Abstract
Background
As an important factor causing end-stage renal disease, diabetic nephropathy is correlated with low-grade chronic inflammation and immune system activation. This study aimed to investigate the protective function of puerarin on the kidneys of diabetic rats.
Material/Methods
A cohort of healthy male SD rats (7 weeks old) were randomly divided into a control group, a model group, and a puerarin treatment group with high (H), moderate (M), and low (L) dosage. After streptozotocin induction, puerarin was applied via intragastric administration for 8 consecutive weeks with dosages of 0.25, 0. 5 and 1.0 mg/(kg·d) for L, M, and H groups, respectively. Fasting blood glucose (BG), creatinine (Scr), urea nitrogen (BUN), and urine albumin excretion rate (UAER) were measured, along with morphological observation of renal cells. The expression of intracellular adhesion molecule 1 (ICAM-1) and tumor necrosis factor α (TNF-α) was determined using immunohistochemical (IHC) staining, while renal cortex cell apoptosis was assayed by in situ end-labeling method.
Results
Model rats had significantly elevated levels of BG, Scr, BUN, and UAER compared to controls (p<0.05). All these increases were partially but significantly suppressed by puerarin (p<0.05), which also caused marked improvement of histopathological damages. Puerarin at each dosage significantly eliminated elevations of ICAM-1 and TNF-α levels in model rats (p<0.05), and decreased apoptotic indexes of renal cortex cells (p<0.05).
Conclusions
Early-stage renal damages can be significantly improved by puerarin, possibly via its suppression of ICAM-1 and TNF-α expression in diabetic rat kidneys.
MeSH Keywords: Diabetic Nephropathies; Pueraria; Receptors, Tumor Necrosis Factor, Type II
Background
As a common complication of diabetes, incidence of diabetic nephropathy (DN) is increasing in China [1]. The pathogenesis of DN has not been clearly illustrated yet. Due to the inherent complexity of metabolic disorders, treatment of end-stage DN is more difficult compared to other renal diseases. Therefore, interference at an early stage is critical for DN patients. Studies have shown that DN is related to various factors, including genetics, abnormal renal hemodynamics, metabolic disorders caused by hyperglycemia, hypertension, and metabolic disorders of vasoactive substances [2–4]. Both intracellular adhesion molecule 1 (ICAM-1) and tumor necrosis factor α (TNF-α) are closely related with diabetes, as the former plays an important role in the development of diabetic atherosclerosis while the latter factor can facilitate the secretion of adhesion factors from endothelial cells. After exogenous stimuli such as inflammatory cytokines and glycosylation end-products, endothelial cells have an increased level of ICAM-1 expression. Therefore, ICAM-1 works as an important index reflecting early-stage pathological changes of diabetes and participates in the pathogenesis of diabetic nephropathy. TNF-α, on the other hand, can exert a synergistic effect with glycosylation end-products to activate the adhesion between endothelial cells and inflammatory cells. The elevation of adhesion molecules and inflammatory factors such as ICAM-1, TNF-α, and IL-1 in diabetic renal tissues can effectively facilitate the adhesion between neutrophils, monocytes, and endothelial cells, which are further induced to release various cytokines, further aggravating inflammatory response and related renal damages [5–7]. Furthermore, oxidative stress and non-enzymatic glycosylation also play critical roles in DN, whose complications can be retarded by the suppression of oxidative stress and non-enzymatic glycosylation [8,9].
Puerarin is the main effective component extracted from the rhizome of Pueraria lobate (Willd.) and has been shown to have multiple functions, including inhibition of aldose reductase activity or non-enzymatic glycosylation of proteins, improvement of microcircuits, and regulating systolic and diastolic function of endothelial cells, as well as anti-free radicals and clearance of free radicals produced by glycosylated reaction and lipid peroxidation [10]. It has also been shown to inhibit the production of glycosylated protein, suppress glycosylated low-density lipoprotein or hemoglobin, and elevate ICAM-1 expression on the surface of vessel; therefore, can be used in the treatment of cardiovascular disease and diabetic nephropathy [10]. This study therefore aimed to investigate the possible mechanism of puerarin in protecting kidneys via the measurement of expressional profiles of ICAM-1 and TNF-α in diabetic rat kidneys.
Material and Methods
Experimental animals
Healthy male SD rats (7 weeks old, body weight=200~250 g) were provided by the Animal Experimental Center of Shandong University. All rats were kept in an SPF grade animal house, with food and water provided ad libitum. Animals were randomly divided into 5 groups: a control group, a diabetic model group, a high-dose puerarin (H) group, a moderate-dose puerarin (M) group, and a low-dose puerarin (L) group (N=10 per group). All procedures performed on living animals were pre-approved by the Ethics Committee of Animal Study in our institute.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of Shandong University.
Diabetic rat models and puerarin application
Twelve hours after fasting, 1% streptozotocin (Sigma, USA) diluted in citric acid buffer was injected at a single dose of 60 mg/kg into intraperitoneal cavity of rats in model and all 3 treatment groups. Control animals received an equal volume of citric acid buffers. The diabetic model was determined by the fasting blood glucose level, which is higher than 16.7 mmol/L at 72 h after the injection. Urine protein levels were quantified from 24-h urine samples collected from the metabolic cages.
Puerarin (Jinnong Biotech, China) as brown powder was re-suspended in 0.9% saline and was given by intra-gastric intubation at various concentrations (0.25 mg/(kg·d) for L group, 0.5 mg/(kg·d) for M group, and 1.0 mg/(kg·d) for H group) each day for 8 consecutive days. An equal volume of saline was administered to control and model rats during the same time period.
Biochemical assays
General healthy conditions, including mental status, food and water intake, and urine volume, were observed and recorded daily. Body weights of all animals were measured every 2 weeks and blood glucose level was quantified each month. At the end of the experiment (8 weeks after the injection), 24-h urine samples were collected from fasting rats for quantification of 24-h urinary proteins. Blood samples were collected from peritoneal veins, centrifuged at 3500 rpm and extracted for serum. Fasting blood glucose (BG), creatinine (Scr), and blood urea nitrogen (BUN) were measured using test kits (Jiancheng Biotech, China) following the manual instructions.
Histopathological assays
All rats were then sacrificed to collect both kidneys. The kidney weight index (KI) was calculated as (left kidney weight)/body weight. The renal cortex were removed and divided into small tissue cubes, which were then fixed in 10% formalin. The expression level of ICAM-1 and TNF-α was semi-quantified by immunohistochemical (IHC) staining methods using rabbit anti-rat ICAM-1 /TNF-α polyclonal antibodies and DAB chromogenic substrates (Boster Biotech, China) followed by the measurement of integral optical density (IOD) values of positive-staining areas. Cell apoptosis was detected using TUNEL in situ end-labelling method by test kits (Boster Biotech, China). The apoptotic index (AI) is calculated as: AI=number of apoptotic cells in five 400× fields/total number of cells ×100%.
Statistical analysis
All collected data were analyzed by SPSS 17.0 software package (IBM, USA). Measurement data are presented as mean ± standard deviation (SD). The comparison among multiple groups was performed using analysis of variance (ANOVA) test and further between-group comparison was done using the LSD-t test. The correlation between parameters was identified using the Spearman test. A statistical significance was defined as p<0.05.
Results
General conditions of animals
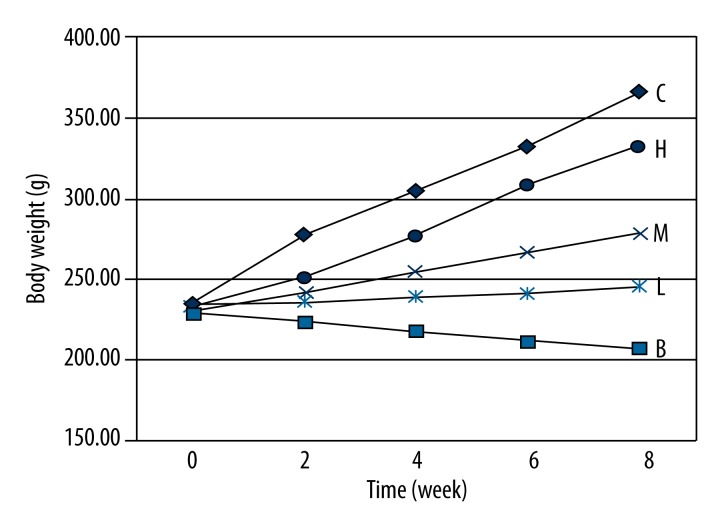
At the end of experiment, control rats had increased body weights, glossy smooth fur, and sensitive response. Diabetic rats in the model group, however, had significantly decreased body weights (Figure 1) and energy, accompanied with polydipsia, polyuria, diminished response, and rough dull fur. Animals with puerarin treatment (H, M and L groups) showed significantly elevated body weights, improved mental status, and sensitive response when compared to model rats. These treated rats, however, still showed impairments to some extents compared to control rats. The effect of puerarin on diabetic rat body weights is illustrated in Figure 1.
Figure 1.
Effects of puerarin on body weights of diabetic rats. Body weights of all rats were measured every 2 weeks and were averaged. The model animals had significantly decreased body weight while the application of puerarin significantly elevated body weights. C, control group; B, model group; H, M&L, high-, moderate-, and low-dose puerarin treatment groups.
The effect of puerarin on blood glucose and kidney function of diabetic rats
Table 1 shows that diabetic rats had significantly elevated levels of BG, Scr, BUN, UAER, and KI (p<0.01 compared to control rats), along with lower body weights (p<0.01). The application of puerarin markedly rescued the higher blood diabetic indexes and all biochemical indexes (p<0.05 compared to model rats), although they were still higher than in control rats.
Table 1.
The effect of puerarin on blood glucose and kidney function indexes.
| Group | Dosage mg/(kg·d) | Body weight (g) | KI (×10−3) | BG (mmol/L) | UAER (μg/24 h) | BUN (mmol/L) | Scr (μmol/L) |
|---|---|---|---|---|---|---|---|
| C | 0 | 366.45±15.66 | 2.43±0.32 | 5.29±0.85 | 123.21±5.97 | 2.88±0.28 | 65.12±3.54 |
| B | 0 | 206.77±11.12** | 4.92±0.27** | 26.78±1.66** | 1602.45±22.34** | 6.42±0.46** | 90.21±4.32** |
| H | 1.00 | 342.88±14.32**,## | 2.66±0.17**,## | 10.04±0.46**,## | 206.78±6.34**,## | 3.15±0.33**,## | 66.55±2.17*,## |
| M | 0.50 | 278.56±13.20**,## | 3.34±0.21**,## | 18.79±0.87**,## | 310.67±11.43**,## | 3.82±0.44**,## | 68.76±1.32** |
| L | 0.25 | 245.57±12.31**,## | 3.76±0.25**,## | 21.65±0.76**,## | 389.34±15.42**,## | 4.46±0.45**,## | 76.14±3.01**,# |
p<0.05,
p<0.01 compared to control group;
, p<0.05,
, p<0.01 compared to model group. N=10 per group.
Histopathological observation of renal cells
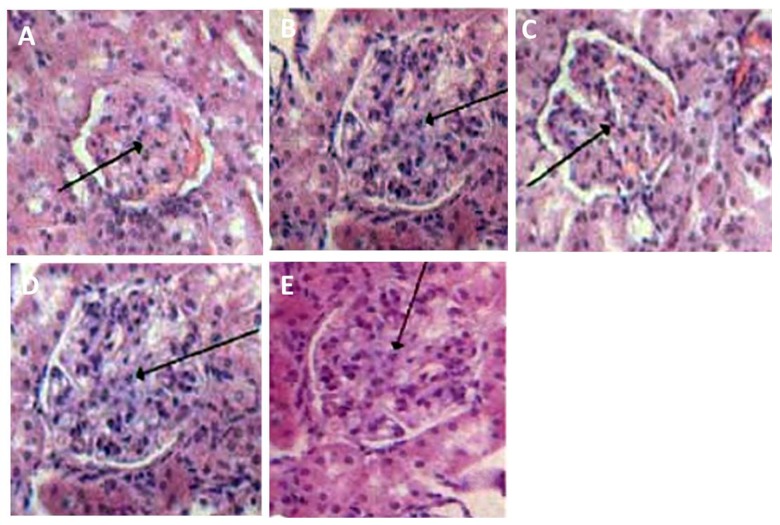
We used H-E staining to check the morphology of renal cells in all rats. As shown in Figure 2, the glomerulus in diabetic rat kidneys was enlarged, and we found adhesions of cystic walls and hyperplasia of glomerular mesangial cells, but these were significantly improved in the puerarin treatment group in a dose-dependent manner.
Figure 2.
Histopathological changes of diabetic rat kidneys. (A) control; (B) model; (C) high puerarin; (D) moderate puerarin; (E) low puerarin.
Protein expression of ICAM-1 and TNF-α in diabetic rat kidneys
Further IHC staining was performed to check the expressional profile of ICAM-1 and TNF-α in diabetic rat kidneys. As shown in Figure 2, model rats had significantly elevated expression of both proteins (p<0.01), and levels were lowered by the application of puerarin at any dose.
A further correlation analysis revealed that expression levels of ICAM-1 and TNF-α were positively correlated with each other (r=0.914, p<0.05). ICAM-1 expression level was also positively related to Scr and BUN (r=0.876, r=0.817, p<0.05 in both cases), which were also positively correlated with TNF-α expression level (r=0.879, r=0.847, p<0.05 in both cases).
Renal cortex cell apoptosis in diabetic rats
As shown in Table 2, diabetic model rats had significantly more apoptotic cells (presented as AI) in the renal cortex. The application of puerarin, however, decreased AI in a dose-dependent manner.
Table 2.
Effects of puerarin on apoptosis of renal cortical cells.
| Group | Dosage (mg/(kg·d)) | Apoptotic index (AI) |
|---|---|---|
| Control | 0 | 1.33±0.11 |
| Model | 0 | 8.67±0.55** |
| High dose | 1.00 | 3.11±0.21**,##,&,$ |
| Moderate dose | 0.50 | 5.35±0.87**,##,& |
| Low dose | 0.25 | 6.85±0.62**,# |
p<0.05
p<0.01 compared to control group;
p<0.05,
p<0.01 compared to model group;
p<0.05 compared to low dose group;
p<0.05 compared to moderate group. N=10 per group.
Discussion
The pathogenesis of DN is not yet fully understood, although studies have shown that inflammatory response plays an important role in the whole disease process. The pathological basis of DN includes renal hyper-filtration, high perfusion, hyperplasia of glomerular mesangial cells, thickening of basal lamina, and hyperplasia of extracellular matrix, all of which lead to granular or diffused sclerosis, further causing hypertension, proteinuria, or even kidney failure [11,12]. Kidney tissues of both type 1 and type 2 diabetic nephropathy patients have infiltration of mononuclear macrophages, which can cause renal tissue damage by the production of free oxygen radicals and inflammatory factors, further facilitating sclerosis of the glomerulus. Renal cells can endogenously produce inflammatory factors such as TNF-α and NO, which can further cause inflammatory cascade reactions in paracrine or autocrine manner [13,14]. All these studies establish the role of inflammatory response in causing kidney damage in DN.
As a common characteristic pathological change of all types of diabetes, hyperglycemia can cause hyperinsulinemia and increase the risk of diabetic angiopathy. Under hyperglycemic condition, end-products of glycosylation and oxidative stress can activate glomerular endothelial cells, which produce adhesion molecules such as ICAM-1, further causing the adhesion, infiltration across vascular walls, and transformation of monocytes into macrophages [15–18]. Activated macrophages can produce large amounts of cytokines, including TNF-α, which facilitates glomerular sclerosis. Macrophages infiltrating into renal interstitial tissues can lead to interstitial fibrosis and produce active oxygen species, TNF-α, and NO, causing further glomerular damage and higher local permeability. In UUO model study in neonatal mice, macrophages were shown to cause irreversible interstitial fibrosis by the direct induction of glomerular cell apoptosis via TNF-α and NO production, or indirectly through the down-regulation of VEGF [19]. In this study, after destroying islet β cells by streptozotocin, model rats had polydipsia, polyuria, hyperglycemia, and elevated urine protein, BUN, and Scr levels, all of which are typical DN symptoms. All these abnormalities were improved by the application of puerarin, indicating that puerarin can decrease early-stage diabetic renal damage and improve kidney functions.
DN patients with significant proteinuria are likely to develop end-stage renal dysfunctions, making it necessary to manage DN at an early stage, although effective treatment has not been developed yet [20]. Currently, the major treatments of DN include the strict control of blood glucose level, management of blood pressure, reversing the insulin resistance, improving hemodynamics, correcting metabolic disorders, and the application of angiotensin II receptor and ACEI to decrease proteinuria. None of these methods, however, prevent the progression of disease. Animal and clinical studies have provided evidence showing that anti-inflammatory treatment can postpone the development of DN. For example, diabetic mice generated on ICAM-1 gene knockout animals showed relieved pathological damage, including renal macrophage infiltration, enlarged glomerulus, elevated protein excretion rate, and proliferation of mesangial matrix when compared to normal mice [21,22]. Traditional Chinese medicines such as anti-inflammatory herbs (e.g., astragalus and rhubarb) achieve satisfactory effects in treating DN, with fewer adverse effects. Puerarin, a flavonoid component extracted from the rhizome of Pueraria lobate (Willd.), showed multiple effects, including lowering blood glucose level, dilating micro-arteries, decreasing blood viscosity, improving microcirculation, clearing free radicals after glycosylation, reducing end-products of glycosylation, and relieving oxidative stress response [23]. Animal studies have revealed that puerarin can suppress blood lipid level and expression of ICAM-1 in diabetic rat aorta, in addition to suppressing ICAM-1 level in rat retinal tissues [23]. The present study has shown significantly suppressed blood levels of BG, Scr, BUN, and UAER in a dose-dependent manner when compared with diabetic model rats (Table 1), suggesting the improvement of diabetes after puerarin treatment. The lower BG level suggests that puerarin may relieve the kidney damage via the reduction of over-deposition of end-products of glycosylation. Further histopathological studies showed improvement of glomerular tissue damage by puerarin treatment (Figure 2). The increase of both inflammatory factors and adhesion molecules can lead to the enlargement of glomerulus and filtration size, causing hyper-filtration and deposition of extracellular matrix, further leading to damage of the infiltration barrier of glomerulus and proteinuria. The suppressed expression levels of renal ICAM-1 and TNF-α protein in renal tissues after puerarin treatment suggest that it can improve kidney function and relieve renal damages in early-stage diabetic rats, possibly via its down-regulation of ICAM-1 and TNF-α protein.
Persistent hyperglycemia can also facilitate the formation of end-products of non-enzymatic glycosylation from glucose, lipid, protein, and DNA molecules. Such end-products can stimulate DN via both the alteration of renal vascular structure by formation of collagen molecule and induction of chronic renal disease by synthesis of extracellular matrix, PDGF, and MCP-1 from mesangial cells by the specific binding onto receptors on mesangial cells [15,19]. In DN patients, the abnormal renal blood flow can cause increased pressure in glomerular capillaries, leading to facilitated expression of ICAM-1 by endothelial cells and chronic inflammation. TNF-α, on the other hand, can facilitate the infiltration of inflammatory factors via the enhancement of adhesion of leucocytes onto endothelial cells following elevated expression of ICAM-1. The binding between ICAM-1 and its receptor can further aggravate renal tissue damage by the aggregation of leucocytes adjacent to renal endothelial cells, thereby blocking microcapillaries [14]. The major targeting cell of TNF-α is an endothelial cell named TNF-R1, where endothelial cells are compromised, causing thrombosis, focal blood stream obstruction, and stimulating local inflammatory response, which can further activate vessel walls to over-express ICAM-1 to aggravate renal tissue damages. Our study shows the protective role of puerarin against diabetic kidneys by the suppression of both ICAM-1 and TNF-α expression (Table 3), inhibition of non-enzymatic glycosylation of proteins, and relieving oxidative stress or inflammatory reaction damage.
Table 3.
Effects of puerarin on ICAM-1 and TNF-α expression levels in diabetic rat kidneys.
| Group | Dosage mg/(kg·d) | ICAM-1 | TNF-α |
|---|---|---|---|
| Control | 0 | 90.83±2.11 | 93.47±3.14 |
| Model | 0 | 128.67±4.65** | 131.76±3.12** |
| High dose | 1.00 | 95.32±2.17**,##,&,$ | 100.14±2.11**,##,&,$ |
| Moderate dose | 0.50 | 110.75±3.84**,##,& | 120.49±3.37**,##,& |
| Low dose | 0.25 | 119.45±3.67**,# | 124.73±3.19**,# |
IOD values of positive-staining areas in each group were calculated and compared.
p<0.05,
p<0.01 compared to control group;
p<0.05,
p<0.01 compared to model group;
p<0.05 compared to low dose group;
p<0.05 compared to moderate group. N=10 per group.
The present study has also completed a correlation analysis in which the expressions of ICAM-1 and TNF-α were positively correlated with each other (r=0.914, p<0.05) while either TNF-α or ICAM-1 were positively correlated with Scr and BUN levels (p<0.05 in all cases). These data collectively suggest the presence of a cascade reaction among all those inflammatory factors, as TNF-α may up-regulate ICAM-1 expression via certain signal transduction pathways. After the stimulus, mesangial and endothelial cells of glomerulus can abundantly express ICAM-1 to enhance the adhesion and aggregation between endothelial cells and leucocytes, further releasing inflammatory factors and aggravating renal damage. We also used the TUNEL assay, which found significant suppression of AI after puerarin treatment, suggesting that the major site of diabetic renal cell apoptosis was the glomerulus, where puerarin can effectively suppress cell apoptosis and relieve renal damage. Overall, puerarin protect kidneys from DN possibly via the inhibition of glomerular cell apoptosis.
Conclusions
This study shows that puerarin can significantly improve early-stage renal damage in diabetic rats, possibly via its down-regulation of TNF-α and ICAM, in addition to its inhibition of cell apoptosis. Puerarin therefore has potential to become a candidate drug in treating early-stage DN, although its detailed mechanism needs further elucidation.
Footnotes
Source of support: Departmental sources
References
- 1.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Qu X, Yao J, et al. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59(10):2612–24. doi: 10.2337/db09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamagishi S, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev. 2010;3(2):101–8. doi: 10.4161/oxim.3.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue K, Wada J, Eguchi J, et al. Urinary fetuin-A is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray. PLoS One. 2013;8(10):e77118. doi: 10.1371/journal.pone.0077118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa T, Tanabe K, Croker BP, et al. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol. 2011;7(1):36–44. doi: 10.1038/nrneph.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto S, Shikata K, Miyasaka K, et al. Cholecystokinin plays a novel protective role in diabetic kidney through anti-inflammatory actions on macrophage: anti-inflammatory effect of cholecystokinin. Diabetes. 2012;61(4):897–907. doi: 10.2337/db11-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmarakby AA, Abdelsayed R, Yao Liu J, Mozaffari MS. Inflammatory cytokines as predictive markers for early detection and progression of diabetic nephropathy. EPMA J. 2010;1(1):117–29. doi: 10.1007/s13167-010-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30(1):49–59. doi: 10.1111/j.1755-5922.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 9.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–40. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 10.Zhong Y, Zhang X1, Cai X, et al. Puerarin attenuated early diabetic kidney injury through down-regulation of matrix metalloproteinase 9 in streptozotocin-induced diabetic rats. PLoS One. 2014;9(1):e85690. doi: 10.1371/journal.pone.0085690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Möllsten A, Prázny M, et al. Genetic influences of the intercellular adhesion molecule 1 (ICAM-1) gene polymorphisms in development of Type 1 diabetes and diabetic nephropathy. Diabet Med. 2006;23(10):1093–99. doi: 10.1111/j.1464-5491.2006.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124(3):139–52. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Cong X, Sun R, et al. Association between the ICAM-1 K469E polymorphism and diabetic retinopathy in Type 2 diabetes mellitus: A meta-analysis. Diabetes Res Clin Pract. 2014;104(2):e46–49. doi: 10.1016/j.diabres.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Kanasaki K, Taduri G, Koya D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol. 2013;4:7. doi: 10.3389/fendo.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramseyer VD, Garvin JL. Tumor necrosis factor-α: regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 2013;304(10):F1231–42. doi: 10.1152/ajprenal.00557.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matavelli LC, Huang J, Siragy HM. (Pro) renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37(3):277–82. doi: 10.1111/j.1440-1681.2009.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YJ, Pan JL, Jiang MJ, et al. Apo E gene polymorphism affects development of type 2 diabetic nephropathy in Asian populations, especially in East Asians: An updated meta-analysis. Med Sci Monit. 2014;20:1596–603. doi: 10.12659/MSM.892111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arık HO, Yalcin AD, Gumuslu S, et al. Association of circulating sTRAIL and high-sensitivity CRP with type 2 diabetic nephropathy and foot ulcers. Med Sci Monit. 2013;19:712–15. doi: 10.12659/MSM.889514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therrien FJ, Agharazii M, Lebel M, Larivière R. Neutralization of tumor necrosis factor-alpha reduces renal fibrosis and hypertension in rats with renal failure. Am J Nephrol. 2012;36(2):151–61. doi: 10.1159/000340033. [DOI] [PubMed] [Google Scholar]
- 20.Hao LN, Zhang YQ, Shen YH, et al. Effect of puerarin on retinal pigment epithelial cells apoptosis induced partly by peroxynitrite via Fas/FasL pathway. Int J Ophthalmol. 2010;3(4):283–87. doi: 10.3980/j.issn.2222-3959.2010.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao LN, Wang M, Ma JL, Yang T. Puerarin decreases apoptosis of retinal pigment epithelial cells in diabetic rats by reducing peroxynitrite level and iNOS expression. Sheng Li Xue Bao. 2012;64(2):199–206. [PubMed] [Google Scholar]
- 22.Luo CF, Hou N, Tian J, et al. Metabolic profile of puerarin in rats after intragastric administration of puerarin solid lipid nanoparticles. Int J Nanomedicine. 2013;8:933–40. doi: 10.2147/IJN.S39349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang CT, Shi D, Zheng Y, et al. Chronopharmacokinetics of puerarin in diabetic rats. Indian J Pharm Sci. 2013;75(3):357–61. doi: 10.4103/0250-474X.117407. [DOI] [PMC free article] [PubMed] [Google Scholar]