Abstract
There is progressive concern about the evolving burden of morbidity and mortality caused by coinfection with HIV-1 and hepatitis B virus (HBV) in sub-Saharan Africa, but the epidemiology and impact of this problem are not well defined. We therefore set out to assimilate more information about the nature of HBV/HIV coinfection in this region by undertaking a retrospective observational study of southern African adult women. We used samples from previously recruited HIV-1 positive women attending antenatal clinics in three settings in South Africa and Botswana (n = 950) and added a small cohort of HIV-negative antenatal South African women for comparison (n = 72). We tested for HBsAg and followed up HBsAg-positive samples by testing for HBeAg, HBV DNA, HBV genotype, presence of drug-resistance associated mutations (RAMs) and HDV. We identified HBsAg in 72 individuals (7% of the whole cohort), of whom 27% were HBeAg-positive, and the majority HBV genotypes A1 and A2. We did not detect any HDV coinfection. HBV prevalence was significantly different between geographically distinct cohorts, but did not differ according to HIV status. Among adults from South Africa, HBV/HIV coinfected patients had lower CD4+ T cell counts compared to those with HIV-monoinfection (p = 0.02), but this finding was not replicated in the cohort from Botswana. Overall, these data provide a snapshot of the coinfection problem at the heart of the HIV/HBV co-epidemic, and are important to inform public health policy, resource allocation, education, surveillance and clinical care.
Introduction
There has been a recent revival of political and clinical interest in the problem of infection with Hepatitis B Virus (HBV) in sub-Saharan African populations in whom Human Immunodeficiency Virus (HIV) is also frequently endemic [1, 2]. The progressive availability and success of antiretroviral therapy (ART) has reduced opportunistic infection and malignancy in individuals with HIV, increasing survival and allowing the emergence of previously unrecognized chronic liver disease [3, 4].
Furthermore, there is increasing evidence that chronic HIV/HBV coinfection is associated with long-term morbidity and mortality that exceeds the impact of infection with either one of these viruses alone in African populations [5–8]. This includes evidence of lower CD4+ T cell counts in HIV/HBV coinfected individuals compared to HIV monoinfected patients [8–10]. Adding to the scale of the problem, many individuals in Africa are particularly vulnerable to liver disease for a variety of other reasons including diet, genetics, and exposure to toxins and other pathogens [6, 7, 11].
Despite these concerns, the burden and impact of HIV/HBV coinfection in sub-Saharan Africa have not been well characterized. As reviewed recently [11], there are regional differences in the prevalence and severity of HIV, HBV and hepatitis delta virus (HDV) infection, and the existing literature is generally based on small studies of disparate populations. Although important information can be gained from studying HBV virological markers, such as hepatitis B e-antigen (HBeAg) status, this information is not available for the majority of studies published from African cohorts [11].
We therefore set out to characterize the prevalence and characteristics of HBV infection in a large cohort of HIV-positive African women, and to determine the influence of HBV coinfection on CD4+ T-cell counts and HIV-specific CD8+ T-cell responses using ELISpot assays. We drew upon a large bank of samples that have been gathered over the past decade for studies of HIV infection, as well as adding a small control group of HIV-negative individuals.
We elected to focus on this geographic region as it represents populations at the epicentre of the global HIV epidemic, in whom HBV also represents a substantial and emerging clinical challenge. Our data provide a detailed picture of HBV/HIV in populations who are highly vulnerable to coinfection, for whom resources for screening, diagnosis and treatment are limited.
Materials and Methods
Study approach
Over the past decade, our group has produced numerous detailed studies of immune responses to HIV in southern African adults (for examples, see references [12–17]). However, to date, the prevalence and nature of coinfection with other chronic viruses in these cohorts has not been described. When we recently undertook a review of the existing literature surrounding HIV/HBV coinfection in these populations [11], we were alerted to the absence of clear data regarding the prevalence and virologic characteristics of HBV in the region. We therefore set out to characterise the epidemiology and nature of HIV/HBV coinfection in this setting, drawing upon our large pre-existing repository of clinical samples and laboratory data as a starting point for further study.
Study populations and ethics statement
We performed a retrospective cross-sectional analysis of 1,022 African women, recruited from antenatal and paediatric clinics (in the latter case, as the mothers of HIV-infected children) between 2004 and 2013. Of these, 950 (93%) were HIV-positive, ART-naïve, recruited from four cohorts described as a list below and also in Table 1, with reference to previous descriptions in the published literature where applicable:
Durban HIV-positive cohort (n = 426): HIV-positive women attending antenatal clinics at Cato Manor and Sinikithemba in Durban, South Africa [18, 19]. Ethics approval was given by the University of KwaZulu-Natal Biomedical Research Ethics Committee (ref. E028/99).
Kimberley HIV-positive cohort (n = 81): HIV-positive women recruited as mothers of children attending paediatric HIV clinics in Kimberley, South Africa [13, 20]. Ethics approval was given by Ethics Committee of the Faculty of Health Science, University of Free State, Bloemfontein, South Africa (ref. ETOVS Nr 08/09).
Gaborone HIV-positive cohort (n = 443): HIV-positive women recruited via the Mma Bana Study [21], attending antenatal clinics in Gaborone, Botswana. Ethics approval was given by the Health Research and Development Division, Ministry of Health, Gaborone (ref. PPME-13/18/1).
Table 1. Prevalence and characteristics of HBV infection in 1,022 adult women from Botswana and South Africa.
| Cohort Name / Location | Masibambisane / Durban | Sinikithemba and Cato Manor / Durban | Kimberley | Mma Bana / Gaborone | Total | |
|---|---|---|---|---|---|---|
| Country of origin | South Africa | South Africa | South Africa | Botswana | South Africa + Botswana | |
| Recruitment source | Antenatal clinics | Antenatal clinics | Paediatric clinics (mothers of HIV-infected children) | Antenatal clinics | All cohorts combined | |
| HIV-status | Negative | Positive | Positive | Positive | Mixed | |
| Number of individuals | 72 | 426 | 81 | 443 | 1,022 | |
| HIV viral load (RNA copies/ml plasma) | Median | n/a | 30,800 | 33,947 | 16,400 | 23,300 |
| IQR | n/a | 6,990–112,500 | 6,400–165,000 | 3,610–70,100 | 4,861–93,625 | |
| CD4 T cell count (cells/mm 3 ) | Median | n/a | 368 | 325 | 344 | 359 |
| IQR | n/a | 258–532 | 225–478 | 228–1,342 | 253–507 | |
| Number with HBsAg (% of all individuals) a | 6 (8.3) | 40 (9.4) | 9 (10.8) | 17 (3.8) | 72 (7.0) | |
| Number with HBeAg (% of HBsAg+ individuals tested) a | 1/6 (16.7) | 12/40 (30.0) | 1/4 (25.0) | 2/10 (20.0) | 16/60 (26.7) | |
| Number with HDV | 0/6 (0) | 0/37 (0) | 0/8 (0) | 0/9 (0) | 0/60 (0) | |
In addition, we investigated a parallel group of HIV-negative women, in order to be able to compare the characteristics of HBV in HIV-positive vs. HIV-negative groups. This group consisted of HIV-negative antenatal women from the Masibambisane cohort, recruited at Prince Mshiyeni Hospital, Durban, South Africa (n = 72). Ethics approval was given by the University of KwaZulu-Natal Biomedical Research Ethics Committee (ref. BF 168.09).
All subjects gave written informed consent for participation.
HIV-1 RNA load and CD4 T cell count
We quantified HIV-1 RNA from plasma using the Roche Amplicor Version 1.5 assay (Rotkreuz, Switzerland), and measured CD4+ T cell counts by flow cytometry. These assays were done on location by the clinical centres recruiting the patients, using fresh plasma and cells.
Serological testing
The South African cohorts underwent testing for Hepatitis B surface antigen (HBsAg) retrospectively from frozen sera using the Biokit enzyme immune assay (Barcelona, Spain). For Botswana, HBsAg results were performed in Gaborone, using the Murex HBsAg v3 (DiaSorin) assay.
HBsAg-positive samples with sufficient volume underwent testing for HBeAg by the chemilumiscence immune assays Architect (Abbott Diagnostics, Maidenhead, UK) (Durban cohorts) and ADVIA Centaur CP (Siemens, Camberley, UK) (Gaborone and Kimberley cohorts). We also investigated for potential HDV coinfection by screening HBsAg-positive samples for total HDV antibody when sufficient sample volume was available, using the DIA.PRO HDV Ab enzyme immune assay (Milan, Italy).
HBV DNA load and sequencing
HBsAg-positive/HBeAg-negative samples for which sufficient sample was available underwent HBV DNA quantification by real-time PCR as previously described [22] (lower limit of quantification 50 IU/mL). Samples with HBV DNA >500 IU/ml underwent population (Sanger) sequencing of the HBV polymerase gene (amino acids 1–344) to determine genotype/sub-genotype and presence of drug-resistance associated mutations (RAMs), as previously described [22, 23]. HBV genotyping was initially done using the online automated subtyping tool Oxford HBV BioAfrica (http://www.bioafrica.net/rega-genotype/html/indexhbv.html) [24, 25]. To provide confirmation of these results and sub-genotyping, we undertook further phylogenetic analysis using a panel of reference sequences with maximum likelihood (ML) as implemented in PhyML 3.0 (1,000 bootstrap replicates) [26, 27].
IFN-gamma ELISpot Assays
We undertook ELISpot assays using samples from the Durban HIV-infected cohort with sufficient available cells (n = 325, of whom 35 were positive for HBsAg (10.7%), and 10 were positive for HBeAg (28.6% of HBsAg-positives)). Ex vivo CD8+ T cell responses to HIV were quantified by testing peripheral blood mononuclear cells (PBMCs) against a panel of 410 overlapping HIV-1 peptides spanning the entire HIV-1 proteome, as previously described [19].
Statistical analysis
Data were analysed using GraphPad Prism v.6.0f. Fisher’s Exact Test was used to compute significance for categorical variables in 2x2 contingency tables. HIV-1 RNA viral loads and CD4+ T cell counts in HBsAg-positive vs. HBsAg-negative subjects were compared using a Mann Whitney U test. HBsAg seroprevalence was quoted with 95% confidence intervals (CI) calculated by the adjusted Wald test (http://www.measuringu.com/wald.htm).
Results
HIV status
This study enrolled a total of 1,022 ART-naïve HIV-positive women from South Africa and Botswana. HIV subtype was not determined, but based on the origin of the patients is likely to have been C-clade in the majority [28]. In addition, we recruited 72 HIV-negative adults from Durban. Raw data for the cohort are available as supplementary data (S1 Table).
There were no significant differences in CD4+ T cell count between different sub-cohorts (S1 Fig; Table 1). HIV-1 RNA viral loads were higher in the South African cohorts (median RNA VL 30,800 copies/ml and 33,947 copies/ml in Durban and Kimberley, respectively) than in Botswana (median RNA VL 16,400 copies/ml; S1 Fig; Table 1).
HBsAg prevalence
The overall prevalence of HBsAg in the combined cohort was 72/1022 (7.0%; 95% CI 5.6–8.8%; Table 1). Prevalence varied geographically: the lowest prevalence was in Botswana, where 17/443 (3.8%, 95% CI 2.3–5.9%) individuals tested positive for HBsAg, in contrast to South Africa (Durban and Kimberley cohorts), where 49/507 (9.7%, 95% CI 6.7–11.5%) were HBsAg positive.
The prevalence of HBsAg was not significantly different between HIV-positive vs. HIV-negative sub-groups (66/950 (6.9%) vs. 6/72 (8.3%); p = 0.6, Fisher’s Exact Test). Restricting this comparison to Durban (from where all our HIV-negative subjects were recruited), the prevalence of HBsAg was 40/426 (9.4%) versus 6/72 (8.3%) in HIV-positive and HIV-negative individuals respectively (p = 1.0).
Virological characteristics of HBV infection in HIV-positive adults
To characterize the HBV replicative status of HIV/HBV coinfected patients, we first screened HBsAg-positive individuals for HBeAg. Overall, 16/60 (26.7%) HIV/HBV coinfected individuals were HBeAg positive (Table 1). Among 30 HBsAg-positive/HBeAg-negative individuals from South Africa and Botswana the HBV DNA load was median 1.8 log10 IU/ml (IQR 1.6–3.7 log10 IU/ml). Of these, 9/30 (30%) had HBV DNA >2,000 IU/ml. HDV total antibody was not detected in any of the 60 HBsAg-positive individuals tested (Table 1).
We sequenced the HBV polymerase gene from 16 individuals with detectable HBV DNA. Of these, 15/16 did not contain HBV drug RAMs, but one specimen harboured the M204I nucleos(t)ide analogue RAM. HBV genotypes were A for the South Africa cohort (9/14 (64%) sub-genotype A1 and 5/14 (36%) A2) and D for the Botswana cohort (2/2 (100%) sub-genotype D3).
Impact of HBV co-infection on HIV-1 RNA load, CD4+ T-cell count and HIV-specific CD8+ T-cell responses
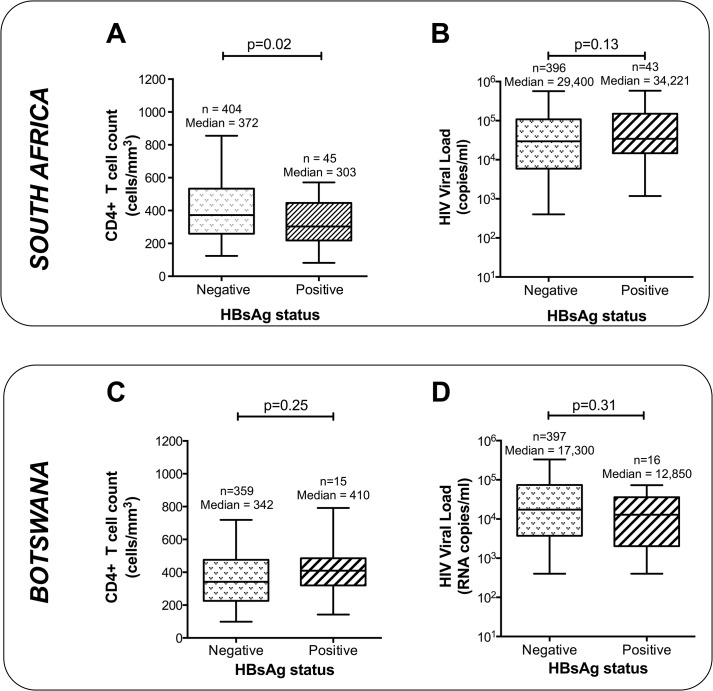
Within our combined HIV-positive South African cohort (Durban and Kimberley), HBsAg-positive individuals had statistically lower CD4+ T cell counts than HBsAg-negative individuals (303 vs. 372 cells/mm3, respectively; p = 0.02, Mann-Whitney test, Fig 1A). The median HIV-1 RNA load was slightly higher in HBsAg-positive individuals than in HBsAg-negative individuals in South Africa although this did not reach statistical significance (34,221 RNA copies/ml vs. 29,400 copies/ml; p = 0.13, Mann Whitney test; Fig 1B). No significant association was observed between HBV status and CD4+ T cell count or HIV-1 RNA load in Botswana (Fig 1C and 1D).
Fig 1. Relationship between HBV status and markers of HIV disease in HIV-positive women from South Africa and Botswana.
Panels (A) and (B): South Africa (Durban + Kimberley cohorts pooled); Panels (C) and (D): Botswana (Gaborone). Left-hand column (panels (A) and (C)) shows CD4+ T cell counts; right-hand column (panels (B) and (D)) shows HIV-1 RNA viral load. In each case, box represents median and 25/75th centiles, whiskers 5-95th centiles. P values by Mann Whitney U test.
As CD8+ T cell responses are well recognized to be an important mediator of HIV disease control [19, 29, 30], we sought evidence of any impact of HBV coinfection on ex vivo CD8+ T cell responses to HIV using IFN-gamma ELISpot assays for 325 subjects from within the Durban cohort. There was no significant difference in the breadth, magnitude, or protein-specificity of IFN-gamma responses to HIV in the presence or absence of HBsAg or HBeAg (S2 Fig).
Discussion
The virological expression of HBV infection varies substantially among HIV-positive individuals in sub-Saharan Africa, and its determinants remain poorly characterized [22]. This study determined the prevalence and virological expression of HBV coinfection in a composite cohort of ART-naïve HIV-positive adult women from South Africa and Botswana. Comparing our HBsAg prevalence data to other published literature is difficult, as data are relatively sparse and epidemiology varies by setting. However, broadly speaking, our current results are comparable to those assimilated from the review we have recently undertaken of this region [11]; the latter incorporated data on 11,346 HIV-positive individuals from southern Africa, of whom 687 (6.1%) were reported to have chronic HBV (median 6.0% across all studies). This study suggests that HDV is unlikely to be a major contributory factor to chronic liver disease in the specific settings we have studied, but further detailed work is needed to establish HDV hotspots and risk factors.
Interestingly, although CD4+ T cell counts were comparable between our geographically distinct sub-cohorts, plasma HIV-1 RNA levels were significantly lower in Botswana than South Africa, likely as a result of multiple influences that include the maturity of the epidemic and fitness of the viral infecting strain [17].
Our data point to a potential association between HBsAg-positivity and lower CD4+ T cell counts (Fig 1A), but this is a weak effect that does not appear consistent across locations; this is in keeping with uncertainty in the published literature to date [11]. Where this effect does operate, the direction of causality is uncertain; it is possible that chronic HBV infection may contribute to the decline of CD4+ T cell populations that are already diminished by HIV, but alternatively the effect could be explained by an increased risk of acquisition or reactivation of chronic HBV infection in HIV-positive individuals with low CD4+ T cell counts. Without individual level data, (e.g. duration of HIV infection), we are unable to draw any certain conclusions about the factors underpinning differences in CD4+ T cell counts between cohorts or by HBV status.
There are several caveats and limitations to our analysis. Due to the cross-sectional nature of the study, we were unable to verify the persistence of HBsAg over time; but for the purposes of this study we made the assumption that HBsAg-positivity represented chronic infection. It should be noted that we enrolled women attending antenatal clinics, and that pregnancy may modulate the nature of the immune response to viral infection; for example, it is recognized that HBV viral loads are typically higher in the second half of pregnancy. Although HIV-positive patients were all recorded as ‘ART-naïve’ at the time of enrollment, we cannot exclude the possibility that a minority of individuals may have accessed short courses of treatment prior to the study. Thus the detection of the M204I substitution may reflect either transmission of a RAM, or prior lamivudine exposure that was unreported by the patient.
It is evident that HIV/HBV coinfection patterns differ substantially by region [11], and an attempt to combine analysis of individuals from disparate settings could be misleading. Detailed demographic data were not recorded for these cohorts at the time of recruitment, so we are not able to draw conclusions regarding the specific relationship of our findings to different communities, regions and countries, or to age or socio-demographic characteristics.
To make the analysis as broad and far-reaching as possible, we avoided limiting ourselves to a single geographic cohort. In the interests of optimizing numbers, we have pursued the multi-centre approach, but to emphasise differences by region we have presented epidemiology data as separate results for individual sub-cohorts by region (Table 1; Fig 1; S1 Fig). Although we made every effort to optimize numbers, analysis of even greater numbers of subjects would be beneficial to form more robust conclusions.
Another influence on the epidemiology of this co-epidemic is the roll-out of the prophylactic HBV vaccination that has been variably introduced across sub-Saharan Africa in the past two decades; HBV prevalence will doubtless continue to change in accordance with the success of this vaccine campaign [31]. A recent review from South Africa points out that although vaccine coverage among children has increased since the mid 1990’s, there has been no ‘catch-up’ campaign, and vulnerable individuals remain unimmunized [32]. Other countries in the region have been slower to introduce routine vaccination [33, 34], and the coverage of vaccine campaigns is uncertain. Furthermore, because we did not test for serological markers of immunity (HBsAb or HBcAb) it is uncertain to what extent the HBsAg-negative individuals here may have acquired vaccine-mediated or natural immunity.
The difficulty of assimilating robust and representative data on the characteristics of HIV/HBV co-infection in this region is highlighted by this study; despite assimilating >1000 individuals over a ten year period, we identified only 72 with HBsAg-positive status. Furthermore, our dataset is incomplete, as small volumes within some of the samples meant that we were able to test only a subset of the whole cohort for HBeAg and HDV. There is no single systematic bias that we can identify that is likely to be clearly associated with this issue, but we are unable to categorically state the influence of missing data. We were also limited by availability of cells for ex vivo T cell studies: it remains possible that HBV-specific CD8+ T cell responses are abrogated by the presence of HIV, but we unfortunately did not have sufficient cells to investigate this possibility.
Despite these caveats and limitations, we believe this dataset to be of utility and importance in providing insight into the extent and characteristics of the HBV/HIV co-epidemic in certain southern African populations. Ongoing efforts are needed to secure prompt diagnosis and appropriate monitoring and treatment for coinfected individuals, in order to avert an emerging crisis of chronic liver disease.
Supporting Information
(A) HIV-1 RNA viral load and (B) CD4+ T cell count. Boxes show 25–75% centiles, whiskers show 5–95% CI. There was significant variation in HIV-1 RNA viral load between cohorts (p = 0.0002, Kruskal-Wallis test), but not in CD4+ T cell counts (ns = not significant).
(TIFF)
(A): Total number of ELISpot responses across the entire HIV proteome according to HBsAg status; (B): Total number of HIV proteins targeted by ELISpot responses according to HBsAg status; (C): Number of Gag-specific ELISpot responses according to HBsAg status; (D): Magnitude of immunodominant ELISpot response according to HBsAg status; (E): Total number of ELISpot responses across the entire HIV proteome according to HBeAg status; (F): Total number of HIV proteins targeted by ELISpot responses according to HBeAg status; (G): Number of Gag-specific ELISpot responses according to HBeAg status; (H): Magnitude of immunodominant ELISpot response according to HBeAg status. Error bars show 95% CI. P-values by Mann Whitney U test.
(TIFF)
Tab 1 contains cohort location, HIV status, HBsAg status, HIV-1 RNA viral load (copies / ml plasma) and CD4+ T cell count (cells/mm3). Tab 2 contains HBeAg status, HDV antibody status, HBV DNA viral load (IU/ml) and HBV genotype.
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
PCM received salary from the NIHR and grants from Oxford University Clinical Academic Graduate School (OUCAGS) and the John Fell Fund to cover the cost of this work. TN is funded through the South African DST/NRF Research Chair in Systems Biology of HIV/AIDS, the Victor Daitz Chair in HIV/TB Research and an International Early Career Scientist Award from the Howard Hughes Medical Institute. PG is funded by the Wellcome Trust (WT 104748 MA). PK is funded by the Wellcome Trust (WT091663 MA) and the Oxford Martin School. Microsoft Research provided support in the form of a salary for author JMC, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the author contributions section.
References
- 1. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global challenge. N Engl J Med. 2012;366(19):1749–52. 10.1056/NEJMp1201796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gravitz L. Introduction: a smouldering public-health crisis. Nature. 2011;474(7350):S2–4. 10.1038/474S2a [DOI] [PubMed] [Google Scholar]
- 3. Bonacini M, Louie S, Bzowej N, Wohl AR. Survival in patients with HIV infection and viral hepatitis B or C: a cohort study. Aids. 2004;18(15):2039–45. [DOI] [PubMed] [Google Scholar]
- 4. Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57(1):249–57. 10.1002/hep.25800 [DOI] [PubMed] [Google Scholar]
- 5. Hawkins C, Christian B, Ye J, Nagu T, Aris E, Chalamilla G, et al. Prevalence of hepatitis B co-infection and response to antiretroviral therapy among HIV-infected patients in Tanzania. Aids. 2013;27(6):919–27. 10.1097/QAD.0b013e32835cb9c8 [DOI] [PubMed] [Google Scholar]
- 6. Stabinski L, Reynolds SJ, Ocama P, Laeyendecker O, Ndyanabo A, Kiggundu V, et al. High prevalence of liver fibrosis associated with HIV infection: a study in rural Rakai, Uganda. Antivir Ther. 2011;16(3):405–11. 10.3851/IMP1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ocama P, Opio KC, Kagimu M, Seremba E, Wabinga H, Colebunders R. Hepatitis B virus and HIV infection among patients with primary hepatocellular carcinoma in Kampala, Uganda. Afr Health Sci. 2011;11 Suppl 1:S20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ladep NG, Agaba PA, Agbaji O, Muazu A, Ugoagwu P, Imade G, et al. Rates and impact of hepatitis on human immunodeficiency virus infection in a large African cohort. World J Gastroenterol. 2013;19(10):1602–10. 10.3748/wjg.v19.i10.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sagoe KW, Agyei AA, Ziga F, Lartey M, Adiku TK, Seshi M, et al. Prevalence and impact of hepatitis B and C virus co-infections in antiretroviral treatment naive patients with HIV infection at a major treatment center in Ghana. J Med Virol. 2012;84(1):6–10. 10.1002/jmv.22262 [DOI] [PubMed] [Google Scholar]
- 10. Thio CL, Smeaton L, Saulynas M, Hwang H, Saravan S, Kulkarni S, et al. Characterization of HIV-HBV coinfection in a multinational HIV-infected cohort. Aids. 2013;27(2):191–201. 10.1097/QAD.0b013e32835a9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matthews PC, Geretti AM, Goulder PJ, Klenerman P. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in Sub-Saharan Africa. J Clin Virol. 2014;61(1):20–33. 10.1016/j.jcv.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 12. Matthews PC, Prendergast A, Leslie A, Crawford H, Payne R, Rousseau C, et al. Central role of reverting mutations in HLA associations with human immunodeficiency virus set point. J Virol. 2008;82(17):8548–59. 10.1128/JVI.00580-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matthews PC, Adland E, Listgarten J, Leslie A, Mkhwanazi N, Carlson JM, et al. HLA-A*7401-mediated control of HIV viremia is independent of its linkage disequilibrium with HLA-B*5703. Journal of immunology. 2011;186(10):5675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leslie A, Matthews PC, Listgarten J, Carlson JM, Kadie C, Ndung'u T, et al. Additive contribution of HLA class I alleles in the immune control of HIV-1 infection. J Virol. 2010;84(19):9879–88. 10.1128/JVI.00320-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawashima Y, Pfafferott K, Frater J, Matthews P, Payne R, Addo M, et al. Adaptation of HIV-1 to human leukocyte antigen class I. Nature. 2009;458(7238):641–5. 10.1038/nature07746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432(7018):769–75. [DOI] [PubMed] [Google Scholar]
- 17. Payne R, Muenchhoff M, Mann J, Roberts HE, Matthews P, Adland E, et al. Impact of HLA-driven HIV adaptation on virulence in populations of high HIV seroprevalence. Proceedings of the National Academy of Sciences of the United States of America. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brumme Z, Wang B, Nair K, Brumme C, de Pierres C, Reddy S, et al. Impact of select immunologic and virologic biomarkers on CD4 cell count decrease in patients with chronic HIV-1 subtype C infection: results from Sinikithemba Cohort, Durban, South Africa. Clin Infect Dis. 2009;49(6):956–64. 10.1086/605503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nature medicine. 2007;13(1):46–53. [DOI] [PubMed] [Google Scholar]
- 20. Matthews PC, Listgarten J, Carlson JM, Payne R, Huang KH, Frater J, et al. Co-operative additive effects between HLA alleles in control of HIV-1. PLoS One. 2012;7(10):e47799 10.1371/journal.pone.0047799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362(24):2282–94. 10.1056/NEJMoa0907736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aoudjane S, Chaponda M, Gonzalez Del Castillo AA, O'Connor J, Noguera M, Beloukas A, et al. Hepatitis B Virus Sub-genotype A1 Infection Is Characterized by High Replication Levels and Rapid Emergence of Drug Resistance in HIV-Positive Adults Receiving First-line Antiretroviral Therapy in Malawi. Clin Infect Dis. 2014;59(11):1618–26. 10.1093/cid/ciu630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stockdale AJ, Phillips RO, Beloukas A, Appiah LT, Chadwick D, Bhagani S, et al. Liver Fibrosis by Transient Elastography and Virologic Outcomes After Introduction of Tenofovir in Lamivudine-Experienced Adults With HIV and Hepatitis B Virus Coinfection in Ghana. Clin Infect Dis. 2015. [DOI] [PubMed] [Google Scholar]
- 24. Alcantara LC, Cassol S, Libin P, Deforche K, Pybus OG, Van Ranst M, et al. A standardized framework for accurate, high-throughput genotyping of recombinant and non-recombinant viral sequences. Nucleic acids research. 2009;37(Web Server issue):W634–42. 10.1093/nar/gkp455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Oliveira T, Deforche K, Cassol S, Salminen M, Paraskevis D, Seebregts C, et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics. 2005;21(19):3797–800. [DOI] [PubMed] [Google Scholar]
- 26. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic biology. 2010;59(3):307–21. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 27. Beloukas A, Magiorkinis E, Magiorkinis G, Zavitsanou A, Karamitros T, Hatzakis A, et al. Assessment of phylogenetic sensitivity for reconstructing HIV-1 epidemiological relationships. Virus research. 2012;166(1–2):54–60. 10.1016/j.virusres.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 28. Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS. 2006;20(16):W13–23. [DOI] [PubMed] [Google Scholar]
- 29. Goulder PJ, Walker BD. HIV and HLA class I: an evolving relationship. Immunity. 2012;37(3):426–40. 10.1016/j.immuni.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Payne RP, Matthews PC, Prado JG, Goulder PJ. HLA-mediated control of HIV and HIV adaptation to HLA. Advances in parasitology. 2009;68:1–20. 10.1016/S0065-308X(08)00601-5 [DOI] [PubMed] [Google Scholar]
- 31. Mutwa PR, Boer KR, Rusine JB, Muganga N, Tuyishimire D, Reiss P, et al. Hepatitis B virus prevalence and vaccine response in HIV-infected children and adolescents on combination antiretroviral therapy in Kigali, Rwanda. The Pediatric infectious disease journal. 2013;32(3):246–51. 10.1097/INF.0b013e318271b93d [DOI] [PubMed] [Google Scholar]
- 32. Burnett RJ, Kramvis A, Dochez C, Meheus A. An update after 16 years of hepatitis B vaccination in South Africa. Vaccine. 2012;30 Suppl 3:C45–51. 10.1016/j.vaccine.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 33. Magoni M, Ekra KD, Aka LN, Sita KS, Kanga K. Effectiveness of hepatitis-B vaccination in Ivory Coast: the case of the Grand Bassam health district. Annals of tropical medicine and parasitology. 2009;103(6):519–27. 10.1179/136485909X451816 [DOI] [PubMed] [Google Scholar]
- 34. Nyirenda M, Beadsworth MB, Stephany P, Hart CA, Hart IJ, Munthali C, et al. Prevalence of infection with hepatitis B and C virus and coinfection with HIV in medical inpatients in Malawi. J Infect. 2008;57(1):72–7. 10.1016/j.jinf.2008.05.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) HIV-1 RNA viral load and (B) CD4+ T cell count. Boxes show 25–75% centiles, whiskers show 5–95% CI. There was significant variation in HIV-1 RNA viral load between cohorts (p = 0.0002, Kruskal-Wallis test), but not in CD4+ T cell counts (ns = not significant).
(TIFF)
(A): Total number of ELISpot responses across the entire HIV proteome according to HBsAg status; (B): Total number of HIV proteins targeted by ELISpot responses according to HBsAg status; (C): Number of Gag-specific ELISpot responses according to HBsAg status; (D): Magnitude of immunodominant ELISpot response according to HBsAg status; (E): Total number of ELISpot responses across the entire HIV proteome according to HBeAg status; (F): Total number of HIV proteins targeted by ELISpot responses according to HBeAg status; (G): Number of Gag-specific ELISpot responses according to HBeAg status; (H): Magnitude of immunodominant ELISpot response according to HBeAg status. Error bars show 95% CI. P-values by Mann Whitney U test.
(TIFF)
Tab 1 contains cohort location, HIV status, HBsAg status, HIV-1 RNA viral load (copies / ml plasma) and CD4+ T cell count (cells/mm3). Tab 2 contains HBeAg status, HDV antibody status, HBV DNA viral load (IU/ml) and HBV genotype.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.