Abstract
Patient-derived cancer xenografts (PDCX) generally represent more reliable models of human disease in which to evaluate a potential drugs preclinical efficacy. However to date, only a few patient-derived gastric cancer xenograft (PDGCX) models have been reported. In this study, we aimed to establish additional PDGCX models and to evaluate whether these models accurately reflected the histological and genetic diversities of the corresponding patient tumors. By engrafting fresh patient gastric cancer (GC) tissues into immune-compromised mice (SCID and/or nude mice), thirty two PDGCX models were established. Histological features were assessed by a qualified pathologist based on H&E staining. Genomic comparison was performed for several biomarkers including ERBB1, ERBB2, ERBB3, FGFR2, MET and PTEN. These biomarkers were profiled to assess gene copy number by fluorescent in situ hybridization (FISH) and/or protein expression by immunohistochemistry (IHC). All 32 PDGCX models retained the histological features of the corresponding human tumors. Furthermore, among the 32 models, 78% (25/32) highly expressed ERBB1 (EGFR), 22% (7/32) were ERBB2 (HER2) positive, 78% (25/32) showed ERBB3 (HER3) high expression, 66% (21/32) lost PTEN expression, 3% (1/32) harbored FGFR2 amplification, 41% (13/32) were positive for MET expression and 16% (5/32) were MET gene amplified. Between the PDGCX models and their parental tumors, a high degree of similarity was observed for FGFR2 and MET gene amplification, and also for ERBB2 status (agreement rate = 94~100%; kappa value = 0.81~1). Protein expression of PTEN and MET also showed moderate agreement (agreement rate = 78%; kappa value = 0.46~0.56), while ERBB1 and ERBB3 expression showed slight agreement (agreement rate = 59~75%; kappa value = 0.18~0.19). ERBB2 positivity, FGFR2 or MET gene amplification was all maintained until passage 12 in mice. The stability of the molecular profiles observed across subsequent passages within the individual models provides confidence in the utility and translational significance of these models for in vivo testing of personalized therapies.
Introduction
Gastric carcinoma (GC) is the third most common cancer worldwide and is the most frequent cancer diagnosed in East Asian countries [1]. Despite recent progress in earlier diagnosis and improved therapeutic regimens, many patients still eventually develop advanced disease and have poor clinical outcomes. The median overall survival is 8–10 months and 5-year survival is less than 7% for metastatic GC [2]. With regard to standard chemotherapy, limited efficacy has spurred research into targeted therapies designed to block signaling via molecular pathways known to be important for gastric tumorigenesis [3, 4]. To date, two monoclonal antibodies have been approved by the FDA for the treatment of GC, namely Trastuzumab [5] and Ramucirumab [6, 7], targeting ERBB2 (HER2) and VEGFR2 respectively. A number of other targeted therapeutics are currently being tested in mid to late stage GC trials including: AZD4547, targeting the FGFR2 gene [8, 9], and Onartuzumab [10], ARQ197 [11], AMG102 [12, 13] and crizotinib [14] all targeting the MET pathway [15]. Within the drug development process, evaluation of preclinical efficacy with relevant in vivo models is an important checkpoint before moving the drug forward into human clinical studies. Accordingly, one of our research goals is to establish appropriate preclinical models which as accurately as possible represent the complexity of human GC and provide predictive power.
In contrast to standard cancer cell line derived xenografts, which may undergo genetic modification as well as subpopulation rearrangements during the cell line’s in vitro culture [16], patient-derived cancer xenograft (PDCX) models are established by directly engrafting surgically resected human tumor tissues into immune deficient mice. Therefore, at least initially, PDCX models inherit the complexity and genetic diversity of the original human tumors [17, 18] and are preferred models for evaluating the anticancer efficacy of targeted therapies [19, 20]. Panels of tumor-specific PDCX models have been established in many cancer types [21] including breast cancer [22], ovarian cancer [23], esophageal carcinoma [17], non small cell lung cancer [18, 24, 25], colorectal cancer [26–28], prostate cancer [29, 30] and pancreatic cancer [31]. The generation of patient-derived gastric cancer xenograft models (PDGCX) has been reported recently in great depth by Zhu and colleagues using gastroscopic biopsy samples from non-resectable advanced GC [32]. However, using surgical GC samples to establish PDGCX models has been more challenging. To our knowledge, not many PDGCX models have been established [33–36] apart from those established by our group [9, 37–40]. In addition, molecular biomarkers have not been well studied.
In the present study, we successfully established 32 PDGCX models from human GC surgical samples and performed histological examination and profiling of genetic biomarkers. These genetic biomarkers, which included ERBB1 (EGFR), ERBB2 (HER2), ERBB3 (HER3), PTEN, FGFR2 and MET, are six genes that are known targets for clinical or pre-clinical targeted therapies in GC. Through comparison to parental patient tumors, we demonstrated that these PDGCX models accurately maintained the histological and genetic characteristics of human GC, thereby underscoring their value and potential predictive power in evaluating oncology drug efficacy in pre-clinical studies.
Material and Methods
Patients and tumor samples
GC tissues from 207 treatment-naïve patients were obtained intraoperatively during gastrectomy resection at Ren Ji Hospital (Shanghai, China) from 2009 to 2012. Prior written informed consent was obtained from all patients and the study protocol was approved by the ethics committee at Ren Ji hospital. Resected tumor samples were separated into two parts. One part was used for in vivo engrafting as described in the next paragraph, while another part was processed to generate formalin-fixed, paraffin-embedded (FFPE) tissues blocks. FFPE sections were stained with hematoxylin and eosin (H&E) and reviewed by pathologist to confirm the GC diagnosis.
Establishment of PDGCX models
All animal experiments were performed in accordance of the guidelines (IACUC protocol NO.1404-ONM-01) approved by AstraZeneca Institutional Animal Care and Use Committee (IACUC). PDGCX mouse models were established using fresh GC tissues surgically removed from GC patients. In brief, surgically removed GC tissues (F0) were immediately placed into FBS-free medium with 50units/ml penicillin and 50ug/ml streptomycin, and then transported to the animal facility within two hours for implantation into immune compromised mice. The tissues were cut into 2 mm3 fragments and subsequently transplanted subcutaneously into the right hind flanks of immune-compromised mice, either 8-10-week-old nude (nu/nu) or SCID mice (Vital River, Beijing, China). In general, tissue from one patient tumor was used to implant into 5~10 mice. Specific standard operation procedure (SOP) and policy have been generated for alleviation of pain, distress or discomfort for routine monitor, care and humanized euthanasia. The criteria for humane euthanasia in mouse experiments, which has been defined in the IACUC protocol and SOP, include animals in a moribund state; ≥20% body weight loss which can’t be recovered in 5 days; tumor mass ≥ 2cm in any dimension or volume over 2cm3 and ulcerated or necrotic masses of engraft, etc. If any clinical signs in implanted animals were found to meet the criteria for humanized euthanasia, euthanasia was performed by excessive CO2 inhalation. Subcutaneous tumor growth in mice was observed daily until 90 days. Xenograft tumors were measured once a week using calipers when tumors started to grow up in mice. Tumor volume was calculated using the formula V = π/6 × length × width × width, with the length and width value being obtained from caliper measurements. When the first generation (F1) of the xenografted tumors reached a size of 1–2 cm3, tumor-bearing mice were sacrificed and the xenograft tumor tissues were resected under aseptic conditions and implanted into nude mice for maintenance and expansion (F2) within 30 minutes of resection. Meanwhile, representative portions of freshly harvested tissues were fixed in 10% formalin buffer for 24 hours and embedded in paraffin (FFPE) for pathological assessment. A PDGCX model was considered successfully established if it was passagable in nude mice for more than three passages (F3). Xenograft tumor tissues from well-established PDGCX models were also harvested every passage for model characterization and biomarker studies.
Immunohistochemistry (IHC)
IHC assays were performed to detect the protein expression of 5 biomarkers. All IHC assays were done on 4um FFPE tissue slides (SuperFrost, ThermoFisher), sectioned from FFPE blocks 24 hours prior to experiment. For ERBB2 staining, a HercepTest kit (K5204, DAKO) was used following the manufacturer’s instructions. MET staining was performed using a rabbit monoclonal anti-total cMET (SP44) antibody (790–4430, Ventana Medical Systems) on a Ventana automatic immunostainer (Discovery XT; Ventana Medical Systems). ERBB1, ERBB3 and PTEN staining were performed manually using the following antibodies: rabbit anti-tERBB1 monoclonal antibody (1:100 dilution, 0.00017ug/ul, CST4267, Cell Signaling Technology), anti-ERBB3 antibody (1:2000 dilution, CST D43D4, 0.0009ug/ul, Cell Signaling Technology) and rabbit anti-PTEN monoclonal antibody (1:100 dilution, 0.00026ug/ul, CST9559, Cell Signaling Technology). The manual IHC procedure is briefly described as follows: slides were dried at 37°C overnight and then baked at 56°C for 30min, then deparaffinized in xylene and rehydrated through a graded series of ethanol concentrations. Antigen retrieval was performed in a pressure cooker using Target Retrieval Solution, pH 9 (K8004, DAKO) for ERBB1 and ERBB3 staining, or pH6 (K8005, DAKO) for PTEN staining for 5 min. Intrinsic peroxidase activity was blocked by peroxidase blocking solution (S2023, DAKO) for 5 min. Slides were then covered with primary antibody solution and incubated at room temperature for 60min. After two washes in TBS-T for 5 min each, slides were incubated with visualization reagent at room temperature for 30 min. Following two additional washes in TBS-T, slides were visualized using DAB substrate-chromagen (K3468, DAKO). Sections were then dehydrated through a graded series of ethanol concentrations, cleared in xylene and coverslipped in DPX mounting medium. Images were taken by Photoshop CS3 using Leica DM2500 microscope under 40X objective.
Fluorescence in situ hybridization (FISH)
Dual-color FISH was performed to assess copy number changes in three oncogenes including ERBB2, FGFR2 and MET. The ERBB2/CEP17 dual color FISH probe (30–171060, Vysis) was purchased from Vysis Company, the FGFR2 and MET FISH probes were generated internally by directly labeling BAC (FGFR2: RP11-62L18; MET: CTD-2270N20) DNA with Red-dUTP (02N34-050, ENZO). The CEP10- Spectrum Green probe (Vysis, Cat # 32–132010) and CEP7- Spectrum Green probe (32–132007, Vysis) for the centromeric regions of chromosome 10 and chromosome 7 were used as internal controls for the FGFR2 and MET probes respectively. Detailed procedures were previously described [41]. In brief, assays were run on 4 micron dewaxed and dehydrated FFPE samples, or 3 micron snap-frozen tissues. The SpotLight Tissue pretreatment Kit (00–8401, Invitrogen) was used for pretreatment according to the manufacturer’s instructions. Sections and probes were codenaturated at 79°C for 6 minutes and then hybridized at 37°C for 48 hours (FFPE) or 24 hours (frozen tissues). After a quick post wash off process (0.3%NP40/2xSSC at 75.5°C for 2 minutes, twice in 2×SSC at room temperature for 2 minutes), sections were mounted with 0.3μg/ml DAPI (H-1200, Vector).
FISH signals were observed using a fluorescence microscope equipped with the appropriate filters to allow visualization of the intense red/green signals and the blue counterstained nuclei. For ERBB2 analysis, tumors with a ratio of ERBB2 to CEP17 ≥2 were defined as amplified; for FGFR2 analysis, tumors with a ratio of FGFR2 to CEP10 ≥2 were defined as amplified and tumors with an average MET gene copy number of greater than 5 was defined as amplified.
Criteria for defining biomarker positivity
Tumors were categorized as ERBB1/ERBB3/MET expression positive when the IHC staining signal intensity was 2+ or 3+ (0–3+ scale) in >10% of tumor cells. For PTEN, tumors with an IHC staining signal intensity of 0 (0–3+ scale) were categorized as ‘PTEN loss’. FGFR2 and MET FISH positivity was defined by gene amplification. ERBB2 positive cases were defined as IHC 3+ or IHC 2+ plus FISH amplification following the criteria used in the gastric TOGA trial [42].
Statistical analysis
The analysis was conducted using R version 3.0.2. The demographic and clinical characteristics of the two groups were compared using the Fisher’s Exact Test for categorical variables or Mann-Whitney U Test for continuous variables. The difference in survival distributions between the two groups were compared using the log-rank test. Two-sided P values <0.05 were considered statistically significant. Biomarker concordance between the primary samples and the xenograft models were assessed using the Cohen’s kappa coefficient. For the extent of agreement of the estimated kappa, the judgment given by Landis J.R. and Koch G.G. was used [43].
Results
PDGCX model establishment
Among 207 human GC tissue engrafted mouse models, patient tumors grew up in 49 (23.7%) immunodeficient mouse models (F1) within three months post implantation, either in 16.9% (14/83) nude mice or in 26.9% (32/119) SCID mice, or from both species (3/5). Furthermore, 32 out of 49 F1 tumor tissues continued growth after implantation into nude mice (F2) and were passageable for more than three passages (≥F3), giving a final success rate of 15.5% (32/207).
To evaluate the potential impact of GC patient clinicopathological parameters on PDGCX model establishment success rate, we compared patients’ clinical parameters by dividing the GC patient tumors into ‘Established model’ and ‘Failed model’ groups as listed in Table 1. Statistical analysis revealed that the success rate of model establishment was independent of most patients’ pathological parameters such as age, tumor grade, clinical stage, TNM status, recurrence status and overall survival. However, mouse models were more likely to be successfully established if derived from a male patient’s GC tissue (p = 0.016) or from an intestinal GC (p = 0.030)
Table 1. Clinical characteristics of primary gastric carcinomas.
| Parameters | Evaluable patient number | Unevaluable patient number* | Established model | Failed Model | p-value | |
|---|---|---|---|---|---|---|
| Gender | 161 | 46 | 0.016 a | |||
| Male | 26 | 83 | ||||
| Female | 4 | 48 | ||||
| Median Age | 195 | 12 | 62 | 62 | 0.532 b | |
| Tumor grade | 158 | 49 | 0.271 a | |||
| 2 | 12 | 33 | ||||
| 3 | 16 | 87 | ||||
| 4 | 2 | 8 | ||||
| Clinical stage | 160 | 47 | 0.587 a | |||
| 1 | 0 | 4 | ||||
| 2 | 10 | 29 | ||||
| 3 | 14 | 70 | ||||
| 4 | 6 | 27 | ||||
| Tumor Size | 161 | 46 | 0.729 a | |||
| T1 | 0 | 2 | ||||
| T2 | 2 | 7 | ||||
| T3 | 28 | 115 | ||||
| T4 | 0 | 7 | ||||
| Lymph node metastases | 158 | 49 | 0.635 a | |||
| N0 | 9 | 27 | ||||
| N1 | 12 | 52 | ||||
| N2 | 4 | 28 | ||||
| N3 | 5 | 23 | ||||
| Distant Metastases | 161 | 46 | 0.643 a | |||
| M0 | 28 | 125 | ||||
| M1 | 2 | 6 | ||||
| Lauren subtype | 191 | 16 | 0.030 a | |||
| Intestinal type | 16 | 41 | ||||
| Diffused type | 11 | 86 | ||||
| Mixed type | 5 | 32 | ||||
| Site | 158 | 49 | 0.415 a | |||
| Pylorus | 15 | 67 | ||||
| Cardia | 3 | 16 | ||||
| Body | 9 | 43 | ||||
| Pylorus and body | 0 | 4 | ||||
| Lesser curvature | 1 | 0 | ||||
| Recurrence | 160 | 47 | 1.000 a | |||
| No recurrence | 28 | 124 | ||||
| With recurrence | 1 | 7 | ||||
| Mean Overall Survival (Month) | 138 | 69 | 0.347 c | |||
| 14.0869 | 21 | |||||
| Std Error | 1.31631 | 12.01009777 |
a: Fisher exact test
b: Mann Whitney U Test
c: Log Rank Test
* patients had missing information
Histological assessment
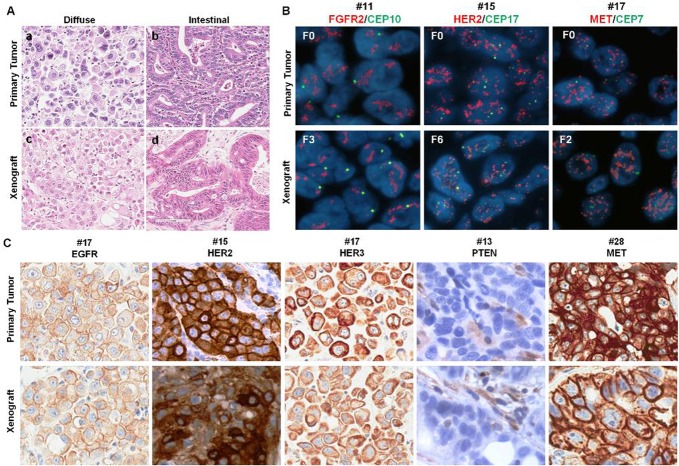
Histological H&E assessment was performed by pathologist for all 207 GC patient tumors and 32 established PDGCX models. 16 models were classified as intestinal subtype, 11 were diffuse subtype and 5 were mixed subtype. Compared to the corresponding human tumors, all 32 PDGCX models perfectly maintained the same histological features as their parental human tumors (Fig 1A).
Fig 1. PDGCX models retain the histological features and genetic profiles of their parental tumors.
(A) shows comparison of histological subtypes of GC observed in primary tumors (a, b) and corresponding PDGCX models (c, d); (B) shows FGFR2, ERBB2 and MET gene amplification in primary tumors (F0) and corresponding PDGCX models. All target gene probes are labeled in red and CEP control probes are in green, nuclei are counterstained in blue by DAPI. (C) shows ERBB1 (+++), ERBB2 (+++), ERBB3 (+++), PTEN (-ve) and MET (+++) expression on primary tumors and corresponding PDGCX models.
Genetic characterization
To explore the genetic characteristics of the established PDGCX models, we focused on analysis of well-characterized driver oncogenes in GC including: the ERBB family members (ERBB1, ERBB2 & ERBB3), PTEN, FGFR2 and MET as listed in S1 Table. Among the 32 PDGCX models, 78% (25/32) of models showed positivity for ERBB1, 22% (7/32) were positive for ERBB2, 78% (25/32) were ERBB3 positive, 66% (21/32) lost PTEN expression, 3% (1/32) harbored FGFR2 amplification, 41% (13/32) were MET expression positive, and 16% (5/32) were defined as MET amplified.
The genetic profile concordance between the PDGCX models and their parental patient tissues was analyzed either by agreement rate according to biomarker positivity, or by statistical analysis using the Cohen’s kappa coefficient. Between the PDGCX models and their corresponding primary tumors, a high degree of concordance was observed at the DNA level for FGFR2 and MET genes (agreement rate = 100%; kappa value = 1) (Fig 1B); ERBB2 status (combining FISH and IHC data together) showed almost perfect agreement (agreement rate = 94%; kappa value = 0.82) (Fig 1B and 1C); MET expression positivity and PTEN loss defined by IHC showed moderate agreement (agreement rate = 78%; kappa value = 0.46~0.56), whilst ERBB1 and ERBB3 expression showed only slight agreement (agreement rate = 59~75%; kappa value = 0.18~0.19) by IHC (Fig 1C) (S1 Table).
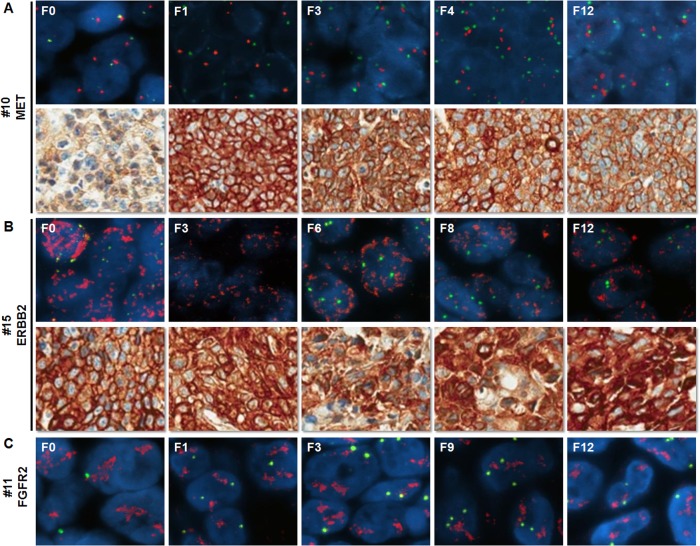
PDGCX model genetic stability
To evaluate the genetic stability of the various biomarkers through sequential passages of established PDGCX models, we examined three biomarkers from different passages of 5 biomarker positive models. ERBB2 and MET protein expression, as well as FGFR2, MET and ERBB2 gene amplification were continuously maintained in all generations of the same models up until the 12th passage (Fig 2).
Fig 2. Biomarker profiles of serial passages of PDGCX models.
Representative images of MET status (A), ERBB2 status (B) and FGFR2 gene amplification (C) on serial passages of PDGCX models by FISH or IHC are shown. All target gene probes for FISH are labeled in red and CEP control probes are in green, nuclei are counterstained in blue by DAPI.
Discussion
PDCX models from a variety of cancer types have been successfully established in recent years. We previously reported successful establishment of a large cohort of PDCX models from non-small cell lung cancer (NSCLC) and esopheageal cancers (EC) [17, 25]. However, the success rates for PDCX model establishment from different tumor types vary considerably, ranging from 5% to 80% [44]. For gastric cancer, only a few successful PDGCX models have been reported, indicating the difficulty of PDGCX establishment using human surgical tumor samples [33–36]. In our present study, we successfully established 32 PDGCX models, passagable to at least F3. To improve this success rate, we implanted human GC tissues simultaneously into nude and SCID mice at F0. Comparison of this data showed a trend towards a higher success rate when using SCID mice, compared to the use of nude mice either for the F1 generation (28.2% vs. 19.3%) or for the final successful establishment (16.9% vs. 13.6%), although these differences were not statistically significant. This data suggests that the more severe immunodeficient species may offer a superior platform for successfully establishing a potential PDGCX model. In the current study, we also observed that GC tissues from male patients (p = 0.016) or of intestinal subtype (p = 0.030) were easier to grow up in mice (Table 1). Interestingly, gender and Lauren subtype were highly correlated (p = 0.02589) in this cohort. The majority of the female patients (40 out of 49) presented with diffuse tumors, while the majority of patients with intestinal subtype tumors were male (38 out 47). Therefore, it is difficult to judge which factor(s) are dominant in determining the primary tumor’s potential to grow in mice.
The biomarkers studied here were selected based on existing known targets or potential new targets for clinical therapeutic strategies in GC. As molecular targets for guided therapeutics, these biomarkers are largely oncogenic drivers in GC, driven by either genomic or protein expression aberrations (or both). Therefore, the consistency of expression of these markers between human GC and PDGCX models, and among different generations within PDGCX models, is of importance in evaluating the utility of targeted therapeutic drugs. To explore how well this panel of 32 PDGCX models represented human GC, we performed further genetic characterization studies using IHC and FISH assays, in order to provide an in situ analysis of the target gene’s copy number and protein expression. The individual PDGCX model’s histological and genetic profiles were compared with the parental human GC tumors and agreement rates determined according to biomarker positivity and by Cohen’s Kappa. Our data revealed either high or perfect agreement in the majority of the biomarkers tested by either method, especially at the DNA level. Although ERBB1 and ERBB3 protein expression were judged to have ‘slight agreement’ according to kappa value, these biomarkers still showed an agreement rate of 59% and 75% between parental tumors and PDGCX models. A closer look at the data revealed that parental tumors and PDGCX models agreed well for both protein biomarkers, when the parental tumor was positive for the biomarker (14/16 for ERBB1 and 22/27 for ERBB3). This link was less obvious when the primary tumor was negative for the biomarker (5/16 for ERBB1 and 2/5 for ERBB3). Thus, the disagreement judged by Cohen’s Kappa could be a consequence of statistical bias due to the relatively small number of primary tumor negative samples. Furthermore, intratumoral heterogeneity of different biomarkers has been frequently reported in surgical GC samples [45–49], and may represent a more intrinsical reason for the inconsistencies between primary tumors and models. Nevertheless, the profiles of all tested biomarkers across the whole panel of 32 PDGCX models accurately reflects that of their prevalence in human GC samples, which is reported as 30~75% for ERBB1 positivity [50, 51], 15~22% for ERBB2 positivity [37, 50, 51], 60~70% for ERBB3 high expression [51, 52], 39~47% for PTEN loss [53, 54], 4–5% for FGFR2 amplification [8, 9, 37, 55], 20~70% for MET high expression [37, 56–59] and 0~10% for MET amplification [37, 56, 58–60]. This high concordance could in part be attributable to the lack of correlation between most of the patient clinicopathological parameters and model success rates (i.e. There appears to be little bias on model success, so most patient tumors should give rise to viable PDGCX models). Importantly, the positivity of ERBB2, FGFR2 and MET, either at the DNA or protein level, were stably maintained in all PDGCX models, out to at least passage F12. This considerable fidelity and duration of genetic maintenance underscores another advantage of using PDGCX models for evaluation of pre-clinical drug efficacy.
The purpose of generating this panel of PDGCX models was to evaluate the anti-tumor efficacy of targeted therapeutic drugs. Indeed, a number of PDGCX models from this panel with different genetic aberrations have been previously used to successfully explore preclinical drug efficacy [9, 37–40]. For example, models with positive ERBB2 (#8 and 16), FGFR2 amplification (# 11), PTEN loss (#2) and MET amplification (#10, 18 and 30) were sensitive to Trastuzumab, AZD4547, AZD5363 and Volitinib, respectively. Taken together, these data highlight the considerable utility of PDGCX models in evaluating preclinical drug efficacy, enabling definition of prospective biomarker selection criteria and modeling of tumor architecture and genetic heterogeneity.
Conclusions
In summary, we have successfully established a panel of 32 PDGCX models and demonstrated that these models faithfully recapitulate the histological characteristics and genetic diversity of the primary human tumors. Furthermore, these PDGCX xenograft models maintain genetic diversity until at least the 12th model passage. Our data also show that the panel in its entirety accurately reflects the tested biomarker profiles present in the wider human GC population. Thus, this panel of PDGCX models represents a valuable tool in understanding this lethal disease and serves as a powerful resource in enabling preclinical efficacy testing in oncology drug discovery.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study is fully funded by AstraZeneca. To clarify, AstraZeneca authors were fully involved in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2. Power DG, Kelsen DP, Shah MA. Advanced gastric cancer—slow but steady progress. Cancer Treat Rev. 2010;36:384–392. 10.1016/j.ctrv.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 3. Asaoka Y, Ikenoue T, Koike K. New targeted therapies for gastric cancer. Expert Opin Investig Drugs. 2011;20:595–604. 10.1517/13543784.2011.566863 [DOI] [PubMed] [Google Scholar]
- 4. de Mello RA, Marques AM, Araujo A. HER2 therapies and gastric cancer: a step forward. World J Gastroenterol. 2013;19:6165–6169. 10.3748/wjg.v19.i37.6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 6. Ramucirumab approved for gastric cancer. Cancer Discov. 2014;4:752–753. [DOI] [PubMed] [Google Scholar]
- 7. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 8. Hong L, Han Y, Liu J, Brain L. Fibroblast growth factor receptor 2: a therapeutic target in gastric cancer. Expert Rev Gastroenterol Hepatol. 2013;7:759–765. 10.1586/17474124.2013.837804 [DOI] [PubMed] [Google Scholar]
- 9. Xie L, Su X, Zhang L, Yin X, Tang L, Zhang X, et al. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res. 2013;19:2572–2583. 10.1158/1078-0432.CCR-12-3898 [DOI] [PubMed] [Google Scholar]
- 10. Salgia R, Patel P, Bothos J, Yu W, Eppler S, Hegde P, et al. Phase I dose-escalation study of onartuzumab as a single agent and in combination with bevacizumab in patients with advanced solid malignancies. Clin Cancer Res. 2014;20:1666–1675. 10.1158/1078-0432.CCR-13-2070 [DOI] [PubMed] [Google Scholar]
- 11. Kang YK, Muro K, Ryu MH, Yasui H, Nishina T, Ryoo BY, et al. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32:355–361. 10.1007/s10637-013-0057-2 [DOI] [PubMed] [Google Scholar]
- 12. Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007–1018. 10.1016/S1470-2045(14)70023-3 [DOI] [PubMed] [Google Scholar]
- 13. Zhu M, Tang R, Doshi S, Oliner KS, Dubey S, Jiang Y, et al. Exposure-response analysis of rilotumumab in gastric cancer: the role of tumour MET expression. Br J Cancer. 2015;112:429–437. 10.1038/bjc.2014.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okamoto W, Okamoto I, Arao T, Kuwata K, Hatashita E, Yamaguchi H, et al. Antitumor action of the MET tyrosine kinase inhibitor crizotinib (PF-02341066) in gastric cancer positive for MET amplification. Mol Cancer Ther. 2012;11:1557–1564. 10.1158/1535-7163.MCT-11-0934 [DOI] [PubMed] [Google Scholar]
- 15. Teng L, Lu J. cMET as a potential therapeutic target in gastric cancer (Review). Int J Mol Med. 2013;32:1247–1254. 10.3892/ijmm.2013.1531 [DOI] [PubMed] [Google Scholar]
- 16. Daniel VC, Marchionni L, Hierman JS, Rhodes JT, Devereux WL, Rudin CM, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–3373. 10.1158/0008-5472.CAN-08-4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, Jiang D, Li X, Lv J, Xie L, Zheng L, et al. Establishment and characterization of esophageal squamous cell carcinoma patient-derived xenograft mouse models for preclinical drug discovery. Lab Invest. 2014;94:917–926. 10.1038/labinvest.2014.77 [DOI] [PubMed] [Google Scholar]
- 18. Zhang XC, Zhang J, Li M, Huang XS, Yang XN, Zhong WZ, et al. Establishment of patient-derived non-small cell lung cancer xenograft models with genetic aberrations within EGFR, KRAS and FGFR1: useful tools for preclinical studies of targeted therapies. J Transl Med. 2013;11:168 10.1186/1479-5876-11-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu X, Zhang J, Zhen R, Lv J, Zheng L, Su X, et al. Trastuzumab anti-tumor efficacy in patient-derived esophageal squamous cell carcinoma xenograft (PDECX) mouse models. J Transl Med. 2012;10:180 10.1186/1479-5876-10-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J, Zhang L, Su X, Li M, Xie L, Malchers F, et al. Translating the therapeutic potential of AZD4547 in FGFR1-amplified non-small cell lung cancer through the use of patient-derived tumor xenograft models. Clin Cancer Res. 2012;18:6658–6667. 10.1158/1078-0432.CCR-12-2694 [DOI] [PubMed] [Google Scholar]
- 21. Jin K, Teng L, Shen Y, He K, Xu Z, Li G. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 2010;12:473–480. 10.1007/s12094-010-0540-6 [DOI] [PubMed] [Google Scholar]
- 22. Zhang H, Cohen AL, Krishnakumar S, Wapnir IL, Veeriah S, Deng G, et al. Patient-derived xenografts of triple-negative breast cancer reproduce molecular features of patient tumors and respond to mTOR inhibition. Breast Cancer Res. 2014;16:R36 10.1186/bcr3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scott CL, Becker MA, Haluska P, Samimi G. Patient-derived xenograft models to improve targeted therapy in epithelial ovarian cancer treatment. Front Oncol. 2013;3:295 10.3389/fonc.2013.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilie M, Nunes M, Blot L, Hofman V, Long-Mira E, Butori C, et al. Setting up a wide panel of patient-derived tumor xenografts of non-small cell lung cancer by improving the preanalytical steps. Cancer Med. 2014;In Press. [DOI] [PMC free article] [PubMed]
- 25. Fichtner I, Rolff J, Soong R, Hoffmann J, Hammer S, Sommer A, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res. 2008;14:6456–6468. 10.1158/1078-0432.CCR-08-0138 [DOI] [PubMed] [Google Scholar]
- 26. Burgenske DM, Monsma DJ, Dylewski D, Scott SB, Sayfie AD, Kim DG, et al. Establishment of genetically diverse patient-derived xenografts of colorectal cancer. Am J Cancer Res. 2014;4:824–837. [PMC free article] [PubMed] [Google Scholar]
- 27. Cho YB, Hong HK, Choi YL, Oh E, Joo KM, Jin J, et al. Colorectal cancer patient-derived xenografted tumors maintain characteristic features of the original tumors. J Surg Res. 2014;187:502–509. 10.1016/j.jss.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 28. Julien S, Merino-Trigo A, Lacroix L, Pocard M, Goere D, Mariani P, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18:5314–5328. 10.1158/1078-0432.CCR-12-0372 [DOI] [PubMed] [Google Scholar]
- 29. Lin D, Xue H, Wang Y, Wu R, Watahiki A, Dong X, et al. Next generation patient-derived prostate cancer xenograft models. Asian J Androl. 2014;16:407–412. 10.4103/1008-682X.125394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin D, Wyatt AW, Xue H, Wang Y, Dong X, Haegert A, et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 2014;74:1272–1283. 10.1158/0008-5472.CAN-13-2921-T [DOI] [PubMed] [Google Scholar]
- 31. Mattie M, Christensen A, Chang MS, Yeh W, Said S, Shostak Y, et al. Molecular characterization of patient-derived human pancreatic tumor xenograft models for preclinical and translational development of cancer therapeutics. Neoplasia. 2013;15:1138–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu Y, Tian T, Li Z, Tang Z, Wang L, Wu J, et al. Establishment and characterization of patient-derived tumor xenograft using gastroscopic biopsies in gastric cancer. Sci Rep. 2015;5:8542 10.1038/srep08542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin K, He K, Han N, Li G, Wang H, Xu Z, et al. Establishment of a PDTT xenograft model of gastric carcinoma and its application in personalized therapeutic regimen selection. Hepatogastroenterology. 2011;58:1814–1822. 10.5754/hge11136 [DOI] [PubMed] [Google Scholar]
- 34. Jin K, Lan H, Cao F, Xu Z, Han N, Li G, et al. Antitumor effect of FP3 in a patient-derived tumor tissue xenograft model of gastric carcinoma through an antiangiogenic mechanism. Oncol Lett. 2012;3:1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Milne AN, Sitarz R, Carvalho R, Polak MM, Ligtenberg M, Pauwels P, et al. Molecular analysis of primary gastric cancer, corresponding xenografts, and 2 novel gastric carcinoma cell lines reveals novel alterations in gastric carcinogenesis. Hum Pathol. 2007;38:903–913. [DOI] [PubMed] [Google Scholar]
- 36. Lu J, Li G, He K, Jiang W, Xu C, Li Z, et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J Transl Med. 2015;13:42 10.1186/s12967-015-0398-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu YJ, Shen D, Yin X, Gavine P, Zhang T, Su X, et al. HER2, MET and FGFR2 oncogenic driver alterations define distinct molecular segments for targeted therapies in gastric carcinoma. Br J Cancer. 2014;110:1169–1178. 10.1038/bjc.2014.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gavine PR, Ren Y, Han L, Lv J, Fan S, Zhang W, et al. Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol Oncol. 2015;9:323–333. 10.1016/j.molonc.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li J, Davies BR, Han S, Zhou M, Bai Y, Zhang J, et al. The AKT inhibitor AZD5363 is selectively active in PI3KCA mutant gastric cancer, and sensitizes a patient-derived gastric cancer xenograft model with PTEN loss to Taxotere. J Transl Med. 2013;11:241 10.1186/1479-5876-11-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Ye Q, Lv J, Ye P, Sun Y, Fan S, et al. Evaluation of Trastuzumab Anti-Tumor Efficacy and its Correlation with HER-2 Status in Patient-Derived Gastric Adenocarcinoma Xenograft Models. Pathol Oncol Res. 2015;In Press. [DOI] [PMC free article] [PubMed]
- 41. Su X, Zhan P, Gavine PR, Morgan S, Womack C, Ni X, et al. FGFR2 amplification has prognostic significance in gastric cancer: results from a large international multicentre study. Br J Cancer. 2014;110:967–975. 10.1038/bjc.2013.802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. 10.1111/j.1365-2559.2008.03028.x [DOI] [PubMed] [Google Scholar]
- 43. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 44. Jin K, Teng L, Shen Y, He K, Xu Z, Li G. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 12:473–480. 10.1007/s12094-010-0540-6 [DOI] [PubMed] [Google Scholar]
- 45. Cho EY, Park K, Do I, Cho J, Kim J, Lee J, et al. Heterogeneity of ERBB2 in gastric carcinomas: a study of tissue microarray and matched primary and metastatic carcinomas. Mod Pathol. 2013;26:677–684. 10.1038/modpathol.2012.205 [DOI] [PubMed] [Google Scholar]
- 46. Lee HE, Park KU, Yoo SB, Nam SK, Park do J, Kim HH, et al. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur J Cancer. 2013;49:1448–1457. 10.1016/j.ejca.2012.10.018 [DOI] [PubMed] [Google Scholar]
- 47. Stahl P, Seeschaaf C, Lebok P, Kutup A, Bockhorn M, Izbicki JR, et al. Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol. 2015;15:7 10.1186/s12876-015-0231-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tajiri R, Ooi A, Fujimura T, Dobashi Y, Oyama T, Nakamura R, et al. Intratumoral heterogeneous amplification of ERBB2 and subclonal genetic diversity in gastric cancers revealed by multiple ligation-dependent probe amplification and fluorescence in situ hybridization. Hum Pathol. 2014;45:725–734. 10.1016/j.humpath.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 49. Yang J, Luo H, Li Y, Li J, Cai Z, Su X, et al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62:221–228. 10.1007/s12013-011-9286-1 [DOI] [PubMed] [Google Scholar]
- 50. Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000. 10.1158/1078-0432.CCR-12-1318 [DOI] [PubMed] [Google Scholar]
- 51. Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL Jr., et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29:3030–3036. 10.1200/JCO.2010.33.6313 [DOI] [PubMed] [Google Scholar]
- 52. Hayashi M, Inokuchi M, Takagi Y, Yamada H, Kojima K, Kumagai J, et al. High expression of HER3 is associated with a decreased survival in gastric cancer. Clin Cancer Res. 2008;14:7843–7849. 10.1158/1078-0432.CCR-08-1064 [DOI] [PubMed] [Google Scholar]
- 53. Chong ML, Loh M, Thakkar B, Pang B, Iacopetta B, Soong R. Phosphatidylinositol-3-kinase pathway aberrations in gastric and colorectal cancer: meta-analysis, co-occurrence and ethnic variation. Int J Cancer. 2014;134:1232–1238. 10.1002/ijc.28444 [DOI] [PubMed] [Google Scholar]
- 54. Tran TN, Brettingham-Moore K, Duong CP, Mitchell C, Clemons NJ, Phillips WA. Molecular changes in the phosphatidylinositide 3-kinase (PI3K) pathway are common in gastric cancer. J Surg Oncol. 2013;108:113–120. 10.1002/jso.23357 [DOI] [PubMed] [Google Scholar]
- 55. Matsumoto K, Arao T, Hamaguchi T, Shimada Y, Kato K, Oda I, et al. FGFR2 gene amplification and clinicopathological features in gastric cancer. Br J Cancer. 2012;106:727–732. 10.1038/bjc.2011.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carneiro F, Sobrinho-Simoes M. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 2000;88:238–240. [DOI] [PubMed] [Google Scholar]
- 57. Ha SY, Lee J, Kang SY, Do IG, Ahn S, Park JO, et al. MET overexpression assessed by new interpretation method predicts gene amplification and poor survival in advanced gastric carcinomas. Mod Pathol. 2013;26:1632–1641. 10.1038/modpathol.2013.108 [DOI] [PubMed] [Google Scholar]
- 58. Janjigian YY, Tang LH, Coit DG, Kelsen DP, Francone TD, Weiser MR, et al. MET expression and amplification in patients with localized gastric cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1021–1027. 10.1158/1055-9965.EPI-10-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK, Lee BL, et al. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer. 2012;107:325–333. 10.1038/bjc.2012.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kawakami H, Okamoto I, Arao T, Okamoto W, Matsumoto K, Taniguchi H, et al. MET amplification as a potential therapeutic target in gastric cancer. Oncotarget. 2013;4:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.