Abstract
Eclampsia, clinically defined as unexplained seizure in a woman with preeclampsia, is a life threatening complication unique to the pregnant state. However, a subpopulation of women with seemingly uncomplicated pregnancies experience de novo seizure without preeclamptic signs or symptoms, suggesting pregnancy alone may predispose the brain to seizure. Here, we hypothesized that normal pregnancy lowers seizure threshold and investigated mechanisms by which pregnancy may affect seizure susceptibility, including neuroinflammation and plasticity of gamma-aminobutyric acid type A receptor (GABAAR) subunit expression. Seizure threshold was determined by quantifying the amount of pentylenetetrazole (PTZ) required to elicit electrical seizure in Sprague Dawley rats that were either nonpregnant (Nonpreg, n = 7) or pregnant (Preg; d20, n = 6). Seizure-induced vasogenic edema was also measured. Further, activation of microglia, a measure of neuroinflammation (n = 6-8/group), and GABAAR δ- and γ2-subunit protein expression in the cerebral cortex and hippocampus (n = 6/group) was determined. Seizure threshold was lower in Preg compared to Nonpreg rats (36.7±9.6 vs. 65.0±14.5 mg/kg PTZ; p<0.01) that was associated with greater vasogenic edema formation (78.55±0.11 vs. 78.04±0.19% water; p<0.05). The % of active microglia was similar between groups; however, pregnancy was associated with downregulation of cortical GABAAR-δ and hippocampal GABAAR-γ2 expression. Overall, pregnancy appears to be a state of increased seizure susceptibility that is not due to neuroinflammation, but rather is associated with reduced expression of GABAAR subunits and greater edema. Understanding neurophysiological changes occurring in normal pregnancy could allow for better prevention and management of de novo seizure, including pathologic states such as eclampsia.
Introduction
Preeclampsia, defined as the new onset of hypertension and proteinuria after the 20th week of gestation, is a life-threatening complication of pregnancy that afflicts 1–7% of all pregnancies [1–4]. Numerous organs are affected by preeclampsia, including the brain in the form of eclampsia [5–9]. Eclampsia is the appearance of unexplained seizure in a woman with preeclampsia and is one of the most dangerous complications of pregnancy [4]. Eclampsia is a leading cause of maternal and fetal morbidity and mortality worldwide that accounts for greater than 50,000 maternal deaths each year [6, 7, 10]. While eclampsia by definition is restricted to women with preeclampsia, seizure during pregnancy does not appear to be a progression from severe preeclampsia to eclampsia [11–13]. In fact, de novo seizure has been reported to occur in 38% of seemingly uncomplicated pregnancies, without hypertension and proteinuria, or the diagnosis of preeclampsia [12]. The finding that de novo seizure occurs in the absence of preeclampsia suggests that pregnancy alone may be a state of increased seizure susceptibility. In addition, women who develop eclampsia are by definition normotensive and asymptomatic prior to pregnancy, with no known underlying conditions contributing to seizure onset, supporting the concept that pregnancy alone may predispose the brain to seizure independently of preeclampsia.
It is well-established that fluctuations in neurosteroids occur during the menstrual cycle, and to a greater extent during pregnancy, that can affect neuronal excitability [14–16]. Specifically, increases in progesterone metabolites, including allopregnanolone, act as positive allosteric modulators of gamma-aminobutyric acid type A receptors (GABAARs), the main inhibitory neurotransmitter receptors in the brain, and decrease neuronal excitability [16]. GABAARs consist of several subunits, however, the main binding site and therefore site of action of allopregnanolone is on the δ-subunit [17]. δ-subunit-containing GABAARs (GABAAR-δ) are located extrasynaptically and are involved in tonic inhibition throughout the brain [16]. Neuroactive steroids such as allopregnanolone reduce neuronal excitability selectively through actions at GABAAR-δ; however, GABAAR-δ are downregulated in response to increased allopregnanolone to maintain the excitatory/inhibitory balance [16, 17]. Plasticity of the δ-subunit of GABAARs occurs in the brain during pregnancy, and is an adaptation that likely functions to maintain a steady state of excitability in the face of increased neurosteroids [17, 18]. In fact, brain slices from pregnant mice were found to be hyperexcitable due to the downregulation of the δ-subunit that was normalized by the presence of allopregnanolone [18]. While these in-vitro studies suggest that the brain is hyperexcitable during pregnancy, it is less clear if the brain is more susceptible to seizure under normal physiological conditions of pregnancy where naturally circulating neurosteroids are present. Further, synaptic γ2-subunit-containing GABAARs (GABAAR-γ2) are involved in phasic inhibition and have also been shown to decrease expression in the brain during pregnancy [19]. Although synaptic GABAAR-γ2 are not affected by neurosteroids [16], pregnancy-induced changes in their expression may also contribute to increased seizure susceptibility during pregnancy.
One mechanism by which brain excitability may be affected during pregnancy is through peripheral inflammation. Pregnancy is considered a state of mild peripheral inflammation and peripheral inflammation has been shown to cause neuroinflammation through the activation of microglia, the resident immune cells in the brain [20, 21]. When microglia become active, they aid in clearance of debris and play a reparative role in the brain; however, for unknown reasons they can become cytotoxic [22]. Active microglia secrete proinflammatory cytokines and increase local neuronal excitability by simultaneously promoting the endocytosis of GABAARs and exocytosis of excitatory α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [21, 23]. In fact, neuroinflammation has recently been shown to be present in a rat model of preeclampsia that was associated with decreased seizure threshold [24]. Whether neuroinflammation is present during normal pregnancy is unknown, however, it may be one mechanism by which the pregnant state is more susceptible to seizure. We hypothesized that pregnancy is a state of increased seizure susceptibility due to decreased expression of GABAARs and/or neuroinflammation that acts to lower seizure threshold.
Onset of eclampsia may also be related to loss of cerebral blood flow autoregulation and decreased cerebrovascular resistance that increases pressure on the microcirculation and subsequent vasogenic edema formation [25]. In fact, vasogenic edema is present in ~ 90% of women with eclampsia, as assessed by diffusion weighted MRI [26, 27]. Further, vasogenic edema formation occurs to a greater extent during pregnancy under pathologic conditions such as acute hypertension, suggesting pregnancy predisposes the brain to edema [28, 29]. Thus, we hypothesized that the maternal brain is more susceptible to seizure-induced vasogenic edema formation than in the nonpregnant state. Understanding if the maternal brain is more susceptible to seizure-induced edema may lead to a greater understanding of the pathophysiological process of de novo seizure during pregnancy.
Methods
Animals and ethics statement
All experiments were conducted using virgin, nonpregnant (Nonpreg) or timed-pregnant (Preg) Sprague Dawley rats that were 14–16 weeks old (Charles River, Canada). Pregnant rats were used experimentally late in gestation (day 20 of a 22 day gestation), a time point when eclampsia occurs most often [12]. Preg rats do not exhibit preeclamptic-like symptoms, as it has previously been reported that Nonpreg and Preg rats have similar blood pressures and urinary protein excretion [30, 31]. All rats were housed singly in the University of Vermont Animal Care Facility, an Association for Assessment and Accreditation of Laboratory Animal Care International accredited facility. All procedures were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Animals were euthanized under either isoflurane or chloral hydrate anesthesia according to NIH guidelines.
Measurement of seizure threshold, susceptibility and severity, and brain water content
Rats that were Nonpreg (n = 9) or Preg (n = 9) were anesthetized initially with isoflurane (1–3% in oxygen) for intubation, electrode placement and instrumentation. Animals were mechanically ventilated to maintain blood gases and pH within normal physiological ranges. Body temperature was monitored with a rectal thermometer and maintained with a heating pad at 37°C throughout the experiment. The dorsal surface of the head was shaved to expose the scalp and silver subdermal corkscrew electrodes (Ambu, Glen Burnie, MD, USA) were implanted under the scalp and secured in place with collodion glue. Electroencephalography (EEG) was recorded unipolarly using a MP150 acquisition system (BIOPAC System Inc., Goleta, CA, USA) to monitor generalized seizure, as previously described [24]. After placement of electrodes, animals were placed in supine position for placement of venous and arterial catheters. Femoral arteries were cannulated to obtain blood samples for blood gas measurements and continuous measurement of arterial blood pressure via a pressure transducer (BIOPAC Systems Inc., Goleta, CA, USA). Femoral veins were cannulated for administration of the anesthetic chloral hydrate and infusion of the chemoconvulsant pentylenetetrazole (PTZ). PTZ was used because it reliably elicits seizure through antagonistic actions at GABAARs, thereby allowing investigation into the direct involvement of GABAAR plasticity in whole brain excitability during pregnancy. After instrumentation, animals were tapered off isoflurane and anesthesia maintained by continuous intravenous infusion of chloral hydrate (41.5 mg / mL in Nonpreg, 50 mg / mL in Preg; 30 μL / min) and seizure threshold measured as previously described [24]. Chloral hydrate was used because it is thought to not depress neural function, and is the preferred anesthetic for studies measuring EEG [32, 33]. Seizure threshold was calculated as the amount of PTZ (mg/kg) required to elicit electrical seizure: Tinfusion * Rinfusion * [PTZ] / bw where Tinfusion is the time of infusion in min, Rinfusion is the rate of infusion in mL/min, [PTZ] is the concentration of PTZ in mg/mL, and bw is the body weight in kg. Seizure susceptibility scores were also calculated: bw * 10/v, where bw is body weight in grams and v is volume of PTZ infused in μL [21]. Baseline blood pressures were taken 30 seconds prior to PTZ infusion and at seizure onset. EEG was recorded for 30 minutes post-PTZ infusion and seizure severity assessed by counting the number of recurring seizures and calculating the percent of the post-infusion period spent in seizure. Two Nonpreg and three Preg rats were excluded because blood gases were outside of the physiological range. Seizure severity was not assessed in one Preg rat because EEG recordings were not available for the entire 30 minutes due to a technical issue with the ground electrode. After 30 minutes animals were euthanized under chloral hydrate anesthesia by decapitation and brains immediately removed. The cerebral cortex was bisected coronally into anterior and posterior sections just posterior to the M2 vertical branch of the middle cerebral artery. The posterior cerebral cortex was isolated and weighed wet (weightwet), then dried in a laboratory oven at 90° C for 24 hours and re-weighed dry (weightdry). The posterior brain region was chosen as it is a primary location of edema in women with eclampsia [34]. Percent water content was determined by wet:dry weights using the following formula: (weightwet—weightdry/weightwet) * 100. Brain water content of one Nonpreg rat was not measured because the 24-hour drying time point was unable to be completed.
Quantification and morphological assessment of microglia
Separate groups of Nonpreg (n = 6) and Preg (n = 8) rats were euthanized under isoflurane anesthesia and brains immediately removed. A 3 mm section (4–7 mm posterior to bregma) was taken of the posterior cerebral cortex and fixed in 10% buffered formalin at 4°C overnight, then transferred to 0.1 M PBS and slices paraffin embedded. Immunohistochemical staining for ionized calcium binding adapter molecule 1 (Iba 1; Wako, Richmond, VA, USA), a marker for microglia was done using standard procedure as described previously [24]. For each brain section, four micrographs of cerebral cortex were captured using an Olympus BX50 microscope at 20X magnification. Each Iba1+ cell was assessed by its morphology and activation state ranked using a graded scale from 1 (relatively inactive) to 4 (relatively active) as previously described [24]. To assess microglia, two analyses were performed. First, the percentage of cells in each activation state was calculated for each micrograph and averaged per group. Second, total number of Iba1+ cells were counted per mm2 and averaged for each group. Two evaluators that were blinded to group performed all morphological assessments.
GABAAR subunit expression by Western blot
Separate groups of Nonpreg and Preg animals (n = 6 / group) were euthanized under isoflurane anesthesia and brains immediately removed. Cerebral cortex and hippocampus were isolated and snap frozen in liquid nitrogen and stored at– 80°C. Cerebral cortex or hippocampus from either Nonpreg or Preg rats were homogenized in homogenization buffer and centrifuged at 100,000 g at 4° C for 30 min. The pellet was resuspended in homogenization buffer containing 1% Triton-X-100 for 1 hour on ice, then centrifuged at 100,000 g at 4°C for 30 min. The supernatant was collected as the membrane fraction as done previously [35]. Protein concentration was determined using a DC Protein Assay (BioRad, Hercules, CA, USA). The supernatant was converted to a gel sample by adding 5X Laemmli sample prep buffer and beta-mercaptoethanol to a final concentration of 280 mM. For determination of membrane protein concentration of GABAAR-δ and GABAAR-γ2, 35 μg or 25 μg of either cerebral cortex or hippocampus was loaded on a 10% Mini-PROTEAN TGX Precast Gel (BioRad, Hercules, CA, USA) and ran at 30 mA, respectively. The gel was then wet transferred to a polyvinyl difluoride membrane at 90 V for 45 min. The membrane was Ponceau-S stained to ensure that protein was properly transferred. The membrane was blocked with 5% non-fat dried milk in TBS-T for 1.5 hrs at room temperature. The membrane was then rinsed with TBS-T and the primary antibody was applied, anti-GABAAR-δ (1:200; Santa Cruz Biotechnology, Dallas, TX, USA) or anti-GABAAR-γ2 (1:1000, Novus, Littleton, CO, USA) overnight at 4°C with rocking. The antibodies were removed and the membrane rinsed with TBS-T and secondary antibody was applied, anti-Goat (1:100,000) (Southern Biotech, Birmingham, AL, USA) for GABAAR-δ or anti-Rabbit (1:100,000, Promega, Madison, WI, USA) for GABAAR-γ2 for 1 hr at room temperature. The secondary antibodies were removed and subsequent TBS-T washes were done. West Pico chemiluminescent substrate (ThermoScientific, Rockford, IL, USA) was applied to the membrane and then exposed to film. β-actin served as a loading control (1:2000, Abcam, Cambridge, MA, USA) and all samples were normalized to a Nonpreg control.
Drugs and solutions
Chloral hydrate and PTZ were purchased from Sigma Aldrich (St Louis, MO, USA) and made daily in sterile lactated Ringer’s solution. Western blot solutions were prepared as follows: Homogenization buffer contained 50 mM Tris HCl, pH 7.4, 5 mM EDTA, 10 mM EGTA, protease inhibitor cocktail (1:200; Sigma Aldrich, St Louis, MO, USA) and 0.5 mM DTT, and TBS-T contained 15.4 mM Tris-HCl, pH 7.4, 137.0 mM NaCl, 0.1% Tween-20.
Statistical Analysis
Data are presented as mean ± standard error of mean. All comparisons were made between groups using Student’s t-test and differences were considered significant at p < 0.05.
Results
The effect of pregnancy on seizure threshold, susceptibility and severity
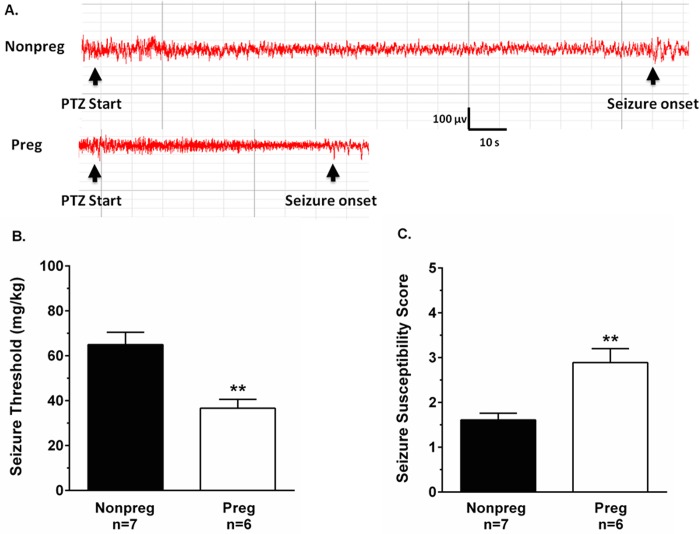
To determine the effect of pregnancy on seizure susceptibility, seizure was induced with PTZ and seizure threshold determined in Nonpreg and Preg rats. Fig 1A shows representative EEG tracings during seizure threshold measurements from Nonpreg (top tracing) and Preg rats (bottom tracing). The black arrowheads indicate when PTZ infusion started and the time of seizure onset. PTZ infusion caused an initial decrease in EEG amplitude in both groups of animals; however, the time to seizure onset was longer in Nonpreg rats compared to Preg rats. When seizure threshold was calculated, the amount of PTZ (mg/kg) required to elicit electrical seizure was significantly lower in Preg compared to Nonpreg rats (Fig 1B). Further, Preg rats had significantly higher seizure susceptibility scores compared to Nonpreg rats (Fig 1C). There were no differences in physiological parameters during seizure threshold measurements between groups, except that, as expected, body weights were significantly higher in Preg compared to Nonpreg rats (Table 1). Baseline arterial blood pressures under chloral hydrate anesthesia were similar between Nonpreg and Preg rats (95 ± 4 vs. 86 ± 4 mm Hg; p > 0.05), and with seizure onset arterial blood pressures remained similar between Nonpreg and Preg rats (85 ± 7 vs. 94 ± 6 mm Hg; p > 0.05).
Fig 1. The effect of normal pregnancy on seizure threshold and susceptibility.
(A) Representative EEG tracings from nonpregnant (Nonpreg) and late-pregnant (Preg) rats during timed-infusion of pentylenetetrazol (PTZ). Black arrows indicate when PTZ infusion started and the onset of spike-wave discharge indicative of electrical seizure. (B) Seizure threshold was significantly lower in Preg rats compared to Nonpreg rats. (C) Preg rats scored higher on the seizure susceptibility scale compared to Nonpreg rats. ** p < 0.01 vs. Nonpreg by Student’s t-test.
Table 1. Physiological parameters of nonpregnant (Nonpreg) and late-pregnant (Preg) rats under chloral hydrate anesthesia for seizure threshold measurements.
| Nonpreg (n = 7) | Preg (n = 6) | |
|---|---|---|
| Body Weight (grams) | 287 ± 7 | 377 ± 10 ** |
| Body Temp ( o C) | 36.3 ± 0.2 | 36.8 ± 0.2 |
| Arterial P O2 (mm Hg) | 114 ± 8 | 111 ± 7 |
| Arterial P CO2 (mm Hg) | 42.1 ± 1.9 | 42.8 ± 1.7 |
** p < 0.01 vs. Nonpreg using Student’s t-test
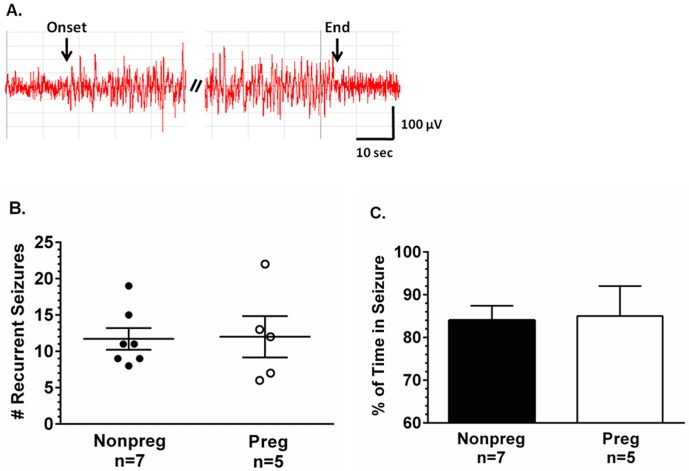
To determine if there were changes in seizure severity during pregnancy, the total number of recurrent seizures was counted in the 30 minutes post-infusion time period, and the percent of time spent in seizure calculated. Fig 2A shows a representative EEG tracing within the 30 minutes post-PTZ infusion recording time period. Indicated on the tracing is the rapid onset of a recurrent seizure, followed by the rapid cessation of the recurring seizure. There was no change in the number of recurrent seizures in the 30 minutes post-PTZ infusion between groups (Fig 2B). There were also no differences in the percent of time spent in seizure between Nonpreg and Preg rats (Fig 2C).
Fig 2. The effect of pregnancy on seizure severity.
(A) Representative EEG tracing of a recurrent seizure during the 30-minute post-pentylenetetrazol (PTZ) infusion time period of a nonpregnant (Nonpreg) rat. Left black arrow indicates seizure onset and right black arrow seizure cessation. (B) Number of recurrent seizures in the 30-minute post-PTZ infusion period was similar between Nonpreg and late-pregnant (Preg) rats. (C) The percent of time spent in seizure during the 30-minute post-PTZ time period was similar between Nonpreg and Preg rats.
The effect of pregnancy on microglial activation and GABAAR subunit expression
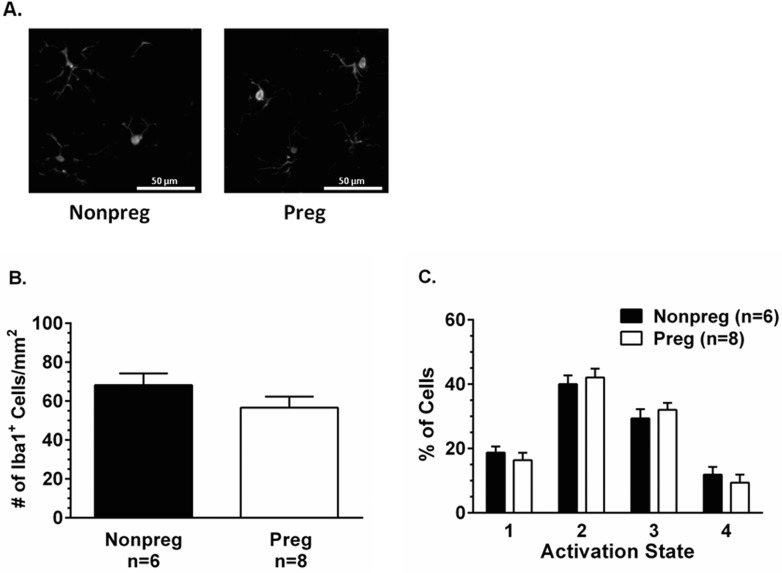
To determine if normal pregnancy was a state of basal neuroinflammation, we used the microglia-specific marker Iba 1 to morphologically assess the activation state of microglia in the posterior cerebral cortex from Nonpreg and Preg rats. Fig 3A shows representative photomicrographs of Iba 1+ microglia in the posterior cerebral cortices of Nonpreg and Preg rats. There was no difference in the total number of microglial cells in the posterior cerebral cortex between groups (Fig 3B). Further, there were no changes in basal microglial activation during pregnancy with no differences in the percent of cells in any activation state between groups (Fig 3C). Thus, it appears that the lower seizure threshold measured in Preg rats was not due to microglial activation.
Fig 3. Basal activation state of microglia in cerebral cortex of nonpregnant (Nonpreg) and pregnant (Preg) rats.
(A) Representative photomicrographs of Iba 1+ microglia in the cerebral cortices of Nonpreg and Preg rats. (B) There was no difference in the number of microglia in the cerebral cortices of Nonpreg and Preg rats. (C) The percent of Iba 1+ microglial cells in each activation state was similar in the cortices of Nonpreg and Preg rats.
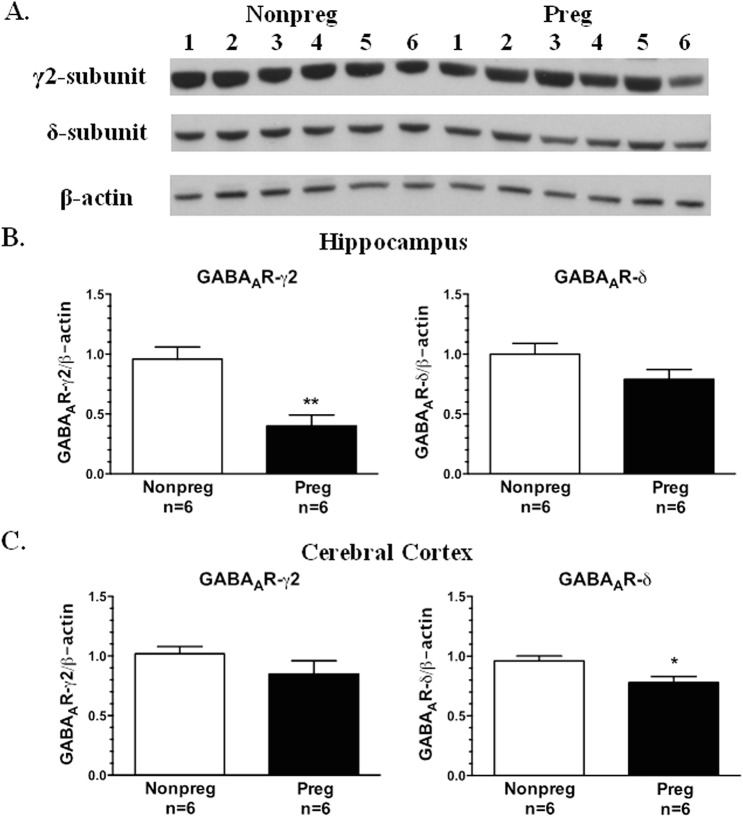
To determine if pregnancy induced changes in protein expression of GABAAR subunits in the hippocampus or cerebral cortex, GABAAR δ- and GABAAR γ2-subunit expression was measured using Western blot, and representative Western blots are shown in Fig 4A. In the hippocampus there was a statistically significant decrease in expression of the GABAAR γ2-subunit in Preg compared to Nonpreg rats, and a similar trend in GABAAR δ-subunit expression (Fig 4B). In the cerebral cortex GABAAR γ2-subunit expression was similar between groups; however, GABAAR δ-subunit expression was significantly decreased in Preg compared to Nonpreg rats (Fig 4C). This pregnancy-induced GABAAR subunit plasticity appears to contribute to the decrease in seizure threshold in Preg rats.
Fig 4. The effect of pregnancy on GABAAR δ- and γ2-subunit protein expression in the hippocampus and cerebral cortex.
(A) Representative Western blots showing protein expression of the GABAAR γ2-subunit and δ-subunit in the cerebral cortex of nonpregnant (Nonpreg) and pregnant (Preg) rats. (B) GABAAR γ2-subunit protein expression was significantly lower in the hippocampus from Preg compared to Nonpreg rats, and GABAAR δ-subunit expression trended towards being decreased in the hippocampus of Preg compared to Nonpreg rats. (C) Cortical GABAAR γ2-subunit expression was similar in Preg and Nonpreg rats, however, GABAAR δ-subunit expression was decreased in the cerebral cortex of Preg compared to Nonpreg rats. ** p < 0.01; * p < 0.05 vs. Nonpreg using Student’s t-test.
The effect of pregnancy on seizure-induced vasogenic brain edema formation
Percent water content of the brain was calculated post-seizure in Nonpreg and Preg rats to determine if there were differences in seizure-induced vasogenic edema formation. The percent of water content was significantly higher in the brains of Preg rats after seizure compared to Nonpreg rats (Fig 5). Thus, the maternal brain seems to be more susceptible to seizure-induced vasogenic edema that the nonpregnant state.
Fig 5. The effect of seizure on vasogenic edema formation in nonpregnant (Nonpreg) and late-pregnant (Preg) rats.
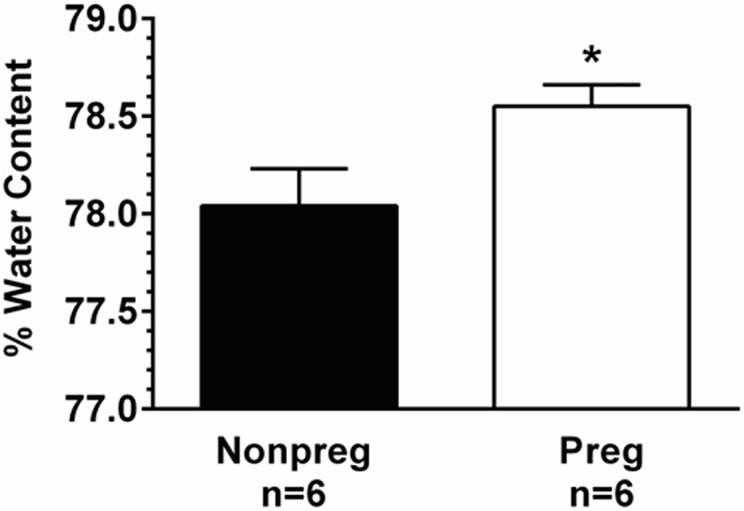
Percent water content of the posterior cerebral cortex was significantly higher after seizure in Preg compared to Nonpreg rats. * p < 0.05 vs. Nonpreg using Student’s t-test.
Discussion
De novo seizure can occur during seemingly uncomplicated pregnancies without preeclamptic symptoms, suggesting that an adaptation to normal pregnancy may increase the sensitivity of the brain to seizure [11, 12]. Therefore, the current study tested the hypothesis that normal pregnancy is a state of increased seizure susceptibility due to basal neuroinflammation and plasticity of GABAARs. The major finding of this study was that normal pregnancy was a state of increased seizure susceptibility that did not appear to be due to basal neuroinflammation; however, there was decreased GABAAR δ-subunit expression in the cerebral cortex and γ2-subunit expression in the hippocampus of pregnant compared to nonpregnant rats that could contribute to the increase in excitability. These findings support the concept that pregnancy may predispose the brain to seizure, whether in seemingly uncomplicated pregnancies or those complicated by preeclampsia, given the maternal brain appears to be in a hyperexcitable state.
The increased seizure susceptibility during pregnancy was not associated with microglial activation. However, we did find decreased inhibitory GABAAR subunit expression in the brains of pregnant rats that could affect seizure threshold. GABAAR-δ are the main inhibitory neurotransmitter receptors in the brain that are tonically active to provide continuous inhibitory input to neurons and tightly regulate neuronal activity and network excitability [16]. Others have shown that during pregnancy, there is plasticity of GABAARs in response to elevated endogenous neurosteroids, including the progesterone metabolite allopregnanolone [16, 18, 19, 36]. Allopregnanolone binds to the δ-subunit and enhances the function of GABAARs [16]. As an apparent compensation to enhanced GABAAR function, GABAAR subunits downregulate to avoid over-inhibition that maintains a normal state of neuronal excitability [16, 18]. Interestingly, despite high levels of naturally circulating neurosteroids present in pregnant animals in the current study that would be expected to maintain normal excitability, seizure threshold was still lower during pregnancy than the nonpregnant state. Thus, it appears that decreased expression of both GABAAR δ- and γ2- subunits during pregnancy were more important for overall excitability than elevated neurosteroids, at least in response to PTZ, a GABAAR antagonist.
It is important to note that while we found that seizure threshold was lower in pregnant animals, these animals (and the majority of women) do not have spontaneous seizure during pregnancy. This suggests that there are mechanisms that protect the brain from seizure during pregnancy despite having a hyperexcitable brain, and that de novo seizure during pregnancy may only occur under certain conditions. For example, seizure-provoking factors are circulating late in gestation that cause epileptiform activity when exposed directly to brain parenchyma [37]. However, these factors do not readily gain access to the brain due to the blood-brain barrier (BBB), suggesting the BBB may be a critical interface in preventing seizure during pregnancy. It is possible that the BBB adapts over the course of gestation to protect the maternal brain from seizure-provoking circulating factors. We speculate that failure of the BBB to adapt during pregnancy may be one mechanism by which de novo seizure occurs during pregnancy by allowing passage of seizure-provoking factors into the hyperexcitable maternal brain.
The brain has been shown to be more susceptible to vasogenic edema formation during pregnancy under certain pathologic conditions, such as acute hypertension [28, 29]. The current study shows that the maternal brain was also more sensitive to seizure-induced brain injury, with seizure causing greater vasogenic edema formation than in the nonpregnant state. It is important to note that previous studies have revealed no differences in basal brain water content between pregnant and nonpregnant rats [28]. Thus, the increase in brain water content found in the present study appeared to be due to an effect of seizure. Although edema formation has been found in ~ 90% of women with eclampsia [26, 27], suggesting it to be an underlying cause of the condition, seizure itself can cause edema formation through disruption of the BBB [38–40]. The finding in the current study that the brain is more susceptible to seizure-induced vasogenic edema formation during pregnancy further supports the concept that the brain is more susceptible to injury-induced edema formation during pregnancy. In addition, sensitivity to seizure-induced brain edema during normal pregnancy may explain the presence of vasogenic edema in eclamptic women who experienced de novo seizure without an acute elevation in blood pressure.
There are several potential contributors to increased susceptibility to cerebral vasogenic edema formation during pregnancy. First, if pregnancy were associated with either more severe seizure or seizure-induced acute hypertension, there may be greater brain injury and edema formation. However, there were no differences in seizure severity between pregnant and nonpregnant rats nor did seizure cause a change in blood pressure in either group, making these possibilities unlikely. Second, capillary density increases in the posterior cerebral cortex during pregnancy [41] that may increase the number of sites of BBB disruption during seizure. Lastly, plasma volume increases 50% during pregnancy resulting in a hemodiluted and hyponatremic state that under conditions of BBB disruption such as seizure may drive water into the brain due to decreased osmolality [42]. Regardless of the mechanism, it appears that the maternal brain is more susceptible to vasogenic edema formation during conditions that cause BBB disruption, highlighting the importance of seizure prevention during pregnancy.
In conclusion, understanding pregnancy-related neurophysiological changes may clarify mechanisms by which eclamptic seizure occurs during seemingly uncomplicated pregnancies when there are failures of other protective mechanisms, such as at the BBB or changes in neurosteroid concentrations involved in maintaining steady-state excitability. Further, clarifying the contribution of normal pregnancy to seizure onset could lead to a greater understanding of pregnancy-specific pathologies such as eclampsia. Understanding these conditions may result in development of specific screenings to identify pregnant women who are at risk of de novo seizure, aiding in seizure prevention and specific treatment during pregnancy.
Acknowledgments
We thank Nicole Bishop in the Microscopy Imaging Center at the University of Vermont for her technical expertise in performing immunohistochemistry.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Institutes of Health, National Institute of Neurologic Disorders and Stroke grant RO1 NS045940 to MJC (URL: http://www.ninds.nih.gov/); National Institutes of Health, National Institute of Neurologic Disorders and Stroke Neural Environment Cluster Supplement RO1 NS 045940-06S1 to MJC (URL: http://www.ninds.nih.gov/); and the American Heart Association Fellowship 14PRE18590005 to ACJ (URL: http://www.heart.org/HEARTORG/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341(8858):1447–51. Epub 1993/06/05. 0140-6736(93)90889-O [pii]. . [DOI] [PubMed] [Google Scholar]
- 2. Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544 e1- e12. 10.1016/j.ajog.2013.08.019 . [DOI] [PubMed] [Google Scholar]
- 3. Rich-Edwards JW, Ness RB, Roberts JM. Epidemiology of Pregnancy-Related Hypertension In: Taylor RN, Roberts JM, Cunningham FG, Lindheimer MD, editors. Chesley's Hypertensive Disorders in Pregnancy. Fourth ed: Academic Press/Elsevier; 2014. [Google Scholar]
- 4. Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121 Suppl 1:14–24. 10.1111/1471-0528.12629 . [DOI] [PubMed] [Google Scholar]
- 5. Donaldson JO. Eclampsia. Adv Neurol. 1994;64:25–33. Epub 1994/01/01. . [PubMed] [Google Scholar]
- 6. Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99(7):547–53. Epub 1992/07/01. . [DOI] [PubMed] [Google Scholar]
- 7. Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–7. Epub 2009/05/26. S0146-0005(09)00021-4 [pii] 10.1053/j.semperi.2009.02.010 . [DOI] [PubMed] [Google Scholar]
- 8. Aukes AM, Wessel I, Dubois AM, Aarnoudse JG, Zeeman GG. Self-reported cognitive functioning in formerly eclamptic women. Am J Obstet Gynecol. 2007;197(4):365 e1-6. Epub 2007/10/02. S0002-9378(07)00820-4 [pii] 10.1016/j.ajog.2007.06.044 . [DOI] [PubMed] [Google Scholar]
- 9. Aukes AM, de Groot JC, Aarnoudse JG, Zeeman GG. Brain lesions several years after eclampsia. Am J Obstet Gynecol. 2009;200(5):504 e1-5. Epub 2009/03/10. S0002-9378(08)02439-3 [pii] 10.1016/j.ajog.2008.12.033 . [DOI] [PubMed] [Google Scholar]
- 10. Donaldson JO. Eclampsia Neurology of Pregnancy. Philadelphia: W.B. Saunders; 1989. p. 269–310. [Google Scholar]
- 11. Katz VL, Farmer R, Kuller JA. Preeclampsia into eclampsia: toward a new paradigm. Am J Obstet Gynecol. 2000;182(6):1389–96. 10.1067/mob.2000.106178 . [DOI] [PubMed] [Google Scholar]
- 12. Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309(6966):1395–400. Epub 1994/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sibai BM. Eclampsia. VI. Maternal-perinatal outcome in 254 consecutive cases. Am J Obstet Gynecol. 1990;163(3):1049–54; discussion 54–5. Epub 1990/09/01. 0002-9378(90)91123-T [pii]. . [DOI] [PubMed] [Google Scholar]
- 14. Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27(9):2155–62. Epub 2007/03/03. 27/9/2155 [pii] 10.1523/JNEUROSCI.4945-06.2007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 2009;34 Suppl 1:S84–90. Epub 2009/07/28. S0306-4530(09)00211-X [pii] 10.1016/j.psyneuen.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100(24):14439–44. Epub 2003/11/19. doi: 10.1073/pnas.2435457100 2435457100 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther. 2007;116(1):20–34. 10.1016/j.pharmthera.2007.03.007 . [DOI] [PubMed] [Google Scholar]
- 18. Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci. 2009;29(30):9592–601. Epub 2009/07/31. 29/30/9592 [pii] 10.1523/JNEUROSCI.2162-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207–13. Epub 2008/08/01. S0896-6273(08)00537-0 [pii] 10.1016/j.neuron.2008.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179(1):80–6. Epub 1998/08/15. S0002937898702546 [pii]. . [DOI] [PubMed] [Google Scholar]
- 21. Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci U S A. 2008;105(44):17151–6. Epub 2008/10/29. 0806682105 [pii] 10.1073/pnas.0806682105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization-new prospects for brain repair. Nature reviews Neurology. 2014. 10.1038/nrneurol.2014.207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25(12):3219–28. 10.1523/JNEUROSCI.4486-04.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson AC, Tremble SM, Chan SL, Moseley J, LaMarca B, Nagle KJ, et al. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PloS one. 2014;9(11):e113670 Epub 2014/11/20. doi: 10.1371/journal.pone.0113670 PONE-D-14-43811 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50(1):14–24. Epub 2007/06/06. HYPERTENSIONAHA.106.079442 [pii] 10.1161/HYPERTENSIONAHA.106.079442 . [DOI] [PubMed] [Google Scholar]
- 26. Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, et al. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol. 2013;208(6):468 e1-6. 10.1016/j.ajog.2013.02.015 . [DOI] [PubMed] [Google Scholar]
- 27. Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol. 2004;190(3):714–20. Epub 2004/03/26. 10.1016/j.ajog.2003.09.015S0002937803011244 [pii]. . [DOI] [PubMed] [Google Scholar]
- 28. Euser AG, Cipolla MJ. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension. 2007;49(2):334–40. Epub 2007/01/04. 01.HYP.0000255791.54655.29 [pii] 10.1161/01.HYP.0000255791.54655.29 . [DOI] [PubMed] [Google Scholar]
- 29. Cipolla MJ, Bishop N, Chan SL. Effect of pregnancy on autoregulation of cerebral blood flow in anterior versus posterior cerebrum. Hypertension. 2012;60(3):705–11. Epub 2012/07/25. HYPERTENSIONAHA.112.198952 [pii] 10.1161/HYPERTENSIONAHA.112.198952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cipolla MJ, DeLance N, Vitullo L. Pregnancy prevents hypertensive remodeling of cerebral arteries: a potential role in the development of eclampsia. Hypertension. 2006;47(3):619–26. Epub 2005/12/29. 01.HYP.0000196948.15019.28 [pii] 10.1161/01.HYP.0000196948.15019.28 . [DOI] [PubMed] [Google Scholar]
- 31. Kanayama N, Tsujimura R, She L, Maehara K, Terao T. Cold-induced stress stimulates the sympathetic nervous system, causing hypertension and proteinuria in rats. J Hypertens. 1997;15(4):383–9. Epub 1997/04/01. . [DOI] [PubMed] [Google Scholar]
- 32. Thoresen M, Henriksen O, Wannag E, Laegreid L. Does a sedative dose of chloral hydrate modify the EEG of children with epilepsy? Electroencephalogr Clin Neurophysiol. 1997;102(2):152–7. Epub 1997/02/01. S0921884X96965091 [pii]. . [DOI] [PubMed] [Google Scholar]
- 33. Olson DM, Sheehan MG, Thompson W, Hall PT, Hahn J. Sedation of children for electroencephalograms. Pediatrics. 2001;108(1):163–5. Epub 2001/07/04. . [DOI] [PubMed] [Google Scholar]
- 34. Schwartz RB, Jones KM, Kalina P, Bajakian RL, Mantello MT, Garada B, et al. Hypertensive encephalopathy: findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 1992;159(2):379–83. Epub 1992/08/01. . [DOI] [PubMed] [Google Scholar]
- 35. Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8(6):797–804. Epub 2005/05/17. nn1469 [pii] 10.1038/nn1469 . [DOI] [PubMed] [Google Scholar]
- 36. Biggio G, Cristina Mostallino M, Follesa P, Concas A, Sanna E. GABA(A) receptor function and gene expression during pregnancy and postpartum. International review of neurobiology. 2009;85:73–94. 10.1016/S0074-7742(09)85006-X . [DOI] [PubMed] [Google Scholar]
- 37. Cipolla MJ, Pusic AD, Grinberg YY, Chapman AC, Poynter ME, Kraig RP. Pregnant serum induces neuroinflammation and seizure activity via TNFalpha. Exp Neurol. 2012;234(2):398–404. Epub 2012/01/28. S0014-4886(12)00006-4 [pii] 10.1016/j.expneurol.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donaldson J. The brain in eclampsia. hypertens Pregnancy. 1994;13:115–33. [Google Scholar]
- 39. Oztas B, Kaya M, Kucuk M, Tugran N. Influence of hypoosmolality on the blood-brain barrier permeability during epileptic seizures. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):701–4. Epub 2003/06/06. S0278-5846(03)00084-8 [pii] 10.1016/S0278-5846(03)00084-8 . [DOI] [PubMed] [Google Scholar]
- 40. Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006;47(11):1761–74. Epub 2006/11/23. EPI817 [pii] 10.1111/j.1528-1167.2006.00817.x . [DOI] [PubMed] [Google Scholar]
- 41. Cipolla MJ, Sweet JG, Chan SL. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J Appl Physiol. 2011;110(2):329–39. Epub 2010/11/13. japplphysiol.01159.2010 [pii] 10.1152/japplphysiol.01159.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clapp JF 3rd, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80(11):1469–73. Epub 1997/12/17. S0002-9149(97)00738-8 [pii]. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.