Abstract
Objective
Fibrosis has been implicated in a number of pathological, organ-based conditions of the liver, kidney, heart, and lungs. The objective of this study was to determine whether biomarkers of fibrosis are associated with vascular disease in the large and/or small vessels.
Methods
We evaluated the associations of two circulating biomarkers of fibrosis, transforming growth factor-β (TGF-β) and procollagen type III N-terminal propeptide (PIIINP), with incident peripheral artery disease (PAD) and subclinical macrovascular (carotid intima-media thickness, flow-mediated vasodilation, ankle-brachial index, retinal vein diameter), and microvascular (retinal artery diameter and retinopathy) disease among older adults in the Cardiovascular Health Study. We measured TGF-β and PIIINP from samples collected in 1996 and ascertained clinical PAD through 2011. Measurements of large and small vessels were collected between 1996–1998.
Results
After adjustment for sociodemographic, clinical, and biochemical risk factors, TGF-β was associated with incident PAD (hazard ratio [HR]=1.36 per doubling of TGF-β, 95% confidence interval [CI]= 1.04, 1.78) and retinal venular diameter (1.63 µm per doubling of TGF-β, CI=0.23, 3.02). PIIINP was not associated with incident PAD, but was associated with carotid intima-media thickness (0.102 mm per doubling of PIIINP, CI=0.029, 0.174) and impaired brachial artery reactivity (−0.20 % change per doubling of PIIINP, CI=−0.39, −0.02). Neither TGF-β nor PIIINP were associated with retinal arteriolar diameter or retinopathy.
Conclusions
Serum concentrations of fibrosis-related biomarkers were associated with several measures of large vessel disease, including incident PAD, but not with small vessel disease. Fibrosis may contribute to large vessel atherosclerosis in older adults.
Keywords: fibrosis, peripheral artery disease, atherosclerosis
Introduction
Reactive interstitial fibrosis is the pathological accumulation of excess collagen and extracellular matrix deposition in response to severe or repetitive tissue injury. Over time, fibrosis can lead to scar formation and organ dysfunction, as seen in end-stage liver disease, kidney disease, and idiopathic pulmonary fibrosis [1]. In older adults, fibrosis has been implicated in age-related ventricular stiffening and diastolic dysfunction [2]; for example, between the third and seventh decade of life, myocardial collagen content increases by almost 50% [3]. Whether fibrosis plays a role in vascular atherogenesis and function in older adults remains an attractive but still unproven hypothesis.
Transforming growth factor-β (TGF-β) is a pleiotropic cytokine and a central mediator of fibrosis. TGF-β activity promotes collagen biosynthesis and the release of collagen byproducts, including procollagen type III N-terminal propeptide (PIIINP). In addition to its pro-fibrotic activity, TGF-β has potent anti-inflammatory effects; it has been found to suppress the expression of pro-inflammatory adhesion molecules by the vascular endothelium, prevent leukocyte and macrophage recruitment, and de-activate T-cells enriched in rupture prone vascular plaques [4]. TGF-β’s dual pro-fibrotic and anti-inflammatory effects complicate its ultimate relationship with vascular disease, as these effects are likely to have opposing effects on atherogenesis.
Although fibrosis is a plausible risk factor for vascular disease, evidence to support this hypothesis has been inconsistent. Previous studies have found an inverse association between TGF-β expression and the probability of aortic atherosclerosis [5], as well as lower circulating levels of TGF-β among individuals with advanced atherosclerosis [6]. In contrast, other studies have shown positive correlations between TGF-β expression and atherogenic stimuli such as shear stress [7], oxidized cholesterol [8], and angiotensin II [9], as well as lower circulating levels of TGF-β among individuals with advanced atherosclerosis [10]. Though relatively few studies of PIIINP have been conducted, one cross-sectional study found that serum PIIINP concentrations were higher among individuals with peripheral arterial disease (PAD) compared to individuals with normal vasculature [11]. A second study found a borderline association between PIIINP and carotid atherosclerosis [12]. Most of the previous studies that have been conducted have been small and cross-sectional. Larger studies, and studies with prospective endpoints, such as clinical PAD, could begin to address existing inconsistencies.
A key question that has not yet been investigated is whether fibrosis-related biomarkers are differentially associated with large vs. small vessel disease. Existing studies of fibrosis-related biomarkers have focused on large vessel disease in the carotid and brachial arteries [11, 13, 14]; no studies of microvascular outcomes have been conducted, despite the fact that these may represent distinct pathophysiologic processes. Large and small vessel disease have been shown to have different biological etiologies and risk factors [15]. For example, large vessel disease primarily results from atherosclerosis [16], while small vessel disease is thought be the result of multiple molecular and cellular mechanisms, including prolonged hyperglycemia, dysregulation of vascular tone, and oxidative stress [16, 17]. These differences highlight the possibility that associations between fibrosis-related biomarkers and vascular parameters may differ in vascular beds of different size.
Herein, we sought to evaluate the associations of TGF-β and PIIINP with clinical PAD and a broad range of measures of vascular structure and function, in both large and small vessels, among participants in the Cardiovascular Health Study (CHS), a population-based study of older adults from four U.S. communities. Because these markers of fibrosis have already been measured in this large, prospective study with both central adjudication of PAD and detailed subclinical vascular phenotyping, we could address multiple dimensions of their association with vascular disease, including possible interactions with inflammation that we observed in studies of other cardiovascular outcomes.
Materials and Methods
Study Design
The design, rationale and examination details of CHS have been published elsewhere [18]. Briefly, 5,201 participants were recruited from Medicare eligibility lists from Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania in 1989–1990. A supplemental cohort of 687 mostly African-American participants was added in 1992–1993. Individuals were eligible to participate if they were at least 65 years old, living in the community, and expected to remain in that community for at least three years after baseline. Individuals under active cancer treatment, and those not able to provide written informed consent, were excluded. Follow-up interviews were conducted during annual visits through 1998–1999 and interim 6-month phone calls that are still ongoing. The present analysis was limited to follow-up through 2011. All participants in our study provided written informed consent, and the institutional review board at each center approved the study protocol.
Exposure Assessment
Our analysis included 1,384 individuals free from clinical PAD, myocardial infarction (MI), and stroke with measured levels of TGF-β and 2,647 with measured levels of PIIINP. TGF-β and PIIINP were measured in 2011–2012 from stored EDTA plasma samples collected during the 1996–1997 CHS visit, which is baseline for these analyses. TFG-β was measured by ELISA (Quantikine Human TGF-β1 Immunoassay; R&D Systems, Minneapolis, MN). PIIINP was measured by the UniQ Intact N-terminal Propeptide of Type III Procollagen radioimmunoassay kit manufactured by Orion Diagnostics (Fountain Hills, AZ). Inter- and intra-assay coefficients of variation (CVs) were between 1.9–2.9% and 6.4–9.3%, respectively for TFG-β. For PIIINP, corresponding values were both less than 7.2%.
In pilot studies, we identified probable platelet contamination resulting in artificially elevated levels of TGF-β [19] at two of our four clinic sites. Hence, a priori, we only measured TGF-β at the two remaining sites. Of the two sites at which TGF-β was not measured, one site (Washington County, Maryland) did not enroll an African American cohort in 1992–1993, leading to modest differences in other participant characteristics across clinic sites (Supplemental Table I).
Ascertainment of Incident PAD
We ascertained incident clinical PAD events from 1996 through 2011 (Figure 1). The assessment criteria for PAD were defined prospectively and applied uniformly across CHS sites. During follow-up, PAD outcomes were identified by any of the following methods: 1) report of a PAD diagnosis by the participant at a clinic visit or during a telephone call; 2) a PAD diagnosis found during review of medical records for another event; 3) active surveillance of CMS records for the ICD-9 codes 400.2 (atherosclerosis of the native arteries of the extremities) and 443.9 (peripheral vascular disease, unspecified). After a potential diagnosis of clinical PAD was identified, medical records were obtained and the CHS Clinical Events Subcommittee adjudicated a final decision. Potential diagnoses required validation by one of the following: 1) ankle-brachial index (ABI) less than 0.90; 2) exertional leg pain relieved by rest and a physician’s diagnosis of PAD; 3) leg pain with confirmatory radiological or sonographic evidence (ultrasound showing an obstruction of ≥75% of the cross-sectional area of the artery or showing an ulcerated plaque or angiography showing ≥50% obstruction of the diameter or ≥75% of the artery cross-sectional area or an ulcerated plaque; or absence of a Doppler pulse in any major vessel); 4) a positive exercise test for claudication; or 5) bypass surgery, angioplasty, amputation, or thrombolysis for the indication of PAD.
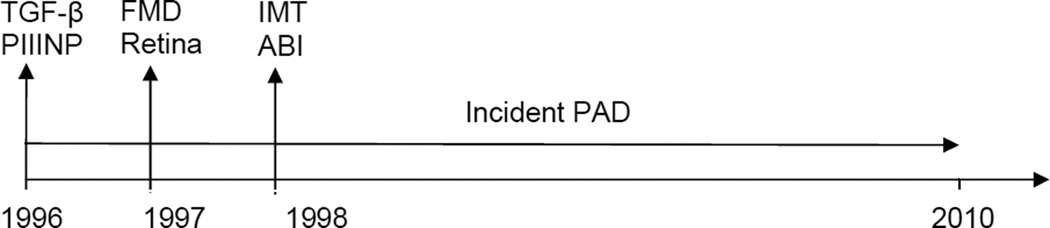
Figure 1.
Timeline of exposure and outcome measurements, Cardiovascular Health Study, 1996–2011
ABI=ankle-brachial index, FMD=flow-mediated vasodilation, IMT=carotid artery intima-media thickness, PAD=peripheral artery disease, PIIINP= procollagen type III N-terminal propeptide, Retina=retinal findings (vascular caliber and retinopathy), TGF-β= transforming growth factor-β
Vascular Measurements
For robustness, we ascertained a wide variety of structural and functional measurements from multiple vascular beds. Measurements of vascular structure and function were collected between 1996–1998 (Figure 1) according to standardized criteria across clinical sites.
Flow-mediated vasodilation (FMD) was measured at the 1997–1998 visit using images of the right brachial artery captured with a 10 MhZ Biosound Phase 2 ultrasound system [20]. %FMD was calculated using the formula 100%*[(maximum diameter after cuff inflation-baseline diameter)/(baseline diameter)] [21].
Retinal photographs were taken during the 1997–1998 visit to determine retinal vessel caliber and presence or absence of retinopathy. A 45° retinal photograph centered between the optic disc and the macula was obtained of one randomly selected eye using a non-mydriatic fundus camera after five minutes of dark adaptation (Canon CR-45UAF; Canon USA, Inc., Lake Success, NY). Photographs were evaluated according to a standardized protocol at the Fundus Photograph Reading Center in Madison, WI. Retinal photographs were evaluated by two certified graders masked to participant characteristics [22].
Retinal arteriolar and venular diameter measurements were summarized as central retinal artery equivalent (µm) and central retinal vein equivalent (µm) [23]. Retinopathy was defined as present if any of the following lesions were graded definite or probable: microaneurysms, retinal hemorrhages (blot or flame-shaped), soft exudates (cotton wool spots), hard exudates, or vitreous hemorrhage; these signs are measures of small vessel disease [24]. Individuals were excluded from all analyses involving retinal variables if they had prevalent age-related macular degeneration.
Carotid artery intima-media thickness (IMT) was evaluated at the 1998–1999 visit with high resolution B-mode ultrasonography by readers blinded to all clinical information [25]. A composite measure of carotid IMT was generated by averaging the maximal common carotid IMT and internal carotid IMT after standardization (subtraction of the mean and division by the standard deviation) [26, 27].
ABI was measured during the 1998–1999 visit by trained technicians according to a standard protocol described in detail previously [28]. The ABI was calculated as the ratio of the average of two systolic blood pressure measurements of the lower and upper extremities. Participants were classified according to the lower of the right or left ABI. ABI is usually ≥0.9 in normal adults; lower ABI indicates more severe arterial disease. We excluded individuals with prevalent clinical PAD in 1996–1997 or with ABI > 1.4 from ABI analyses as clinical studies have indicated that these individuals have arterial rigidity that prevents arterial occlusion and results in falsely high ABIs [29].
On the basis of vessel size, location, and previous associations, we grouped vascular structural functions into macrovascular and microvascular categories. Macrovascular measurements included carotid IMT, ABI, FMD, and retinal vein diameter; microvascular measurements included retinal artery diameter and retinopathy. Retinal vein diameter and retinal artery diameter were categorized differently despite their similar location due to previous associations with other forms of vascular disease. Previously, smaller retinal arteriolar diameter has been associated with systemic small vessel disease; in particular, lacunar stroke [30], MRI-defined cerebral small vessel disease [31–33], and coronary microvascular disease [34]. In contrast, larger retinal venular equivalent has been associated with measures of large vessel disease such carotid IMT [35, 36], incident stroke, and coronary heart disease [22, 37].
Covariate assessment
When possible, covariate measurements were taken from the 1996–1997 visit. If 1996–1997 data were unavailable, we used data from the 1997–1998 or 1995–1996 visits (or earlier, depending on data availability). A uniform set of assessment criteria were used across all clinical sites. Participants self-reported age, sex, race, smoking history, alcohol intake, leisure time physical activity, and use of anti-hypertensive medications (including ACE inhibitors, angiotensin receptor blockers, or aldosterone receptor inhibitors), and use of oral hypoglycemic agents, insulin, and statins using a validated medication inventory [38]. We used technician-measured height and weight to calculate body mass index (BMI, the weight in kilograms divided by the square of the height in meters). Trained study personnel measured systolic blood pressure (SBP). Blood and urine samples were obtained from participants for measurement of total cholesterol, albumin, high sensitivity C-reactive protein (CRP), fasting glucose, 2-hour glucose (measured two hours after 75 g of oral glucose administration), urine albumin/creatinine ratio, N-terminal type B pro-brain natriuretic peptide (NT-proBNP), and cystatin-C based estimated glomerular filtration rate (eGFR). We defined diabetes as fasting glucose ≥ 126 mg/dL (fasting), 2-hour glucose ≥200 mg/dL, or use of insulin or oral hypoglycemic medications.
Statistical analysis
We examined the distribution of TGF-β and PIIINP by the above listed covariates and calculated bivariate associations using the Kruskal-Wallis test, categorizing categorical variables according to clinically-relevant cutpoints. We used Cox proportional hazards models to examine associations of TGF-β and PIIINP with incident PAD, using follow-up time since the 1996–1997 visit as the time scale. We used multivariable linear or logistic regression to evaluate the cross-sectional associations of TGF-β and PIIINP with carotid IMT, ABI, FMD, retinal vein diameter, retinal artery diameter, and retinopathy. The distributions of TGF-β and PIIINP displayed right skew. We transformed TGF-β and PIIINP (log base 2) and observed no departures from linearity on this scale. Estimates on the log base 2 scale can be interpreted as per doubling of TGF-β or PIIINP. For Cox models, we checked the proportional hazards assumption in fully-adjusted models using Schoenfeld residuals and found no violations.
We created sequential models. Where necessary, we categorized covariates according to clinically-relevant cutpoints. Our minimally-adjusted model adjusted for age, sex, race, and clinic site. Multivariable-adjusted model 1 additionally adjusted for BMI, smoking (never, former, current), ln(pack years+1), alcohol consumption (none, ≤1/week, >1 to ≤7/week, >7 to ≤14/week and >14/week), physical activity (ln(kilocalories/week+1)), SBP, total cholesterol, albumin, ln(CRP), ln(fasting glucose), and use of medications for hypertension, diabetes, and hypercholesterolemia. In sensitivity analysis (multivariable-adjusted model 2), we adjusted for variables not available in the full cohort, specifically replacing fasting glucose with 2-hr glucose, and additionally adjusting for urinary albumin/creatinine ratio, ln(NT-proBNP), and ln(eGFR). No individuals with diabetes were included in multivariable-adjusted model 2, as those individuals did not undergo oral glucose tolerance testing, so diabetes medication use was dropped as a covariate in model 2.
Using our fully-adjusted model 1, we assessed multiplicative interaction between TGF-β and PIIINP and by age, sex, race, CRP, and diabetes status in 1996–1997. Where we found evidence of an interaction, we conducted stratified analyses accordingly. For CRP, we created categories according to clinically-relevant cutpoints (1, 3, 10 mg/L). We found no evidence of interaction by age, sex, race, or diabetes status, and therefore did not pursue stratified analyses for these variables.
All analyses were conducted in STATA, version 12 (College Station, TX). P-values < 0.05 were considered statistically significant for all analyses, including interaction terms.
Results
Participant characteristics
Among 1,384 individuals free from PAD, MI, and stroke, TGF-β and PIIINP levels were higher among blacks, individuals with diabetes, and those with higher BMI, higher CRP, or worse kidney function (Table 1). TGF-β was weakly positively correlated with PIIINP (Spearman r=.07, P=.007).
Table 1.
Baseline characteristics and bivariate associations with TGF-β and PIIINP, Cardiovascular Health Study, 1996–1997
| TGF-β, ng/mL | PIIINP, ng/mL | ||||||
|---|---|---|---|---|---|---|---|
| Risk factor | N | Median (Interquartile range) |
P- value* |
N | Median (Interquartile range) |
P- value* |
|
| Age: | <80 | 969 | 3.3 (2,0, 6.0) | 0.24 | 1,820 | 4.4 (3.6, 5.4) | <0.001 |
| ≥80 | 415 | 3.4 (2.0, 6.4) | 827 | 4.7 (3.9, 5.7) | |||
| Sex: | male | 512 | 3.4 (2.0, 6.0) | 0.58 | 977 | 4.6 (3.9, 5.6) | <0.001 |
| female | 872 | 3.3 (2.0, 6.2) | 1,670 | 4.4 (3.6, 5.4) | |||
| Race: | black | 300 | 3.9 (2.0, 7.4) | 0.01 | 422 | 4.8 (4.0, 6.1) | <0.001 |
| nonblack | 1,084 | 3.3 (2.0, 5.8) | 2,225 | 4.4 (3.7, 5.4) | |||
| Clinic: | NC | 710 | 2.4 (1.7, 4.0) | <0.001 | 685 | 4.6 (3.9, 5.7) | <0.001 |
| CA | 0 | 744 | 4.5 (3.7, 5.5) | ||||
| MD | 0 | 544 | 4.2 (3.5, 5.2) | ||||
| PA | 674 | 4.7 (3.1, 7.7) | 674 | 4.5 (3.7, 5.5) | |||
| Body mass index (kg/m2): | <25 | 503 | 3.3 (1.9, 5.6) | 0.05 | 914 | 4.2 (3.5, 5.2) | <0.001 |
| 25–30 | 593 | 3.3 (2.0, 6.2) | 1,152 | 4.5 (3.7, 5.4) | |||
| >30 | 288 | 3.7 (2.1, 7.1) | 581 | 4.8 (4.0, 6.0) | |||
| Smoking status: | never | 662 | 3.2 (1.9, 5.9) | 0.12 | 1,330 | 4.4 (3.7, 5.5) | 0.22 |
| former | 594 | 3.4 (2.1, 6.1) | 1,119 | 4.5 (3.7, 5.5) | |||
| current | 128 | 3.8 (1.8, 7.7) | 198 | 4.4 (3.5, 5.4) | |||
| Drinks/week: | 0 | 821 | 3.3 (2.0, 6.1) | 0.45 | 1,520 | 4.5 (3.7, 5.6) | 0.02 |
| ≤7 | 426 | 3.5 (2.0, 6.0) | 847 | 4.5 (3.7, 5.4) | |||
| >7 | 137 | 3.8 (2.3, 6.1) | 280 | 4.3 (3.5, 5.2) | |||
| Hypertension: | no | 572 | 3.4 (2.0, 5.7) | 0.80 | 1,014 | 4.4 (3.6, 5.4) | 0.11 |
| yes | 812 | 3.3 (2.0, 6.4) | 1,633 | 4.5 (3.7, 5.5) | |||
| Diabetes : | no | 1,191 | 3.3 (2.0, 5.9) | 0.01 | 2,294 | 4.4 (3.7, 5.4) | 0.01 |
| yes | 193 | 4.1 (2.1, 7.1) | 353 | 4.7 (3.8, 5.8) | |||
| Statins: | no | 1,258 | 3.4 (2.0, 6.1) | 0.87 | 2,442 | 4.5 (3.7, 5.5) | 0.67 |
| yes | 126 | 3.2 (2.0, 6.0) | 205 | 4.6 (3.7, 5.4) | |||
| C-reactive protein (mg/L): | <3 | 803 | 3.2 (2.0, 5.8) | 0.03 | 1,543 | 4.4 (3.7, 5.4) | 0.02 |
| ≥3 | 581 | 3.5 (2.0, 6.8) | 1,104 | 4.6 (3.7, 5.6) | |||
| eGFR (mL/min/1.73m2): | <60 | 319 | 3.7 (2.2, 6.4) | 0.02 | 643 | 5.0 (4.2, 6.2) | <0.001 |
| ≥60 | 1065 | 3.3 (1.9, 6.0) | 2004 | 4.3 (3.6, 5.3) | |||
| Urinary ACR (mg/g): | <30 | 1034 | 3.4 (2.0, 6.1) | 0.16 | 1913 | 4.4 (3.7, 5.4) | <0.001 |
| >=30 | 192 | 3.5 (2.1, 6.5) | 398 | 4.9 (3.9, 6.0) | |||
ACR= albumin/creatinine ratio, eGFR=estimated glomerular filtration rate, PIIINP=procollagen type III N-terminal propeptide, TGF-β=transforming growth factor-β
Kruskal-Wallis rank test
Incident PAD
There were 63 incident PAD events over a median follow-up time of 10.4 years (range 0.06–15.5) among the 1,384 individuals with measured TGF-β. Among the 2,646 individuals without PAD who had measured PIIINP there were 109 incident PAD events over a median follow-up of 10.5 years (range 0.06–15.6). Individuals with higher levels of TGF-β were more likely to develop incident PAD (hazard ratio=1.36 per doubling of TGF-β, 95% confidence interval=1.04, 1.78). The magnitude of the association between TGF-β and incident PAD was changed by approximately 10% and remained statistically significant after additional adjustment for sensitivity analysis variables in the limited subset of individuals for whom measurements were available (Table 2). We did not observe a significant association between PIIINP and risk of incident PAD.
Table 2.
Associations of fibrosis biomarkers with measures of subclinical and clinical vascular disease, Cardiovascular Health Study, 1996–2010
| log2(TGF-β) | log2(PIIINP) | |||||
|---|---|---|---|---|---|---|
| N | Estimate* | P | N | Estimate* | P | |
| PAD (HR) | ||||||
| Age, sex, race, clinic-adjusted | 1,384 | 1.45 (1.12, 1.88) | .005 | 2,646 | 1.29 (0.85, 1.96) | .23 |
| Multivariable Model 1 | 1,384 | 1.36 (1.04, 1.78) | .02 | 2,646 | 1.18 (0.78, 1.79) | .42 |
| Multivariable Model 2 | 892 | 1.49 (1.02, 2.17) | .04 | 1,682 | 0.85 (0.46, 1.59) | .61 |
| Carotid IMT (mm) | ||||||
| Age, sex, race, clinic-adjusted | 1,088 | 0.055 (0.006, 0.103) | .03 | 2,050 | 0.110 (0.036, 0.184) | .004 |
| Multivariable Model 1 | 1,088 | 0.043 (−0.004, 0.090) | .07 | 2,050 | 0.102 (0.029, 0.174) | .006 |
| Multivariable Model 2 | 740 | 0.031 (−0.023, 0.086) | .26 | 1,378 | 0.110 (0.017, 0.203) | .02 |
| Ankle-Arm Index | ||||||
| Age, sex, race, clinic-adjusted | 1,107 | −0.005 (−0.016, −0.006) | .37 | 1,981 | 0.017 (−0.000, 0.034) | .05 |
| Multivariable Model 1 | 1,107 | −0.003 (−0.013, 0.008) | .63 | 1,981 | 0.009 (−0.006, 0.026) | .25 |
| Multivariable Model 2 | 747 | −0.005 (−0.017, 0.008) | .45 | 1,332 | 0.020 (−0.0003, 0.041) | .05 |
| FMD (% change) | ||||||
| Age, sex, race, clinic-adjusted | 1,078 | −0.006 (−0.13, 0.12) | .92 | 2,120 | −0.20 (−0.39, −0.02) | .03 |
| Multivariable Model 1 | 1,078 | −0.013 (−0.14, 0.12) | .84 | 2,120 | −0.20 (−0.39, −0.02) | .03 |
| Multivariable Model 2 | 719 | −0.075 (−0.24, 0.09) | .36 | 1,402 | −0.16 (−0.41, 0.09) | .21 |
| Retinal arteriolar equivalent, trunk (µm) | ||||||
| Age, sex, race, clinic-adjusted | 657 | 1.27 (−0.07, 2.61) | .06 | 1,270 | −0.33 (−2.39, 1.72) | .75 |
| Multivariable Model 1 | 657 | 1.15 (−0.22, 2.52) | .10 | 1,270 | −0.38 (−2.48, 1.71) | .72 |
| Multivariable Model 2 | 438 | 0.80 (−1.05, 2.65) | .40 | 829 | −0.68 (−3.57, 2.21) | .64 |
| Retinal venular equivalent (µm) | ||||||
| Age, sex, race, clinic-adjusted | 657 | 2.26 (0.89, 3.64) | .001 | 1,270 | 0.07 (−1.99, 2.13) | .95 |
| Multivariable Model 1 | 657 | 1.63 (0.23, 3.02) | .02 | 1,270 | −0.56 (−2.65, 1.53) | .60 |
| Multivariable Model 2 | 438 | 2.08 (0.35, 3.81) | .02 | 829 | 0.54 (−2.24, 3.32) | .70 |
| Retinopathy (OR) | ||||||
| Age, sex, race, clinic-adjusted | 654 | 0.91 (0.65, 1.27) | .58 | 1,272 | 1.47 (0.90, 2.38) | .12 |
| Multivariable Model 1 | 654 | 0.84 (0.59, 1.20) | .35 | 1,272 | 1.18 (0.71, 1.94) | .53 |
| Multivariable Model 2 | 418 | 1.05 (0.63, 1.74) | .86 | 827 | 1.67 (0.76, 3.69) | .20 |
FMD=flow-mediated vasodilation, HR=hazard ratio, IMT=intima-media thickness, OR=odds ratio, PAD=peripheral artery disease, PIIINP=procollagen type III N-terminal propeptide, TGF-β=transforming growth factor-β
Entries in table are regression coefficients per unit change in exposure (per doubling of TGF-β or PIIINP), except for the outcomes of retinopathy (odds ratio) and incident PAD (hazard ratio).
Multivariable model 1 is adjusted for age, sex, race, clinic, body mass index, smoking status, pack years, alcohol consumption, physical activity, systolic blood pressure, total cholesterol, albumin, C-reactive protein, fasting glucose, use of medications for hypertension, diabetes, or hypercholesterolemia.
Multivariable model 2 replaces fasting glucose with 2-hr glucose, and additionally adjusts for albumin/creatinine ratio, N-terminal type B pro-brain natriuretic peptide, and estimated glomerular filtration rate. No individuals with diabetes were included in the model, so diabetes medication use was dropped.
Macrovascular parameters
TGF-β was not associated with carotid IMT, ABI, or brachial artery reactivity. However, TGF-β was positively associated with larger retinal venular equivalent, which we considered a measure of macrovascular disease because of its previous correlation with several forms of large vessel disease including carotid IMT [35, 36], incident stroke, and coronary heart disease [22, 37].
Higher PIIINP was associated with greater carotid IMT and impaired brachial artery reactivity (Table 2). We detected several modestly significant interactions between CRP and PIIINP and subsequently conducted stratified analyses accordingly (Table 3). For the relationship between PIIINP and carotid IMT, the magnitude of the association and the degree of statistical significance was greater among individuals with higher CRP. Interactions with CRP were robust to excluding individuals with CRP > 50mg/L or using untransformed (linear) CRP as the interacting variable.
Table 3.
Stratified analyses of the association between log2(PIIINP) and vascular measurements by level of C-reactive protein (mg/L), Cardiovascular Health Study, 1996–1997
| log2(PIIINP) | ||||
|---|---|---|---|---|
| N | Estimate | P | P-int* | |
| Carotid intima-media thickness (mm) | 0.02 | |||
| CRP≤1 | 500 | 0.034 (−0.111, 0.179) | .65 | |
| 1<CRP≤3 | 723 | 0.045 (−0.077, 0.167) | .47 | |
| 3<CRP≤10 | 658 | 0.117 (−0.008, 0.242) | .07 | |
| CRP>10 | 169 | 0.369 (0.160, 0.579) | .001 | |
| Retinal arteriolar equivalent, trunk (µm) | 0.04 | |||
| CRP≤1 | 312 | 1.39 (−2.84, 5.62) | .52 | |
| 1<CRP≤3 | 445 | 2.71 (−0.78, 6.20) | .13 | |
| 3<CRP≤10 | 405 | −4.54 (−8.28, −0.80) | .02 | |
| CRP>10 | 108 | −2.30 (−8.04, 3.44) | .43 | |
| Retinal venous equivalent, (µm) | 0.03 | |||
| CRP≤1 | 312 | 1.78 (−2.44, 6.00) | .41 | |
| 1<CRP≤3 | 445 | 1.81 (−1.68, 5.30) | .31 | |
| 3<CRP≤10 | 405 | −3.46 (−7.20, 0.28) | .07 | |
| CRP>10 | 108 | −4.40 (−10.1, 1.34) | .13 | |
Eq=equivalent, CRP=C-reactive protein, PIIINP= procollagen type III N-terminal propeptide
P-interaction for PIIINP and CRP
Entries in table are regression coefficients per unit change in log2(PIIINP)
Adjusted for age, sex, race, clinic, body mass index, smoking status, pack years, alcohol consumption, physical activity, systolic blood pressure, total cholesterol, albumin, C-reactive protein, fasting glucose, use of medications for hypertension, diabetes, or hypercholesterolemia.
Microvascular parameters
We considered smaller retinal arteriolar diameter and the presence of retinopathy as indicators of microvascular disease. There were 43 cases of retinopathy among individuals with measured TGF-β, and 82 cases among individuals with measured PIIINP. Neither TGF-β nor PIIINP were associated with retinal arteriolar equivalent or retinopathy in the full cohort (Table 2), but we observed an association between PIIINP and smaller retinal arteriolar equivalent among individuals with elevated CRP (Table 3).
Discussion
In this study of older adults, TGF-β was associated with a higher risk of incident PAD over a median of 10.4 years of follow-up, and both TGF-β and PIIINP were associated with multiple measures of macrovascular structure and function, including retinal venular diameter, a novel marker of large vessel disease. In contrast, TGF-β and PIIINP were not associated with measures of microvascular disease, specifically retinal arteriolar diameter and retinopathy. Our study provides preliminary evidence that serum concentrations of biomarkers of fibrosis, measured late in life, are directly associated with atherosclerosis and impaired vascular reactivity in large, but not small, vessels.
At this time, no definitive pathophysiological data is available to explain the differential association of these fibrosis markers with large vs. small vessel disease; however, previous studies have demonstrated important differences in the biological etiologies and risk factors of large and small vessel disease [15]. For example, while atherosclerosis is thought to be the primary driver of large vessel disease [16], small vessel disease is more strongly related to risk factors such as prolonged hyperglycemia, dysregulation of vascular tone, and oxidative stress [16, 17]. In the case of diabetes, for example, tight glycemic control reduces microvascular complications, but its effect on macrovascular disease is less clear. These differences corroborate our finding that associations between fibrosis-related biomarkers and vascular parameters may differ in different vascular beds.
TGF-β
Although several studies have been published on TGF-β and cross-sectional measures of subclinical vascular disease, no studies have examined prospective associations with incident PAD outcomes. In our study, we observed an approximately 40% higher risk of incident PAD per doubling of TGF-β. There are a number of potential biological mechanisms whereby TGF-β could increase the risk of PAD. For example, TGF-β is known to stimulate vascular smooth muscle proliferation and extracellular matrix production, which may contribute to atherosclerotic plaque formation in the peripheral arteries [39]. Although we observed higher levels of TGF-β among individuals with black race, obesity, diabetes, inflammation, and poor renal function in bivariate analyses, our results were robust to multivariable adjustment for these variables. Simultaneously, TGF-β was not associated with subclinical vascular measurements such as carotid IMT, ABI, or brachial artery reactivity. Carotid IMT, ABI, and other measures of vascular structure and function are ultimately imperfect measures of the spectrum of clinical PAD. The association between TGF-β and clinical PAD may instead reflect biological mechanisms beyond those captured by these subclinical phenotypes, such as arterial stiffness or angiogenic potential. Our results should stimulate further studies that clarify this discrepancy. Among the multiple forms of retinal vascular disease that we investigated, TGF-β was positively associated with wider retinal venular diameter, but not narrower retinal arteriolar diameter. Wider retinal venular diameter is now recognized as a marker of large vessel disease, [22, 35–37], while retinal arteriolar narrowing and retinopathy signs are considered markers of microvascular disease [24, 31–33]. Few studies have investigated the associations between fibrosis and retinal vascular disease, but extracellular matrix expansion and basement membrane thickening are quintessential histological features of diabetic retinopathy [40]. In diabetic rats, inhibition of protein kinase C (an upstream regulator of TGF-β transcription) improves retinal blood flow, a marker for retinal vascular integrity. Protein kinase C inhibition also improves visual acuity among individuals with diabetes [41], in whom levels of TGF-β are often elevated [42]. Our findings build on this previous research and indicate a need for further investigation of the relationship between TGF-β and retinal vascular disease as well as ongoing work on small-molecule inhibitors of TGF-β and other fibrogenic agents [43–47].
PIIINP
Although PIIINP was not associated with the risk of incident PAD in our study, higher PIIINP was associated with several other measures of macrovascular disease, including carotid IMT and FMD. Of note, the association between PIIINP and carotid IMT was larger in magnitude among individuals with elevated CRP, similar to our previous findings on PIIINP and mortality risk in this cohort [48]. Because CRP stimulates multiple genes involved in collagen deposition [49], pro-fibrotic pathways may have synergistic and more pronounced effects on the vasculature in the context of inflammation. Overall, the observed associations between PIIINP, carotid IMT, and FMD suggest that excess collagen type III deposition may affect both vascular structure and function in the larger vessels.
Although we were unable to find an association between PIIINP and retinal venular equivalent in our study, this was not unexpected as collagen type III is not highly expressed in the retinal vessels compared to expression in other vessels [50]. Future studies with markers of other collagen subtypes in addition to collagen type III may shed further light on this issue.
Strengths and Limitations
Strengths of our study include a large, well-characterized, community-based population and the availability of a diverse range of adjustment variables to minimize confounding in multivariable-adjusted models. We examined several different measurements to assess vascular disease, including clinical and subclinical outcomes, from both large and small vessels. Finally, we had access to two biomarkers of fibrosis, which together provide complementary snapshots of the process of collagen biosynthesis and accumulation.
Several limitations also merit consideration. First, we measured TGF-β on a smaller sample of participants, and thus had more limited statistical power to detect small associations of TGF-β with vascular outcomes. We also had limited statistical power to detect associations with retinopathy due to the relatively small number of cases. We have presented results for a number of outcomes in this analysis without correction for multiple comparisons given their high degree of interdependence. Additionally, serial measurements of TGF-β and PIIINP could not be performed and therefore the relationships of the longitudinal trajectory of change in these measures over time with vascular outcomes remains uncertain. We are also unable to infer temporality from our cross-sectional studies. Finally, associations in this study may be biased towards the null because of measurement error resulting from the long hiatus between sample collection in 1996–1997 and biomarker measurement in 2011–2012, although we have previously been able to detect associations of these biomarkers with other outcomes, including mortality.
The exact mechanisms underlying the differential associations with large vs. small vessel disease remain uncertain, though the observed associations with large vessel disease are likely mediated by atherogenesis. In previous studies, other circulating factors such as matrix metalloproteinases and cathepsins have been implicated in the process of atherosclerosis [12]. These factors regulate extracellular matrix turnover and have a number of substrates, including type III collagen, from which PIIINP is liberated. Our study provides further support for the link between extracellular matrix turnover and atherosclerosis.
Given the relatively modest observed hazard ratios for TGF-β and PIIINP and the fact that assays for these biomarkers are not routinely available or easy to measure in clinical practice, neither biomarker is likely to be immediately useful for prognosis of vascular disease in clinical care. Circulating TGF-β and PIIINP are imperfect measures of underlying tissue fibrosis. Other methods of detecting fibrosis, such as tissue biopsy and histological analysis or gadolinium-based imaging, can detect tissue-specific fibrosis with higher sensitivity and specificity. These methods have not been widely used in longitudinal human studies, however, due to cost and technical difficulty. In comparison to these methods, plasma biomarkers such as TGF-β and PIIINP provide readily-available and non-invasive estimates of vascular fibrosis. Because TGF-β and PIIINP only imperfectly reflect underlying fibrosis, the potential benefits of targeting fibrosis itself may be larger than the observed associations for these biomarkers might suggest. Given that several anti-fibrotic agents are already in development or testing [43–47], clinical trials to specifically target fibrosis and determine its effects on clinical and subclinical vascular disease may be feasible in the near future.
Conclusion
In conclusion, TGF-β is associated with a higher risk of incident PAD and both TGF-β and PIIINP are positively associated with several measures of large vessel disease among community-living older adults. Our findings strongly support ongoing and future research efforts to understand and intervene on fibrosis and evaluate its potential impacts on atherosclerosis and vascular disease.
Supplementary Material
Highlights.
We measured serum concentrations of two complementary fibrosis-related biomarkers.
These fibrosis-related biomarkers were associated with incident peripheral artery disease and several cross-sectional measures of large vessel disease.
Our results suggest that fibrosis may be related to the development of large vessel atherosclerosis in older adults.
Acknowledgements
None.
Funding Sources
This research was supported by contracts HL118775, HL094555, N01HC85084, N01HC35129, N01HC85085, N01HC45133, HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Abbreviations
- ABI
ankle-brachial index
- CHS
Cardiovascular Health Study
- FMD
flow-mediated vasodilation
- IMT
intima-media thickness
- PAD
peripheral artery disease
- PIIINP
procollagen type III N-terminal propeptide
- TGF-β
transforming growth factor-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biernacka A, Frangogiannis NG. Aging and Cardiac Fibrosis. Aging Dis. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- 3.Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 4.Grainger DJ. Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol. 2004;24:399–404. doi: 10.1161/01.ATV.0000114567.76772.33. [DOI] [PubMed] [Google Scholar]
- 5.Borkowski P, Robinson MJ, Kusiak JW, Borkowski A, Brathwaite C, Mergner WJ. Studies on TGF-beta 1 gene expression in the intima of the human aorta in regions with high and low probability of developing atherosclerotic lesions. Mod Pathol. 1995;8:478–482. [PubMed] [Google Scholar]
- 6.Grainger DJ, Kemp PR, Metcalfe JC, Liu AC, Lawn RM, Williams NR, et al. The serum concentration of active transforming growth factor-beta is severely depressed in advanced atherosclerosis. Nat Med. 1995;1:74–79. doi: 10.1038/nm0195-74. [DOI] [PubMed] [Google Scholar]
- 7.Negishi M, Lu D, Zhang YQ, Sawada Y, Sasaki T, Kayo T, et al. Upregulatory expression of furin and transforming growth factor-beta by fluid shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:785–790. doi: 10.1161/01.atv.21.5.785. [DOI] [PubMed] [Google Scholar]
- 8.Leonarduzzi G, Sevanian A, Sottero B, Arkan MC, Biasi F, Chiarpotto E, et al. Up-regulation of the fibrogenic cytokine TGF-beta1 by oxysterols: a mechanistic link between cholesterol and atherosclerosis. FASEB J. 2001;15:1619–1621. doi: 10.1096/fj.00-0668fje. [DOI] [PubMed] [Google Scholar]
- 9.Siegert A, Ritz E, Orth S, Wagner J. Differential regulation of transforming growth factor receptors by angiotensin II and transforming growth factor-beta1 in vascular smooth muscle. J Mol Med (Berl) 1999;77:437–445. doi: 10.1007/s001090050374. [DOI] [PubMed] [Google Scholar]
- 10.Panutsopulos D, Papalambros E, Sigala F, Zafiropoulos A, Arvanitis DL, Spandidos DA. Protein and mRNA expression levels of VEGF-A and TGF-beta1 in different types of human coronary atherosclerotic lesions. Int J Mol Med. 2005;15:603–610. [PubMed] [Google Scholar]
- 11.Migdalis IN, Kalogeropoulou K, Zachariadis D, Koutoulidis K, Samartzis M. Serum levels of type III procollagen peptide and peripheral vascular disease in diabetic patients. Diabetes Res Clin Pract. 1994;23:179–182. doi: 10.1016/0168-8227(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 12.Romero JR, Vasan RS, Beiser AS, Polak JF, Benjamin EJ, Wolf PA, et al. Association of carotid artery atherosclerosis with circulating biomarkers of extracellular matrix remodeling: the Framingham Offspring Study. J Stroke Cerebrovasc Dis. 2008;17:412–417. doi: 10.1016/j.jstrokecerebrovasdis.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrosius W, Kazmierski R, Michalak S, Kozubski W. Anti-inflammatory cytokines in subclinical carotid atherosclerosis. Neurology. 2006;66:1946–1948. doi: 10.1212/01.wnl.0000219808.28678.48. [DOI] [PubMed] [Google Scholar]
- 14.Deng HB, Jiang CQ, Tomlinson B, Liu B, Lin JM, Wong KS, et al. A polymorphism in transforming growth factor-beta1 is associated with carotid plaques and increased carotid intima-media thickness in older Chinese men: the Guangzhou Biobank Cohort Study-Cardiovascular Disease Subcohort. Atherosclerosis. 2011;214:391–396. doi: 10.1016/j.atherosclerosis.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Criqui MH, Browner D, Fronek A, Klauber MR, Coughlin SS, Barrett-Connor E, et al. Peripheral arterial disease in large vessels is epidemiologically distinct from small vessel disease. An analysis of risk factors. Am J Epidemiol. 1989;129:1110–1119. doi: 10.1093/oxfordjournals.aje.a115233. [DOI] [PubMed] [Google Scholar]
- 16.Fowler M. Microvascular and Macrovascular Complications of Diabetes. Clinical Diabetes. 2008;26:77–82. [Google Scholar]
- 17.Dokken B. The Pathophysiology of Cardiovascular Disease and Diabetes: Beyond Blood Pressure and Lipids. Diabetes Spectrum. 2008;21:160–165. [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 19.Fredericks S, Holt DW. TGF-beta quantitation can be tricky. Transplantation. 1999;68:468–469. doi: 10.1097/00007890-199908270-00002. [DOI] [PubMed] [Google Scholar]
- 20.Herrington DM, Fan L, Drum M, Riley WA, Pusser BE, Crouse JR, et al. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk. 2001;8:319–328. doi: 10.1177/174182670100800512. [DOI] [PubMed] [Google Scholar]
- 21.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 22.Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166:2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 24.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288:67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 25.O'Leary DH, Polak JF, Wolfson SK, Jr, Bond MG, Bommer W, Sheth S, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 26.O'Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, et al. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke. 1996;27:224–231. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 27.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 29.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48:1197–1203. doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Lindley RI, Wang JJ, Wong MC, Mitchell P, Liew G, Hand P, et al. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet Neurol. 2009;8:628–634. doi: 10.1016/S1474-4422(09)70131-0. [DOI] [PubMed] [Google Scholar]
- 31.Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, et al. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke. 2006;37:82–86. doi: 10.1161/01.STR.0000195134.04355.e5. [DOI] [PubMed] [Google Scholar]
- 32.Longstreth W, Jr, Larsen EK, Klein R, Wong TY, Sharrett AR, Lefkowitz D, et al. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: the Cardiovascular Health Study. Am J Epidemiol. 2007;165:78–84. doi: 10.1093/aje/kwj350. [DOI] [PubMed] [Google Scholar]
- 33.Wong TY, Mosley TH, Jr, Klein R, Klein BE, Sharrett AR, Couper DJ, et al. Retinal microvascular changes and MRI signs of cerebral atrophy in healthy, middle-aged people. Neurology. 2003;61:806–811. doi: 10.1212/01.wnl.0000086372.05488.8d. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Wong TY, Sharrett AR, Klein R, Folsom AR, Jerosch-Herold M. Relationship between retinal arteriolar narrowing and myocardial perfusion: multi-ethnic study of atherosclerosis. Hypertension. 2008;51:119–126. doi: 10.1161/HYPERTENSIONAHA.107.098343. [DOI] [PubMed] [Google Scholar]
- 35.Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 36.De Silva DA, Liew G, Wong MC, Chang HM, Chen C, Wang JJ, et al. Retinal vascular caliber and extracranial carotid disease in patients with acute ischemic stroke: the Multi-Centre Retinal Stroke (MCRS) study. Stroke. 2009;40:3695–3699. doi: 10.1161/STROKEAHA.109.559435. [DOI] [PubMed] [Google Scholar]
- 37.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009;170:1323–1332. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45:683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, et al. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem. 1996;44:1469–1479. doi: 10.1177/44.12.8985139. [DOI] [PubMed] [Google Scholar]
- 41.Aiello LP, Clermont A, Arora V, Davis MD, Sheetz MJ, Bursell SE. Inhibition of PKC beta by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients. Invest Ophthalmol Vis Sci. 2006;47:86–92. doi: 10.1167/iovs.05-0757. [DOI] [PubMed] [Google Scholar]
- 42.Pfeiffer A, Middelberg-Bisping K, Drewes C, Schatz H. Elevated plasma levels of transforming growth factor-beta 1 in NIDDM. Diabetes Care. 1996;19:1113–1117. doi: 10.2337/diacare.19.10.1113. [DOI] [PubMed] [Google Scholar]
- 43.Fukasawa H, Yamamoto T, Suzuki H, Togawa A, Ohashi N, Fujigaki Y, et al. Treatment with anti-TGF-beta antibody ameliorates chronic progressive nephritis by inhibiting Smad/TGF-beta signaling. Kidney Int. 2004;65:63–74. doi: 10.1111/j.1523-1755.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 44.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD., Jr TGF-beta-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L151–L161. doi: 10.1152/ajplung.00389.2006. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247–255. doi: 10.1053/jhep.2000.9109. [DOI] [PubMed] [Google Scholar]
- 46.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, et al. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol. 2011;22:1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richeldi L, du Bois RM. Pirfenidone in idiopathic pulmonary fibrosis: the CAPACITY program. Expert Rev Respir Med. 2011;5:473–481. doi: 10.1586/ers.11.52. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal I, Glazer NL, Barasch E, Biggs ML, Djousse L, Fitzpatrick AL, et al. Fibrosis-related Biomarkers and Risk of Total and Cause-Specific Mortality: The Cardiovascular Health Study. American Journal of Epidemiology. 2014 doi: 10.1093/aje/kwu067. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang R, Zhang YY, Huang XR, Wu Y, Chung AC, Wu EX, et al. C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin II-induced hypertensive cardiac disease. Hypertension. 2010;55:953–960. doi: 10.1161/HYPERTENSIONAHA.109.140608. [DOI] [PubMed] [Google Scholar]
- 50.Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008;4:575–596. doi: 10.2147/vhrm.s1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.