Abstract
The vast majority of new HIV infections in male-to-female transmission occurs through semen, where HIV-1 is present in two different forms: as free and as cell-associated virus. In the female lower genital tract, semen mixes with female genital secretions that contain various factors, some of which facilitate or inhibit HIV-1 transmission. Next, HIV-1 crosses the genital epithelia, reaches the regional lymph nodes, and disseminates through the female host. Cervico-vaginal mucosa contains multiple barriers, resulting in a low probability of vaginal transmission. However, in some cases HIV-1 is able to break these barriers. Although the exact mechanisms of how these barriers function remain unclear, their levels of efficiency against cell-free and cell-associated HIV-1 are different, and both cell-free and cell-associated virions seem to use different strategies to overcome these barriers. Understanding the basic mechanisms of HIV-1 vaginal transmission is required for the development of new antiviral strategies to contain HIV-1 epidemics.
Keywords: Cell, HIV-1, mucosa, semen, transmission
INTRODUCTION
The vast majority of new HIV infections occurs in women through male-to-female sexual transmission1, 2. In such intercourse, HIV is transferred from semen of an infected male to an uninfected female partner and overcomes some host’s defensive barriers. The presence of mucus, epithelial layers, secreted neutralizing antibodies, defensive proteins (eg. α-defensins, immunoglobulins, complement, antimicrobial peptides, lysozyme and lactoferrin) in genital tracts, are examples of the host barriers against these microbes3-5
Although the mechanisms of vaginal HIV-1 transmission are widely studied (see6-8), important aspects of this process remain to be elucidated. In particular, it is not known what the source of transmitted HIV is: does it come from infected cells present in semen, or from a cell-free HIV-1? What are the roles of seminal cytokines and other seminal compounds in HIV-1 transmission? What are the protective barriers against HIV transmission in the female lower genital tract? These questions and their possible answers are discussed in the present review.
In particular, we discuss below the biological mechanisms of the male-to-female vaginal HIV-1 transmission, the role of seminal components in this process, the barriers to HIV-1 transmission present in the female genital tract and the strategies HIV uses to overcome them. In our review, we specifically address data regarding the roles of cell-free and cell-associated viruses in HIV-1 vaginal transmission.
SEMEN AS A VEHICLE FOR HIV-1 TRANSMISSION
Since the discovery of HIV-1 in genital secretions, several groups have evaluated the relation between viral concentrations in these fluids and the risk of transmission and found that higher concentration in genital secretions augments the probability of HIV-1 sexual transmission1, 9-11. The level of seminal HIV-1 depends on the stage of the infection: for example more viral particles are detected in semen of HIV-1-infected men in the acute phase of the infection11, 12 and the risk of HIV transmission varies from 8 cases per 1000 vaginal coital acts in the acute to 1-2 per 1,000 in chronic phase10, 13-18. Administration of antiviral therapy decreases seminal viral load, consequently diminishing the risk of transmission19-22.
The origin of HIV-1 in semen has been debated for years. Blood does not seem to be the only source of seminal HIV-1, since genetic discordances were observed between viruses isolated from blood and from semen22-25. In particular, some genetic signatures are present only in seminal HIV-1 (e.g., specific glycosylation patterns in the viral envelope gp120)25. Other genetic analysis of HIV-1 demonstrated that seminal HIV-1 originates predominantly from male genital tract tissues22-26. This was confirmed in macaques: male genital organs, such as testis, epididymis, prostate, and seminal vesicles were infected by SIV in both acute and chronic phases of the infection and thus may be the source of HIV-1 found in semen27, 28. Moreover, cultures of human testis and prostate contained cells that can be productively infected by HIV-1, i.e., CD4+ CCR5+ T cells and macrophages29, 30. On the basis of the phylogenetic analysis of viral sequences in the blood and semen, Anderson et al. proposed that viral populations in semen derive from multiple sources including direct import of virus from blood and an oligoclonal amplification within the male genital tract31. However, vasectomy does not preclude the presence of virus in ejaculate, indicating that most cell-free HIV in seminal plasma arises distally to the vas deferens32, 33. Thus, seminal HIV-1 is composed of an heterogeneous population of viruses produced by semen-producing organs and blood.
Whichever the source of the virus in semen is, HIV-1 exists in two different forms: free and cell-associated12, 34-39. Monocytes/macrophages and CD4+ T cells are the major populations infectable by HIV-1 and can both produce free virus and carry it in semen. Both forms of viruses were proved to be infectious (reviewed in12). Anderson and colleagues estimated that roughly 0.2% of macrophages and CD4+ T cells are infected in human semen of HIV-1 therapy naïve infected patients12. These results are in general agreement with the enumeration of infected cells in semen of SIV infected macaques40.
SEMEN AFFECTS HIV ACQUISITION
In the course of heterosexual intercourse, HIV-1 carried by semen is deposited in the vaginal mucosa. Semen is more than a mere carrier of HIV-1, since it contains many biological factors that may facilitate or inhibit HIV-1 transmission41. For instance, semen neutralizes the acidic cervical mucus increasing HIV-1 diffusion42 otherwise trapped in mucus43. Furthermore, semen can significantly modify the chemical and physical properties of the female genital mucus. For example, semen contains amyloid fibrils derived from the prostatic alkaline phosphatase (SEVI)44 and semenogelins (SEM1 & SEM2)45 that promote viral attachment to target cells enhancing HIV-1 transmission. These fibrils increase the infectivity of cell free HIV-1 but should have no effect on cell-associated HIV-1 infectivity. Also, semen contains inhibitors of the complement system, such as CD59, which could facilitate viral escape from complement-mediated virucidal activity46.
Other seminal factors affecting HIV-1 transmission are cytokines. The modulation of the cytokine network in semen affects viral transmission. Indeed, it was showed that seminal plasma induces chemokine (C-C motif) ligand (CCL)-2 secretion by ectocervical epithelial cells. CCL-2, also referred as MCP-1, may in turn recruit immune cells to the female genital tract following ejaculation, providing new targets for HIV infection47. More recently, we48, and others49, reported that interleukin (IL)-7, one of the most prominent seminal cytokine is upregulated in the seminal plasma of HIV-1-infected individuals. We used an ex vivo system of human cervical tissues to investigate the role of IL-7 in HIV-1 transmission. We found that, ex vivo, IL-7 facilitates HIV-1 transmission predominantly by preventing apoptosis of HIV-1 infected cells. IL-7 is not the only cytokine that modulates HIV transmission, since Olivier et al. reported that concentrations of G-CSF in semen significantly predicted both HIV shedding and T-cell activation49.
Upregulation of different cytokines in semen of HIV-1 infected individuals may be related to a local inflammation or immunoactivation in the male genital tract caused by HIV-1 itself of by HIV-associated infections. By residing and replicating in the genital tract of HIV-1 infected individuals, copathogens such as Chlamydia trachomatis, Trichomonas vaginalis, Neisseria gonorrhoeae, Human papillomavirus, cytomegalovirus (CMV) and herpes simplex viruses (HSV)48, 50-54 may promote subclinical inflammation, therefore modulating HIV-1 replication via cytokine network alteration or/and the recruitment of new target cells55. As examples, CMV, HSV, and Neisseria gonorrhoeae reactivation in the seminal compartment augment HIV shedding in semen53, 56-59. In contrast, GBV-C has recently been showed to decerease T cell activation and inflammation and therefore may decrease HIV-1 transmission60.
Furthermore, some genital pathogens, such as Trichomonas or HSV-2, can facilitate HIV acquisition directly by disrupting the mucosal epithelia or by inducing the infiltration of susceptible cells50, 52, 61. In summary, male-to-female transmission of HIV-1 is a multiregulated process affected by various seminal factors and other pathogens.
SEMINAL FREE AND CELL-ASSOCIATED VIRUS IN THE ESTABLISHMENT OF HIV-1 INFECTION
Most experiments on HIV-1 or SIV transmission were performed with cell-free viruses. However, the literature regarding the role of cell-associated virus in HIV-1 transmission is now growing. Operatively, it is often difficult to distinguish between virus deposited on the vaginal mucosa in cell-free or cell-associated form, since HIV-1 can be temporarily adsorbed on the cell surface and subsequently released as free virus. We believe that virus adsorbed to seminal cells should be considered as cell-associated only if it remains on the cell surface (e.g., spermatozoa62, 63) during its contact with female genital epithelia.
The idea of infection transmitted by cells containing pathogens (“Trojan Horses”) predates the discovery of HIV as the agent of AIDS64, 65. Later, two independent groups found in vivo evidence that mouse spleen mononuclear cells are able to cross mouse vaginal epithelium after atraumatic inoculation in the vaginal lumen66, 67. Furthermore, it was demonstrated in hu-SCID mice that HIV-infected human cells migrate transepithelially and transmit infection68, 69. Similarly, in a non-human primate model, intravaginal inoculation of SIV-infected cells resulted in persistent infection of exposed animals70-72. In humans, a longitudinal study reported that the HIV-1 genotype found in women in acute infection matched the viruses integrated in the seminal cells of their infected male partners, suggesting that HIV originated from infected cells present in semen23, mainly lymphocytes and macrophages. This is in agreement with experiments showing that intravaginal inoculation of semen simulant containing 111In-radiolabeled autologous leukocytes together with 99mTc-radiolabeled nanoparticles result in migration of both labeled components in the human cervical tract73. Thus it seems that not only free virus but also infected cells are able to interact with cervico-vaginal tissue, transmitting infection in heterosexual intercourse.
Whether free or cell-associated HIV-1 is more prone to overcome the multiple barriers that defend the female tract from HIV-1 transmission remains to be elucidated. Free virus seems to diffuse where water diffuses74. Thomas Hope’s group found that different cell-free HIV-1 clones penetrated on average approximately 7 to 9 μm and in some cases up to 50 μm in ecto- or endocervixes74. Unlike cell-free HIV-1 particles, which move passively, cells are capable of active locomotion through barriers such as epithelia.
Let us consider how these barriers (“gatekeepers”) insure a low probability of HIV-1 transmission through vaginal sex1. The notion of biological barriers for HIV sexual transmission evolved when it was noticed that the only HIV strain detected at the early stages of HIV-1 sexual transmission was of the R5 (CCR5 coreceptor-using) phenotype, while in semen both R5 and X4 (CXCR4 coreceptor-using) HIV-1 variants were present. While these viruses use different co-receptors often expressed by the same cells, their physiological features are dramatically different. At least in B-clade HIV-1 R5 dominates early stages of transmission/infection while X4 HIV-1 often evolves at the later stage. Since both R5 and X4 HIV-1 are present in semen, female host’s barriers seem to block X4 viruses as R5 HIV-1 are found ubiquitously in almost all reported HIV-1 sexual transmission events75. Moreover, it seems that there are barriers that not only select R5 over X4 but also may operate among R5 HIV-1 variants. Genetic analysis of HIV-1 diversity at the earliest stages of HIV infection indicates that, in majority of cases, infection is transmitted by a single R5 viral particle76, 77. HIV-1 transmission by a single virion can be explained by stochastic mechanisms78. However, several characteristics of the transmitted virus that distinguish them from the bulk have been reported (i.e., glycosylation pattern79). If, only these selected HIV-1 virions are transmitted, the gatekeeping mechanism is even more selective than previously anticipated.
Where do these gatekeepers reside? The genital mucus is the first barrier against HIV on its way to dissemination. Mucus can protect underlying epithelia by decreasing HIV infectivity via various soluble factors and by temporarily trapping virions or infected cells in the protein mesh, slowing their movement by several orders of magnitude compared with water42, 43, 80, 81. Since free HIV, due to its fragility, cannot remain outside of cells for a long time, its infectivity may be significantly decreased if mucus slows viral penetration81. However, if transmission is mediated by direct contact of an infected cell with a target cell, the slowing of its movement by mucus may not be critical for infection. While virus can be protected inside the cell, HIV-1 may be protected from antiviral compounds as well in the mucus because virions may be covered by seminal proteins and other seminal colloid constituents. This aspect of HIV-semen interactions has not been exhaustively studied yet.
Viral particles or virus-infected cells that go through the cervical mucus reach the epithelial layer, which constitutes another major barrier to efficient transmission of HIV and other pathogens. According to some data82, 83, HIV crosses the epithelial barrier predominantly through lesions that commonly occur as a result of various infections or coital/sexual abrasion84. However, the efficiency of epithelial protection doesn’t seem to be uniform through the entire surface of the female lower genital tract. It is believed that the main site for HIV transmission in the female genital tract is the cervix, especially the endocervix and the transitional zone, which are covered by a single-layer columnar epithelium. Such a layer is less protective against HIV-1 than the stratified epithelia of the vagina85, 86; reviewed in87). Also, the ectocervix, together with the transition zone, contains a high number of potential cell targets for HIV82. However, it is known that HIV-1 transmission through the vaginal mucosa does happen as well, as HIV genital transmission to women with a congenital absence of cervix has been reported88. Similarly, SIV has been transmitted intravaginally to hysterectomized rhesus macaques12, 34.
HIV-1 transmission through epithelia has been simulated in various models ex vivo. Conventional cultures of cell lines or peripheral blood mononuclear cells have been sucessfully used in many areas of HIV research. However. these cultures have an important limitation: they neither reproduce the morphology nor mimic the functions of living tissues, and therefore they lack the potential to predict tissue responses to viral challenges. In contrast, cervico-vaginal tissue explants have several advantages: first, these explants preserve tissue architecture for 2–3 weeks; second, they retain the majority of cell types, which express key cell surface molecules relevant for HIV infection89-91; third, unlike single-cell cultures they do not require exogenous activation or stimulation to support productive HIV infection. Cervico-vaginal tissue ex vivo as a model to study early events in HIV-1 infection has been extensively reviewed by Merbah et al.,201192.
In particular, several studies using polarized epithelial monolayers exposed to HIV-1-infected cells showed that HIV-1-infected cells were able to transcytose and infect underlying susceptible target cells93-95. Also, it was reported that in vitro cells are able to migrate through ecto-/endocervical tissues when placed on the luminal side12, 96-98. Collins’group used cervical tissue polarized in 3% agarose and reported that HIV-1-infected cells cross cervical explants and transmit infection to susceptible cells underneath96. However, the methodology of this study was criticized because of the problems with reliable tissue polarization in their experiments: HIV-1 could potentially penetrate tissue explant from the (wounded) lateral sides99. Later, Maher and colleagues observed that seminal cells penetrate beneath the most external layer of ectocervix97. More recently, Anderson et al. observed macrophages protruding their membranes into interepithelial spaces of endocervical tissues, thus potentially carrying and delivering HIV to sub-epithelial cells12. Finally, Soto-Rivera et al. (2013) exposed cervical explants to HIV-1-infected cells and reported that these cells transmit HIV-1 to isolated tonsillar cells located in the lower chamber in a transwell system98. Although it has been shown in ex vivo models that HIV-1-infected cells can penetrate and cross the cervical epithelium, it remains to be demonstrated whether such processes account for HIV-1 infection in vivo, especially in vaginal and cervical multilayered epithelium.
Adhesion proteins may play an important role in the transmission of virus through epithelia. Immune cells use adhesion proteins to migrate from the apical to the basal part of epithelia100. The expression of these proteins is a process coordinated by chemokines and inflammatory mediators, which are present in high concentration in the vaginal mucosal and submucosal tissues9, 83. For example, LFA-1 adhesion molecules on seminal macrophages and on T cells from SIV-infected macaques could interact with intercellular adhesion molecules (ICAMs) on mucosal epithelia, triggering the penetration of infected cells into the epithelia40, 49, 101, 102. Acordingly, semen from HIV-1-infected patients is enriched in the chemokine CXCL-1248 (SDF-1), which enables the activation of LFA-1102. These reports showed the possible role of adhesion proteins in cell-associated virus penetration through epithelia but the role of these proteins in cell-free HIV-1 penetration remains to be elucidated. LFA-1 was demonstrated on the surface of HIV-1103 (reviewed in104) and recently Arakelyan et al.105 reported that LFA-1 is present on selected virions. Thus, it is conceivable that these particular cell-free viruses cross epithelia utilizing adhesion molecules as conduits, similar to cell-associated virions do.
Moreover, free HIV-1 may interact with molecules on the surface of host epithelial cells, such as cell surface heparan sulfate proteoglycans and glycosphingolipids106, 107. It has been shown that virions attachment to glycosphingolipids promotes their endocytosis in cervico-vaginal tissue cells74, 108-112. On the other hand, the trapping of HIV-1 by epithelium surface-molecules may constitute another barrier for HIV transmission.
Once the epithelial barrier has been overcome, migrant free HIV-1 or HIV-1-infected cells reach the submucosal tissue, wherein they can interact with HIV-1 target cells, such as activated T cells and macrophages113. The first (founding) infected cells seem to be CD4 lymphocytes114, 115 rather than macrophages. In submucosa, infected cells transmit HIV-1 to uninfected cells through a cell-to-cell specific structure called a virological synapse and through nanotubes and filopodia116-119.
Cell-to-cell transfer seems to be efficient120, 121 because it facilitates contacts between virus and its receptor(s) on the target cells, as this contact occurs in the intercellular space of a synapse that also may protect HIV-1 from extracellular soluble antiviral compounds122-127. Another mechanism that may protect virus from extracellular soluble antiviral compounds is HIV uptake by host cervical dendritic cells (DCs). DCs are specialized cells that take up antigens, and transfer them to local lymph nodes128. This way they transfer HIV-1 to these lymph nodes, where they transmit viruses to T cells, contributing to viral disseminatation8, 112, 129. It is still under debate whether DCs are infected by HIV-1 or just carry virions without being productively infected130-134. Although free HIV virions are capable to be transmitted by this mechanism via binding to DC’s C-type lectin DC-SIGN, or langerin in the case of Langerhans cells135-137, recently it was suggested that DCs can capture and transmit cell-associated virus63, 138.
CONCLUSION
In semen of infected men, HIV-1 is present as free virions and as cell-associated ones. In heterosexual vaginal intercourse, semen carrying HIV-1 is deposited in the female lower genital tract. In case of efficient transmission, HIV-1 penetrates the genital epithelia, reaches the draining lymph nodes, and disseminates through the female host. Cervico-vaginal tissues provide efficient multiple barriers against the vast majority of cell-free and cell-associated HIV virions, resulting in a low probability of vaginal transmission. Although the exact mechanisms by which these barriers function are unclear, their levels of efficiency against cell-free and cell-associated HIV-1 are different, and both free and cell-associated virions seem to use different strategies to overcome them. Seminal components play an important role in HIV transmission, both facilitating and inhibiting transmission.
Data from in vivo and ex vivo studies convincingly show that both free and cell-associated HIV virions are able to penetrate the female genital mucosa, although by different pathways, and reach the regional lymph nodes. However, the relative contribution of free and cell-associated HIV-1 in transmission of HIV-1 infection from an infected man to his uninfected female partner remains to be understood. Towards this goal, it is necessary (i) to evaluate and characterize cells that carry HIV in semen as well as to characterize free virions that are preferentially transmitted; (ii) to investigate the effects of female genital mucus mixed with semen on free or cell-associated HIV-1; (iii) to identify seminal factors and evaluate their effect on free or cell-associated HIV in vaginal transmission; and (iv) to investigate the distinct strategies used by free HIV and HIV-carrying seminal cells to penetrate vaginal epithelial layers and reach regional lymph nodes.
As these strategies seem to be different, different counter-measures, should be developed to prevent transmission of free and cell-associated HIV-1. A better knowledge of these strategies will lead to the development of new approaches to prevent HIV-1 transmission.
Fig. Overcoming barriers to vaginal transmission by free or cell-associated HIV-1.
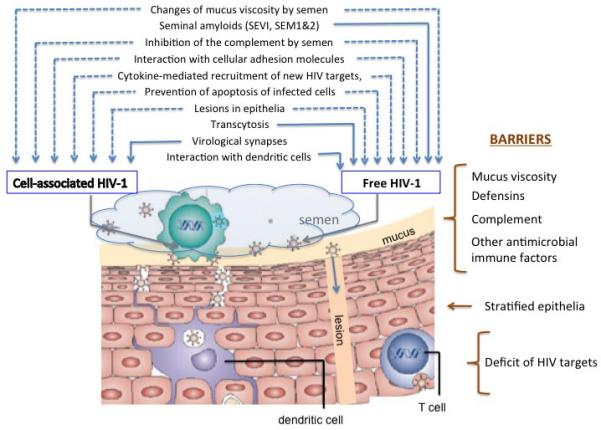
Female host’s natural barriers prevent HIV-1 vaginal transmission, (text in brown) HIV-1 is overcoming these barriers via various mechanisms. Some of these mechanisms seem to be common for free and cell associated viruses (dashed blue arrows) whereas others predominantly facilitate either free or cell-associated HIV-1 transmission (solid blue arrows).
Table.
The role of cell-free and cell-associated in HIV-1 vaginal transmission: Questions to answer
| Free and cell-associated HIV-1 transmission |
Question to answer |
|---|---|
| Cell-associated HIV-1 is present in semen | Which types of cells carry HIV-1 in semen? What is the relative proportions of these type of these cells? |
| Free and cell-associated HIV-1 are present in semen |
What are the sources of free HIV-1 and cell-associated HIV-1 in semen |
| Both free and cell-associated HIV-1 transmit infection |
What are the relative contributions of free and cell-associated virus to HIV-1 transmission? |
| Different cytokines differentially affect HIV-1 transmission/infection |
What are the effects of various cytokine spectra on HIV-1 transmission |
| Sexually transmitted pathogens facilitate HIV-1 transmission |
What are the mechanisms of this facilitation? |
| DCs transmit HIV-1 infection | Are these DCs productively infected or do they passively carry HIV-1? |
| Female genital barriers decrease the probability of HIV-1 transmission |
Which is more apt to overcome barriers that defend the female genital tract: free or cell-associated HIV-1? |
| Mucus slows penetration of free HIV, probably diminishing its infectivity. |
Is the same true for cell-associated virus? |
| Free HIV particles cross epithelia through lesions. |
Can HIV-infected cells penetrate mucosal epithelia via a similar pathway? |
| Cell-associated HIV transmitted through virological synapsis is protected from many soluble factors |
Are there antiviral factors (e.g., neutralizing antibodies, antiretrovirals) that penetrate virological synapses? |
| Most experiments on protection from HIV-1 transmission were performed with free virus |
Would the results be the same for cell- associated virus? |
ACKNOWLEDGEMENTS
This work was supported by the NICHD intramural programs. VBS was partially supported by fellowships from the Brazilian Ministry of Education/CAPES and the Brazilian Ministry of Science and Technology/CNPq. We are also grateful to Dr. Jean-Charles Grivel for his critical review of the manuscript and helpful suggestions.
REFERENCES
- 1.Royce RA, Sena A, Cates W, Jr., Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 2.Beyrer C, Abdool Karim Q. The changing epidemiology of HIV in 2013. Curr Opin HIV AIDS. 2013;8:306–310. doi: 10.1097/COH.0b013e328361f53a. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011;118:839–846. doi: 10.1182/blood-2010-12-325860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 7.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 8.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iversen AK, Larsen AR, Jensen T, Fugger L, Balslev U, Wahl S, Gerstoft J, Mullins JI, Skinhoj P. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J Infect Dis. 1998;177:1214–1220. doi: 10.1086/515266. [DOI] [PubMed] [Google Scholar]
- 10.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 11.Pilcher CD, Joaki G, Hoffman IF, Martinson FE, Mapanje C, Stewart PW, Powers KA, Galvin S, Chilongozi D, Gama S, Price MA, Fiscus SA, Cohen MS. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. Targeting Trojan Horse leukocytes for HIV prevention. AIDS. 2010;24:163–187. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 14.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 15.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 16.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, Alary M. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, de Bruyn G, Kiarie J, Inambao M, Kilembe W, Farquhar C, Celum C. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamed KA, Winters MA, Holodniy M, Katzenstein DA, Merigan TC. Detection of human immunodeficiency virus type 1 in semen: effects of disease stage and nucleoside therapy. J Infect Dis. 1993;167:798–802. doi: 10.1093/infdis/167.4.798. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Dornadula G, Beumont M, Livornese L, Jr., Van Uitert B, Henning K, Pomerantz RJ. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 21.Leruez-Ville M, Dulioust E, Costabliola D, Salmon D, Tachet A, Finkielsztejn L, De Almeida M, Silbermann B, Sicard D, Jouannet P, Rouzioux C. Decrease in HIV-1 seminal shedding in men receiving highly active antiretroviral therapy: an 18 month longitudinal study (ANRS EP012) AIDS. 2002;16:486–488. doi: 10.1097/00002030-200202150-00023. [DOI] [PubMed] [Google Scholar]
- 22.Shen C, Ding M, Craigo JK, Tarwater P, Chatterjee R, Roy P, Guha SK, Saha B, Modak D, Neogi D, Chen Y, Gupta P. Genetic characterization of HIV-1 from semen and blood from clade C-infected subjects from India and effect of therapy in these body compartments. Virology. 2010;401:190–196. doi: 10.1016/j.virol.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu T, Wang N, Carr A, Nam DS, Moor-Jankowski R, Cooper DA, Ho DD. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. Journal of virology. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paranjpe S, Craigo J, Patterson B, Ding M, Barroso P, Harrison L, Montelaro R, Gupta P. Subcompartmentalization of HIV-1 quasispecies between seminal cells and seminal plasma indicates their origin in distinct genital tissues. AIDS Res Hum Retroviruses. 2002;18:1271–1280. doi: 10.1089/088922202320886316. [DOI] [PubMed] [Google Scholar]
- 25.Pillai SK, Good B, Pond SK, Wong JK, Strain MC, Richman DD, Smith DM. Semen-specific genetic characteristics of human immunodeficiency virus type 1 env. Journal of virology. 2005;79:1734–1742. doi: 10.1128/JVI.79.3.1734-1742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, Ross SO, Dragavon J, Peterson G, Hooton TM, Collier AC, Corey L, Koutsky L, Krieger JN. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 27.Miller CJ, Vogel P, Alexander NJ, Dandekar S, Hendrickx AG, Marx PA. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab Invest. 1994;70:255–262. [PubMed] [Google Scholar]
- 28.Le Tortorec A, Le Grand R, Denis H, Satie AP, Mannioui K, Roques P, Maillard A, Daniels S, Jegou B, Dejucq-Rainsford N. Infection of semen-producing organs by SIV during the acute and chronic stages of the disease. PLoS One. 2008;3:e1792. doi: 10.1371/journal.pone.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roulet V, Satie AP, Ruffault A, Le Tortorec A, Denis H, Guist’hau O, Patard JJ, Rioux-Leclerq N, Gicquel J, Jegou B, Dejucq-Rainsford N. Susceptibility of human testis to human immunodeficiency virus-1 infection in situ and in vitro. Am J Pathol. 2006;169:2094–2103. doi: 10.2353/ajpath.2006.060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Tortorec A, Satie AP, Denis H, Rioux-Leclercq N, Havard L, Ruffault A, Jegou B, Dejucq-Rainsford N. Human prostate supports more efficient replication of HIV-1 R5 than X4 strains ex vivo. Retrovirology. 2008;5:119. doi: 10.1186/1742-4690-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JA, Ping LH, Dibben O, Jabara CB, Arney L, Kincer L, Tang Y, Hobbs M, Hoffman I, Kazembe P, Jones CD, Borrow P, Fiscus S, Cohen MS, Swanstrom R. HIV-1 Populations in Semen Arise through Multiple Mechanisms. PLoS pathogens. 2010;6:e1001053. doi: 10.1371/journal.ppat.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson DJ, Politch JA, Martinez A, Van Voorhis BJ, Padian NS, O’Brien TR. White blood cells and HIV-1 in semen from vasectomised seropositive men. Lancet. 1991;338:573–574. doi: 10.1016/0140-6736(91)91139-l. [DOI] [PubMed] [Google Scholar]
- 33.Krieger JN, Nirapathpongporn A, Chaiyaporn M, Peterson G, Nikolaeva I, Akridge R, Ross SO, Coombs RW. Vasectomy and human immunodeficiency virus type 1 in semen. J Urol. 1998;159:820–825. discussion 825-826. [PubMed] [Google Scholar]
- 34.Miller CJ, Alexander NJ, Vogel P, Anderson J, Marx PA. Mechanism of genital transmission of SIV: a hypothesis based on transmission studies and the location of SIV in the genital tract of chronically infected female rhesus macaques. J Med Primatol. 1992;21:64–68. [PubMed] [Google Scholar]
- 35.Van Voorhis BJ, Martinez A, Mayer K, Anderson DJ. Detection of human immunodeficiency virus type 1 in semen from seropositive men using culture and polymerase chain reaction deoxyribonucleic acid amplification techniques. Fertil Steril. 1991;55:588–594. [PubMed] [Google Scholar]
- 36.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 37.Xu C, Politch JA, Tucker L, Mayer KH, Seage GR, 3rd, Anderson DJ. Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J Infect Dis. 1997;176:941–947. doi: 10.1086/516539. [DOI] [PubMed] [Google Scholar]
- 38.Tachet A, Dulioust E, Salmon D, De Almeida M, Rivalland S, Finkielsztejn L, Heard I, Jouannet P, Sicard D, Rouzioux C. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS. 1999;13:823–831. doi: 10.1097/00002030-199905070-00012. [DOI] [PubMed] [Google Scholar]
- 39.Ghosn J, Viard JP, Katlama C, de Almeida M, Tubiana R, Letourneur F, Aaron L, Goujard C, Salmon D, Leruez-Ville M, Rouzioux C, Chaix ML. Evidence of genotypic resistance diversity of archived and circulating viral strains in blood and semen of pre-treated HIV-infected men. AIDS. 2004;18:447–457. doi: 10.1097/00002030-200402200-00011. [DOI] [PubMed] [Google Scholar]
- 40.Bernard-Stoecklin S, Gommet C, Corneau AB, Guenounou S, Torres C, Dejucq-Rainsford N, Cosma A, Dereuddre-Bosquet N, Le Grand R. Semen CD4(+) T Cells and Macrophages Are Productively Infected at All Stages of SIV infection in Macaques. PLoS pathogens. 2013;9:e1003810. doi: 10.1371/journal.ppat.1003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doncel GF, Joseph T, Thurman AR. Role of semen in HIV-1 transmission: inhibitor or facilitator? Am J Reprod Immunol. 2011;65:292–301. doi: 10.1111/j.1600-0897.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- 42.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. Journal of virology. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boukari H, Brichacek B, Stratton P, Mahoney SF, Lifson JD, Margolis L, Nossal R. Movements of HIV-virions in human cervical mucus. Biomacromolecules. 2009;10:2482–2488. doi: 10.1021/bm900344q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Gimenez-Gallego G, Sanchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel JC, Margolis L, Keppler OT, Forssmann WG, Kirchhoff F. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Roan NR, Muller JA, Liu H, Chu S, Arnold F, Sturzel CM, Walther P, Dong M, Witkowska HE, Kirchhoff F, Munch J, Greene WC. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe. 2011;10:541–550. doi: 10.1016/j.chom.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly RW. Immunosuppressive mechanisms in semen: implications for contraception. Hum Reprod. 1995;10:1686–1693. doi: 10.1093/oxfordjournals.humrep.a136156. [DOI] [PubMed] [Google Scholar]
- 47.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13:491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 48.Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X, Grivel JC, Singh S, Margolis L. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97–105. doi: 10.1093/infdis/jir700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olivier AJ, Masson L, Ronacher K, Walzl G, Coetzee D, Lewis DA, Williamson AL, Passmore JA, Burgers WA. Distinct Cytokine Patterns in Semen Influence Local HIV Shedding and HIV Target Cell Activation. J Infect Dis. 2014 doi: 10.1093/infdis/jit649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 51.Kokab A, Akhondi MM, Sadeghi MR, Modarresi MH, Aarabi M, Jennings R, Pacey AA, Eley A. Raised inflammatory markers in semen from men with asymptomatic chlamydial infection. J Androl. 2010;31:114–120. doi: 10.2164/jandrol.109.008300. [DOI] [PubMed] [Google Scholar]
- 52.Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426–433. doi: 10.1136/sextrans-2012-051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gianella S, Anderson CM, Vargas MV, Richman DD, Little SJ, Morris SR, Smith DM. Cytomegalovirus DNA in semen and blood is associated with higher levels of proviral HIV DNA. J Infect Dis. 2013;207:898–902. doi: 10.1093/infdis/jis777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gianella S, Morris SR, Vargas MV, Young JA, Callahan B, Richman DD, Little SJ, Smith DM. Role of seminal shedding of herpesviruses in HIV Type 1 Transmission. J Infect Dis. 2013;207:257–261. doi: 10.1093/infdis/jis683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lisco A, Introini A, Munawwar A, Vanpouille C, Grivel JC, Blank P, Singh S, Margolis L. HIV-1 imposes rigidity on blood and semen cytokine networks. Am J Reprod Immunol. 2012;68:515–521. doi: 10.1111/aji.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Speck CE, Coombs RW, Koutsky LA, Zeh J, Ross SO, Hooton TM, Collier AC, Corey L, Cent A, Dragavon J, Lee W, Johnson EJ, Sampoleo RR, Krieger JN. Risk factors for HIV-1 shedding in semen. Am J Epidemiol. 1999;150:622–631. doi: 10.1093/oxfordjournals.aje.a010061. [DOI] [PubMed] [Google Scholar]
- 57.Rotchford K, Strum AW, Wilkinson D. Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: systematic review and data synthesis. Sex Transm Dis. 2000;27:243–248. doi: 10.1097/00007435-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 58.McClelland RS, Wang CC, Overbaugh J, Richardson BA, Corey L, Ashley RL, Mandaliya K, Ndinya-Achola J, Bwayo JJ, Kreiss JK. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16:2425–2430. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 59.Sheth PM, Danesh A, Sheung A, Rebbapragada A, Shahabi K, Kovacs C, Halpenny R, Tilley D, Mazzulli T, MacDonald K, Kelvin D, Kaul R. Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J Infect Dis. 2006;193:45–48. doi: 10.1086/498576. [DOI] [PubMed] [Google Scholar]
- 60.Stapleton JT, Martinson JA, Klinzman D, Xiang J, Desai SN, Landay A. GB virus C infection and B-cell, natural killer cell, and monocyte activation markers in HIV-infected individuals. AIDS. 2013;27:1829–1832. doi: 10.1097/QAD.0b013e328363089f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piomboni P, Baccetti B. Spermatozoon as a vehicle for HIV-1 and other viruses: a review. Mol Reprod Dev. 2000;56:238–242. doi: 10.1002/(SICI)1098-2795(200006)56:2+<238::AID-MRD5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 63.Ceballos A, Remes Lenicov F, Sabatte J, Rodriguez Rodrigues C, Cabrini M, Jancic C, Raiden S, Donaldson M, Agustin Pasqualini R, Jr., Marin-Briggiler C, Vazquez-Levin M, Capani F, Amigorena S, Geffner J. Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells. The Journal of experimental medicine. 2009;206:2717–2733. doi: 10.1084/jem.20091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson DJ, Yunis EJ. “Trojan Horse” leukocytes in AIDS. N Engl J Med. 1983;309:984–985. [PubMed] [Google Scholar]
- 65.Shearer GM. Allogeneic leukocytes as a possible factor in induction of aids in homosexual men. N Engl J Med. 1983;308:223–224. doi: 10.1056/NEJM198301273080415. [DOI] [PubMed] [Google Scholar]
- 66.Ibata B, Parr EL, King NJ, Parr MB. Migration of foreign lymphocytes from the mouse vagina into the cervicovaginal mucosa and to the iliac lymph nodes. Biol Reprod. 1997;56:537–543. doi: 10.1095/biolreprod56.2.537. [DOI] [PubMed] [Google Scholar]
- 67.Zacharopoulos VR, Perotti ME, Phillips DM. A role for cell migration in the sexual transmission of HIV-1? Curr Biol. 1997;7:534–537. doi: 10.1016/s0960-9822(06)00225-9. [DOI] [PubMed] [Google Scholar]
- 68.Di Fabio S, Giannini G, Lapenta C, Spada M, Binelli A, Germinario E, Sestili P, Belardelli F, Proietti E, Vella S. Vaginal transmission of HIV-1 in hu-SCID mice: a new model for the evaluation of vaginal microbicides. AIDS. 2001;15:2231–2238. doi: 10.1097/00002030-200111230-00003. [DOI] [PubMed] [Google Scholar]
- 69.Khanna KV, Whaley KJ, Zeitlin L, Moench TR, Mehrazar K, Cone RA, Liao Z, Hildreth JE, Hoen TE, Shultz L, Markham RB. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J Clin Invest. 2002;109:205–211. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaizu M, Weiler AM, Weisgrau KL, Vielhuber KA, May G, Piaskowski SM, Furlott J, Maness NJ, Friedrich TC, Loffredo JT, Usborne A, Rakasz EG. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J Infect Dis. 2006;194:912–916. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- 71.Weiler AM, Li Q, Duan L, Kaizu M, Weisgrau KL, Friedrich TC, Reynolds MR, Haase AT, Rakasz EG. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated simian immunodeficiency virus SIVmac239. Journal of virology. 2008;82:4154–4158. doi: 10.1128/JVI.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salle B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, Le Grand R. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis. 2010;202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 73.Louissaint NA, Fuchs EJ, Bakshi RP, Nimmagadda S, Du Y, Macura KJ, King KE, Wahl R, Goldsmith AJ, Caffo B, Cao YJ, Anderson J, Hendrix CW. Distribution of cell-free and cell-associated HIV surrogates in the female genital tract after simulated vaginal intercourse. J Infect Dis. 2012;205:725–732. doi: 10.1093/infdis/jir841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, Hope TJ. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. Journal of virology. 2013;87:11388–11400. doi: 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keele BF, Derdeyn CA. Genetic and antigenic features of the transmitted virus. Curr Opin HIV AIDS. 2009;4:352–357. doi: 10.1097/COH.0b013e32832d9fef. [DOI] [PubMed] [Google Scholar]
- 77.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. The Journal of experimental medicine. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boeras DI, Hraber PT, Hurlston M, Evans-Strickfaden T, Bhattacharya T, Giorgi EE, Mulenga J, Karita E, Korber BT, Allen S, Hart CE, Derdeyn CA, Hunter E. Role of donor genital tract HIV-1 diversity in the transmission bottleneck. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E1156–1163. doi: 10.1073/pnas.1103764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gnanakaran S, Bhattacharya T, Daniels M, Keele BF, Hraber PT, Lapedes AS, Shen T, Gaschen B, Krishnamoorthy M, Li H, Decker JM, Salazar-Gonzalez JF, Wang S, Jiang C, Gao F, Swanstrom R, Anderson JA, Ping LH, Cohen MS, Markowitz M, Goepfert PA, Saag MS, Eron JJ, Hicks CB, Blattner WA, Tomaras GD, Asmal M, Letvin NL, Gilbert PB, Decamp AC, Magaret CA, Schief WR, Ban YE, Zhang M, Soderberg KA, Sodroski JG, Haynes BF, Shaw GM, Hahn BH, Korber B. Recurrent signature patterns in HIV-1 B clade envelope glycoproteins associated with either early or chronic infections. PLoS pathogens. 2011;7:e1002209. doi: 10.1371/journal.ppat.1002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shukair SA, Allen SA, Cianci GC, Stieh DJ, Anderson MR, Baig SM, Gioia CJ, Spongberg EJ, Kauffman SM, McRaven MD, Lakougna HY, Hammond C, Kiser PF, Hope TJ. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013;6:427–434. doi: 10.1038/mi.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes Infect. 2003;5:59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 83.Rodriguez-Garcia M, Patel MV, Wira CR. Innate and adaptive anti-HIV immune responses in the female reproductive tract. J Reprod Immunol. 2013;97:74–84. doi: 10.1016/j.jri.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Norvell MK, Benrubi GI, Thompson RJ. Investigation of microtrauma after sexual intercourse. J Reprod Med. 1984;29:269–271. [PubMed] [Google Scholar]
- 85.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 86.O’Connor DM. A tissue basis for colposcopic findings. Obstet Gynecol Clin North Am. 2008;35:565–582. viii. doi: 10.1016/j.ogc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 87.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 88.Kell PD, Barton SE, Edmonds DK, Boag FC. HIV infection in a patient with Meyer-Rokitansky-Kuster-Hauser syndrome. J R Soc Med. 1992;85:706–707. doi: 10.1177/014107689208501119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grivel JC, Margolis LB. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nature medicine. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- 90.Grivel JC, Malkevitch N, Margolis L. Human immunodeficiency virus type 1 induces apoptosis in CD4(+) but not in CD8(+) T cells in ex vivo-infected human lymphoid tissue. Journal of virology. 2000;74:8077–8084. doi: 10.1128/jvi.74.17.8077-8084.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grivel JC, Penn ML, Eckstein DA, Schramm B, Speck RF, Abbey NW, Herndier B, Margolis L, Goldsmith MA. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. Journal of virology. 2000;74:5347–5351. doi: 10.1128/jvi.74.11.5347-5351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Merbah M, Introini A, Fitzgerald W, Grivel JC, Lisco A, Vanpouille C, Margolis L. Cervico-vaginal tissue ex vivo as a model to study early events in HIV-1 infection. Am J Reprod Immunol. 2011;65:268–278. doi: 10.1111/j.1600-0897.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alfsen A, Yu H, Magerus-Chatinet A, Schmitt A, Bomsel M. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol Biol Cell. 2005;16:4267–4279. doi: 10.1091/mbc.E05-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Herrewege Y, Michiels J, Waeytens A, De Boeck G, Salden E, Heyndrickx L, van den Mooter G, de Bethune MP, Andries K, Lewi P, Praet M, Vanham G. A dual chamber model of female cervical mucosa for the study of HIV transmission and for the evaluation of candidate HIV microbicides. Antiviral Res. 2007;74:111–124. doi: 10.1016/j.antiviral.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 95.Lawrence P, Portran D, Terrasse R, Palle S, Olivier T, Fantini J, Bourlet T, Pozzetto B, Delezay O. Selective transmigration of monocyte-associated HIV-1 across a human cervical monolayer and its modulation by seminal plasma. AIDS. 2012;26:785–796. doi: 10.1097/QAD.0b013e328351426e. [DOI] [PubMed] [Google Scholar]
- 96.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nature medicine. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 97.Maher D, Wu X, Schacker T, Horbul J, Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soto-Rivera J, Patterson BK, Chen Y, Shen C, Ratner D, Ding M, Tumne A, Gupta P. Study of HIV-1 transmission across cervical mucosa to tonsil tissue cells using an organ culture. Am J Reprod Immunol. 2013;69:52–63. doi: 10.1111/aji.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shattock RJ, Griffin GE, Gorodeski GI. In vitro models of mucosal HIV transmission. Nature medicine. 2000;6:607–608. doi: 10.1038/76138. [DOI] [PubMed] [Google Scholar]
- 100.Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985;230:1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- 101.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nature medicine. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 102.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, Zipori D, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 103.Orentas RJ, Hildreth JE. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 104.Ott DE. Cellular proteins in HIV virions. Rev Med Virol. 1997;7:167–180. doi: 10.1002/(sici)1099-1654(199709)7:3<167::aid-rmv199>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 105.Arakelyan A, Fitzgerald W, Margolis L, Grivel JC. Nanoparticle-based flow virometry for the analysis of individual virions. J Clin Invest. 2013;123:3716–3727. doi: 10.1172/JCI67042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Z, Chen Z, Phillips DM. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ Cells: implications for mechanisms of sexual transmission. J Infect Dis. 2003;188:1473–1482. doi: 10.1086/379248. [DOI] [PubMed] [Google Scholar]
- 107.Connell BJ, Lortat-Jacob H. Human Immunodeficiency Virus and Heparan Sulfate: From Attachment to Entry Inhibition. Front Immunol. 2013;4:385. doi: 10.3389/fimmu.2013.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nature medicine. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 109.Dezzutti CS, Guenthner PC, Cummins JE, Jr., Cabrera T, Marshall JH, Dillberger A, Lal RB. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J Infect Dis. 2001;183:1204–1213. doi: 10.1086/319676. [DOI] [PubMed] [Google Scholar]
- 110.Yeaman GR, Asin S, Weldon S, Demian DJ, Collins JE, Gonzalez JL, Wira CR, Fanger MW, Howell AL. Chemokine receptor expression in the human ectocervix: implications for infection by the human immunodeficiency virus-type I. Immunology. 2004;113:524–533. doi: 10.1111/j.1365-2567.2004.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. Journal of virology. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nature medicine. 2003;9:847–852. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 115.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. Journal of virology. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 117.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. Journal of virology. 2009;83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nobile C, Rudnicka D, Hasan M, Aulner N, Porrot F, Machu C, Renaud O, Prevost MC, Hivroz C, Schwartz O, Sol-Foulon N. HIV-1 Nef inhibits ruffles, induces filopodia, and modulates migration of infected lymphocytes. Journal of virology. 2010;84:2282–2293. doi: 10.1128/JVI.02230-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ladinsky MS, Kieffer C, Olson G, Deruaz M, Vrbanac V, Tager AM, Kwon DS, Bjorkman PJ. Electron tomography of HIV-1 infection in gut-associated lymphoid tissue. PLoS pathogens. 2014;10:e1003899. doi: 10.1371/journal.ppat.1003899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–1747. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, Tager AM, Luster AD, Mempel TR. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012;490:283–287. doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen P, Hubner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. Journal of virology. 2007;81:12582–12595. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–98. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 124.Abela IA, Berlinger L, Schanz M, Reynell L, Gunthard HF, Rusert P, Trkola A. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS pathogens. 2012;8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Durham ND, Yewdall AW, Chen P, Lee R, Zony C, Robinson JE, Chen BK. Neutralization resistance of virological synapse-mediated HIV-1 Infection is regulated by the gp41 cytoplasmic tail. Journal of virology. 2012;86:7484–7495. doi: 10.1128/JVI.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duncan CJ, Williams JP, Schiffner T, Gartner K, Ochsenbauer C, Kappes J, Russell RA, Frater J, Sattentau QJ. High multiplicity HIV-1 infection and neutralizing antibody evasion mediated by the macrophage-T cell virological synapse. Journal of virology. 2013 doi: 10.1128/JVI.03245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Poli G. Cell-to-cell vs. cell-free HIV-1 transmission from macrophages to CD4+ T lymphocytes: lessons from the virology textbook. AIDS. 2013;27:2307–2308. doi: 10.1097/QAD.0b013e328363619a. [DOI] [PubMed] [Google Scholar]
- 128.Luban J. Innate immune sensing of HIV-1 by dendritic cells. Cell Host Microbe. 2012;12:408–418. doi: 10.1016/j.chom.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. Journal of virology. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cavrois M, Neidleman J, Kreisberg JF, Greene WC. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS pathogens. 2007;3:e4. doi: 10.1371/journal.ppat.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.VanCompernolle SE, Taylor RJ, Oswald-Richter K, Jiang J, Youree BE, Bowie JH, Tyler MJ, Conlon JM, Wade D, Aiken C, Dermody TS, KewalRamani VN, Rollins-Smith LA, Unutmaz D. Antimicrobial peptides from amphibian skin potently inhibit human immunodeficiency virus infection and transfer of virus from dendritic cells to T cells. Journal of virology. 2005;79:11598–11606. doi: 10.1128/JVI.79.18.11598-11606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang TL, Teleshova N, Rapista A, Paluch M, Anderson RA, Waller DP, Zaneveld LJ, Granelli-Piperno A, Klotman ME. SAMMA, a mandelic acid condensation polymer, inhibits dendritic cell-mediated HIV transmission. FEBS Lett. 2007;581:4596–4602. doi: 10.1016/j.febslet.2007.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Izquierdo-Useros N, Blanco J, Erkizia I, Fernandez-Figueras MT, Borras FE, Naranjo-Gomez M, Bofill M, Ruiz L, Clotet B, Martinez-Picado J. Maturation of blood-derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. Journal of virology. 2007;81:7559–7570. doi: 10.1128/JVI.02572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang JH, Janas AM, Olson WJ, Wu L. Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. Journal of virology. 2007;81:8933–8943. doi: 10.1128/JVI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 136.de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TB. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nature medicine. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 137.Moris A, Pajot A, Blanchet F, Guivel-Benhassine F, Salcedo M, Schwartz O. Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood. 2006;108:1643–1651. doi: 10.1182/blood-2006-02-006361. [DOI] [PubMed] [Google Scholar]
- 138.Izquierdo-Useros N, Esteban O, Rodriguez-Plata MT, Erkizia I, Prado JG, Blanco J, Garcia-Parajo MF, Martinez-Picado J. Dynamic imaging of cell-free and cell-associated viral capture in mature dendritic cells. Traffic. 2011;12:1702–1713. doi: 10.1111/j.1600-0854.2011.01281.x. [DOI] [PubMed] [Google Scholar]


