lowest protection level: 1-National Park, 2- Marine Reserve, 3- Natural Park, 4- Sites of Community Importance (SCI) and 5- Non protected areas. Regarding sewage effluents we assigned a value of 1 when no effluents were in the area, and 2 if effluents were present in a radius of 1 km from the sampling locality [27]. We classified Fishing activity in two groups: with value of 1 for fishing forbidden and 2 when the activity was allowed. Finally, we categorized the diving activity based in expert criteria in two groups according to areas with common diving activity in a radius of 1 km: absence-1 and presence-2.
Data analyses
Spatial variation of fan mussel P. nobilis population
We performed a Permutational multivariate analysis of variance (PERMANOVA) to test differences on: living P. nobilis densities (ind/100m2), maximum shell width of living individuals (cm), dead P. nobilis densities, maximum shell width of dead individuals; and percentage of living and dead P. nobilis individuals (excluding transects with zero individuals for percentage data) across islands, depths and localities. The experimental design comprised 3 factors: “Island” fixed with 5 levels of variation: Mallorca, Menorca, Cabrera, Ibiza and Formentera; “Depth” fixed with two levels of variation: 10 and 20 meters; and “Locality” random and nested with both main factors. We transformed data on Pinna densities to fourth root, while non-transformed data were analyzed for shell size and percentage. We applied a Bray-Curtis similarity to fan mussel densities and shell size, while Euclidean distance was calculated for percentage data with 999 permutations. We examined significant terms in the full model applying a posterior pair wise comparisons [28]. We calculated all statistical analyses using software PRIMER v6 [29] with the add-on package PERMANOVA+ [30].
Relationship between fan mussel P. nobilis population and environmental and human variables
We applied a distance-based linear model routine (DistLM) in order to test relationships among tested variables: living P. nobilis densities, maximum shell width of living individuals, and percentage of dead P. nobilis individuals (considered as a proxy of a rate of mortality) with environmental and human stressors (explanatory variables) [31]. The analysis was based on log (x+1) data transformed for densities, and untransformed data for size and mortality; and in all cases similarity matrices were constructed using Euclidean Distance. Additionally, for living P. nobilis density we conducted a DistLM analysis based on major variables (environmental versus human) based on log (x+1) data transformed and Euclidean Distances. In all cases, we applied the method specified selection and adjusted R2 criterion with 999 permutations. We calculated draftsman plots with the corresponding Pearson correlation coefficients to explore the relationships between the environmental and human variables [29] and to detect strongly correlated variables. We excluded no variable due to low correlations between them. We plotted the output of the dbRDA axis and the tested variable through a PCA, superimposing the original regressor variables as vectors (multiple correlation) in order to visualize the relationships between tested variables and explanatory variables (we analyzed a matrix of a single tested variable: Anderson, pers. comm.). We performed all statistical analyses with PRIMER v6 [29] with the add-on package PERMANOVA+ [30].
Results
Fan mussel P. nobilis populations
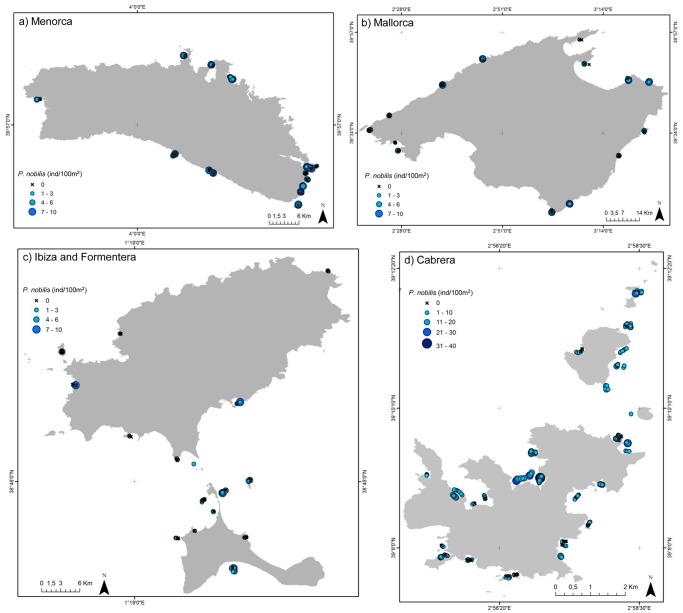
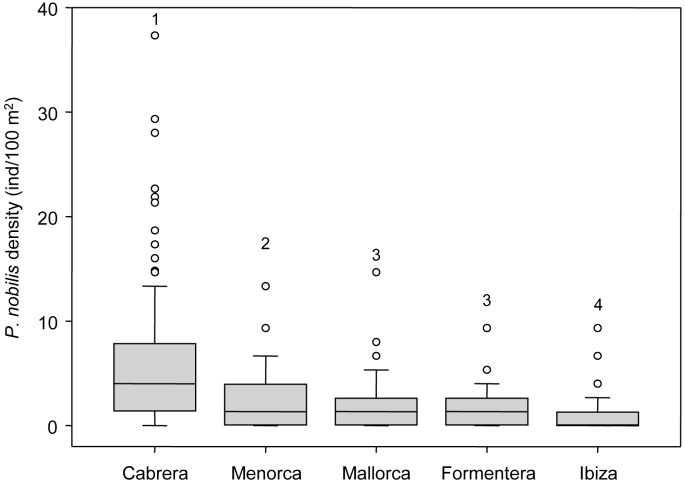
We recorded a total of 2081 specimens of P. nobilis by visual census along 49,575 m2 surveyed. Regarding spatial allocation among islands, we censed 1328 individuals in Cabrera, 113 in Formentera, 70 in Ibiza, 247 in Mallorca and 323 in Menorca. Overall dead individuals represent 17.78% of the counted fan shell, with relative values among the islands of 18.5%, 19.5%, 30%, 15.8% and 13%, respectively. Total observed average densities among islands were 3.21±0.13 ind/100 m2, varying among islands and among localities (Fig 2, for further details see S1 Table). Regarding living individuals, we found the highest density values in Cabrera 5.74 value ind/100 m2 and the lowest in Ibiza 0.83 mean ind/100 m2 (Fig 3, Table 1, Is: p <0.01).
Fig 2. Density of P. nobilis populations.
Densities of living P. nobilis (ind/100m2) found across islands and localities. (Note that density scale differs for Cabrera).
Fig 3. Mean values (±standard error) of living P. nobilis density (individuals/100m2) among islands.
Median values are included, the rectangles contain values between the first and the third quartiles, the bars represent the variability outside the upper and lower quartiles, outliers are shown as circles. SNK groups are shown by numbers.
Table 1. Results of the PERMANOVA analysis for data on P. nobilis density, shell size and % of presence.
| Living P. nobilis density | Living P. nobilis size | Dead P. nobilis density | |||||||
| Source | df | MS | P (perm) | df | MS | P (perm) | df | MS | P (perm) |
| Is | 4 | 11.98 | 0.0001** | 4 | 2287.6 | 0.003** | 4 | 2.96 | 0.001** |
| De | 1 | 1.34 | 0.2523 | 1 | 15396 | 0.001** | 1 | 0.81 | 0.207 |
| IsxDe | 4 | 0.86 | 0.5016 | 4 | 645.34 | 0.253 | 4 | 0.53 | 0.36 |
| Lo(IsxDe) | 111 | 1.15 | 0.0001** | 101 | 1042.8 | 0.001** | 111 | 0.52 | 0.001** |
| Residual | 540 | 0.25 | 1599 | 175.79 | 540 | 0.25 | |||
| Total | 660 | 1709 | 660 | ||||||
| SNK | Is: C ≠ Me ≠ Ma = F ≠ I | Is: C = Me ≠ Ma, F, I; De: 20 ≠ 10 | Is: C ≠ Me, Ma, F, I | ||||||
| Dead P. nobilis size | % Living P. nobilis | % Dead P. nobilis | |||||||
| Source | df | MS | P (perm) | df | MS | P (perm) | df | MS | P (perm) |
| Is | 4 | 3100.5 | 0.001** | 4 | 716.98 | 0.186 | 4 | 10236 | 0.001** |
| De | 1 | 13134 | 0.001** | 1 | 12.26 | 0.879 | 1 | 316.03 | 0.54 |
| IsxDe | 4 | 2026.4 | 0.001** | 4 | 585.11 | 0.326 | 4 | 1037.3 | 0.384 |
| Lo(IsxDe) | 82 | 480.57 | 0.001** | 101 | 553.3 | 0.001** | 82 | 1239.6 | 0.001** |
| Residual | 278 | 146.33 | 336 | 287.14 | 126 | 418.88 | |||
| Total | 369 | 446 | 217 | ||||||
| SNK | IsxDe: C, F, I: 20≠10;Ma, Me: 20 = 10 | Is: C ≠ Ma, F, I; I ≠ Ma, Me, F | |||||||
Data on living P. nobilis density (individuals/100m2), of living P. nobilis shell size (maximum width), dead P. nobilis density, dead P. nobilis shell size, and % of living and dead P. nobilis per transect (without transects with zero individuals).
Is = Islands; De = Depth; Lo = Locality; df = degrees of freedom; MS = mean square; P = level of significance; C = Cabrera; Me = Menorca; Ma = Mallorca; F = Formentera and I = Ibiza.
** = significant (P< 0.01).
In terms of shell size structure we found significant differences for both main factors island and depth. Regarding factor island, P. nobilis shells were bigger in Cabrera and Menorca and smaller in Mallorca, Formentera and Ibiza (Fig 4a, Table 1, Is: p <0.01). With respect to depth, individuals were bigger at 20 meters depth (Fig 4b, Table 1, De: p <0.01; for further details on size structure see S1 Fig).
Fig 4. Mean values of P. nobilis shell size across: a) Islands and b) Depth.
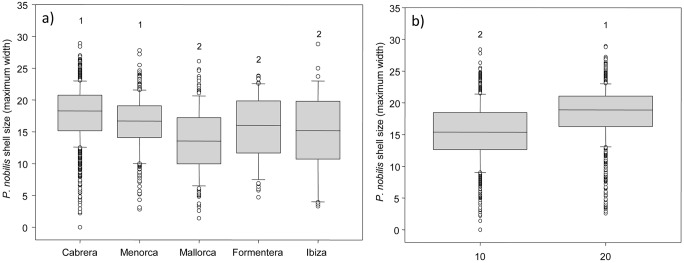
Mean values (±standard error) of living P. nobilis shell size (maximum width). Median values are included, the rectangles contain values between the first and the third quartiles, the bars represent the variability outside the upper and lower quartiles, outliers are shown as circles. SNK groups are shown by numbers.
Regarding dead individuals, density was higher in the protected island of Cabrera (Table 1, Is: p <0.01) and shell sizes of dead individuals were different across islands and depths. At Cabrera, Formentera and Ibiza shell size were bigger at 20 meters depth (Table 1, IsxDe: p <0.01). Regarding ratio of living and dead P. nobilis density, percentage of living individuals varied among localities; however, percentage of dead individuals (such a rate of mortality) was significant different among islands being higher in Ibiza (Table 1, Is: p <0.01).
Relationship between fan mussel P. nobilis population and environmental and human variables
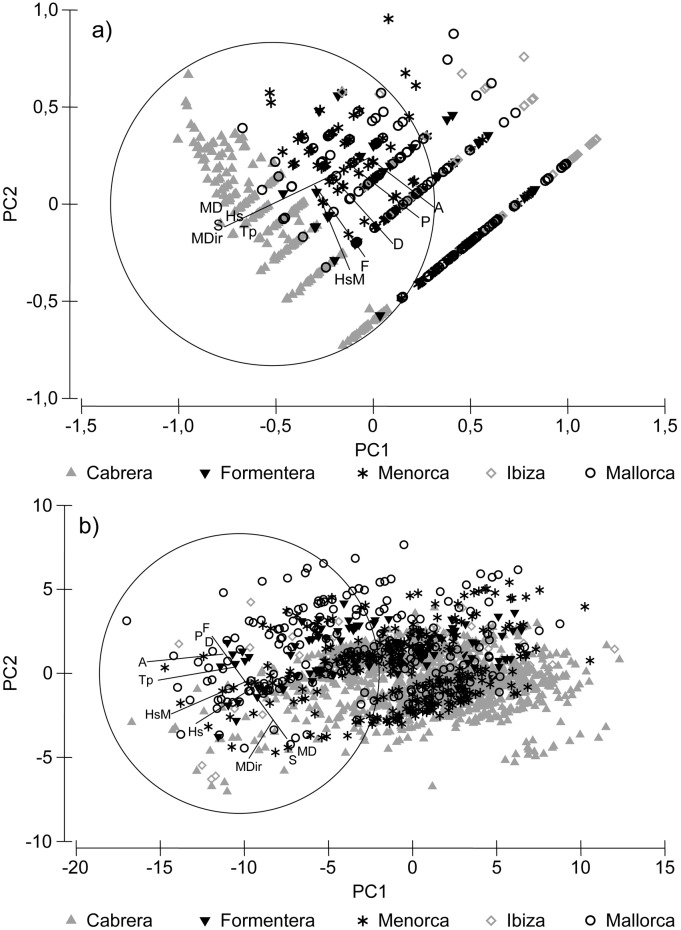
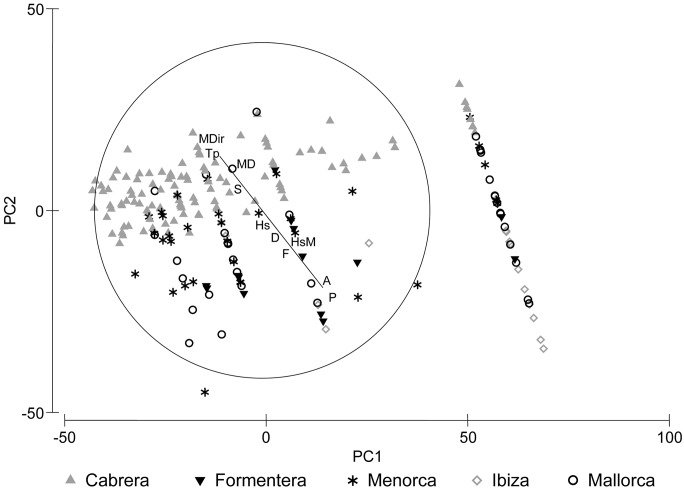
The DistLM analysis calculated on living P. nobilis density showed significant relationship with major variables. The model exhibited the highest percentages of variability explained by the group of human stressors (21.6%), while environmental variables explained only 6.6% of variability in the model (Table 2, Fig 5a). Moreover, DistLM analysis for living P. nobilis density contrasted with the ten explanatory variables showed significant relationships. The highest percentages of variability were explained by anchoring (19.92%) followed by protection status (11.71%); being also significant in the model the mean wave direction, fishing activity and diving. The model explained altogether the 22.93% of variation. Regarding living P. nobilis shell sizes the overall explained variation was 24.16%, being the most contributing variables mean depth, mean direction and anchoring (Table 2, Fig 5b). The model explained the 26.18% of variability in the mortality ratio (% dead individuals). Considering the variables individually, anchoring (17.29%) and protection status (13.71%) explained the highest percentage of variability (Table 3, Fig 6). In all cases, the less contributing variables were maximum wave height Hs, sewage effluents and mean period of the waves with higher energy Tp.
Table 2. Results of DistLM for: a) living P. nobilis densities, major variables; b) living P. nobilis densities; and c) living P. nobilis sizes.
| a) Living P. nobilis densities | |||
| Major Variable | Pseudo-F | p | % var. |
| Environmental | 9.2603 | 0.001 | 6.602 |
| Human | 36.039 | 0.001 | 21.575 |
| b) Living P. nobilis densities | |||
| Variable | Pseudo-F | p | % var. |
| Mean depth | 0.0079 | 0.9658 | <0.01 |
| Hs mean | 4.3023 | 0.0204 | 0.81 |
| Hs maximum | 0.8431 | 0.3182 | 0.16 |
| Mean Tp | 0.9655 | 0.2789 | 0.18 |
| Mean direction | 13.579 | 0.0001 | 2.55 |
| Anchoring | 106.16 | 0.0001 | 19.93 |
| Protection status | 62.412 | 0.0001 | 11.72 |
| Sewage effluents | 0.1216 | 0.7044 | <0.01 |
| Fishing activity | 23.343 | 0.0001 | 4.38 |
| Diving | 32.255 | 0.0001 | 6.05 |
| c) Living P. nobilis size | |||
| Variable | Pseudo-F | p | % var. |
| Mean depth | 130.6 | 0.01 | 7.1 |
| Hs mean | 3.12 | 0.072 | 0.18 |
| Hs maximum | 1.063 | 0.304 | <0.01 |
| Mean Tp | 28.117 | 0.001 | 1.62 |
| Mean direction | 71.3 | 0.001 | 4.007 |
| Anchoring | 69.656 | 0.001 | 3.918 |
| Protection status | 58.214 | 0.001 | 3.296 |
| Sewage effluents | 15.904 | 0.001 | 0.922 |
| Fishing activity | 68.897 | 0.001 | 3.877 |
| Diving | 22.985 | 0.001 | 1.33 |
DistLM (distance-based linear model routine) marginal test for relationships between environmental and human variables. Environmental variables: mean depth, Hs mean, Hs maximum, mean Tp and mean direction. Human variables: anchoring, protection status, sewage effluents, fishing activity and diving.
Fig 5. Relationship between the ordination of (A) living P. nobilis densities, and (B) living P. nobilis size, and environmental and human variables.
Environmental variables: MD: mean depth, Hs: Hs mean, HsM: Hs maximum, Tp: mean Tp, MDir: mean direction. Human variables: A: anchoring, P: protection status, S: sewage effluents, F: fishing activity and D: diving.
Table 3. Results of DistLM for mortality (percentage of dead P. nobilis).
| Mortality | |||
|---|---|---|---|
| Variable | Pseudo-F | p | % var. |
| Mean depth | 1.501 | 0.216 | 0.69 |
| Hs mean | 1.5502 | 0.206 | 0.71 |
| Hs maximum | 2.5205 | 0.119 | 1.153 |
| Mean Tp | 0.0024 | 0.889 | 0.01 |
| Mean direction | 10.209 | 0.002 | 4.513 |
| Anchoring | 45.156 | 0.001 | 17.29 |
| Protection status | 34.314 | 0.001 | 13.71 |
| Sewage effluents | 0.953 | 0.337 | 0.44 |
| Fishing activity | 8.434 | 0.004 | 3.76 |
| Diving | 5.653 | 0.016 | 2.55 |
DistLM (distance-based linear model routine) marginal test for relationships between environmental and human variables. Environmental variables: mean depth, Hs mean, Hs maximum, mean Tp and mean direction. Human variables: anchoring, protection status, sewage effluents, fishing activity and diving).
Fig 6. Relationship between the ordination of P. nobilis mortality (% dead P. nobilis individuals) and environmental and human variables.
Environmental variables: MD: mean depth, Hs: Hs mean, HsM: Hs maximum, Tp: mean Tp, MDir: mean direction. Human variables: A: anchoring, P: protection status, S: sewage effluents, F: fishing activity and D: diving.
Discussion
In the Mediterranean Sea cumulative impacts of human-derived activities are steering biodiversity loss. Endemic and vulnerable species are the target of those impacts with irreversible consequences on biological fitness and species survival. Coastal degradation, especially in high touristic areas is of high concern for vulnerable species. Some species such as the largest bivalve within the Mediterranean Sea are under high pressure [20]. Understanding drivers of species losses and ultimately extinctions are crucial to assure their conservation.
This large-scale study performed with a high spatial resolution demonstrates that the spatial distribution of Pinna nobilis is affected by human stressors more than environmental variables. Anchoring is the main factor affecting density of the fan mussel in the studied sites. The anchoring effect on P. nobilis densities have been already stated in previous works [11, 13]. Legal protection of habitats is crucial in maintaining population structure of large, long-lived and sessile benthic organisms such as the fan mussels. This study shows that protection is widely affecting densities of P. nobilis at the studied geographical extent. Contrasted densities are two-fold in the MPA (Cabrera) where no-take reserves have been effectively set for more than 20 years. Those results indicate that MPAs guarantee conservation demonstrating that a combination of protection size and age of the MPAs [32, 33] seems to set the optimal conditions for growth and development of the species.
Mean densities in this study are within the range of previous studies [34–36, 11], indicating that protection through MPA establishment represents a highly effective measure for conservation of this species. The same states for the regression of seagrass beds Posidonia oceanica, preferential habitat for Pinna nobilis, which degradation has been vastly documented to be under regression [37,38].
Density-dependent processes are probably limiting dispersion of propagules of the fan mussel. There might be a threshold of minimum conspecific densities of P. nobilis in the seabed to ensure gamete release and fecundity probabilities within the water masses. In addition, reproductive aspects of the fan mussel’s densities might induce larval recruitment nearby existing adults by chemical clues and ensuring juvenile replenishment [39]. Those aspects reinforce the need to keep and maintain certain density levels to guarantee population survival while ensuring genetic variability.
Eutrophication does not modify spatial distribution of the species as observed by the low variability explained by the sewage factor. Instead, sewage effluents increase nutrient loads in the oligotrophic waters of the Balearics boosting growth at juvenile stages, as previously demonstrated by stable isotopes signatures [40].
Conversely, fisheries alter fan mussel size and densities. Artisanal fisheries, mainly trammel nets entangle fan mussel’s shells and dislodge the individuals from the benthic substrate as already reported in Greece [10]. Specific management measures to avoid those conflicts could be to exclude fisheries at the extent of the P. nobilis distribution only allowing fisheries activities at the lower limit of its distribution as it is the case for Posidonia oceanica seagrass meadows.
Diving affects density of fan mussels in this study. Many authors have demonstrated diving impacts on sessile organisms corals, bryozoans, gorgonians [41,42]. The studied P. nobilis populations inhabit seagrass beds, and very little work has been carried out on diving impacts on seagrass and soft-bottom habitats. In our case, diving along with poaching disturbance might be altering fan mussel’s spatial distribution and densities. Foster of environmental education programs addressing conservation issues and getting to know protected species to tourist and society might help to reduce impacts of recreational activities (mainly boating and diving) on sessile organism in coastal shores.
The way to assess population structure should be carefully considered. In the studied fan mussels populations, density and size respond to different drivers. Interesting results highlight that P. nobilis densities are tightly linked to human-driven activities, while size of the individuals are responding to environmental variables. Anchoring and protection mainly drive densities, while shell size are more influenced by depth and wave direction. Additionally, significantly larger individuals are found at deeper depths, indicating cross-effects with hydrodynamics. Those responses follow previous data on shell orientation and distribution and depth effects [17].
Climate change and increase in storms and wave intensity might disturb fan mussels by dislodging the individuals from the seabottom and impinging large individuals to deeper depths. Instead, bays and sheltered areas might be protected from intense wave action [19] along with minimum thresholds in wave intensity and direction for Pinna shell hydrodynamics [18].
Our results prove that activities related with recreational activities (anchoring, protection, diving, fishing) have large effects on species distribution. Future scenarios at coastal areas depict a high increase in coastal activities disturbing the habitats and ecosystems. This study reveals that ensuring fan mussels populations in coastal habitats needs a wise spatial management, linking MPAs with the establishment comprehensive measures like no anchoring areas or regulated permanent mooring systems [43, 13] to avoid free anchoring. The results suggest that fan mussel P. nobilis population structure (size, densities and spatial distribution patterns) can be properly considered precise indicators of human impacts in coastal habitats by reflecting human derived activities and cumulative impacts along with environmental responses.
Supporting Information
Percentage of individuals of each size classes among islands (maximum shell width).
(TIF)
Mean density, minimum and maximum density of living and dead P. nobilis (ind/100m2); mean percentage, minimum and maximum percentage of living and dead P. nobilis (ind/100m2) among islands, localities and depth (table also includes mean depth in strata). N = number of strip transects.
(DOCX)
Acknowledgments
We acknowledge Cabrera National Park, CIM, OBSAM, for permission and facilities and several colleagues for fieldwork assistance and data collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was from Organismo Autonomo Parques Nacionales, Ministerio de Medio Ambiente for the project “Estado de conservación del bivalvo amenazado Pinna nobilis en el Parque Nacional del Archipiélago de Cabrera” (024/2010)”, and partly funded from European Community’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement No.287844 project ‘Towards Coast to COast NETworks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential’ (COCONET). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schultz PW, Huber M. Revision of the worldwide Recent Pinnidae and some remarks of fossil European Pinnidae. Acta Conch. 2013; 13: 1–164. [Google Scholar]
- 2. Lemer S, Buge B, Bemis A, Giribet G. First molecular phylogeny of the circumtropical bivalve family Pinnidae (Mollusca, Bivalvia): Evidence for high levels of cryptic species diversity. Molecular Phylogenetics and Evolution 2014; 75: 11–23. 10.1016/j.ympev.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 3.WoRMS, World Register of Marine Species. 2014. Available: http://www.marinespecies.org at VLIZ.
- 4. Zavodnik D, Hrs-Brenko M, Legac M. Synopsis on the fan shell Pinna nobilis L. in the eastern Adriatic Sea In: Boudouresque C.F., Avon M., Gravez V., (eds) Les Espèces Marines à Protéger en Méditerranée. Gis Posidonie publ; Marseille: 1991; 169–178. [Google Scholar]
- 5. Butler AJ, Vicente N, De Gaulejac B. Ecology of the pteroid bivalves Pinna bicolor Gmelin and Pinna nobilis Linnaeus. Marine Life 1993; 3: 37–45. [Google Scholar]
- 6. Vicente N, Moreteau JC. Statut of Pinna nobilis L. en Méditerranée (Mollusque Eulamellibranche) In, Boudouresque C.F., Avon M., Garvez M., (Ed) Les Espèces Marines à Protèger en Méditerranée. GIS Posidonie Publ; 1991; 159–168. [Google Scholar]
- 7. Richardson CA, Peharda M, Kennedy H, Kennedy P, Onofri V. Age, growth rate and season of recruitment of Pinna nobilis (L) in the Croatian Adriatic determinated from Mg: Ca and Sr: Ca shell profiles. Journal of Experimental Marine Biology and Ecology. 2004; 299: 1–16. [Google Scholar]
- 8.SPA/BD Protocol. Annex II of SPA and Biodiversity Protocol. Barcelona Convention Protocol concerning Specially Protected Areas and Biological Diversity in the Mediterranean. 1995. Available: http://rac-spa.org/sites/default/files/annex/annex_2_en_2013.pdf
- 9.EU Habitats Directive, 2007. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Annex IV: animal and plant species of community interest in need of strict protection. Available: http://ec.europa.eu/environment/nature/legislation/habitatsdirective/index_en.htm
- 10. Katsanevakis S. Growth and mortality rates of the fan mussel Pinna nobilis in Lake Vouliagmeni (Korinthiakos Gulf, Greece): a generalized additive modelling approach. Marine Biology. 2007; 152: 1319–1331. [Google Scholar]
- 11. Hendriks I, Tenan S, Tavecchia G, Marbà N, Jordà G, Deudero S, et al. Boat anchoring impacts coastal populations of the pen shell, the largest bivalve in the Mediterranean. Biological Conservation. 2013; 160: 105–113. [Google Scholar]
- 12. Prado P, Caiola N, Ibáñez C. Habitat use by a large population of Pinna nobilis in shallow waters. Scientia Marina. 2014; 78(4): 555–565. [Google Scholar]
- 13. Vázquez-Luis M, Borg JA, Morell C, Banach-Esteve G, Deudero S. Influence of boat anchoring on Pinna nobilis: a field experiment using mimic units. Marine and Freshwater Research. 2015. Available: 10.1071/MF14285 [DOI] [Google Scholar]
- 14. Brown JH. On the relationship between abundance and distribution of species. The American Nauralist. 1984; 124 (2): 255–279. [Google Scholar]
- 15. Claudet J, Fraschetti F. Human-driven impacts on marine habitats: A regional meta-analysis in the Mediterranean Sea. Biological Conservation 1. 2010; 43(9): 2195–2206. [Google Scholar]
- 16. Katsanevakis S, Poursanidis D, Issaris Y, Panou A, Petza D, Vassilopoulou V, et al. "Protected" marine shelled molluscs: thriving in Greek seafood restaurants. Mediterranean Marine Science. 2011;12(2): 429–438. [Google Scholar]
- 17. García-March JR, Pérez-Rojas L, García-Carrascosa AM. Influence of hydrodynamic forces on population structure of Pinna nobilis L., 1758 (Mollusca: Bivalvia): The critical combination of drag force, water depth, shell size and orientation. Journal of Experimental Marine Biology and Ecology. 2007; 342: 202–212. [Google Scholar]
- 18. Hendriks IE, Cabanellas-Reboredo M, Bouma TJ, Deudero S, Duarte CM. Seagrass meadows modify drag forces on the Shell of the Fan Mussel Pinna nobilis . Estuaries and Coasts. 2011; 34: 60–67. [Google Scholar]
- 19. Coppa S, Andrea de Lucia G, Magni P, Domenici P, Antognarelli F, Satta A, Cucco A. The effect of hydrodynamics on shell orientation and population density of Pinna nobilis in the Gulf of Oristano (Sardinia, Italy). Journal of Sea Research. 2013; 76: 201–210. [Google Scholar]
- 20. Basso L, Vázquez-Luis M, García-March JR, Deudero S, Alvarez E, Vicente N, et al. The pen shell Pinna nobilis, state-of-the-art, research priorities and best practices. Advances in Marine Biology. 2015: in press. [DOI] [PubMed] [Google Scholar]
- 21. Marbà N, Duarte CM, Díaz-Almela E, Terrados J, Álvarez E, Martínez R, et al. Direct evidence of imbalanced seagrass (Posidonia oceanica) shoot population dynamics along the Spanish Mediterranean. Estuaries 2005; 28: 53–62. [Google Scholar]
- 22. Procaccini G, Buia MC, Gambi MC, Perez M, Pergent G, Pergent-Martini C, Romero J. The seagrasses of the Western Mediterranean, In: World Atlas of Seagrasses, Green E.P.; Short F.T. 2003; 48–58. UNEP World Conservation Monitoring Centre, University of California Press, Berkeley, ISBN 0-520-24047-2. [Google Scholar]
- 23.Deudero S, Vallespir J, Obrador M. Atlas de Biodiversidad Marina del Mar Balear. 2011. Available: http://www.ba.ieo.es
- 24. Vázquez-Luis M, March D, Alvarez E, Alvarez-Berastegui D, Deudero S. Spatial distribution modelling of the endangered bivalve Pinna nobilis in a Marine Protected Area. Mediterranean Marine Science. 2014; 15/3: 626–634. [Google Scholar]
- 25.Historical Governamental dataset. WANA provide temporal series from numeric modeling. Puertos del Estado. Available: http://www.puertos.es/es-es/oceanografia/Paginas/portus.aspx
- 26.Data obtained from local knowledge and Google Earth historical images.
- 27.Database sewage effluents Available: http://dgrechid.caib.es/www/ajuda_fondeig/visor/es.html
- 28. Anderson MJ. PERMANOVA_2 factor: a FORTRAN computer program for permutational multivariate analysis of variance (for any two-factor ANOVA design) using permutation test. Department of Statistics, University of Auckland, Auckland: 2004. [Google Scholar]
- 29.Clarke KR, Gorley RN. PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth. 2006.
- 30.Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth. 2008.
- 31. Legendre P, Anderson MJ. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs. 1999; 69: 1–24. [Google Scholar]
- 32. Claudet J, Osenberg CW, Benedetti-Cecchi L, Domenici P, García-Charton J-A, Pérez-Ruzafa Á, et al. Planes S. Marine reserves: Size and age do matter. Ecology Letters. 2008; 11(5): 481–489. 10.1111/j.1461-0248.2008.01166.x [DOI] [PubMed] [Google Scholar]
- 33. Fraschetti S, Guarnieri G, Bevilacqua S, Terlizzi A, Boero F. Protection Enhances Community and Habitat Stability: Evidence from a Mediterranean Marine Protected Area. PlosONE. 2013; 8(12): e81838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Addis P, Secci M, Brundu G, Manunza A, Corrias S, Cau A. Density, size structure, shell orientation and epibiontic colonization of the fan mussel Pinna nobilis L. 1758 (Mollusca: Bivalvia) in three contrasting habitats in an estuarine area of Sardinia (W Mediterranean). Scientia Marina. 2009; 73(1): 143–152. [Google Scholar]
- 35. Coppa S., Guala I., de Lucia G. A., Massaro G., & Bressan m. Of Oristano (Italy). Università degli studi della Tuscia di Viterbo, 2010; 90(5), 885–894. [Google Scholar]
- 36. Rabaoui L, Tlig-Zouari S, Katsanevakis S, Ben Hassine OK. Modelling population density of Pinna nobilis (Bivalvia) on the eastern and southertern coast of Tunisia. Journal of Molluscan Studies. 2010; 76: 340–347. [Google Scholar]
- 37. Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL Jr, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams SL. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. PNAS, Proceedings of the National Academy of Sciences. 2009; 106(30): 12377–12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boudouresque CF, Bernard G, Pergent G, Shili A, Verlaque M. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: a critical review. Botanica Marina. 2009; 52(5): 395–418. [Google Scholar]
- 39. Cabanellas-Reboredo M, Deudero S, Alós J, Valencia JM, March D, Hendriks I, Alvarez E. Recruitment of Pinna nobilis (Mollusca: Bivalvia) on artificial structures. Marine Biodiversity Records. 2009; (2):126. [Google Scholar]
- 40. Alomar C, Vázquez-Luis M, Magraner K, Lozano L, Deudero S. Evaluating stable isotopic signals at bivalve Pinna nobilis under different human pressures. Journal of Experimental Marine Biology and Ecology. 2015; 467: 77–86. [Google Scholar]
- 41. Luna B, Valle C, Sanchez-Lizaso JL. Benthic impacts of recreational divers in a Mediterranean Marine Protected Area. ICES Journal of Marine Science. 2009; 66:517–523. [Google Scholar]
- 42. Milazzo M, Chemello R, Badalamenti F, Riggio RC, Riggio S. The impact of human recreational activities in marine protected areas: What lessons should be learnt in the Mediterranean sea? Marine Ecology. 2002; 23 (SUPPL. 1), 280–290. [Google Scholar]
- 43. Balaguer P, Diedrich A, Sardá R, Fuster M, Cañellas B, Tintoré J. Spatial analysis of recreational boating as a first key step for marine spatial planning in Mallorca (Balearic Islands, Spain).Ocean and Coastal Management. 2011; 54: 241–249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percentage of individuals of each size classes among islands (maximum shell width).
(TIF)
Mean density, minimum and maximum density of living and dead P. nobilis (ind/100m2); mean percentage, minimum and maximum percentage of living and dead P. nobilis (ind/100m2) among islands, localities and depth (table also includes mean depth in strata). N = number of strip transects.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.