Abstract
AIMS
Urodynamic studies (UDS) are generally recommended prior to surgical treatment for stress urinary incontinence (SUI), despite insufficient evidence that it impacts treatment plans or outcomes in patients with uncomplicated SUI. This analysis aimed to calculate the cost incurred when UDS was performed as a supplement to a basic office evaluation and to extrapolate the potential savings of not doing UDS in this patient population on a national basis.
METHODS
This is a secondary analysis from the Value of Urodynamic Evaluation (ValUE) trial, a multicenter non-inferiority randomized trial to determine whether a basic office evaluation (OE) is non-inferior in terms of SUI surgery outcomes to office evaluation with addition of urodynamic studies (UDS). All participants underwent an OE; those patients who randomized to supplementary UDS underwent non-instrumented uroflowmetry, filling cystometry, and a pressure flow study. Costs associated with UDS were calculated using 2014 U.S. Medicare allowable fees. Models using various patient populations and payor mixes were created to obtain a range of potential costs of performing UDS in patients undergoing SUI surgery annually in the US.
Results
630 women were randomized to OE or OE plus UDS. There was no difference in surgical outcomes between the two groups. The per patient cost of UDS varied from site to site, and included complex cystometrogram $314-$343 (CPT codes 51728-51729) plus complex uroflowmetry $16 (CPT code 51741). Extrapolating these costs for US women similar to our study population, 13 to 33 million US dollars could be saved annually by not performing preoperative urodynamics.
Conclusion
For women with uncomplicated SUI and a confirmatory preoperative basic office evaluation, tens of millions of dollars US could be saved annually by not performing urodynamic testing. In the management of such women, eliminating this preoperative test has a major economic benefit.
Introduction
Over 260,000 surgeries for stress urinary incontinence (SUI) in women were performed in the U.S. in 2010. (1) Although there is significant geographic and professional variability in practice, urodynamic studies (UDS) are often performed prior to surgery for SUI despite absence of data that findings from UDS actually alter surgical plans or improve outcomes. A survey in the U.K found that 66% of specialists considered urodynamics essential in a clinical scenario of pure, demonstrable SUI and 89% of specialists felt it was essential in a setting of stress predominant mixed urinary incontinence (UI) (2) In a Medicare claims analysis in the U.S. from 1999 to 2001, urodynamic studies were done within 6 months preoperatively in 27% of women receiving a sling procedure. (3)
The role of UDS in the evaluation and treatment of SUI has been investigated in several recent studies (4). The ValUE (Value of Urodynamic Evaluation) study (5) is a multicenter trial that reported 12-month outcomes in women with uncomplicated stress predominant UI planning surgery, and showed that women with office evaluation (OE) alone had non-inferior outcomes compared to those with OE supplemented by UDS. A secondary analysis of VaLUE showed that while UDS after OE commonly changed the secondary clinical diagnosis, UDS information rarely influenced surgeons to cancel, change or modify their planned surgery (6). A complementary Dutch study reinforced these conclusions (7). In recognition of these findings, the American Urologic Association / Society of Urodynamics Female Pelvic Medicine and Urogenital Reconstruction considers UDS as “optional” in the evaluation of SUI with an evidence strength of “C” (8).
While UDS has been a useful clinical and research tool that has increased our understanding of continence, incontinence, and voiding dysfunction, its use can add substantial costs and patient time. The need to control health care costs is critical in the US, and cost-savings that preserve desired patient outcomes is desirable. The aim of this planned secondary analysis wasto calculate the additional cost incurred when UDS was performed as a supplement to OE using data from the ValUE study. Additionally, we calculated the potential savings to the US health care system when these costs were extrapolated to all women with uncomplicated SUI considering surgical treatment.
Materials and methods
This is a secondary analysis from the ValUE trial, a multicenter non-inferiority randomized trial to determine whether SUI surgical outcomes were non-inferior amongst women who underwent a basic office evaluation versus those who were assessed by office evaluation supplemented by urodynamic studies. (4) Briefly, women planning surgery for uncomplicated stress predominant UI were recruited at eleven U.S. sites. Inclusion criteria included stress predominant UI as evidenced by all of the following: Self-reported stress predominant UI symptoms of duration >3 months, and Medical, Epidemiologic and Social Aspects of Aging (MESA) stress symptom index (percent of total possible stress score) greater than MESA urge symptom index (percent of total possible urge score) (9). The OE included the MESA questionnaire, provocative stress test, post-void residual (PVR), dipstick urinalysis, assessment of urethral mobility, and a standing, straining prolapse exam. After completing the OE, subjects were randomized to OE only group or OE plus urodynamic testing group.
Those patients who were randomized to the UDS group underwent non-instrumented uroflowmetry (NIF), filling cystometry with valsalva leak point pressures, and a pressure flow study (PFS). Urethral pressure profilometry (UPP) was neither required nor restricted by the protocol. Seventy percent of the ValUE participants undergoing UDS had filling cystometry/PFS without UPP, and 30% with UPP. Results of UDS were recorded using ICS recommended nomenclature and diagnoses.(10)
Costs associated with UDS were calculated using published 2014 U.S. Medicare allowable payments (11), with the primary procedure paid at 100% and additional procedures paid at 50% for the second procedure, and 25% for additional procedures. To estimate potential cost savings that could result from decreasing the use of UDS testing, we performed a cost simulation from the perspective of Medicare and private insurance payers to estimate the total amount of payments in the U.S. We performed a simulation of costs associated with UDS if 10%, 30%, and 90% of the women that met the criteria for uncomplicated stress predominant UI underwent UDS testing (2,3). Our model for the estimated cost of UDS testing assumed that 70% of women randomized to OE plus UDS in the ValUE trial underwent complex uroflowmetry with pressure flow study (CPT code 51728) and 30% had a complex cystometry with pressure flow study and urethral pressure profiles (CPT code 51729), which was the approximate proportion for each test reported in the ValUE trial.
All participants randomized to OE plus UDS underwent complex uroflowmetry (CPT code 51741). UDS costs were based on 2014 CMS reimbursement rates: $314.50 for CPT code 51728, $343.90 for CPT code 51729, and $15.80 for CPT code 51741. Private or commercial insurers do not publish their reimbursements, but we compared reimbursements for UDS at representative sites and based on these, we made the assumption that private insurance costs are 2-3 times the Medicare allowable rate. In addition to testing different assumptions about the percent of women who had UDS testing, we also varied the mix of CMS and private insurance payments.
Results
We screened 4,083 women for enrollment; of these, 2199 did not meet inclusion criteria (1032 women did not meet criteria for stress-predominant urinary incontinence, 639 had significant pelvic organ prolapse, and 528 had a history of surgery for urinary incontinence or other conditions.) Thus, 46% of women screened in tertiary referral practices met the definition of uncomplicated stress-predominant SUI. For the purposes of the calculation, we used 50% as the approximate number of women undergoing stress incontinence surgery who would meet the criteria in the U.S. Using the estimate of 260,000 surgeries for urinary incontinence in 2010, we assumed that half of these women fit the criteria for uncomplicated stress predominant UI (130,000). Six hundred thirty women were randomized , and primary outcome data was available for 539 subjects undergoing OE (n=259) and OE plus UDS n=264.) There were no differences in outcomes between the two groups (table 1), thus the only difference in cost between the groups was the cost associated with UDS.
Table I.
Outcomes in the ValUE trial (from Nager et al 2012)
| OUTCOMES | URODYNAMIC TESTING n=272 | OFFICE EVALUATION ONLY n=266 | P value |
|---|---|---|---|
| PRIMARY | |||
| 70% reduction in Urogenital Distress Inventory Score-no.(%) | 210 (77.2) | 210 (78.9) | 0.63 |
| “very much better” or “much better” on Patient Global Impression of Improvement-no./total no.(%) | 248/270 (91.9) | 238/262 (90.8) | 0.68 |
| SECONDARY | |||
| Overall patient satisfaction score at 12 mo | 79.5±30.4 | 82.2±28.6 | 0.28 |
| Positive provocative stress test at 12 mo — no./total no. (%) | 36/225 (16.0) | 26/222 (11.7) | 0.19 |
The cost of the UDS varied from site to site, so we used the national CMS reimbursement rates to enhance generalizability. Based on the estimate that 70% of patients undergo complex cystometry with pressure flow study and 30% undergo complex cystometry with pressure flow study and urethral pressure profiles, the total CMS urodynamic unit cost was calculated as $338.3 $338.3 [(.7 × $314.52) + (.3 × $341.390) + $15.76.] The calculated cost of UDS reimbursed by private insurers was assumed to be 250% that of the CMS rate, or $846 in 2014.
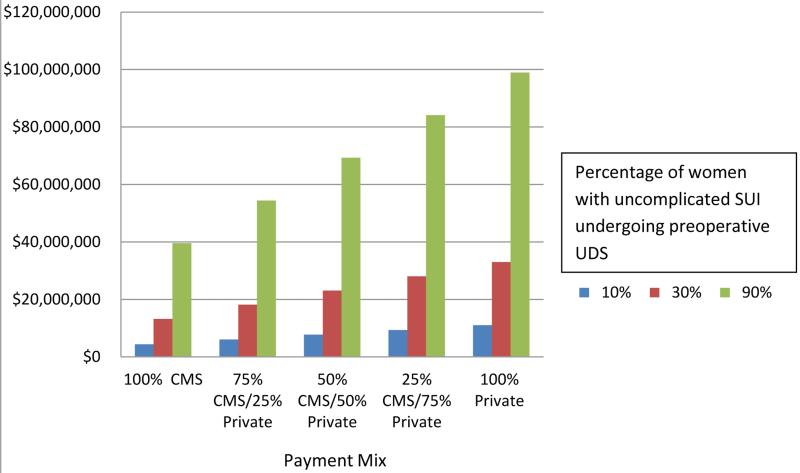
Table II represents the calculated cost of performing urodynamics based on average 2014 reimbursements assuming a wide range of practice patterns (10% to 90% of such patients undergoing testing) although the most likely percentages range from a low of 27% (3) to as high as 89% of patients (2). In most communities there is a mixture of public and private insurers, and the amount spent on urodynamic testing on patients such as those in the ValUE trial has been estimated to range from as low as $ 4.4 million U.S. annually to almost $99 million (Figure 1.) Extrapolating for US women similar to our study population and assuming 30 and 90% estimates for percentage of women undergoing UDS before SUI surgery, 13 to 33 million US dollars could be saved annually by not performing preoperative urodynamics in these cases. If we use the more conservative figure of 30% of women undergoing UDS in these circumstances, and assume either 100% CMS insured or 100% private insured, a similar 13 and 33 million US dollars could be saved annually, respectively.
Table II.
Cost Simulation for estimated cost of urodynamic studies in U.S. in 2014, by percentage of surgical patients undergoing preoperative studies.
| Percent of SUI Surgeries which include preoperative urodynamics | |||||
|---|---|---|---|---|---|
| 10% | 30% | 50% | 75% | 90% | |
| All CMS reimbursed | |||||
| 2014 | $4,398,433 | $13,195,299 | $21,992,165 | $32,988,248 | $39,585,897 |
| All private reimbursed | |||||
| 2014 | $10,996,083 | $32,988,248 | $54,980,413 | $82,470,619 | $98,964,743 |
Estimated annual cost of preoperative urodynamic testing in women with uncomplicated stress urinary incontinence, in millions of dollars U.S. (2014 costs).
Discussion
For women with uncomplicated stress-predominant UI undergoing incontinence surgery, the ValUE trial found that preoperative UDS testing adds additional cost without benefit over a basic OE, and this analysis calculates the cost saving to be tens of millions of dollars US. We postulate that if 50% of women who undergo SUI surgery in the U.S every year met our definition of uncomplicated stress urinary incontinence, and if 30 % of these women who currently receive urodynamic studies do not undergo such testing, then 13 to 33 million dollars could be saved annually by not performing preoperative urodynamics. These are calculated estimates, and real costs will vary by location, practice, and source of payment. For example, private practices may see a higher prevalence of uncomplicated stress incontinence in women compared to referral based university practices that made up many of the clinical sites in the ValUE trial.
The ValUE trial found that urodynamic studies rarely changed the primary diagnosis of SUI (<1%) but frequently changed the listing of secondary diagnoses of overactive bladder-wet (OAB-wet), OAB-dry, voiding dysfunction, or suspected intrinsic sphincter deficiency in over half of women undergoing UDS (6). However, these changes in secondary diagnoses rarely changed treatment plans and therefore, no treatment benefit was realized by the additional UDS. In carefully selected women, avoiding urodynamics is both safe and cost effective.
We emphasize that the ValUE data should be considered as applicable only to adult women with uncomplicated stress predominant UI planning to undergo surgery, and do not affect recommendations for UDS in the setting of complicated incontinence and voiding dysfunction. (12). The role of UDS testing in women who do not meet the ValUE eligibility criteria (women with prior incontinence surgeries, pelvic organ prolapse, elevated post void residual urine, or mixed incontinence without stress predominance) cannot be determined from this dataset.
The strength of our analysis is that we have use study-derived cost estimates to understand approximate real-life costs. Collection of individual costs in the ValUE trial could not be supported once we determined that outcomes were comparable in the two groups. In the U.S., private or commercial insurance considers reimbursements to be contractual agreements that may not be published, so these rates were carefully investigated and confirmed as personal communications. Another limitation of our analysis is that we do not know what proportion of U.S. women with stress predominant urinary incontinence would have met our inclusion criteria.
There are many factors to influence the use of UDS in the United States, including geographic and practitioner differences, reimbursements, and historical practice. But additional testing with costly equipment and clinician time can add considerable burden to the care of incontinent women. The need to control health care costs is a well recognized priority in the United States, where health care spending has skyrocketed, reaching $2.7 trillion in 2011. (14) Changes in health care coverage for evaluation and management fees, tests and treatments will undoubtedly be necessary to begin to control costs. To avoid arbitrary reductions, the health care system needs to evaluate which studies have the desired impact on patient outcomes. Cost-savings that preserve optimal patient outcomes are logical and reasonable. Data from the current study demonstrate that private and government insurers will realize substantial cost savings, without resulting in any deleterious impact on patient care.
Conclusion
Urodynamic testing adds considerable, and apparently unnecessary, costs over a basic office evaluation of women with uncomplicated stress predominant UI. This additional cost is not associated with improvement in surgical outcomes. In such women, a basic office evaluation consisting of questionnaire, urinalysis, physical exam, provocative stress test, and post void residual assessment is a sufficient preoperative workup prior to planning incontinence surgery. Thorough office evaluation without UDS has the potential to save tens of millions of dollars in the United States each year without any detriment to patient care and clinical outcomes.
Acknowledgments
Funding sources: Supported by cooperative agreements (U01 DK58225, U01 DK58229, U01 K58234, U01 DK58231, U01 DK60379, U01 DK60380, U01 DK60393, U01 DK60395, U01 K60397, and U01DK60401) from the National Institute of Diabetes and Digestive and Kidney Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
ClinicalTrials.gov ID Registration Number: NCT00803959
References
- 1.Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. Food and Drug Administration; Silver Spring, MD: Jul, 2011. [Google Scholar]
- 2.Hilton P, et al. Assessing professional equipoise and views about a future clinical trial of invasive urodynamics prior to surgery for stress urinary incontinence in women: a survey within a mixed methods feasibility study. Neurourol Urodyn. 2012;31(8):1223–30. doi: 10.1002/nau.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anger JT, et al. The role of preoperative testing on outcomes after sling surgery for stress urinary incontinence. J Urol. 2007;178(4 Pt 1):1364–8. doi: 10.1016/j.juro.2007.05.139. [DOI] [PubMed] [Google Scholar]
- 4.Nager C, FitzGerald MPM,S, Kraus S, Chai T, Zyczynski H, Sirls L. Urodynamic Measures Do Not Predict Stress Continence Outcomes after Surgery for Stress Urinary incontinence in Selected Women. J Urol. Apr. 2008;179(4):1470–4. doi: 10.1016/j.juro.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 5.Nager CW, Brubaker L, Litman H, et al. A Randomized Trial of Urodynamic Testing before Stress-Incontinence Surgery. NEJM. 2012 doi: 10.1056/NEJMoa1113595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sirls LT, Richter HE, Litman HJ, et al. The Effect of Urodynamic Testing on Clinical Diagnosis, Treatment Plan and Outcomes in Women Undergoing Stress Urinary Incontinence Surgery. J Urol. 2013;189(1):204–9. doi: 10.1016/j.juro.2012.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Leijsen SA, Kluivers KB, Mol BW, Hout Ji, Milani AL, Roovers JP, Boon Jd, van der Vaart CH, Langen PH, Hartog FE, Dietz V, Tiersma ES, Hovius MC, Bongers MY, Spaans W, Heesakkers JP, Vierhout ME. Dutch Urogynecology Consortium. Value of urodynamics before stress urinary incontinence surgery: a randomized controlled trial. Obstet Gynecol. 2013;121(5):999–1008. doi: 10.1097/AOG.0b013e31828c68e3. [DOI] [PubMed] [Google Scholar]
- 8.Winters JC, Dmochowski RR, Goldman HB, et al. Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188:2464. doi: 10.1016/j.juro.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 9.Herzog AR, Diokno AC, Brown MB, Normolle DP, Brock BM. Two-year incidence, remission, and change patterns of urinary incontinence in noninstitutionalized older adults. J Gerontol. 1990;45:M67–74. doi: 10.1093/geronj/45.2.m67. [DOI] [PubMed] [Google Scholar]
- 10.Abrams P, Andersson KE, Birder L, et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010;29:213. doi: 10.1002/nau.20870. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Medicare and Medicaid Services (CMS) www.cms.gov/fee schedules.
- 12.NICE clinical guideline 40. Urodynamic Testing before Stress-Incontinence Surgery. National Institute for Health and Clinical Excellence; London: 2006. Urinary incontinence: the management of urinary incontinence in women. pp. 1–36. [Google Scholar]
- 13.Reynolds WS, et al. Patterns and Predictors of Urodynamics Use in the United States. J Urol. 2012 doi: 10.1016/j.juro.2012.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/Proj2011PDF.pdf.