Abstract
Objectives
Pancreatitis is considered a possible risk factor for and a presentation of pancreatic adenocarcinoma (PA). We aimed to evaluate a large PA patient registry to determine whether prior history of pancreatitis influenced survival.
Methods
We retrospectively analyzed the Mayo Clinic Biospecimen Resource for Pancreas Research database from January 1992 to September 2011. Data collected included demographic characteristics, history of tobacco or alcohol use, diabetes mellitus (DM), cholelithiasis, pseudocyst, and details regarding PA. Clinical characteristics and outcomes of PA patients with pancreatitis were compared to PA patients without pancreatitis history.
Results
We analyzed 2,573 patients with PA diagnosis. Among these patients, 195 (8%) were identified who had pancreatitis diagnosis ≥10 days before PA diagnosis. The cohort with pancreatitis history included more patients with DM (30% vs. 18%; P<.001) and more smokers (68% vs. 58%; P=.02). Compared with patients without pancreatitis history, these patients received diagnoses of PA at a younger age (63 vs. 65 years; P=.005) and earlier stage (stage I and II; 52% vs. 37%; P<.001). A greater percentage had history of surgery with curative intent (50% vs. 43%; P=.001) and significantly better survival (median [range], 387 [314–460] days vs. 325 [306–344] days; P=.003).
Conclusions
Patients with PA and pancreatitis had more weight loss and DM, but had PA diagnosis at an earlier stage, were more likely to have pancreatic surgery, and therefore better survival than PA patients without pancreatitis, likely due to the earlier diagnosis. Further studies are needed to evaluate whether screening for PA in patients with pancreatitis history would provide survival benefit.
Keywords: pancreatic adenocarcinoma, pancreatitis, survival analysis
Introduction
More than 43,000 people receive a diagnosis of pancreatic adenocarcinoma (PA) each year in the United States and almost 37,000 die of the disease (1). Even though extensive research is directed at early detection of PA, the majority of cases are still diagnosed at an advanced stage. Consequently, the 5-year survival for PA continues to be dismal (1).
An association between PA and both acute (2–8) and chronic (9–11) pancreatitis has been reported. Studies have found that idiopathic pancreatitis can be an early manifestation of PA or that a history of pancreatitis confers an important risk of subsequent PA development. However, these studies have not evaluated whether PA patients with a history of pancreatitis have different outcomes from those without a history of pancreatitis. We hypothesized that a prior history of pancreatitis is associated with better PA survival, possibly because of earlier medical evaluation and diagnosis of PA due to pancreatitis.
The primary aim of our study was to determine whether prior history of pancreatitis influences survival in patients with PA. This evaluation involved a large, prospectively maintained research registry of PA patients and control subjects. Secondary aims of our study were to evaluate associations between pancreatitis and both PA stage at presentation and PA resectability.
Methods
Cases of PA were identified through review of the prospective Mayo Clinic Biospecimen Resource for Pancreas Research, which is supported by the National Cancer Institute’s Specialized Program of Research Excellence (SPORE; P50 CA102701) and approved by the Mayo Clinic Institutional Review Board. All subjects enrolled in the SPORE gave written consent and data were prospectively recorded. Subjects for this retrospective analysis were selected from the dates January 1992 through September 2011. Patients were identified through hospital admissions and in pancreas and oncology clinics. They were consented into the registry, allowed access to their medical records, and completed a risk factor questionnaire. The data used for final diagnosis (World Health Organization Histological Classification of Tumors of the Exocrine Pancreas; International Classification of Diseases [ICD] for Oncology; 8500/3) of PA included pathologic findings, medical records, death certificate, or self-report. Data were de-identified by SPORE staff, and each patient was assigned a unique identifier number.
All research registry patients with a history of pancreatitis were included in the present study; subjects were identified using final ICD-9 diagnostic codes, as well as the self-reported questionnaire (ie, question “diagnosed with pancreatitis?” [0=No, 1=Yes, 9=Do not know]).
Data retrieved from the research registry database included the following: demographic characteristics (eg, age at diagnosis of pancreatitis and PA, sex, race, vital status); presence of pancreatitis; verification method of PA diagnosis; PA stage at diagnosis; days of follow-up after PA diagnosis (until death or last known alive date); weight loss; history of diabetes mellitus (DM), cholelithiasis, alcoholism, pseudocysts, and tobacco use (smokers were defined as patients who smoked >100 cigarettes in their life time); adjuvant chemotherapy; adjuvant irradiation; history of surgical procedure with curative intent; and carbohydrate antigen (CA) 19–9 level at diagnosis.
Patients with pancreatitis history were analyzed for temporal association with PA. Pancreatitis patients with a diagnosis ≥10 days before diagnosis of PA were included in the primary analysis. The reference group contained PA patients with no known history of pancreatitis. For temporal association analysis, the interval of ≥10 days prior was chosen to exclude patients who presented with pancreatitis and PA simultaneously; this subgroup was evaluated separately. Additional secondary analyses were conducted of patients with history of pancreatitis diagnosed at any time point.
Because the research registry dataset consisted of de-identified information released without conditions to investigators, the Mayo Clinic Institutional Review Board did not require additional approval for our retrospective analysis and our study was granted an exemption.
Statistical Analyses
Data were entered manually and assessed statistically with SAS System for Windows Version 9.2 (SAS Institute, Inc) or IBM SPSS, Release Version 20.0 (SPSS Inc). Continuous data were presented as mean (SD) or as median (range) or odds ratio (OR) (95% CI). Categorical data were summarized as frequency and percentage. Normality of data sets was evaluated using SPSS Explore and Descriptive functions. Stem-and-leaf plots and histograms were used to evaluate the distributions and assess outliers. Where appropriate, the Kolmogorov-Smirnov test was used for determining normality. Group differences in continuous variables were assessed using the t test or analysis of variance for normally distributed data sets or the Mann-Whitney test or Kruskal-Wallis test for data sets not meeting the assumptions for normality. The χ2 test was used to assess differences in distributions of categorical variables. In cases that failed to meet the assumptions for χ2 test, the Fisher exact test was used. Survival function was evaluated with Kaplan-Meier estimator and log-rank test to assess statistical differences in survival between groups of patients with and without history of pancreatitis. Multivariable Cox proportional hazards models were performed to calculate hazard ratios and assess independent prognostic factors that might be associated with length of survival. Statistical significance was defined as a P value <.05.
Results
Of the 2,573 PA patients included in the research registry, pathological diagnosis was available in 2,281 (89%); PA diagnosis was established through medical record review in 209 (8%), through self-report in 51 (2%), and through death certificate in 32 (1%). The demographic and clinical characteristics of patients are shown in Table 1. The majority of study patients were male and white. The median (range) duration of follow-up after PA diagnosis was 327 days (0–4,390 days) for the pancreatitis group and 279 days (0–5,487 days) for the reference group (P=.04). Patients with a history of pancreatitis received a diagnosis of PA and DM at a younger age, were more likely to have DM and pseudocysts, had more weight loss, and included more smokers. Distributions of sex, race, gallstones, and alcohol abuse were not statistically significant between the 2 groups.
Table 1.
Demographic Characteristics of PA Patients With and Without Prior Pancreatitis
| Patientsa,b
|
|||
|---|---|---|---|
| Demographic Characteristic | PA Only (n=2,156) | PA and Prior History of Pancreatitis (n=195) | P Value |
| Age at PA diagnosis, mean (SD), y | 65 (10) | 63 (12) | .005 |
| Male sex | 1,207 (56) | 109 (56) | >.99 |
| Race | .81 | ||
| American Indian/Alaskan | 7 (0) | 1 (1) | |
| Native | 12 (1) | 0 (0) | |
| Asian | 48 (2) | 4 (2) | |
| Black | 2,053 (96) | 183 (95) | |
| White | 10 (0) | 4 (1) | |
| Other | |||
| Weight loss, mean (SD), lb | 25 (17) | 30 (20) | .001 |
| Gallstones | 228 (21) | 36 (26) | .19 |
| Pseudocyst | 15 (2) | 20 (17) | <.001 |
| DM | 396 (18) | 59 (30) | <.001 |
| Age at DM diagnosis, mean (SD), y | 62 (11) | 58 (12) | .02 |
| Smoking | 818 (58) | 116 (68) | .01 |
| Alcohol use | 194 (55) | 18 (56) | >.99 |
Abbreviations: DM, diabetes mellitus; PA, pancreatic adenocarcinoma.
Values are expressed as No. (%) unless specified otherwise.
Percentages in parentheses represent a valid percent unless specified otherwise.
A history of pancreatitis was identified in 16.1% (413/2,573) of patients, and 47.2% (195/413) of these received a diagnosis of pancreatitis ≥10 days before diagnosis of PA. Pancreatitis was diagnosed via self report in 38% (75/195) and via ICD-9 diagnostic codes in 62% (120/195) of patients. Diagnosis of prior pancreatitis was made at a median of 109 days (range, 10–15,352 days) before diagnosis of PA, with most patients (94%) receiving a diagnosis within 365 days (41% were diagnosed within 1st 30 days: 38% were diagnosed between 30 – 180 days).
Data regarding clinical characteristics of PA patients with and without history of pancreatitis are shown in Table 3. Patients with a pancreatitis history had a diagnosis of PA at an earlier stage than patients without a pancreatitis history; they had lower levels of CA 19–9 at diagnosis, and a greater proportion of patients underwent surgery with curative intent. Most commonly, PA was located in the head of the pancreas in both groups.
Table 3.
Multivariable Survival Analysis for Potential Risk Factors Associated With Long-Term Survival in Patients With PA
| Independent Prognostic Factor | Odds Ratio (95% CI) | P Value |
|---|---|---|
| History of pancreatitis | 0.94 (0.79–1.1) | .53 |
| Age | 1.2 (1.2–1.3) | <.001 |
| Diabetes mellitus | 0.92 (0.8–1.0) | .16 |
| CA 19–9 level | 1.00 (1.0–1.0) | <.001 |
| PA stage I–II | 2.1 (1.7–2.8) | <.001 |
| PA stage I–III | 4.6 (3.5–6.1) | <.001 |
| PA stage I–IV | 5.6 (4.3–7.3) | <.001 |
Abbreviations: CA, carbohydrate antigen; PA, pancreatic adenocarcinoma.
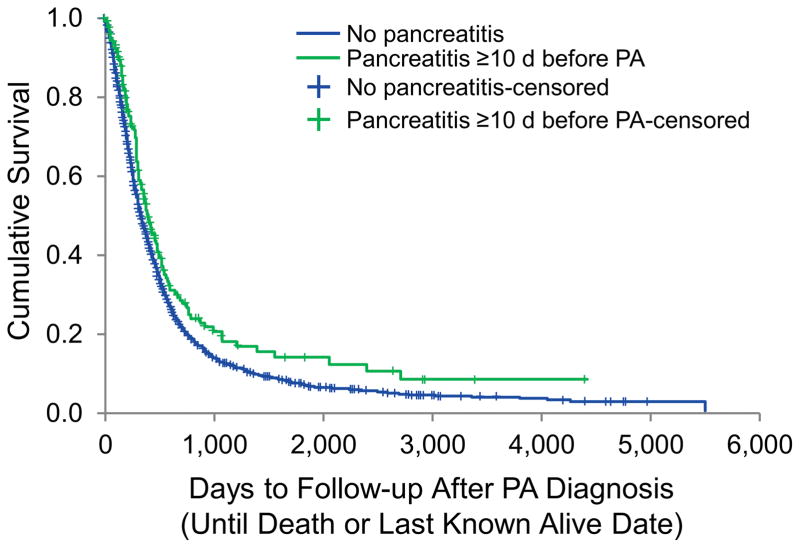
Median survival for the overall study population was 327 days (95% CI, 309–345 days). Patients with a history of pancreatitis had significantly better survival than PA patients without a history of pancreatitis (median [95% CI], 387 days [314–460] days vs. 325 days [306–344 days]; P=.003) (Figure 1).
Figure 1.
Kaplan-Meier Analysis of the Overall Survival of PA Patients With and Without Prior History of Pancreatitis. Data shown are overall survival of individual groups of patients (n=2,160 for patients with PA only; n=195 for patients with PA and prior history of pancreatitis). The median survival period in patients with PA and prior history of pancreatitis was significantly longer than in patients with PA only (median [range], 387 [313–460] days vs. 325 [306–344] days; P=.003, log-rank test). PA indicates pancreatic adenocarcinoma.
History of pancreatitis was significantly associated with reduced mortality on univariable analysis (OR [95% CI], 0.78 [0.65–0.93]; P=.006). In multivariable analysis, history of pancreatitis (OR [95% CI], 0.83 [0.70–0.99]; P=.046) and DM (OR [95% CI], 0.85 [0.76–0.95]; P=.004) were positively associated with survival, while increasing age in decades (OR [95% CI], 1.14 [1.1–1.2]; P<.001) correlated with increased mortality. Smoking was not an independent predictor of survival (1.03 [0.7–1.4]; P = 0.87). Subgroup analysis examining the interval between pancreatitis and PA diagnosis (in 6 month intervals) and survival did not show a significant association, but was limited by a small number of patients with pancreatitis >6 months from PA (data not shown). Multivariable survival analysis with the addition of PA stage and CA 19–9 level indicated that PA stages I and II were independent predictors of favorable prognosis and older age was associated with poor prognosis. After adjustment for PA stage, history of pancreatitis and history of DM were not independent predictors of survival (Table 4).
Table 4.
Demographic Characteristics of PA Patients With and Without Pancreatitis Diagnosed at Any Time Point
| Patientsa,b
|
|||
|---|---|---|---|
| Characteristic | PA Only (n=2,156) | PA and Prior History of Pancreatitis (n=413) | P Value |
| Age at PA diagnosis, mean (SD), y | 65 (10) | 64 (12) | .02 |
| Male sex | 1,207 (56) | 231 (56) | .99 |
| Race | .30 | ||
| American Indian/Alaskan | 7 (0) | 2 (1) | |
| Native | 12 (1) | 1 (0) | |
| Asian | 48 (2) | 6 (2) | |
| Black | 2,053 (96) | 395 (97) | |
| White | 10 (0) | 5 (1) | |
| Other | |||
| Weight loss, mean (SD), lb | 25 (17) | 29 (19) | <.001 |
| Gallstones | 228 (21) | 82 (27) | .02 |
| Pseudocyst | 15 (2) | 37 (15) | <.001 |
| DM | 396 (18) | 123 (30) | <.001 |
| Age at DM diagnosis, mean (range), y | 62 (11) | 60 (29–84) | .02 |
| Smoking | 818 (58) | 247 (67) | .002 |
| Alcohol use | 194 (55) | 18 (56) | .92 |
Abbreviations: DM, diabetes mellitus; PA, pancreatic adenocarcinoma.
Values are expressed as No. (%) unless specified otherwise.
Percentages in parentheses represent a valid percent unless specified otherwise.
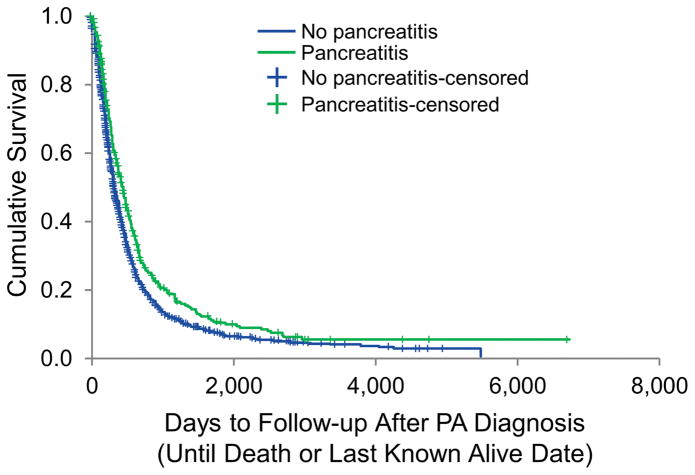
Subgroup analyses including patients with any history of pancreatitis (diagnosed before, during, or after the time of PA diagnosis) are presented in Tables 5 and 6. All patients who had history of any diagnosis of pancreatitis (n=413) were included in the subgroup analyses. Similar to the study cohort (ie, patients with prior history of pancreatitis), patients with any history of pancreatitis had improved survival compared to patients with PA only (median [range] survival, 446 days [378–485 days] vs. 325 days [307–343 days]; P<.001). The Kaplan-Meier curve is shown in Figure 2.
Table 5.
Clinical Characteristics of PA Patients With and Without Pancreatits Diagnosed at Any Time Point
| Patientsa,b
|
|||
|---|---|---|---|
| Clinical Characteristic | PA Only (n=2,156) | PA and Prior History of Pancreatitis (n=413) | P Value |
| CA 19-9, median (range), U/mL | 327 (0–3,715,150) | 161 (0–235,000) | <.001 |
| Chemotherapy | 1,135 (81) | 265 (81) | .85 |
| Irradiation | 654 (47) | 166 (51) | .27 |
| Vital status, alive | 433 (20) | 92 (22) | .31 |
| Site of PA mass | .06 | ||
| Head | 1,147 (55) | 243 (61) | |
| Body | 312 (15) | 40 (10) | |
| Tail | 223 (11) | 30 (8) | |
| Head and body | 143 (7) | 30 (8) | |
| Body and tail | 139 (7) | 28 (7) | |
| Uncinate | 72 (3) | 19 (4) | |
| Not specified | 39 (2) | 8 (2) | |
| PA stage | <.001 | ||
| I | 90 (4) | 36 (9) | |
| II | 665 (33) | 174 (45) | |
| III | 436 (21) | 65 (17) | |
| IV | 860 (42) | 113 (29) | |
| Surgical resection | 802 (43) | 228 (59) | <.001 |
Abbreviations: CA, carbohydrate antigen; PA, pancreatic adenocarcinoma.
Values are expressed as No. (%) unless specified otherwise.
Percentages in parentheses represent a valid percent unless specified otherwise.
Figure 2.
Kaplan-Meier Analysis of the Overall Survival of PA Patients With and Without Any History of Pancreatitis. Data shown are the overall survival of individual groups of patients (n=2,160 for patients with PA only; n=413 for patients with PA and pancreatitis). The median (range) survival period in PA patients was significantly shorter than in patients with PA and pancreatitis diagnosed at any time point (median [range], 446 [378–485] days vs. 325 [306–344] days; P<.001, log-rank test). PA indicates pancreatic adenocarcinoma.
We further analyzed a subgroup of 100 patients who received a diagnosis of pancreatitis and PA concomitantly, defined as occurrence of pancreatitis within 10 days before and 10 days after the diagnosis of PA, and compared them to PA patients without a pancreatitis diagnosis. The PA was diagnosed at a similar mean (SD) age of 64 (13) years vs. 65 (11) years (P=.33) in patients with and without a history of pancreatitis. In addition, DM was diagnosed at a similar mean (SD) age of 62 (11) years vs. 60 (12) years (P=.06) in patients with and without a history of pancreatitis. No statistically significant differences were found for vital status (P=.25), CA 19–9 level (P=.30), weight loss (P=.46), smoking (P=.18), or median (range) survival (450 [324–576] days vs. 325 [306–344] days; P=.07). Patients with concomitant pancreatitis had statistically significant differences, with greater proportions diagnosed with DM (28 [28%] vs. 396 [18%]; P=.03), undergoing surgical procedure with curative intent (60 [62%] vs. 802 [43%]; P=.003), and in stages of PA at diagnosis (stage I, 9 [9%] vs. 90 [4%]; stage II, 48 [50%] vs. 665 [32%]; P<.001).
Discussion
In a prospectively maintained cohort of 2,573 patients, we found that PA patients with a history of pancreatitis diagnosed ≥10 days before the diagnosis of PA were more likely to present at an earlier stage of PA, have surgical resection with curative intent, and therefore improved overall survival.
Chronic pancreatitis is an established risk factor for PA (2,4,10–15), and commonly, histopathologic evidence of chronic pancreatitis is found adjacent to the PA area during surgery (6). Clinical acute pancreatitis is an uncommon but important initial presentation of PA (5,16–18). A widely held clinical axiom states that an episode of acute pancreatitis in patients without identifiable risk factors should prompt concern for an underlying pancreatic malignancy. Studies thus far have evaluated both acute and chronic pancreatitis as either a presentation of or a risk factor for PA. However, they have not evaluated the influence of acute and chronic pancreatitis on survival in PA patients.
To the best of our knowledge, this is the first study to evaluate the influence of a history of pancreatitis on PA patients’ survival and is the largest single institution study to date on the clinical characteristics of PA patients with a history of pancreatitis. In our cohort, 8% of patients with PA had prior history of pancreatitis diagnosed ≥10 days before the PA diagnosis.
The reason for the survival advantage in patients with the history of pancreatitis is likely that the PA of these patients was diagnosed at an earlier stage (I or II) than in patients without a history of pancreatitis. There are multiple possible explanations for this finding. Patients with pancreatitis may seek medical attention earlier, thus prompting investigation and therefore earlier diagnosis. Some patients who report a history of acute pancreatitis may also have an underlying history of chronic pancreatitis and thus may be receiving periodic surveillance examinations. Our data also suggest that patients with pancreatitis are more likely to be younger, which may reflect the earlier stage of PA at diagnosis.
A prior history of pancreatitis in our main study cohort was found to be a predictor of reduced mortality in the univariable analysis; however, in the multivariable analysis, history of pancreatitis was not found to be an independent predictor of survival. It is likely that pancreatitis leads to PA diagnosis at an earlier stage conferring improved survival. This result may also indicate that pancreatitis is a marker of early disease in these patients; that is, when pancreatitis occurs, it occurs early in the disease course of patients with PA. Alternatively, the lack of statistical independence could be due to the PA stage being so strong a predictor of positive outcome that it overcomes the small, but statistically significant, influence of pancreatitis on overall survival. Whether this improved survival is due to earlier administration of therapy (e.g. surgical resection) or to an earlier diagnosis without a subsequent change in natural history (e.g. lead time bias) cannot be stated with certainty.
A known risk factor for PA, DM was present in significantly more patients with prior history of pancreatitis. We did not find DM to be an independent predictor of survival in the multivariable analysis, which is in contrast to previously published studies (19,20). In addition, we did not find that tobacco use influenced survival, which is consistent with previously published studies (22).
The main strengths of our study include a large sample size, a longitudinal database, and histological confirmation of the PA diagnosis in almost 90% of patients. In addition, although the study was a retrospective analysis, the research registry database was prospectively collected and maintained.
One of the important limitations of our study is the lack of accurate differentiation of acute vs. chronic pancreatitis. Even though this is a common weakness in larger studies on this topic (16,21), we do not feel that it nullifies the basic observation of a generally more favorable outcome likely due to the earlier diagnosis in this population group. Recall bias could have overestimated the number of patients with pancreatitis, because 38% of cases were self-reported. Prospective studies should be done to validate our findings. In addition, because of the limitations in the research registry database, some associations could not be explored in detail (eg, alcohol use).
In conclusion, our study demonstrates that a substantial proportion of PA patients have a history of pancreatitis and that PA patients with a history of pancreatitis are more likely to present at an earlier stage, have surgical resection, and therefore have improved survival. Prospective studies should be performed validate our findings. In addition, future studies should also be done to assess the temporal and clinical association between the type of pancreatitis (acute vs. chronic) and PA and evaluate whether screening of pancreatitis patients with idiopathic pancreatitis would provide survival benefit and be cost-effective.
Table 2.
Clnical Characteristics of PA Patients With and Without Prior Pancreatitis
| Patientsa,b
|
|||
|---|---|---|---|
| Clinical Characteristic | PA Only (n=2,156) | PA and Prior History of Pancreatitis (n=195) | P Value |
| CA 19–9, median (range), U/mL | 327 (0–3,715,150) | 183 (0–235,000) | .001 |
| Chemotherapy | 1,135 (81) | 126 (81) | >.99 |
| Irradiation | 654 (47) | 79 (51) | .40 |
| Vital status, alive | 433 (20) | 63 (32) | <.001 |
| Site of PA mass | .36 | ||
| Head | 1,147 (55) | 112 (58) | |
| Body | 312 (15) | 23 (12) | |
| Tail | 223 (11) | 12 (6) | |
| Head and body | 143 (7) | 17 (9) | |
| Body and tail | 139 (7) | 16 (8) | |
| Uncinate | 72 (3) | 7 (4) | |
| Not specified | 39 (2) | 5 (3) | |
| PA stage | <.001 | ||
| I | 90 (4) | 20 (11) | |
| II | 665 (33) | 79 (41) | |
| III | 436 (21) | 34 (18) | |
| IV | 860 (42) | 57 (30) | |
| Surgical resection | 802 (43) | 92 (50) | .001 |
Abbreviations: CA, carbohydrate antigen; PA, pancreatic adenocarcinoma.
Values are expressed as No. (%) unless specified otherwise.
Percentages in parentheses represent a valid percent unless specified otherwise.
Acknowledgments
We gratefully acknowledge the Mayo Clinic SPORE in Pancreatic Cancer (National Institutes of Health grant CA102701) for access to these data and for permission to publish results based on these data.
Funding: None
Abbreviations
- CA
carbohydrate antigen
- DM
diabetes mellitus
- ICD
International Classification of Diseases
- OR
odds ratio
- PA
pancreatic adenocarcinoma
- SPORE
Specialized Program of Research Excellence
Footnotes
Conflict of interest: None.
Guarantor of the article: Ivana Dzeletovic, MD
Author contributions: Design of research—I.D., D.O.F., and M.A.H.; draft of manuscript—I.D., D.O.F., and M.A.H.; statistical analysis—M.D.C. and Q.W; data collection—R.P., and C.C.N.; edit and approval of final manuscript—I.D., M.D.C., R.P., C.C.N., Q.W., and D.O.F.
Financial support: None.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Chung SD, Chen KY, Xirasagar S, et al. More Than 9-Times Increased Risk for Pancreatic Cancer Among Patients With Acute Pancreatitis in Chinese Population. Pancreas. 2012;41:142–146. doi: 10.1097/MPA.0b013e31822363c3. [DOI] [PubMed] [Google Scholar]
- 3.Balthazar EJ. Pancreatitis associated with pancreatic carcinoma. Preoperative diagnosis: role of CT imaging in detection and evaluation. Pancreatology. 2005;5:330–44. doi: 10.1159/000086868. [DOI] [PubMed] [Google Scholar]
- 4.Ekbom A, McLaughlin JK, Karlsson BM, et al. Pancreatitis and pancreatic cancer: a population-based study. J Natl Cancer Inst. 1994;86:625–7. doi: 10.1093/jnci/86.8.625. [DOI] [PubMed] [Google Scholar]
- 5.Lin A, Feller ER. Pancreatic carcinoma as a cause of unexplained pancreatitis: report of ten cases. Ann Intern Med. 1990;113:166–7. doi: 10.7326/0003-4819-113-2-166. [DOI] [PubMed] [Google Scholar]
- 6.Gambill EE. Pancreatitis associated with pancreatic carcinoma: a study of 26 cases. Mayo Clinic Proceedings. 1971;46:174–7. [PubMed] [Google Scholar]
- 7.Tsai MJ, Liao KS, Shih PM, et al. Relapsed acute pancreatitis as the initial presentation of pancreatic cancer in a young man: a case report. Kaohsiung J Med Sci. 2010;26:448–55. doi: 10.1016/S1607-551X(10)70072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mujica VR, Barkin JS, Go VL, et al. Acute pancreatitis secondary to pancreatic carcinoma. Study Group Participants Pancreas. 2000;21:329–32. doi: 10.1097/00006676-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Talamini G, Falconi M, Bassi C, et al. Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol. 1999;94:1253–60. doi: 10.1111/j.1572-0241.1999.01075.x. [DOI] [PubMed] [Google Scholar]
- 10.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 11.Karlson BM, Ekbom A, Josefsson S, et al. The risk of pancreatic cancer following pancreatitis: an association due to confounding? Gastroenterology. 1997;113:587–92. doi: 10.1053/gast.1997.v113.pm9247480. [DOI] [PubMed] [Google Scholar]
- 12.Appelros S, Borgstrom A. Incidence, aetiology and mortality rate of acute pancreatitis over 10 years in a defined urban population in Sweden. The British journal of surgery. 1999;86:465–70. doi: 10.1046/j.1365-2168.1999.01049.x. [DOI] [PubMed] [Google Scholar]
- 13.Bansal P, Sonnenberg A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology. 1995;109:247–51. doi: 10.1016/0016-5085(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 14.Bracci PM, Wang F, Hassan MM, et al. Pancreatitis and pancreatic cancer in two large pooled case-control studies. Cancer causes & control : CCC. 2009;20:1723–1731. doi: 10.1007/s10552-009-9424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2012 doi: 10.1093/annonc/mds140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maes B, Hastier P, Buckley MJ, et al. Extensive aetiological investigations in acute pancreatitis: results of a 1-year prospective study. European journal of gastroenterology & hepatology. 1999;11:891–6. [PubMed] [Google Scholar]
- 17.Tummala P, Tariq SH, Chibnall JT, et al. Clinical Predictors of Pancreatic Carcinoma Causing Acute Pancreatitis. Pancreas. 2012 doi: 10.1097/MPA.0b013e318254f473. [DOI] [PubMed] [Google Scholar]
- 18.Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. The American journal of gastroenterology. 2012;107:1096–103. doi: 10.1038/ajg.2012.126. [DOI] [PubMed] [Google Scholar]
- 19.Chu CK, Mazo AE, Goodman M, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Annals of surgical oncology. 2010;17:502–13. doi: 10.1245/s10434-009-0789-6. [DOI] [PubMed] [Google Scholar]
- 20.Ben Q, Xu M, Jiang Y, et al. Clinical profiles and long-term outcomes of patients with pancreatic ductal adenocarcinoma and diabetes mellitus. Diabetes/metabolism research and reviews. 2012;28:169–76. doi: 10.1002/dmrr.1284. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez E, La Vecchia C, Porta M, et al. Pancreatitis and the risk of pancreatic cancer. Pancreas. 1995;11:185–9. doi: 10.1097/00006676-199508000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Dandona M, et al. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas. 2011;40(6):931–7. doi: 10.1097/MPA.0b013e318215a9b1. [DOI] [PubMed] [Google Scholar]