Abstract
The evaluation of concussed athletes, including testing to determine if and when they may return to play, has become an important task of athletic trainers and team physicians. Currently, concussion protocols are in place, which depend largely upon assessments based upon neurocognitive testing (NCT). The authors have evaluated the use of a biomarker of brain trauma, marinobufagenin (MBG), and compared its application in concussed athletes with the performance of NTC. We found a disparity between these two testing procedures. In this communication, the findings of these comparative data are presented. We noted that athletes whose NCT evaluations had returned to baseline and who were allowed to again participate in play then showed a recurrence of elevated urinary MBG excretion. These observations raise concern as to the processes currently in effect with regard to the decision as to returning athletes to the full activity. They suggest a need for further evaluation.
Keywords: athletic brain trauma, concussion, bufodienolides, marinobufagenin, neurocognitive testing
Introduction
Head injury is a growing concern within the athletic community, especially with regard to mild traumatic brain injury (or concussion). Despite substantial leaps forward in defining the exact parameters of a concussion, the line between healthy and recovering is not yet clear. This dilemma becomes particularly problematic when deciding if an athlete should return to play;1,2 to wit, minor issues can become much more serious if a recovering player rejoins his teammates too soon. While neurocognitive testing (NCT), such as the ImPact test, has shown some promise in determining whether a player should be allowed back on the field, there is much debate as to how effective and reliable these neurocognitive tests actually are in making that determination.3–6 Our laboratory has focused on revealing player status in a quantitative manner that allows for better understanding of the individual athlete’s overall health and safety.
Although concussions are multisymptomatic, they are all characterized by varying levels of inflammation7,8 and the production of vascular leak, including increased permeability in the blood–brain barrier.9 This damage causes an elevation in the concentration of a circulating cardiotonic steroid substance called marinobufagenin (MBG), which both participates in the initiation and the maintenance of the inflammatory response to the trauma and subsequently influences the permeability of brain endothelial cells.9 MBG has been demonstrated to upregulate apoptosis, leading to alterations in the gap junctions between endothelial cells. This leakage can lead to further brain damage, which could have severe long-lasting effects.
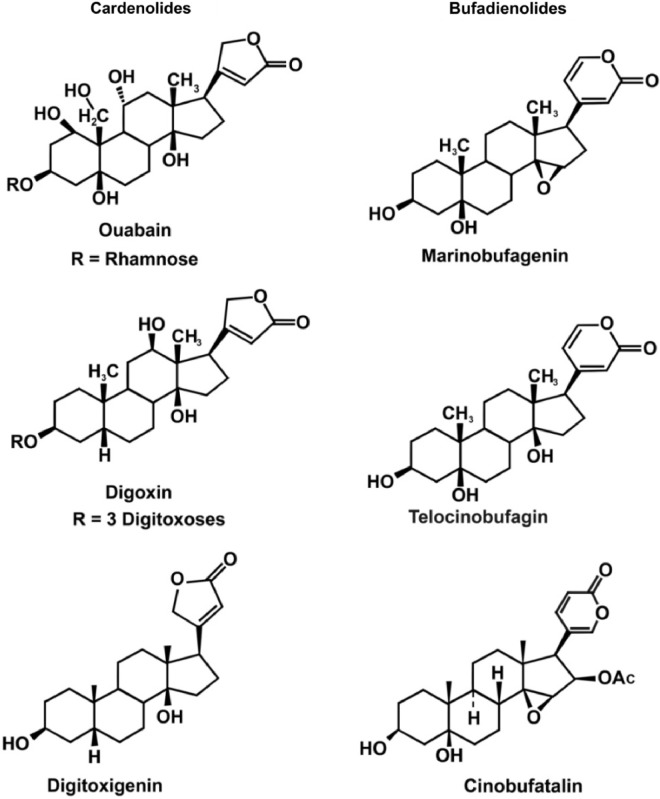
MBG is a member of a class of steroid compounds referred to as the cardiac glycosides or cardiotonic steroids, which consists of two groups, the bufodienolides (which includes MBG) and the cardenolides (Fig. 1). While these two groups are similar (but different) in their chemical structures, they demonstrate similarities in their actions. Both groups inhibit the ubiquitous enzyme sodium/potassium adenosine triphosphatase (Na+/K+ ATPase).10,11 Because of this inhibition, they can cause hypertension through vasoconstriction, alter heart muscle function (acting as cardiac inotropes), and lead to elevated sodium in the urine.10,11 These effects occur both because of (1) the actions of the bufodienolides to upregulate the MAPK-signaling system, leading to alterations in intracellular calcium concentration,11 and (2) their ability to impair the integrity of the vascular endothelium.9,12,13 MBG has also been linked to tissue injury.14,15 Our investigation includes quantitative urinary measurements of MBG concentrations at given time points before and following concussion, which indicate its potential importance in its capacity to distinguish between healthy, recovered concussed athletes and those who should be withheld from participation in organized athletic activities.
Figure 1.
Chemical structures of the “cardiotonic steroids” (cardiac glycosides). The cardenolides are on the left and the bufodienolides on the right.
Materials and Methods
Ethics
Ethical approval for the research was granted by the Office of Research Compliance, Texas A&M University (TAMU IRB2011-0757). Subjects gave their written, informed consent to participate in the research. The study was conducted in accordance with the Declaration of Helsinki.
Neurocognitive examination
Because neurocognitive tests are the most common method of the analysis of concussion recovery time, they were also used in our study. Also, because these examinations tend to be subjective, two types were administered. When a player first arrives to join the football program, a preliminary ImPact test16 was performed as a baseline. During any concussive incident, another, less formal, test called the C3 Logix was performed.17 C3 Logix is a new application for Apple’s iPad that allows for a patient to perform tasks ranging from simple reaction time and shape matching with more mentally demanding tasks such as complex hand-and-arm coordination movements (Neurologix Technology, Inc., Cleveland, OH, USA). In the vast majority of cases, before a player is cleared to return to practice, he must pass both the C3 Logix and a repeat ImPact examination. While C3 Logix has many components, the most easily quantifiable is the symptom score. A symptom score is based upon a number of physiological characteristics as determined by the training staff (ie, sleep patterns, soreness, fatigue, blurry vision, etc). Because the C3 Logix and the ImPact symptom score criteria are similar in nature, only the C3 Logix score was used for analysis. The two tests had very comparable scores when they were given at the same time, but the C3 Logix test is administered much more frequently and thus provided more data points for examination. Before a player was cleared to return to practice, he was required to pass both the C3 Logix and a repeat ImPact examination.16,17
Acquisition and analysis of samples
During the preseason (spring of 2014), 110 urine samples were obtained from football athletes to act as baseline values for MBG excretion (Fig. 2). In addition, the initial measurements of MBG after a concussion have also been plotted in Figure 2. In the event of a concussion at any point during the season, multiple samples were again taken from the individual at 24, 48, 72, and 96 hours with a final sample at 2 weeks postconcussion. However, due to the demanding schedule of a D1 football program, these time points varied slightly. Values were graphed in Microsoft Excel and plotted to best reveal trends of MBG concentration that occur during the recovery period (Figs. 3–8).
Figure 2.
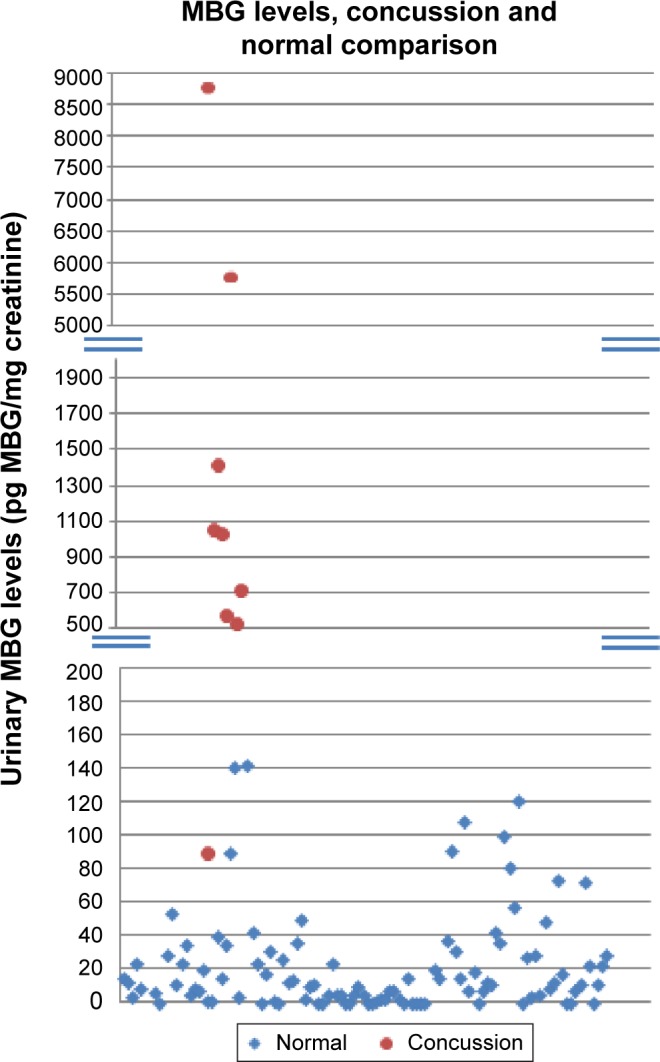
MBG levels in concussed athletes (filled circles) compared to those obtained prior to spring training (filled diamonds).
Figure 3.
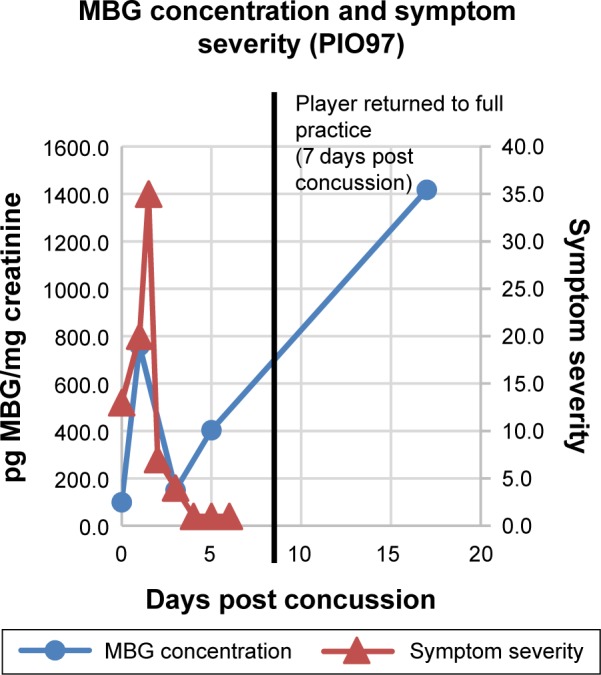
MBG levels (in blue) and symptom scores (in red) in patient PIO97 before and after concussion. The vertical black line represents the return of the athlete to full participation.
Figure 4.
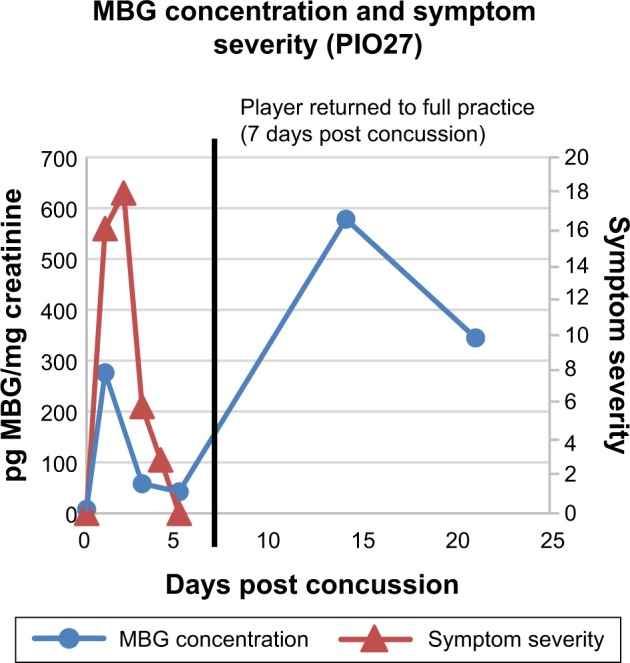
MBG levels (in blue) and symptom scores (in red) in patient PIO27 before and after concussion. The vertical black line represents the return of the athlete to full participation.
Figure 5.
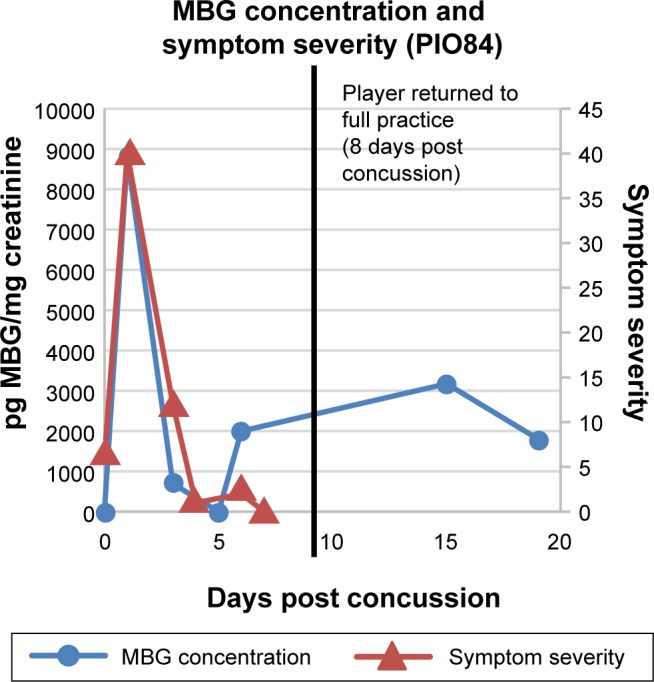
MBG levels (in blue) and symptom scores (in red) in patient PIO84 before and after concussion. The vertical black line represents the return of the athlete to full participation.
Figure 6.
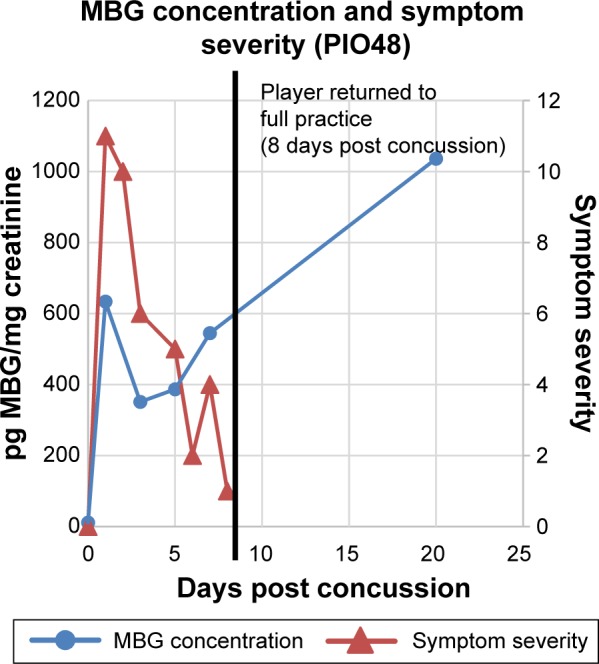
MBG levels (in blue) and symptom scores (in red) in patient PIO48 before and after concussion. The vertical black line represents the return of the athlete to full participation.
Figure 7.
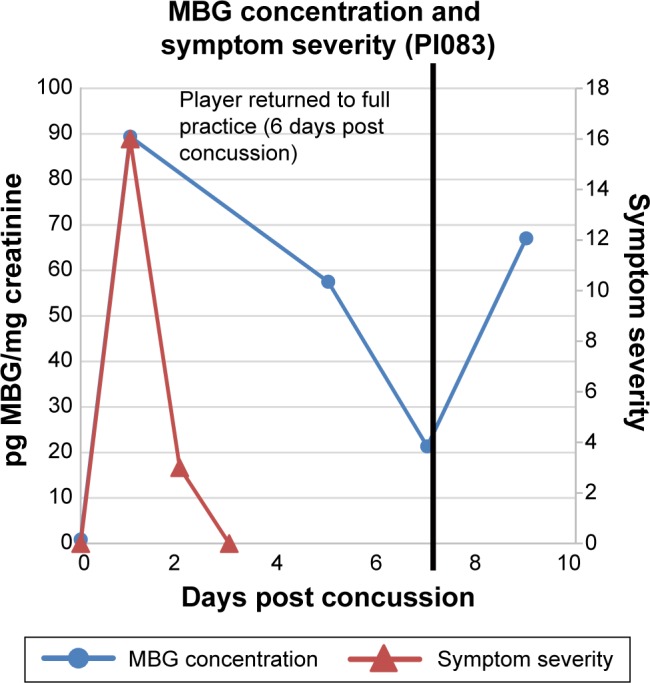
MBG levels (in blue) and symptom scores (in red) in patient PIO83 before and after concussion. The vertical black line represents the return of the athlete to full participation.
Figure 8.
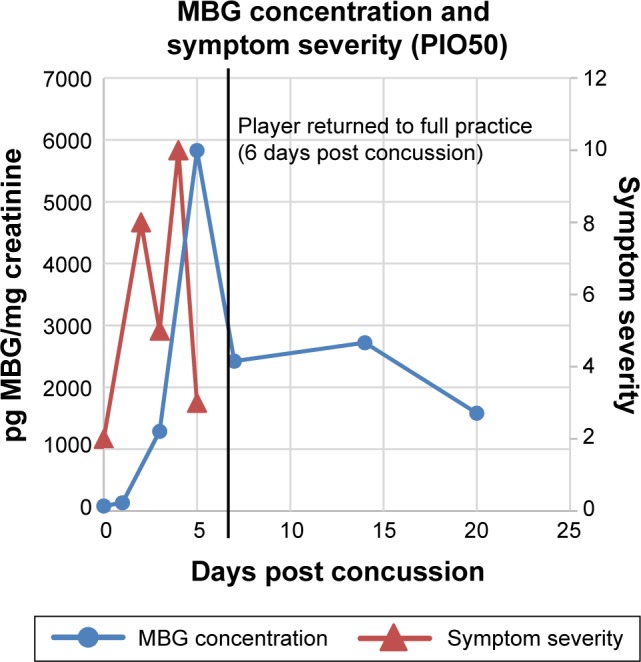
MBG levels (in blue) and symptom scores (in red) in patient PIO50 before and after concussion. The vertical black line represents the return of the athlete to full participation.
Quantification of MBG
In order to accurately measure MBG in the urine of the athletes, an enzyme-linked immunosorbent assay was performed employing polyclonal antibodies.18 A standard curve of MBG was prepared in starting block buffer that had a concentration range from 9 pg/mL to 2 mg/mL. Plates were coated with a MBG–beta lactoglobulin complex for the baseline concentration and washed with Tris-buffered saline and Tween 20. Each sample and MBG dilution were added at 50 µL/well and allowed to incubate with the primary antibodies at room temperature for 3 hours. After the first addition of antibodies, the plate was washed again before adding the secondary antibodies and allowed to incubate for another hour. After a final washing, the addition of QuantaRed (Thermo Scientific, Rockford, IL, USA) enhanced chemifluorescence, and horseradish peroxidase substrate (Thermo Scientific) was combined and fluorescence with 544 excitation and 600 emission was measured using a BioTek plate reader (Biotek Instruments Inc., Winooski, VT, USA). The urinary concentration of creatinine was also measured (NOVA 16 autoanalyzer, Nova Biomedical, Waltham, MA, USA). The urinary concentration of MBG in pg/mL of urine was corrected for urinary flow rate and alterations in glomerular filtration rate by dividing its urinary excretion (in picogram per milliliter) by the urinary creatinine concentration (in milligrams of creatinine per milliliter), yielding the MBG concentration in pg/mg creatinine. The value of MBG obtained was plotted versus symptom score. The latter was obtained at several time points during the first week to 10 days postconcussion until the value had returned to baseline. The MBG determinations were continued for several days postconcussion.
Results
In Figure 2 are presented data for 110 athletes for whom urinary MGB concentrations were measured prior to the beginning of spring practice and for 6 athletes beginning shortly after they suffered a concussion. All the values for the pretraining specimens were 140 pg/mg creatinine or less (Fig. 2). With one exception, all the values for the concussed athletes exceeded 500 pg/mg creatinine, including several in the thousands. In the single instance in which the postconcussion value was within the normal range (it was 89 pg/mg creatinine), that athlete’s baseline level of MBG was almost undetectable. In Figures 3–8 are presented the individual values of six concussed athletes in whom both symptom score (in red) and MBG values (in blue) were obtained. In each case, there was a divergence between the two measurements. In patient PIO197 (Fig. 3), the MGB measurement, which like the symptom score began to decline postconcussion, again rose to a rather high value (1,400 pg MBG/mg creatinine) at a time when the symptom score had returned to normal (baseline) level. A similar situation was seen in patient PIO27 (Fig. 4), PIO84 (Fig. 5), and PIO48 (Fig. 6). In patient PIO83 (Fig. 7) the MBG value remained elevated throughout the 9-day postconcussion period during which it was measured, although the symptom score returned to baseline at 3 days postconcussion. In the case of patient PIO50 (Fig. 8), the MBG value declined but remained at a relatively high level (>1,500 pg MBG/mg creatinine) for 20 days postconcussion. Comparisons of NCT and MBG levels were not available once the athletes returned to plan. This was a result of the fact that NCT was discontinued once the athletes were cleared to resume full football activities.
Discussion
For many years, it has been standard practice to evaluate head trauma patients with NCT both to diagnose the potential seriousness of their injury as well as to follow them posttrauma over time.1,19–25 This has been the case in both civilian and military head trauma cases.21,26–28 Recently, evidence has accumulated indicating that this approach may not represent the “gold standard” as had been thought to be the case previously.2–5,29 However, because NCT was not continued after contused athletes returned to play, in the current report, no comparison with MBG levels could be performed. However, the imaging technique diffusion tensor imaging (DTI) has been identified as a potentially more reliable measure of the degree of seriousness of the brain insult as well, perhaps, as its longevity.3–5,30,31 Although DTI was not performed in the studies reported in this communication, it is the basis for a planned study in which NCT, MBG levels, and DTI will be compared in mild TBI. Should the results of such a study indicate that MBG measurements and DTI results agree as to both the presence and longevity of brain abnormalities, these two test methodologies could provide important new indications of the presence and/or degree of import of the traumatic brain insult. In particular, these techniques could obviate the possibility that the second impact syndrome could be present.32
In other clinical and experimental settings, MBG has been demonstrated to serve both as an indicator and as a prognosticator as well as a pathogenetic agent of injury. Thus, in an animal model of the pregnancy-dependent hypertensive syndrome, preeclampsia (PE), MBG was elevated before the advent of the hypertension and proteinuria.33,34 In addition, the antagonist of MBG, resibufagenin (RBG), corrected the hypertension in the rat model of PE and prevented hypertension, proteinuria, and intrauterine growth restriction if given early in pregnancy to animals destined to develop this syndrome.35–37 Furthermore, in a small series of PE patients (n = 19), MBG was elevated in 85% of the samples compared to those obtained in normal pregnant patients (n = 34).38
In rats in which traumatic brain injury had been induced, urinary MBG was elevated above that obtained in sham animals and was reduced to sham levels in rats treated with RBG 24 hours after the imposition of a brain contusion.39 Furthermore, on histologic examination of the brain, RBG reduced gliosis and vascular injury and prevented scar formation.39 In in vitro studies, MBG caused hyperpermeability of human brain endothelial cell monolayers9 by altering apoptotic signaling.13 MBG causes oxidative stress,40 which is prevented, in the rat PE model, by RBG administration.41
Finally, in preliminary experiments, MBG was elevated in patients with acute respiratory distress syndrome42 and caused vascular leak in rat lung endothelial cell monolayers.13
Based upon the observations previously obtained in both in vivo and in vitro studies and those described above in concussed athletes, we believe that MBG is an excellent candidate to evaluate the presence and status of the inflammation associated with the brain pathology observed in TBI. The coupling of the measurement of MBG and the results of DTI scanning should provide powerful information for both the diagnosis and potential therapy of TBI.
Acknowledgments
The authors are grateful to Ms. Lonnie Doyle for the production of this article.
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
FUNDING: These studies were funded (in part) by the Jules B. Puschett, MD Research Endowment, by a grant from the Huffhines Institute, and by the Texas A&M University School of Veterinary Medicine. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: JBP. Analyzed the data: JO, KA, JTL, KB, BC, JH, and JBP. Wrote the first draft of the manuscript: JO. Contributed to the writing of the manuscript: JO, KA, JTL, KB, BC, JH, and JBP. Agreed with manuscript results and conclusions: JO, KA, JTL, KB, BC, JPB, DW, JH, and JBP. Jointly developed the structure and arguments for the paper: JO, KA, JTL, KB, BC, JPB, DW, JH, and JBP. Made critical revisions and approved the final version: JBP. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.West T, Marion DW. Current recommendations for the diagnosis and treatment of concussion in sport: a comparison of three new guidelines. J Neurotrauma. 2014;31(2):159–168. doi: 10.1089/neu.2013.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139–154. [PMC free article] [PubMed] [Google Scholar]
- 3.Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- 4.Resch J, Driscoll A, McCaffrey N, et al. ImPact test-retest reliability: reliably unreliable? J Athl Train. 2013;48(4):506–511. doi: 10.4085/1062-6050-48.3.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh R, Meier TB, Kuplicki R, et al. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA. 2004;311:1883–1888. doi: 10.1001/jama.2014.3313. [DOI] [PubMed] [Google Scholar]
- 6.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the Fourth International Conference in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 7.Hinson HE, Rowell S, Schreiber M. Clinical evidence of inflammation driving secondary brain injury: a systematic review. J Trauma Acute Care Surg. 2014;78:184–191. doi: 10.1097/TA.0000000000000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling JM, Klimaj S, Toulouse T, Mayer AR. A perspective study of gray matter abnormalities in mild traumatic brain injury. Neurology. 2013;81:2121–2127. doi: 10.1212/01.wnl.0000437302.36064.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ing NH, Berghman L, Abi-Ghanem D, et al. Marinobufagenin regulates permeability and gene expression of brain endothelial cells. Am J Physiol Regul Integr Comp Physiol. 2014;306(12):R918–R924. doi: 10.1152/ajpregu.00499.2013. [DOI] [PubMed] [Google Scholar]
- 10.Schoner W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem. 2002;269(10):2440–2448. doi: 10.1046/j.1432-1033.2002.02911.x. [DOI] [PubMed] [Google Scholar]
- 11.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293(2):C509–C536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 12.Uddin MN, McLean LB, Hunter FA, et al. Vascular leak in a rat model of preeclampsia. Am J Nephrol. 2009;30:26–33. doi: 10.1159/000193220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uddin M, Horvat D, Childs E, Puschett J. Marinobufagenin causes endothelial cell monolayer hyperpermeability by altering apoptotic signaling. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1726–R1734. doi: 10.1152/ajpregu.90963.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Giovanni S, Movsesyan V, Ahmed F, et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Nat Acad Sci U S A. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israelsson C, Bengtsson H, Kylberg A, et al. Distinct cellular patterns of upregulated chemokine expression supporting a prominent inflammatory role in traumatic brain injury. J Neurotrauma. 2008;25:959–974. doi: 10.1089/neu.2008.0562. [DOI] [PubMed] [Google Scholar]
- 16.Thoma RJ, Cook JA, McGrew C, et al. The effect of days since last concussion and number of concussions on cognitive functioning in division one athletes. Brain Inj. 2015;19:1–6. doi: 10.3109/02699052.2014.999352. [DOI] [PubMed] [Google Scholar]
- 17.Ferris C, Schatz P. C-46A new iPad version of ImPACT is resistant to “sand bagging”. Arch Clin Neuropsychol. 2014;29:591. [Google Scholar]
- 18.Abi-Ghanem D, Lai X, Berghman LR, et al. A chemifluorescent immunoassay for the determination of marinobufagenin in body fluids. J Immunoassay Immunochem. 2011;32:31–46. doi: 10.1080/15321819.2010.538107. [DOI] [PubMed] [Google Scholar]
- 19.Erlanger D, Kaushik T, Cantu R, et al. Symptom-based assessment of the severity of a concussion. J Neurosurg. 2003;98:477–484. doi: 10.3171/jns.2003.98.3.0477. [DOI] [PubMed] [Google Scholar]
- 20.Lovell M, Collins M, Iverson G, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98:296–301. doi: 10.3171/jns.2003.98.2.0296. [DOI] [PubMed] [Google Scholar]
- 21.Lovell M, Collins M, Iverson G, Johnston K, Bradley J. Grade 1 or ‘ding’ concussions in high school athletes. Am J Sports Med. 2004;32:47–54. doi: 10.1177/0363546503260723. [DOI] [PubMed] [Google Scholar]
- 22.McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13:27–35. doi: 10.1097/00001199-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Levin H, O’Donnell V, Grossman R. The Galveston orientation and amnesia test. A practical scale to assess cognition after head injury. J Nerv Ment Dis. 1979;167:675–684. doi: 10.1097/00005053-197911000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Rabinowitz A, Levin H. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am. 2014;37(1):1–11. doi: 10.1016/j.psc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryant RA, Nickerson A, Creamer M, et al. Trajectory of post-traumatic stress following traumatic injury: 6-year follow-up. Br J Psychiatry. 2015;206(5):417–423. doi: 10.1192/bjp.bp.114.145516. [DOI] [PubMed] [Google Scholar]
- 26.Logan B, Goldman S, Zola M, Mackey A. Concussive brain injury in the military: September 2001 to the present. Behav Sci Law. 2013;31:803–813. doi: 10.1002/bsl.2092. [DOI] [PubMed] [Google Scholar]
- 27.Nelson NW, Anderson CR, Thuras P, et al. Factors associated with inconsistency in self-reported mild traumatic brain injury over time among military personnel in Iraq. Br J Psychiatry. 2015;206(3):237–244. doi: 10.1192/bjp.bp.114.149096. [DOI] [PubMed] [Google Scholar]
- 28.Williams WW, Rapport LJ, Hanks RA, Millis SR, Greene HA. Incremental validity of neuropsychological evaluations to computed tomography in predicting long-term outcomes after traumatic brain injury. Clin Neuropsychol. 2013;27:356–375. doi: 10.1080/13854046.2013.765507. [DOI] [PubMed] [Google Scholar]
- 29.Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 30.Hasan K, Moeller F, Naryana P. DTI-based segmentation and quantification of human brain lateral ventricular CSF volumetry and mean diffusivity: validation, age, gender effects and biophysical implications. Magn Reson Imaging. 2014;32:405–412. doi: 10.1016/j.mri.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamali A, Hasan KM, Adapa P, et al. Distinguishing and quantification of the human visual pathways using high-spatial-resolution diffusion tensor tractography. Magn Reson Imaging. 2014;32:796–803. doi: 10.1016/j.mri.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wetjen NM, Pichelmann MA, Atkinson JLD. Second impact syndrome: concussion and second injury brain complications. J Am Coll Surg. 2010;211:553–557. doi: 10.1016/j.jamcollsurg.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Ianosi-Irimie M, Nadig J, Williams M, et al. A rat model of preeclampsia. Clin Exp Hypertens. 2005;8:605–617. doi: 10.1080/10641960500298608. [DOI] [PubMed] [Google Scholar]
- 34.Vu HV, Ianosi-Irimie MR, Pridjian CA, et al. Involvement of marinobufagenin in a rat model of human preeclampsia. Am J Nephrol. 2005;25:520–528. doi: 10.1159/000088461. [DOI] [PubMed] [Google Scholar]
- 35.Vu H, Ianosi-Irimie M, Danchuk S, et al. Resibufogenin corrects hypertension in a rat model of human preeclampsia. Exp Biol Med. 2006;231:215–220. doi: 10.1177/153537020623100212. [DOI] [PubMed] [Google Scholar]
- 36.Horvat D, Severson J, Uddin M, Mitchell B, Puschett J. Resibufogenin prevents the manifestations of preeclampsia in an animal model of the syndrome. Hypertens Pregnancy. 2010;29:1–9. doi: 10.3109/10641950802629709. [DOI] [PubMed] [Google Scholar]
- 37.Puschett J. Marinobufagenin predicts and resibufagenin prevents preeclampsia: a review of the evidence. Am J Perinatol. 2012;29:777–785. doi: 10.1055/s-0032-1316447. [DOI] [PubMed] [Google Scholar]
- 38.Agunanne E, Horvat D, Harrison R, et al. Marinobufagenin levels in preeclamptic patients: a preliminary report. Am J Perinatol. 2011;28:509–514. doi: 10.1055/s-0031-1272965. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro L, Foresti M, Arisi G, et al. American Society for Clinical Pharmacology and Therapeutics. Meeting Program Booklet; 2011. Marinobufagenin diagnoses and resibufogenin ameliorates traumatic brain injury; p. 78. (Abstract), 2011. [Google Scholar]
- 40.Mitchell B, Cook L, Danchuk S, Puschett J. Uncoupled endothelial nitric oxide synthase and oxidative stress in a rat model of pregnancy-induced hypertension. Am J Hypertens. 2007;20:1297–1304. doi: 10.1016/j.amjhyper.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Uddin M, Agunanne E, Horvat D, Puschett J. Resibufogenin administration prevents oxidative stress in a rat model of human preeclampsia. Hypertens Pregnancy. 2012;31:70–78. doi: 10.3109/10641955.2010.525275. [DOI] [PubMed] [Google Scholar]
- 42.Farjo B, Morin K, Patel B, Abbas M, Puschett J. Marinobufagenin identifies patients with acute respiratory distress syndrome. J Invest Med. 2014;62:544–545. [Google Scholar]