Abstract
Purpose of review
We summarize recent mechanistic and physiological studies related to the role of perilipin 5 in regulating lipid droplet accumulation and protection to fatty acids (FAs) in tissues with high lipid oxidative metabolism.
Recent findings
Perilipin 5 (Plin5) is a lipid droplet (LD) targeting protein that promotes association of LDs with mitochondria and is most highly expressed in oxidative tissues, including cardiac and skeletal muscle. Recent in vivo and in vitro data indicate an important role for Plin5 in the regulation of cardiac lipid storage and function. Targeted overexpression of Plin5 in heart causes cardiac steatosis and mild mitochondria dysfunction and hypertrophy, but without affecting cardiac function. In contrast, whole body ablation of Plin5 (Plin5−/− mice) reduces cardiac lipid droplet formation, increases cardiac fatty acid oxidation, and promotes cardiac dysfunction; cardiac defects can be prevented with anti-oxidative therapy. These data suggest a cytoprotective role for Plin5 to promote lipid storage but to limit FA toxicity, parameters critical for tissues with high lipid oxidative metabolism.
Summary
In vivo and in vitro data suggest that Plin5 is part of a cell adaptive response to high lipid oxidative metabolism to protect LD storage against neutral lipases and, so, limit FA accumulation. While the specific mechanisms that underlie Plin5 LD storage protection in oxidative tissues remain to be fully elucidated, Plin5 provides a basis for the novel cytoprotective nature of LDs.
Keywords: ATGL, steatosis, β-oxidation, lipolysis, FA toxicity
INTRODUCTION
The pandemic rise in lipid-associated metabolic disorders has focused interest on the mechanistic actions of cytosolic lipid droplets (LDs), the organelles essential for regulation of lipid homeostasis in both adipose and non-adipose tissues, [1,2▪,3▪,4▪,5,6]. Although the accumulation of excess LDs in non-adipose tissues (ectopic fat), e.g. liver, heart, and skeletal muscle, is highly correlated with dyslipidemia, insulin resistance, type 2 Diabetes, and cardiovascular disease, studies in mice and humans have demonstrated a more complex relationship, where the presence of ectopic fat is more than a simple predictor of metabolic disorder [7,8,9▪,10▪,11▪,12]. Thus, LD function, which balances neutral lipid storage and utilization and is tightly regulated in a cell type-specific manner, is suggested to also have an essential protective role in the sequestration of cytotoxic fatty acids (FAs) in non-adipose tissues.
Cytosolic LDs are unique storage structures comprised of a core of neutral lipids, triacylglycerols (TAG) and/or cholesteryl ester (CE), surrounded by a phospholipid monolayer. The contrasting chemical natures of hydrophilic lipid metabolic enzymes and their hydrophobic substrates have directed attention to the LD surface as the regulatory interface between the aqueous cytosol and the hydrophobic lipid core. Specifically, perilipin proteins (Plins) are the definitive abundant proteomic markers of LD surfaces in both adipose and non-adipose cells and function as primary mediators for neutral lipid storage/hydrolysis [13–15].
The mammalian genome encodes five Plin genes, with unique tissue-dependent patterns of transcription and splice variation, although individual cells often express more than a single Plin type [16]. Perilipin 1 (Plin1) is most abundant in white and brown adipose tissue (WAT, BAT). Perilipin 2 (Plin2) and perilipin 3 (Plin3) are more widely distributed, with Plin2 highly expressed in hepatocytes. Perilipin 4 (Plin4) is observed in adipocytes, cardiomyocytes, and myocytes, and perilipin 5 (Plin5) is generally restricted to tissues/cells that utilize lipids for energy through mitochondrial β-oxidation, e.g. cardiomyocytes, brown and inducible brown adipocytes (also referred to beige or bright adipocytes), liver, and skeletal myocytes [17–19], Here, we review recent progress toward understanding the specialization of Plin5 in the mechanistic interaction of the two critical organelles, LDs and mitochondria, that balance oxidative cellular energy, lipid homeostasis, and cytoprotection.
TRANSCRIPTIONAL REGULATION OF PLIN5 CORRELATES WITH A SPECIALIZED FUNCTION IN CELLS THAT UTILIZE LIPID OXIDATION FOR ENERGY
When exposed to an increase in circulating FAs, organs such as heart, skeletal muscle, and liver, respond by inducing genes that regulate FA metabolism. One primary pathway involves the transcription factor family of peroxisome proliferator-activated receptors (PPARs) [20]. Upon activation, through direct interaction with FA-derived ligands, PPARs, in combination with their heterodimerization partners, retinoid X receptors (RXRs), bind at specific genomic sequences (PPAR regulatory elements, PPREs). Cell-specific expression of PPAR variants, in concert with transcriptional co-factors, such as PGC-1 (PPAR coactivator-1) family members, directs expression of appropriate metabolic enzymes for FA utilization/storage [21]. Accordingly, PPARα and PPARβ/δ are highly expressed in tissues with elevated rates of FA oxidative metabolism (e.g. heart, skeletal muscle, and liver), whereas PPARγ is more preferential in lipogenic tissues (e.g. adipose and liver) [20].
As an LD target protein, Plin5 expression is enhanced under physiological or pharmacological conditions that promote systemic FA elevation, e.g. fasting (liver, heart), endurance exercise (skeletal muscle), and chronic β3-adrenergic stimulation (liver) [17–19, 22,23▪,24]. Exogenous FAs can also stimulate Plin5 expression in cell culture [25]. A functionally conserved PPRE site maps to the first intron of Plin5, and Plin5 expression can be induced in liver, skeletal, and cardiac muscle by PPARα agonists, but also in WAT by pioglitazone, a PPARγ agonist [17–19, 26▪▪]. Some agonists, however, are not exclusive, but can cross-activate different PPAR family members.
Although basal Plin5 mRNA levels (liver, heart) are severely suppressed in PPARα−/− mice, Plin5 induced expression is responsive to fasting, suggesting additional regulatory control. Indeed, PPARβ/δ appears the more potent regulator of Plin5 than PPARα in skeletal muscle [26▪▪]. Plins 1, 2, and 4 are similarly induced through FA-ligand activation of PPARs, but their expression is more directly influenced by PPARγ action, than by PPARα or PPARβ/δ [27–30].
The preferential activation of Plin5 expression by PPARα and PPARβ/δ provides a mechanism for selectivity in mammalian tissues that utilize FA for β-oxidation to provide energy or heat. PGC-1α will drive formation of oxidative muscles, fine tune the energy-generating machinery in response to nutrient availability, and promote cellular defenses to metabolic stress [31▪]. Overexpression of PGC-1α in skeletal muscle will also increase Plin5 transcription [23▪]. The restricted presence of Plin5 to LDs of oxidative tissues may be required for the physical and functional interactions between LDs and mitochondria and the interplay of FA substrate availability to enable exquisite regulation of β-oxidation for both energy (heart, skeletal muscle) and heat (BAT) [32,33▪▪].
UNIQUE PLIN5 REGULATION OF OXIDATIVE LD STORAGE
Cytosolic LDs are often viewed only as storage depots to provide regulated availability of the lipid moieties required for essential and various cellular functions, including β-oxidation, membrane phospholipid synthesis, cell signaling, and steroid production. While true, it is now further recognized that LDs also serve a protective function, by sequestering cytotoxic FA and cholesterol, as TAG and CE, respectively [34–36]. The dynamic nature of LDs balances sequestration and storage, with the regulated cleavage of long-chain TAG to provide sufficient metabolic precursors as polar lipids, while also minimizing cytotoxic effects. The mammalian Plins are not required for LD biogenesis per se, but, as primary regulators of lipolysis, they modulate cellular TAG/CE levels. In general, overexpression of any Plin form can promote LD storage [13–15].
Four major proteins, in addition to Plin1, have been identified in the neutral lipid catabolic pathway of adipocytes, hormone sensitive lipase (HSL), adipose triglyceride lipase (ATGL), comparative gene identification 58 (CGI-58), a positive regulator of ATGL, and G0/G1 Switch (G0S2), a negative regulator of ATGL [37,38]. In adipocytes, access to LD surfaces can be regulated by Plin1. Unphosphorylated Plin1 restricts ATGL and HSL from LDs and suppresses lipolysis [39], whereas PKA-phosphorylated Plin1 permits ATGL/CGI-58 and HSL association with LD surfaces to activate lipolysis. Thus, under stimulatory conditions that activate adenylyl cyclase (AC) and elevate intracellullar cAMP levels, lipolytic rates rise ~50-fold [40]. G0S2 inhibits lipolysis through direct interaction with and inhibition of ATGL [38].
ATGL, HSL, CGI-58 are also essential regulators of lipid hydrolysis in non-adipose tissue and Plin5 can interact with all 3 proteins [41–44]. FRET experiments indicate that ATGL and CGI-58 bind toward the C-terminal half of Plin5, whereas the HSL binding sites are thought to reside in the N-terminal PAT-1 domain that is common to all perilipins [42]. The precise interactive sites on Plin5 are not known, but binding of ATGL and CGI-58 to Plin5 appears to be mutually exclusive [41,43]. However, in vivo associations have not been comparatively explored in oxidative cells that are lipolytically dormant or activated. Although G0S2 is expressed in oxidative tissues, a functional role in control of lipolysis is not yet defined.
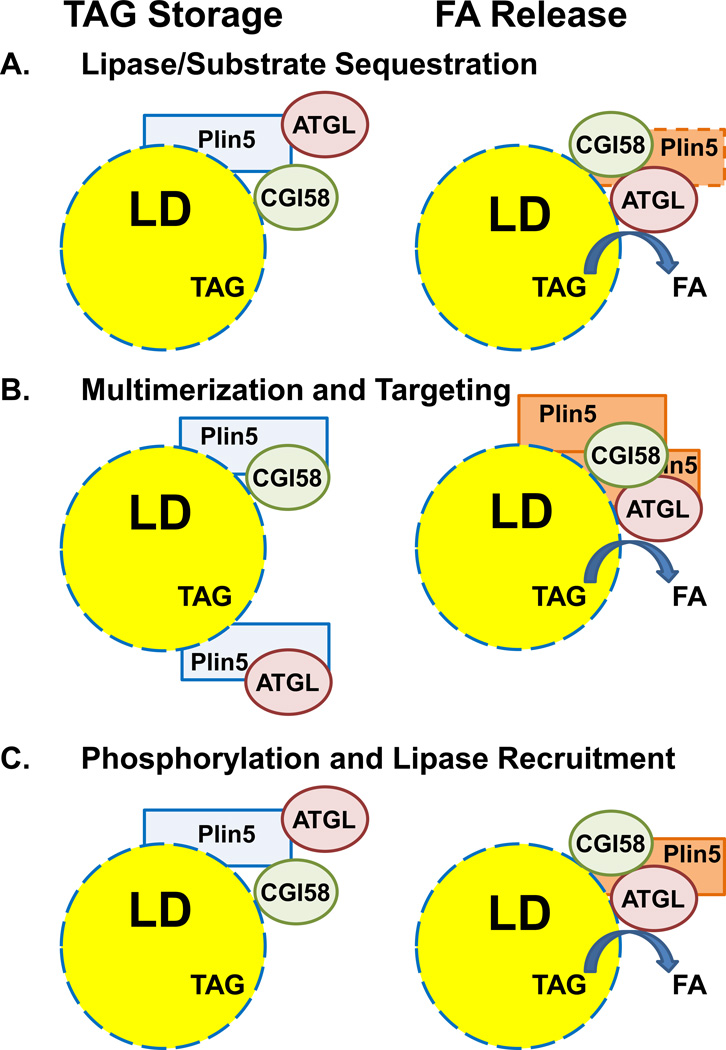
Plin5 acts to protect LD stores, likely by suppressing lipolysis. Thus, cardiomyoctes that overexpress Plin5 have a lipid phenotype similar to those deficient in ATGL, increased LD accumulation [45▪▪,46▪▪,47,48], and cardiac lipid droplet depletion in Plin5−/− mice can be reversed by a lipase inhibitor [49▪▪]. Although Plin5 only minimally affects ATGL activity in reconstituted lipolytic in vitro assays using free triolein as a substrate [45▪▪,46▪▪], ATGL-mediated lipolysis is significantly inhibited using Plin5-coated LD substrates in contrast to LD controls, isolated from cells that do not express Plin5 [46▪▪]. Thus, Plin5 is modeled as a regulated lipolytic barrier that sequesters LD substrates from ATGL [46▪▪]. Cellular lipolytic activation could elicit a structural re-organization of Plin5 at the LD surface to facilitate ATGL/CGI-58 (and perhaps HSL) access to lipid substrates (Figure 1A). In this context, ATGL/CGI-58 interactions are elevated in contracted muscle cells relative to resting cells, without an accompanying alteration in relative Plin5 association with either ATGL or CGI-58 [50]. A potential role for HSL is not defined, but HSL phosphorylation at serine 600 is increased during endurance exercise [51▪].
Figure 1. Models for Plin5 regulation of lipolysis.
In basal cells, Plin5 coats LDs, which limits TAG access of ATGL; TAG is stored. Plin5 is able to bind both ATGL and CGI-58, but the precise relationships and relative cellular localizations of all the components are not known; representations are diagrammatic, but CGI-58 is presumed to be at the LD surface in basal cells. Several models postulate how Plin5/ATGL/CGI-58 associations with LDs become altered in activated cells, where FAs are released. The models do not incorporate additional positive or negative regulators (e.g. G0S2), which may be contributory.
A. Structural re-organization of Plin5 promotes ATGL/substrate access and ATGL/CGI-58 interactions.
B. ATGL and CGI-58 bindings to Plin5 are mutually exclusive. Plin5-multimerization allows proximate interaction of ATGL and CGI-58.
C. Plin5 is a phospho-protein. By analogy to Plin1 regulation of lipolysis in adipocytes, differential phosphorylation of Plin5 facilitates ATGL/substrate access and ATGL/CGI-58 interactions.
Several observations suggest possible mechanistic targets that could modulate ATGL/LD access. Since the binding of ATGL and CGI-58 to Plin5 is mutually exclusive, ATGL may associate with LDs in basal oxidative cells, while being sequestered from its co-activator CGI-58 (Figure 1B). A regulatory path that multimerizes Plin5 could facilitate interactions of proximal ATGL and CGI-58 to elicit efficient lipolysis [43]. Plin5 may also reside in non-LD cellular compartments [52,53]. Although it is suggested that Plin5 may undergo re-partitioning to or from LDs to facilitate lipolytic activation, a differential recruitment of Plin5 to lipid droplets has not been observed in contracted muscle [54].
Recent proteomic [55] and phospho-labeling [44] studies indicate phosphorylation target sites on Plin5. Differential phosphorylation of Plin5 could alter interactions with ATGL and/or CGI-58, thus limiting or activating TAG hydrolysis [44,56] (Figure 1C). Although this has analogy to Plin1 function, functional differences to Plin1 are evident. Unlike Plin1, which does not interact with ATGL regardless of phospho-state, Plin5 readily binds ATGL. In addition, although the Plin5 kinase(s) have yet to be identified, PKA may be less significantly involved. Although mouse (and human) Plin5 does have a potential PKA phosphorylation site, phospho-incorporation into Plin 5 and lipolysis are elevated <2-fold upon AC activation of a cultured Plin5-cell system [44].
The precise molecular mechanism(s) by which Plin5 controls oxidative LD storage remains to be further clarified and the postulated models are not mutually exclusive. Important clues will require definitive identification of Plin5 phosphorylation sites and regulatory kinases. FRET approaches may be considered to probe Plin5 and LD surfaces. DAG enrichment at the surface of LDs will recruit Plin5 [53], and Plin5 is preferentially localized to TAG-enriched LDs, in contrast to CE organelles [57▪]. Studies focused to characterize Plin5/LD surfaces will help evaluate the ability of Plin5 to regulate substrate/lipase sequestration. However, difficulties in purifying the Plins have limited their use in classical biophysical techniques to investigate Plin/LD surfaces and regulation of lipase activity [58]. An inhibitory role for G0S2 in oxidative tissue is suggested, but must be investigated mechanistically.
SPECIFIC ROLE OF PLIN5 IN OXIDATIVE CELLS, INVOLVING PHYSICAL LD-MITOCHONDRIAL ASSOCIATIONS
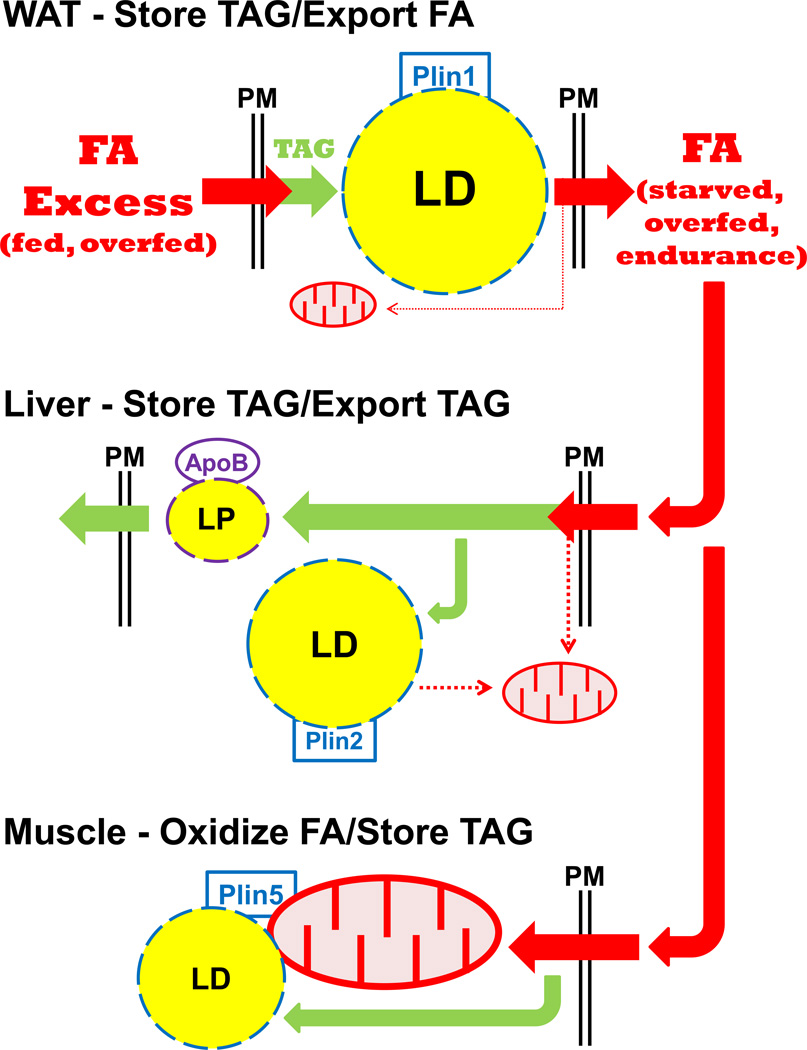
Plin1, Plin2, and Plin5 regulate LD storage by separate mechanisms, reflecting their unique adaptations to cell/lipid utilization (Figure 2). Plin1 facilitates TAG storage in adipocytes for systemic energy homeostasis, whereas Plin2 is expressed more globally to allow TAG storage (ectopic fat) under conditions of starvation or obesity. Plin5 in myocytes and other oxidative cells balances FA availability for mitochondrial oxidation with sequestration to protect against cytotoxicity. Loss-of-function mutants for Plin1, 2, or 5 all have decreased LD stores and increased β-oxidation in their respective tissues, compared to WT [49▪▪,59–60,62▪▪], despite compensating expression by other Plin forms; Plin1−/− adipocytes have increased Plin2 at their LD surface, but elevated rates of lipolysis. Since Plin2 is unable to compensate for Plin1 for lipolytic regulation, Plin1−/− mice are protected to diet-induced obesity [59,60]. Plin2−/− hepatocytes have increased Plin5, but also a marked reduction in LD content in response to starvation or high-fat diet [61,62▪▪]. Although Plin5 may be less protective to LD accumulation in hepatocytes than is Plin2, overexpression of Plin5 in Plin2+/+ hepatocytes can increase TAG storage [63]. Certainly, Plin5 (and the other Plin forms) may respond and function with extreme cell-type specificity, but, importantly, Plin1, Plin2, and Plin5 are not simply operationally redundant for LD storage and regulation.
Figure 2. Plin/tissue-specific utilization of FA; a cytoprotective role for Plin5 in oxidative tissue.
Plin1 and WAT: During fed conditions, excess energy as FA (fatty acid) is stored in Plin1-coated LDs. Upon fasting, endurance exercise, or overfeeding, adipose tissue secretes FAs for use by other organs. Limited intracellular FA is utilized for mitochondrial oxidation.
Plin2 and liver: Excess FA supply leads to an increase in TAG biogenesis, stored as Plin2-coated LDs (ectopic fat) or secreted as ApoB-coated LP (lipoprotein) particles. Some intracellular FA is available for mitochondrial oxidation.
Plin5 and cardiac/skeletal muscle: A significant degree of excess FA supply is utilized for mitochondrial oxidation. Additional FAs are directed to TAG biogenesis, which is stored as Plin5-coated LDs, tethered to mitochondria. TAG storage in Plin5-LDs may protect mitochondria from FA toxicity and promote mitochondrial function.
Plin5 is localized at the surface of LDs in oxidative cells, but also in connection with mitochondria [32,33▪▪]. Overexpression of Plin5 in fibroblasts directs a close association of LDs and mitochondria [32]. Although Plin2 alone is unable to promote a similar re-organization, a Plin2 chimera with the C-terminal 67 amino acids of Plin5 (including 20 essential amino acids) has the ability to tether mitochondria to LDs [32] in fibroblasts.
The physiological significance to promote the association of LDs and mitochondria in oxidative tissue is yet to be determined. However, mitochondria/LD associations, observed in heart and skeletal muscle cells where lipids are utilized for energy, are further enhanced by exercise [64]. In accord, exercise in mice and humans increases PGC-1α expression in skeletal muscle, which promotes expression of Plin5 and other genes involved in LD assembly and mobilization and remodeling of mitochondria [23▪].
An intimate physical association between LDs and mitochondria in oxidative tissue may be essential to coordinate cellular energy homeostasis, but also to protect mitochondria against locally elevated toxic levels of FA and lipid intermediates. Ultimately, LDs can utilize the same substrates as mitochondria (Figure 2), so increased LD storage can be protective to FA cytotoxicity [65▪]. In addition, elevated rates of FA β-oxidation contribute to increased mitochondrial reactive oxygen species (ROS) [65▪]. Plin5 may reduce ROS production by transiently channeling excess FA into LDs. Although LD storage is a hallmark of cellular stress, it may serve more to ameliorate cytotoxicity, rather than to be a causal agent.
The regulated hydrolysis of TAG in Plin5-LDs may not only direct FAs to the mitochondria, but may also provide functional signals for PPARα and PPARβ/δ activation. More specifically, a PPAR ligand has been proposed that is derived from ATGL-mediated LD hydrolyses [48] and that may regulate expression of genes involved in FA metabolism, mitochondrial biogenesis, and protective anti-oxidative pathways [48,66▪▪,67–69].
PLIN5 IN CARDIAC/SKELETAL MYOCYTE FUNCTION AND SYSTEMIC HOMEOSTASIS
Although there is direct relationship between Plin5 expression levels and LD accumulation, connections among β-oxidation, ROS, and cardiac function are less well-defined. Whole body loss-of-function Plin5−/− mice have depleted cardiac LDs, increased β-oxidation, but also increased ROS production and cardiac dysfunction, which may be minimized with anti-oxidative therapy [49▪▪]. Cardiac-specific overexpression of Plin5 in mice results in massive steatosis, a mild defect in mitochondria β-oxidation, but also increased ROS, without cardiac dysfunction [45▪▪]. Although here, the Nrf2-antioxidant response pathway is activated, which increases expression of the protective gluthatione enzymes [45▪▪], a mechanistic link of Plin5, increased LDs, and Nrf2 activation is yet to be established; the ROS sources in these respective models also remained to be clarified.
While cardiac steatosis may be associated with cellular dysfunction, enhanced LD accumulation is not always detrimental. Thus, genetic deficiencies in ATGL or CGI-58 compromise lipase function and cause cardiac steatosis and cardiomyopathy [47,70▪,71▪] whereas cardiac overexpression of diacylglyceride acyltransferase 1 (DGAT-1), the rate-limiting TAG-synthesizing enzyme, promotes steatosis, but without corresponding cardiac defects during a similar time-frame [72]. The Plin5 mouse models [45▪▪,46▪▪,49▪▪] further exemplify the dissociation of cardiac steatosis and cardiac tissue dysfunction.
Overall results suggest a cytoprotective role of Plin5 to cardiac steatosis and FA toxicity, involving the structural re-organization of oxidative LDs and mitochondria [11▪,15,32,33▪▪,73]. Still, the relationship of Plin5 levels to FA β-oxidation and connection to cardiac ROS remains to be resolved. Potentially, the protective nature of Plin5 overexpression may partially derive from compensatory adaptive mechanisms that activate anti-oxidative Nrf2 functions [45▪▪,49▪▪].
The whole body Plin5−/− mouse does not exhibit changes in systemic lipid and glucose homeostasis on a standard chow diet [49▪▪]. Systemic and tissue-specific functions remain to be investigated under conditions of obesity, exercise, and cold challenge [49▪▪]. Relation to insulin sensitivity can only be partially inferred. Selective skeletal muscle Plin5 overexpression results in increased muscle LD content, a gene expression profile favoring β-oxidation, but without compromising or improving muscle sensitivity for insulin-mediated glucose uptake during diet induced obesity [74▪▪]. By contrast, overexpression of Plin2 in skeletal muscle also leads to increased LD stores, but suppressed β-oxidation and partially improved muscle insulin-mediated glucose uptake in response to diet-induced obesity [75▪]. Plin specificity at LD surfaces may differentially impact lipid stores in oxidative tissue (Figure 2). Plin5 may participate more in an adaptive cell mechanism in response to higher rates of FA β-oxidation by mitochondria.
CONCLUSIONS
The Plin5 studies underscore the concept for specialized pools of “oxidative” LDs in highly energetic mammalian cells. In contrast to other Plin forms, Plin5 has the unique ability for LD storage during energetic demands of FA oxidation and to promote the close proximity of LDs with mitochondria, the oxidative site. It may be speculated that LD-mitochondrial associations assure advantageous fuel delivery for energetic efficiency but also to provide a localized cytoprotective sink for excess toxic FA and lipid intermediates. The mechanistic functions of Plin5 are still to be fully elaborated, but Plin5 remains a focus to understand the adaptive cellular mechanisms to high lipid oxidative metabolic states and their failure in earlier stages of metabolic disease.
KEY POINTS.
Plin5 is metabolically and physically linked to LDs and mitochondria in tissues with high β-oxidative activity.
Overexpression of Plin5 in heart promotes steatosis, by inhibiting lipolysis, but in the absence of corresponding cardiac dysfunction.
Cardiac muscle in Plin5−/− mice have reduced LD stores, but lipotoxicity that induces cardiac dysfunction.
Plin5 may exert a cytoprotective role against FA-induced lipotoxicity by channeling FA into LD stores.
Acknowledgments
We thank Dr. Dean Londos for helping pioneer the study of Lipid Droplets as unique cytosolic organelles, for training many in the field, and for continuously inspiring “lipid droplet” investigators.
This research was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases (A.R.K.), a grant from NIH 1RO1 DK 075017 (to C.S.), grant in aid 11GRNT7600027 from the American Heart Association (to C.S.), and the Geriatric Research, Education and Clinical Center, Baltimore Veterans Affairs Health Care Center, the Clinical Nutrition Research Unit of Maryland (DK072488).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Krahmer N, Farese RV, Jr, Walther TC. Balancing the fat: lipid droplets and human disease. EMBO Mol Med. 2013;5:905–915. doi: 10.1002/emmm.201100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kohlwein SD, Veenhuis M, van der Klei IJ. Lipid droplets and peroxisomes: key players in cellular lipid homeostasis or a matter of fat - store 'em up or burn 'em down. Genetics. 2013;193:1–50. doi: 10.1534/genetics.112.143362. ▪ This review highlights the metabolic and physical interactions between LDs and peroxisomes and their importance in cellular lipid homeostasis in yeast.
- 3. Kienesberger PC, Pulinilkunnil T, Nagendran J, Dyck JR. Myocardial triacylglycerol metabolism. J Mol Cell Cardiol. 2013;55:101–110. doi: 10.1016/j.yjmcc.2012.06.018. ▪ This review discusses the growing interest in the role of myocardial triacylglycerol stores in cardiac lipid metabolism and function.
- 4. Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab. 2012;238:391–398. doi: 10.1016/j.tem.2012.05.009. ▪ This review presents the paradoxical data for intramyocellular LDs in skeletal muscle relative to insulin sensitivity.
- 5.Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konige M, Wang H, Sztalryd C. Role of adipose specific lipid droplet proteins in maintaining whole body energy homeostasis. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.05.007. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipid. 2009;20:50–56. doi: 10.1097/mol.0b013e328321b3a8. [DOI] [PubMed] [Google Scholar]
- 8.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 9. Sun Z, Lazar MA. Dissociating fatty liver and diabetes. Trends Endocrinol Metab. 2013;24:4–12. doi: 10.1016/j.tem.2012.09.005. ▪ This review highlights studies in mice and human concerning LDs levels in liver in relation to insulin sensitivity.
- 10. Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. ▪ This review emphasizes the importance of specific lipid intermediates in the development of insulin resistance in liver, skeletal muscle, and adipose tissues.
- 11. Bosma M, Kersten S, Hesselink MK, Schrauwen P. Re-evaluating lipotoxic triggers in skeletal muscle: relating intramyocellular lipid metabolism to insulin sensitivity. Prog Lipid Res. 2012;51:36–49. doi: 10.1016/j.plipres.2011.11.003. ▪ This review highlights the relationships among intramyocellar lipid stores metabolism, insulin resistance, and muscle function in humans and mice.
- 12.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, Eiriksdottir G, Garcia ME, Launer LJ, Nalls MA, Clark JM, Mitchell BD, Shuldiner AR, Butler JL, Tomas M, Hoffmann U, Hwang SJ, Massaro JM, O'Donnell CJ, Sahani DV, Salomaa V, Schadt EE, Schwartz SM, Siscovick DS, NASH CRN. GIANT Consortium. MAGIC Investigators. Voight BF, Carr JJ, Feitosa MF, Harris TB, Fox CS, Smith AV, Kao WH, Hirschhorn JN, Borecki IB GOLD Consortium. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sztalryd C, Kimmel AR. Perilipins: Lipid droplet coat proteins adapted for tissue-specific energy storage and utilization, and lipid cytoprotection. Biochimie. 2013 doi: 10.1016/j.biochi.2013.08.026. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, Kimmel AR. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome. 2001;12:741–749. doi: 10.1007/s00335-01-2055-5. [DOI] [PubMed] [Google Scholar]
- 17.Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, Nebb HI. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771:210–227. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi T, Matsushita S, Motojima K, Hirose F, Osumi T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem. 2006;281:14232–14240. doi: 10.1074/jbc.M601682200. [DOI] [PubMed] [Google Scholar]
- 19.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A, Rasouli N, Kern PA, Finck BN, Bickel PE. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 20.Poulsen Ll, Siersbæk M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23:631–639. doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 22.Amati F, Dubé JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koves TR, Sparks LM, Kovalik JP, Mosedale M, Arumugam R, DeBalsi KL, Everingham K, Thorne L, Phielix E, Meex RC, Kien CL, Hesselink MK, Schrauwen P, Muoio DM. PPARγ coactivator-1α contributes to exercise-induced regulation of intramuscular lipid droplet programming in mice and humans. J Lipid Res. 2013;54:522–534. doi: 10.1194/jlr.P028910. ▪ This study demonstrates the importance in the coordinated expression of genes involved in β-oxidation and in LD storage during exercise via PGC-1α as a key regulatory node.
- 24.Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (PLIN5) with adipose trigylceride lipase (ATGL) J Biol Chem. 2010;886:5126–5135. doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockridge JB, Sailors ML, Durgan DJ, Egbejimi O, Jeong WJ, Bray MS, Stanley WC, Young ME. Bioinformatic profiling of the transcriptional response of adult rat cardiomyocytes to distinct fatty acids. J Lipid Res. 2008;49:1395–1408. doi: 10.1194/jlr.M700517-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bindesbøll C, Berg O, Arntsen B, Nebb HI, Dalen KT. Fatty acids regulate perilipin5 in muscle by activating PPARδ. J Lipid Res. 2013;54:1949–1963. doi: 10.1194/jlr.M038992. ▪▪ This study is the first to identify a PPRE site for Plin5 transcription and to demonstrate that Plin5 is a preferential target gene for PPARβ/δ.
- 27.Dalen KT, Schoonjans K, Ulven SM, Weedon-Fekjaer MS, Bentzen TG, Koutnikova H, Auwerx J, Nebb HI. Adipose tissue expression of the lipid droplet-associating proteins S3–12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes. 2004;53:1243–1252. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 28.Bildirici I, Roh CR, Schaiff WT, Lewkowski BM, Nelson DM, Sadovsky Y. The lipid droplet-associated protein adipophilin is expressed in human trophoblasts and is regulated by peroxisomal proliferator-activated receptor-gamma/retinoid X receptor. J Clin Endocrinol Metab. 2003;88:6056–6062. doi: 10.1210/jc.2003-030628. [DOI] [PubMed] [Google Scholar]
- 29.Nagai S, Shimizu C, Umetsu M, Taniguchi S, Endo M, Miyoshi H, Yoshioka N, Kubo M, Koike T. Identification of a functional peroxisome proliferator-activated receptor responsive element within the murine perilipin gene. Endocrinology. 2004;145:2346–2356. doi: 10.1210/en.2003-1180. [DOI] [PubMed] [Google Scholar]
- 30.Arimura N, Horiba T, Imagawa M, Shimizu M, Sato R. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J Biol Chem. 2004;279:10070–10076. doi: 10.1074/jbc.M308522200. [DOI] [PubMed] [Google Scholar]
- 31. Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. ▪ This review provides a general background to the transcriptional regulation of mitochondrial biogenesis and function.
- 32.Wang H, Sreenevasan U, Hu H, Saladino A, Polster BM, Lund LM, Gong DW, Stanley WC, Sztalryd C. Perilipin 5, lipid droplet associated protein provides physical and metabolic linkage to mitochondria. J Lipid Res. 2011;52:2159–2168. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bosma M, Minnaard R, Sparks LM, Schaart G, Losen M, de Baets MH, Duimel H, Kersten S, Bickel PE, Schrauwen P, Hesselink MK. The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem Cell Biol. 2012;137:205–216. doi: 10.1007/s00418-011-0888-x. ▪▪ This and [32] show the unique physical location of Plin5 at LD and mitochondrial surfaces.
- 34.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturley SL, Hussain MM. Lipid droplet formation on opposing sides of the endoplasmic reticulum. J Lipid Res. 2012;53:1800–1810. doi: 10.1194/jlr.R028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraemer FB, Khor VK, Shen WJ, Azhar S. Cholesterol ester droplets and steroidogenesis. Mol Cell Endocrinol. 2013;371:15–19. doi: 10.1016/j.mce.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Viswanadha S, Londos C. Determination of lipolysis in isolated primary adipocytes. Methods Mol Biol. 2008;456:299–306. doi: 10.1007/978-1-59745-245-8_22. [DOI] [PubMed] [Google Scholar]
- 41.Granneman JG, Moore HP, Mottillo EP, Zhu Z. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J Biol Chem. 2009;284:3049–3057. doi: 10.1074/jbc.M808251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Hu L, Dalen K, Dorward H, Marcinkiewicz A, Russell D, Gong D, Londos C, Yamaguchi T, Holm C, Rizzo MA, Brasaemle D, Sztalryd C. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J Biol Chem. 2009;284:32116–32125. doi: 10.1074/jbc.M109.006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (PLIN5) with adipose trigylceride lipase (ATGL) J Biol Chem. 2010;886:5126–5135. doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Bell M, Sreenevasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong D, Brasaemle D, Sztalryd C. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem. 2011;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang H, Sreenivasan U, Gong DW, O'Connell KA, Dabkowski ER, Hecker PA, Ionica N, Konig M, Mahurkar A, Sun Y, Stanley WC, Sztalryd C. Cardiomyocyte-specific perilipin 5 overexpression leads to myocardial steatosis and modest cardiac dysfunction. J Lipid Res. 2013;54:953–965. doi: 10.1194/jlr.M032466. ▪▪ This study indicates that cardiac-specific overexpression of Plin5 in vivo results in severe increases in LD stores, with mild consequences on mitochondrial or cardiac function. It is proposed that lack of a more severe cardiac dysfunctional phenotype is due to the compensatory activation of the Nrf2 anti-oxidant pathway. It is shown that Plin5 can very modestly inhibit ATGL in presence of CGI-58 in a reconstituted system in vitro.
- 46. Pollak NM, Schweiger M, Jaeger D, Kolb D, Kumari M, Schreiber R, Kolleritsch S, Markolin P, Grabner GF, Heier C, Zierler KA, Rülicke T, Zimmermann R, Lass A, Zechner R, Haemmerle G. Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier. J Lipid Res. 2013;58:1092–1102. doi: 10.1194/jlr.M034710. ▪▪ Cardiac-specific overexpression of Plin5 results in a phenotype similar to that of [45]. Experiments show that Plin5 does not directly inhibit ATGL activity in a reconstituted in vitro system, but is able to prevent hydrolysis of Plin5-coated LDs.
- 47.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 48.Haemmerle G, Moustafa T, Woelkart G, Büttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rülicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, Matsumura S, Inoue K, Fushiki T, Kojima Y, Hashimoto T, Sakai F, Hirose F, Osumi T. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem. 2012;287:23852–23863. doi: 10.1074/jbc.M111.328708. ▪▪ This manuscript describes the phenotypic consequences of Plin−/− mice. The phenotype is characterized by depletion of cardiac lipid stores, increase in β-oxidation, and development of cardiac dysfunction with age, which is preventable with anti-oxidant therapy.
- 50.MacPherson RE, Ramos SV, Vandenboom R, Roy BD, Peters SJ. Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction. Am J Physiol Regul Integr Comp Physiol. 2013;304:R644–R650. doi: 10.1152/ajpregu.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Louche K, Badin PM, Montastier E, Laurens C, Bourlier V, de Glisezinski I, Thalamas C, Viguerie N, Langin D, Moro C. Endurance exercise training up-regulates lipolytic proteins and reduces triglyceride content in skeletal muscle of obese subjects. J Clin Endocrinol Metab. 2013;98:4863–4871. doi: 10.1210/jc.2013-2058. ▪ These results demonstrate an upregulation of Plin5, despite a reduction in LD levels.
- 52.Bartholomew SR, Bell EH, Summerfield T, Newman LC, Miller EL, Patterson B, Niday ZP, Ackerman WE, 4th, Tansey JT. Distinct cellular pools of perilipin 5 point to roles in lipid trafficking. Biochim Biophys Acta. 2012;1821:268–278. doi: 10.1016/j.bbalip.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skinner JR, Shew TM, Schwartz DM, Tzekov A, Lepus CM, Abumrad NA, Wolins NE. Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization. J Biol Chem. 2009;284:30941–30948. doi: 10.1074/jbc.M109.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacPherson RE, Herbst EA, Reynolds EJ, Vandenboom R, Roy BD, Peters SJ. Subcellular localization of skeletal muscle lipid droplets and PLIN family proteins OXPAT and ADRP at rest and following contraction in rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2012;302:R29–R36. doi: 10.1152/ajpregu.00163.2011. [DOI] [PubMed] [Google Scholar]
- 55.Villén J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci U S A. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brasaemle DL. Perilipin 5: putting the brakes on lipolysis. J Lipid Res. 2013;54:876–877. doi: 10.1194/jlr.E036962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hsieh K, Lee YK, Londos C, Raaka BM, Dalen KT, Kimmel AR. Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets. J Cell Sci. 2012;125:4067–4076. doi: 10.1242/jcs.104943. ▪ Results show that the different Plins can selectively target TAG- or CE-LDs, suggesting functional distinctions.
- 58.Ransac S, Ivanova M, Panaiotov I, Verger R. Monolayer techniques for studying lipase kinetics. Methods Mol Biol. 1999;109:279–302. doi: 10.1385/1-59259-581-2:279. [DOI] [PubMed] [Google Scholar]
- 59.Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet. 2000;4:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 60.Tansey JT, C Sztalryd C, J Gruia-Gray J, DL Roush DL, JV Zee JV, O Gavrilova O, ML Reitman ML, CX Deng CX, C Li C, AR Kimmel AR, C Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;3:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McManaman JL, Bales ES, Orlicky DJ, Jackman M, MacLean PS, Cain S, Crunk AE, Mansur A, Graham CE, Bowman TA, Greenberg AS. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res. 2013;54:1346–1359. doi: 10.1194/jlr.M035063. ▪▪ This manuscript describes the consequences of the complete ablation of Plin2 in mice. The phenotype is characterized by a marked decrease in hepatic LD stores and resistance to diet-induced obesity in hepatic and adipose tissue. Adipose tissue resistance occurs only after a long-term, high-fat diet and results in increased brown-like fat cells in the absence of overall thermogenic changes.
- 63.Li H, Song Y, Zhang LJ, Gu Y, Li FF, Pan SY, Jiang LN, Liu F, Ye J, Li Q. LSDP5 enhances triglyceride storage in hepatocytes by influencing lipolysis and fatty acid β-oxidation of lipid droplets. PLoS One. 2012;7:e36712. doi: 10.1371/journal.pone.0036712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipidand mitochondrial ultrastructure, substrate use, mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1271–R1278. doi: 10.1152/ajpregu.00472.2006. [DOI] [PubMed] [Google Scholar]
- 65. Lee SJ, Zhang J, Choi AM, Kim HP. Mitochondrial dysfunction induces formation of lipid droplets as a generalized response to stress. Oxid Med Cell Longev. 2013;2013:327167. doi: 10.1155/2013/327167. ▪ These studies support the concept that increased LD stores is a compensatory mechanism against mitochondrial oxidative stress.
- 66. Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. ▪▪ This review discusses recent studies for the importance of transcriptional signaling molecules generated by LD hydrolysis for lipid and mitochondrial metabolism.
- 67.Mottillo EP, Bloch AE, Leff T, Granneman JG. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) α and δ in brown adipocytes to match fatty acid oxidation with supply. J Biol Chem. 2012;287:25038–25048. doi: 10.1074/jbc.M112.374041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res. 2009;50:1621–1629. doi: 10.1194/jlr.M800614-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zierler KA, Jaeger D, Pollak NM, Eder S, Rechberger GN, Radner FP, Woelkart G, Kolb D, Schmidt A, Kumari M, Preiss-Landl K, Pieske B, Mayer B, Zimmermann R, Lass A, Zechner R, Haemmerle G. Functional cardiac lipolysis in mice critically depends on comparative gene identification-58. J Biol Chem. 2013;288:9892–9904. doi: 10.1074/jbc.M112.420620. ▪ This manuscript demonstrates the importance of CGI-58 in cardiac function and highlights differential impact of CGI-58 deficiency on cardiac and skeletal muscles. Absence of CGI-58 results in severe cardiac steatosis, decrease in cardiac β-oxidation, and cardiac dysfunction. By contrast, there was no change in mitochondrial function in skeletal muscle, despite increased LD.
- 71. Kienesberger PC, Pulinilkunnil T, Nagendran J, Young ME, Bogner-Strauss JG, Hackl H, Khadour R, Heydari E, Haemmerle G, Zechner R, Kershaw EE, Dyck JR. Early structural and metabolic cardiac remodelling in response to inducible adipose triglyceride lipase ablation. Cardiovasc Res. 2013;99:442–451. doi: 10.1093/cvr/cvt124. ▪ This manuscript shows that deletion of ATGL in adult cardiomyocytes of mice results in cardiac steatosis, but with a less severe cardiac dysfunction and unchanged PPARα gene expression than previously reported for the germline ATGL−/− mouse [47–48].
- 72.Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H, Schaffer JE, Yu YH, Goldberg IJ. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Sztalryd C. Oxidative tissue: perilipin 5 links storage with the furnace. Trends Endocrinol Metab. 2011;22:197–203. doi: 10.1016/j.tem.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bosma M, Sparks LM, Hooiveld GJ, Jorgensen JA, Houten SM, Schrauwen P, Kersten S, Hesselink MK. Overexpression of PLIN5 in skeletal muscle promotes oxidative gene expression and intramyocellular lipid content without compromising insulin sensitivity. Biochim Biophys Acta. 2013;1831:844–852. doi: 10.1016/j.bbalip.2013.01.007. ▪▪ These studies show that in vivo specific overexpression of Plin5 in skeletal muscle results in increased LD stores and increased β-oxidation profiles, but without compromising or improving muscle insulin sensitivity during diet induced obesity.
- 75. Bosma M, Hesselink MK, Sparks LM, Timmers S, Ferraz MJ, Mattijssen F, van Beurden D, Schaart G, de Baets MH, Verheyen FK, Kersten S, Schrauwen P. Perilipin 2 improves insulin sensitivity in skeletal muscle despite elevated intramuscular lipid levels. Diabetes. 2012;61:2679–2690. doi: 10.2337/db11-1402. ▪ These studies show that in vivo specific overexpression of Plin2 in skeletal muscle results in increased LD stores, decreased β-oxidation profiles, but improved muscle insulin sensitivity during diet induced obesity.