Abstract
Children with attention-deficit hyperactivity disorder (ADHD) consistently show impaired response control, including deficits in response inhibition and increased intrasubject variability (ISV) compared to typically-developing (TD) children. However, significantly less research has examined factors that may influence response control in individuals with ADHD, such as task or participant characteristics. The current study extends the literature by examining the impact of increasing cognitive demands on response control in a large sample of 81children with ADHD (40 girls) and 100 TD children (47 girls), ages 8–12 years. Participants completed a simple Go/No-Go (GNG) task with minimal cognitive demands, and a complex GNG task with increased cognitive load. Results showed that increasing cognitive load differentially impacted response control (commission error rate and tau, an ex-Gaussian measure of ISV) for girls, but not boys, with ADHD compared to same-sex TD children. Specifically, a sexually dimorphic pattern emerged such that boys with ADHD demonstrated higher commission error rate and tau on both the simple and complex GNG tasks as compared to TD boys, whereas girls with ADHD did not differ from TD girls on the simple GNG task, but showed higher commission error rate and tau on the complex GNG task. These findings suggest that task complexity influences response control in children with ADHD in a sexually dimorphic manner. The findings have substantive implications for the pathophysiology of ADHD in boys versus girls with ADHD.
Keywords: Intrasubject variability, cognitive control, sex differences, response inhibition, attention deficit hyperactivity disorder
Attention-deficit/hyperactivity disorder (ADHD) is the most common psychiatric disorder of childhood affecting approximately 8–10 % of school-age children, and is characterized by developmentally inappropriate levels of inattention, hyperactivity and impulsivity (American Psychiatric Association 2013; Getahun et al. 2013). In addition to behavioral symptoms, individuals with ADHD often present with a number of neurocognitive deficits including impaired response inhibition, attentional control, working memory (WM), and planning ability (Castellanos and Tannock 2002; Kofler et al. 2013; Nikolas and Nigg 2013; Willcutt et al. 2005). In individuals with ADHD, these neurocognitive deficits have been associated with increased academic impairments (grade retention, lower levels of achievement), interpersonal difficulties, difficulties with emotion regulation, and general reduced quality of life. Thus, it is critical to gain a more comprehensive understanding of the mechanisms underlying these neurocognitive deficits in individuals with ADHD (Biederman et al. 2004; Brown and Landgraf 2010; Diamantopoulou et al. 2007; Kofler et al. 2011; Sjowall et al. 2013).
In particular, early theoretical models of ADHD suggested that core deficits in response inhibition were associated with subsequent deficits in executive functioning as well as behavioral symptoms of ADHD (Barkley 1997). Response inhibition refers to the ability to inhibit a prepotent response to a stimulus or the deliberate suppression of actions in order to achieve a goal. Assessment of response inhibition can be accomplished using a variety of behavioral paradigms, including Go/No-Go (GNG) tasks (i.e., responding to one or more stimuli while withholding response to another stimuli) and stop signal tasks (i.e., responding to a stimulus until cued by a separate signal not to respond; Barkley 1997). While impaired response inhibition is one of the most common deficits associated with ADHD, with 40–50 % of children with ADHD exhibiting poor response inhibition, there is significant heterogeneity in the neurocognitive deficits implicated in ADHD (Nigg et al. 2005; Sonuga-Barke 2002; Willcutt et al. 2005; Willcutt et al. 2008). In addition, it has been suggested that deficits in response inhibition in children with ADHD, particularly as measured by the stop signal task, may be the result of alternative cognitive mechanisms, such as working memory and inattention (Alderson et al. 2007; Lijffijt et al. 2005). Further, heterogeneity in neurocognitive deficits mirrors the variability in behavioral symptom presentation both within individual children with ADHD, but also between children with ADHD. For instance, fluctuations in symptoms have been shown across settings, caregivers, and reinforcement schedules (e.g., Guevremont and Barkley 1992). As a result of being unable to characterize all children with ADHD with a singular neurocognitive deficit or behavioral phenotype, there has been a shift from single core deficit to multi-process theoretical models of ADHD, emphasizing alterations in cognition, motivation, and self-regulation (Castellanos et al. 2005; Nigg et al. 2005; Sonuga-Barke and Sergeant 2005; Willcutt et al. 2008). However, response inhibition remains a central feature in leading models of ADHD (Barkley 1997; Kuntsi et al. 2010).
In line with these multiple pathway models, research has focused on examination of intra-subject variability (ISV) as a core feature in ADHD. Broadly, ISV refers to moment-to moment (within-subject) fluctuations in behavior or task performance occurring over a period of seconds or milliseconds rather than hours or days (Kofler et al. 2013). ISVis often measured as reaction time (RT) variability during behavioral tasks, which is the focus of the current study. Compared to their typically-developing (TD) peers, individuals with ADHD consistently demonstrate increased ISV of RT across a variety of cognitive measures, including response inhibition tasks (Castellanos et al. 2005; Epstein et al. 2011; Klein et al. 2006; Kofler et al. 2013; Leth-Steensen et al. 2000; Tamm et al. 2012). The vast majority of the ADHD literature has assessed ISVof RT using the standard deviation of RT (SDRT) or the coefficient of variation (CVRT), which reflects global variability after accounting for overall RT (Kofler et al. 2013; Tamm et al. 2012). However, these methods of assessment are limited by the assumption that reaction times are normally distributed, which does not appear a valid assumption (Schmiedek et al. 2007). Specifically, evidence from a recent meta-analysis of 319 studies of ISV showed that individuals do not have slower RTs overall after accounting for the occurrence of infrequent, longer RTs, indicative of greater variability in responding compared to TD individuals (Kofler et al. 2013). Therefore, use of ex-Gaussian modeling which separates the RT distribution into the normal (Gaussian) component which includes the mean (mu) and standard deviation (sigma) as well as the exponential component of the RT distribution and its mean (tau), reflecting a subset of abnormally slow responses, may be more advantageous for examining ISV in relation to ADHD (Hervey et al. 2006; Leth-Steensen et al. 2000). The use of ex-Gaussian parameters has shown that ADHD-related ISVappears to principally be the result of a subset of abnormally slow responses (tau) rather than variable responding during the entire task (sigma) (Epstein et al. 2011; Hervey et al. 2006; Leth-Steensen et al. 2000; Vaurio et al. 2009).
Increased ISVin individuals with ADHD has been explained in various etiological models as reflecting: deficits in attentional processes (e.g., lapses of attention) (Leth-Steensen et al. 2000), state regulation deficits (Sergeant 2005), insufficient suppression of the default mode network (Castellanos et al. 2005), temporal processing deficits (Sonuga-Barke and Halperin 2010), and the result of impairments in other higher order cognitive processes (e.g., working memory) (Rapport et al. 2008). Indeed, the precise mechanisms underlying increased ISV in individuals with ADHD are unknown. Further, while it has been hypthothesized that tau reflects excessive lapses in attention which may be particularly salient in understanding ISV in individuals with ADHD (e.g., Attention Lapse Model), such lapeses in attention do not account for all of the variance in ISV suggesting alternative explanations may be required (Epstein et al. 2010; Kofler et al. 2013; Schmiedek et al. 2007). For example, while related to symptoms of inattention, hyperactivity and impulsivity, increased ISV has been shown to be more strongly correlated with symptoms of hyperactivity relative to inattention which suggests ISV may reflect more than just periodic lapses in attention (for reviews see Kofler et al. 2013; Tamm et al. 2012). Regardless, ISV has been suggested as a potential endophenotype of ADHD (Castellanos et al. 2005; Sonuga-Barke and Castellanos 2007) indicating the importance of better understanding ISV in individuals with ADHD.
While there is a large and growing literature on impaired response control, or consistent and accurate execution of a motor response as reflected in measures of response inhibition and ISV of RT, in ADHD, significantly less research has examined factors that may influence response control in individuals with ADHD. For example, while deficient response control in children with ADHD has been demonstrated across a variety of cognitive tasks, there is inconsistent evidence as to how task characteristics, such as complexity, influence response control (Buzy et al. 2009; Epstein et al. 2011; Klein et al. 2006; Vaurio et al. 2009). In one of the first examinations of ISV across multiple cognitive tasks, Klein et al. (2006) reported notable effect size differences between the ADHD and TD groups based on task complexity. In particular, the authors reported a significant effect of WM load on ISV that was greater in ADHD compared to control participants when assessed using SDRT, but not for the CVRT; ex-Gaussian measures were not examined. Using ex-Gaussian measures, Buzy and colleagues (2009) found increases in tau in ADHD compared to TD participants during a WM task, but no increase in tau associated with increased working memory demands. In addition, Epstein and colleagues (2011) reported increased ISV (SDRT, CVRT and tau) in children with ADHD compared to TD controls across five different cognitive tasks that varied in their complexity and response requirements, although they did not report a differential effect of task for children with ADHD compared to TD children. These contradictory findings indicate the need for additional research examining the influence of cognitive load on response control in children with ADHD.
Indeed, inconsistencies within this literature may be the result of methodological limitations. Specifically, studies have compared tasks probing a variety of neurocognitive functions that also vary in the type of stimulus being presented, the amount of time the stimulus is presented for, or the time between stimulus presentations (e.g., Epstein et al. 2011; Klein et al. 2006), all of which may influence RT estimates. Instead, comparing response control on nearly identical tasks with the same stimuli and response requirements while varying the cognitive load provides a more controlled assessment. Two prior studies have applied such an approach to examine the impact of increased WM demand on response control in children with ADHD using two GNG tasks with different cognitive loads (e.g., simple GNG with minimal cognitive demands versus complex GNG with increased cognitive demands), but identical stimuli, inter-stimulus interval, and response demands (Vaurio et al. 2009; Wodka et al. 2007). Results across these studies showed that children with ADHD exhibited poorer response control (more commission errors and increased ISV [measured by CVRT and tau]) on both tasks and similar increases in commission error rate and ISV with increasing task complexity as did TD children.
One significant limitation of these prior studies is the lack of examination of sex differences in regards to impairments in response control in the context of increasing cognitive load in children with ADHD. Previous studies of response control have shown little evidence of change in ISV as related to cognitive load (e.g., Vaurio et al. 2009; Wodka et al. 2007); however, these studies primarily examined boys with ADHD. Therefore, the effect of cognitive load on ISV in girls with ADHD remains unknown as few studies to date have examined participant sex in relation to response control (ISV and response inhibition) in children with ADHD (Kofler et al. 2013; Tamm et al. 2012). However, within the broader ADHD literature, significant differences between boys and girls with ADHD have been shown in symptom presentation, associated comorbidity, and psychosocial and cognitive functioning suggesting that sex differences may be important in understanding response control in ADHD (Gaub and Carlson 1997; Gershon 2002; Rucklidge 2008). For example, prior studies have shown that motor control deficits are more prominent in boys than in girls with ADHD (Cole et al. 2008) whereas impairments in WM and related executive dysfunctions are present in both boys and girls with the disorder (Rucklidge 2010). Taken together, these studies support the importance of examining sex differences in regards to impairments in response control in the context of increasing cognitive load in children with ADHD.
Of particular interest to the current study, research has suggested sex differences in children with ADHD in relation to neurocognitive function and neuroanatomical structure (Almeida Montes et al. 2013; Balint et al. 2009; Hasson and Fine 2012; Mahone et al. 2011; Rucklidge 2006; Seidman et al. 2005). For example, a meta-analysis of performance on continuous performance tasks (CPT), a measure of sustained attention, among individuals with ADHD, showed that boys with ADHD made significantly more commission errors than girls, and the magnitude of the differences between ADHD and TD boys was greater than that observed between ADHD and TD girls (Hasson and Fine 2012). These results complement structural neuroimaging studies, which suggest sex-based differences in ADHD particularly in frontal regions (Mahone et al. 2011). Recent findings also suggest a predominance of abnormalities in premotor (PM)-basal ganglia circuits (PM cortex, putamen, globus pallidus) in boys with ADHD compared to TD boys (Peterson et al. 2014; Tang 2014), while girls have abnormalities in prefrontal (PF) and limbic circuits (Dirlikov et al. 2013; Seymour et al. 2014). Yet in regards to response control in ADHD, particularly as reflected in ISV, there remains a scarcity of literature exploring sex differences. While some studies have included sex as a covariate in analysis of response control in ADHD, they have been limited by small female sample sizes, and may therefore have been insufficiently powered to detect group differences (Antonini et al. 2013; Epstein et al. 2011).
Therefore, the current study builds upon the extant literature on response control in children with ADHD by extending the research examining the impact of varying cognitive demand on response control in a large sample of boys and girls with ADHD. Further, our study oversampled for girls (n=40 with ADHD; twice the number of girls with ADHD as in previous similar studies (e.g., Vaurio et al. 2009; Wodka et al. 2007) enabling us to evaluate the role of sexual dimorphism in regards to response control in children with ADHD compared to their same-sex TD peers. Based on prior research, we hypothesized that children with ADHD would demonstrate worse response inhibition and increased ISV compared to TD children regardless of task complexity (Kofler et al. 2013; Vaurio et al. 2009; Wodka et al. 2007). We also hypothesized that the magnitude of diagnostic group differences in response inhibition and ISV would increase with task complexity (i.e., increased cognitive demands) based on prior work (Klein et al. 2006). Despite evidence demonstrating the importance of sex differences in executive functioning in children with ADHD (Balint et al. 2009; Hasson and Fine 2012; Rucklidge 2006), there has been a significant lack of research examining the effect of sex on response control. Therefore, we evaluated whether girls and boys with ADHD demonstrate similar impairments in response control with increasing cognitive demand compared to their same-sex TD peers. We hypothesized that boys with ADHD would show impaired response control regardless of cognitive demands relative to TD boys whereas impaired response control in girls with ADHD compared to TD girls would be task-dependent based on prior work suggesting primary motor control deficits in boys with ADHD, but WM impairments in both boys and girls with ADHD.
Method
Participants
A total of 257 children between the ages of 8–12 years participated in the study including 119 children diagnosed with ADHD and 138 typically developing (TD) controls. Participants were primarily recruited through local schools, with additional recruitment resources including community-wide advertisement, volunteer organizations, medical institutions, and word of mouth. This study was approved by the Johns Hopkins Medical Institutional Review Board. Written consent was obtained from a parent/guardian and assent was obtained from the participating child.
Children were initially screened to determine whether they met inclusion criteria through a brief telephone interview with a parent. At this time, children with a history of intellectual disability, seizures, traumatic brain injury or other neurological illnesses were excluded from participation. Eligible participants completed all remaining study procedures including: (1) a diagnostic interview with parents to assess for the presence of psychopathology (Diagnostic Interview for Children and Adolescents, Fourth Edition [DICA-IV] (Reich 2000), (2) assessment of intellectual ability and academic achievement (Wechsler Intelligence Scale for Children-IV [WISC-IV] (Wechsler 2003) and Wechsler Individual Achievement Test-II [WIAT-II] (Wechsler 2002), (3) completion of parent report measures of ADHD (Conners’ Parent Rating Scales-Revised Long Version [CPRS-R:L] (Conners 2002); ADHD Rating Scale-IV, home versions [ADHD-RS] (DuPaul et al. 1998), and (4) completion of computerized cognitive tasks. If children were taking medication for ADHD, parents were instructed on both the diagnostic interview and report forms to make ratings based on their children's symptoms off medication. Teachers were asked to complete the Conners’ Teacher Rating Scales-Revised Long Version [CTRS-R:L] and ADHD-RS school versions. Teacher report was available for 66.5 % of the sample.
For all participants, FSIQ scores below 80 on the WISC-IV were exclusionary. In addition, participants with a scaled score on the Word Reading subtest of the WIAT-II below 85 were excluded as a screening for a potential reading disorder.
For inclusion in the ADHD group, children had to meet full DSM-IV criteria for ADHD based on the following criteria: (1) an ADHD diagnosis according to the DICA-IV including presence of symptoms and cross-situational impairment criteria, and (2) T-score of 60 or higher on scale L (DSM-IV: inattentive) or M (DSM-IV: hyperactive-impulsive) on the CPRS-R:L or CTRS-R:L, when available, or a score of 2 or 3 (i.e., symptoms rated as occurring often or very often) on at least 6/9 items on the Inattentive or Hyperactivity/Impulsivity scales of the ADHD-RS. If teacher reports were not available, cross-situational impairment was determined based on parent-report during the DICA-IV (e.g., presence of academic or social difficulties, etc.). This information was then reviewed by a child neurologist (S.H.M.) for further confirmation of an ADHD diagnosis. Children taking psychotropic medications other than stimulant medication were excluded from participation and all children taking stimulant medication were asked to withhold medication on the day prior to and day of testing. Children with ADHD were also excluded for the presence of comorbid psychiatric diagnoses on the DICA-IV including major depression, bipolar disorders, conduct disorder, adjustment disorder, obsessive-compulsive disorder and other anxiety disorders. However, children with ADHD presenting with comorbid Oppositional Defiant Disorder (ODD) were included in the study given the high prevalence rates of these disorders in children with ADHD (e.g., Jensen et al. 2001); we also included children with simple phobia (n=11) but no other anxiety disorder given the minimal impact of simple phobia in isolation on generalized functioning is much less consequential than the impact of generalized or other anxiety disorders (Schneier et al. 2014).1
For inclusion in the TD group, participant's scores had to be below the clinical cutpoints on parent and teacher (when available) report measures of ADHD. In addition, participants in the TD group could not meet diagnostic criteria for any psychiatric disorder (aside from isolated specific phobia, n= 6) based on the DICA-IV nor could they have history of neurological disorder, have been diagnosed with a learning disability or be taking psychotropic medication.
Procedures
Participants completed two computer-based GNG paradigms similar to the tasks described in previous studies: a simple GNG paradigm and a complex GNG paradigm (Mostofsky et al. 2003; Shiels Rosch et al. 2013; Vaurio et al. 2009; Wodka et al. 2007).
Simple GNG Paradigm
The task stimuli consisted of green spaceships for “Go” trials (80 % of trials) and red spaceships for “No-Go” trials (20 % of trials), presented one at a time. Participants were instructed to push the spacebar with their index finger as quickly as possible in response to green spaceships only. The use of familiar stimulus–response associations (green for “go”; red for “no-go”) minimized the perceptual and cognitive demands of the tests. Presentation cues were weighted towards green spaceships at a ratio of 4:1, intensifying the need to inhibit a habituated motor response. Go and no-go trials appeared in pseudorandom order with the restrictions that there were never fewer than three go trials before a no-go cue and never more than two no-go trials in a row. There were 11 practice trials (8 go cues; 3 no-go cues) followed by 217 experimental trials (173 go cues; 44 no-go cues). Stimuli were present on-screen for 300 ms with an interstimulus interval of 2000 ms (trial length = 2300 ms) during which a fixation cross was present on-screen. Responses and reaction times (RT) were recorded for the entire trial duration. The task duration was 8 min, 19 s.
Complex GNG Paradigm
The trial structure of the complex GNG task was nearly identical to that of the simple GNG task but there were additional cognitive demands. The stimuli were identical to those in the standard GNG task, consisting of red or green spaceships presented for 300 ms, followed by a blank screen for 2000 ms (trial length = 2300 ms). Children were instructed to push the button as quickly as possible in response to a green spaceship and in response to a red spaceship preceded by an even number of green spaceships. They were to refrain from responding to red spaceships preceded by an odd number of green spaceships. There were five practice trials to demonstrate an even sequence, six practice trials to demonstrate an odd sequence, and 11 practice trials with each type of sequence. The task consisted of 207 experimental trials including 163 green go cues; 21 red go cues (i.e., preceded by an even number of green spaceships) and 23 red no-go cues (i.e., preceded by an odd number of green spaceships). Responses and reaction times (RT) were recorded for the entire trial duration. The total time of this task was 7 min, 56 s.
Statistical Analysis
Primary dependent variables for each of the GNG tasks included commission error rate and ex-Gaussian measures of RT: mu, sigma and tau, with the latter being the primary measure of ISV. Responses faster than 200 ms were excluded from all RT analyses. Commission error rate was defined as the proportion of no-go trials (red spaceship for simple GNG and red spaceship preceded by an even number of green spaceships for complex GNG) on which the participant responded. Mu, sigma and tau were derived using ex-Gaussian analysis of correct go trial RTs (responses to green spaceships in both tasks). On average, the ex-Gaussian estimates for the simple GNG task were based on 165.5 trials for the TD group and 162.8 trials for the ADHD group out of 173 go trials. For the complex GNG task, ex-Gaussian estimates were based on 152.6 trials for the TD group and 148.9 trials for the ADHD group out of 163 go trials. Mu represents the mean of the normal distribution (i.e., mean RT in the normal distribution) and sigma represents the variation of the normal distribution. Tau, which was the primary measure of ISV, represents the mean of the exponential component of the distribution. Ex-Gaussian RT estimates and a goodness-of-fit value, with lower values indicating better fit to the ex-Gaussian model, were computed in Matlab version 7.1 (Hodge et al. 2010) using the DISTRIB toolbox (Lacouture and Cousineau 2008), during which data converged for all participants. Although commission errors and tau were the focus of these analyses, other performance measures are presented in Table 2 including omission error rate (proportion of go trials on which the participant did not respond), RT mean, RT standard deviation, and RT coefficient of variation (RT standard deviation/RT mean). Individuals with poor fit to the ex-Gaussian model were excluded if their goodness-of-fit value, which was generated with the “eglike” function, was greater than 3 standard deviations above the mean for their respective diagnostic group. This resulted in the exclusion of 3 participants from the initial sample of n=257.
Table 2.
Performance on the simple and complex Go/No-Go tasks for boys and girls in the ADHD and TD groups
| Simple GNG task |
Complex GNG task |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Girls (n=87) |
Boys (n=94) |
Girls (n=87) |
Boys (n=94) |
|||||||||||||
| TD (n=47) |
ADHD (n=40) |
TD (n=53) |
ADHD (n=41) |
TD (n=47) |
ADHD (n=40) |
TD (n=53) |
ADHD (n=41) |
|||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Omi | 0.03 | (0.05) | 0.04 | (0.06) | 0.03 | (0.03) | 0.04 | (0.05) | 0.02 | (0.03) | 0.03 | (0.05) | 0.03 | (0.03) | 0.05 | (0.08) |
| Com | 0.33 | (0.19) | 0.36 | (0.19) | 0.35 | (0.20) | 0.48 | (0.20) | 0.39 | (0.20) | 0.50 | (0.18) | 0.42 | (0.18) | 0.54 | (0.20) |
| MRT | 405.4 | (97.2) | 421.3 | (79.3) | 389.6 | (94.7) | 400.0 | (94.6) | 401.4 | (104.8) | 447.2 | (110.5) | 396.0 | (110.2) | 409.0 | (126.4) |
| SDRT | 130.1 | (71.3) | 154.2 | (73.7) | 122.7 | (67.9) | 169.2 | (103.5) | 142.4 | (72.7) | 180.9 | (87.8) | 152.4 | (110.9) | 185.0 | (130.0) |
| CVRT | 0.31 | (0.12) | 0.36 | (0.15) | 0.31 | (0.12) | 0.40 | (0.19) | 0.35 | (0.16) | 0.39 | (0.14) | 0.36 | (0.20) | 0.42 | (0.20) |
| Mu | 291.0 | (52.0) | 297.5 | (53.2) | 287.3 | (60.5) | 266.4 | (52.5) | 286.3 | (66.4) | 293.9 | (77.5) | 275.3 | (76.6) | 254.8 | (60.8) |
| Sigma | 31.1 | (18.6) | 34.8 | (20.4) | 31.0 | (20.4) | 28.4 | (19.2) | 34.4 | (30.8) | 39.9 | (32.1) | 32.2 | (25.9) | 26.8 | (30.9) |
| Tau | 114.4 | (65.6) | 123.8 | (58.2) | 102.3 | (56.0) | 133.6 | (77.1) | 115.1 | (53.1) | 153.3 | (78.4) | 120.7 | (77.5) | 155.5 | (102.1) |
M mean, SD standard deviation, Omi omission error rate (proportion of “Go” trials), Com commission error rate (proportion of “No-Go” trials), MRT mean reaction time (RT), SDRT standard deviation of RT, CVRT coefficient of variation (SDRT/MRT)
Repeated measures ANOVAs with the between-subjects factors of diagnosis (ADHD vs. TD) and sex (boys vs. girls) and a within-subjects factor of task (simple vs. complex GNG) were employed for each of the GNG task dependent variables (commission error rate, mu, sigma, and tau) to determine whether cognitive demands affected response inhibition and ISV. Cohen's d is reported as a measure of effect size for the primary outcome measures, with small, medium, and large effect sizes as Cohen's d 0.3–0.5, 0.5–0.8, and≥0.8, respectively (Cohen 1988).
Results
Sample Characteristics
In order to reduce group differences as a result of confounding demographic factors (e.g., age, sex, IQ), groups were balanced (i.e., no group differences) on important factors. Specifically, initial group comparisons of cognitive ability revealed that TD children had significantly higher scores on most WISC-IV indices, including: full scale intelligence quotient (FSIQ), F(1248)=14.0, p<0.001; verbal comprehension index scores (VCI), F(1248)=5.6, p=0.019; processing speed index (PSI), F(1248)=18.4, p<0.001, and working memory index (WMI), F(1248)=5.6, p=0.019. The perceptual reasoning index (PRI) score did not significantly differ between diagnostic groups, F(1248)=3.03, p=0.083. As a result, 38 TD children (13 girls) were excluded from the initial sample due to exceptionally high scores on the WISC-IV (i.e., scores of ≥130 [98–99 percentile] on the Verbal Comprehension Index [VCI] or Perceptual Reasoning Index [PRI]). TD children with higher scores on PSI or WMI compared to the ADHD group were not excluded because weaker processing speed and working memory are often found in children with ADHD (Jacobson et al. 2011).
Furthermore, given our interest in sex differences and evidence that girls diagnosed with ADHD often present with more significant symptoms and impairments than boys with ADHD relative to same-sex TD children, we examined whether boys and girls with ADHD differed in ADHD symptom severity. In the full sample, girls and boys with ADHD did not differ in raw ADHD total symptoms as rated by parents on the DuPaul RS (p=0.906). However, girls with ADHD had significantly higher symptom severity as reflected in T-scores normalized in reference to same-sex peers (p<0.001). Therefore, we excluded 38 boys with ADHD with a T-score below 69 on the Conners DSM Total ADHD scale to eliminate the difference in symptom severity for boys and girls with ADHD and made the sample of girls (n=40) and boys (n= 41) with ADHD more comparable in size (see Table 1).
Table 1.
Participant demographic and clinical characteristics
| TD |
ADHD |
TD v ADHD p-value 0.750 | |||||
|---|---|---|---|---|---|---|---|
| Sex | Boys (n=53) | Girls (n=47) | All (n=100) | Boys (n=41) | Girls (n=40) | All (n=81) | |
| Age (years) | 10.4 (1.5) | 10.4 (1.6) | 10.4 (1.6) | 10.7 (2.1) | 10.4 (1.8) | 10.6 (1.9) | 0.525 |
| Race (% caucasian) | 77 % | 70 % | 73 % | 71 % | 83 % | 77 % | 0.586 |
| SES | 51.8 | 54.2 | 51.3 | 51.0 | 53.0 | 52.2 | 0.564 |
| FSIQ | 111.3 (9.4) | 109.8 (9.6) | 110.6 (9.5) | 107.5 (12.6) | 110.1 (13.8) | 108.9 (13.2) | 0.304 |
| VCI | 113.6 (10.2) | 110.4 (10.5) | 112.1 (10.4) | 113.0 (14.2) | 114.1 (15.5) | 113.5 (14.8) | 0.444 |
| PRI | 110.7 (10.8) | 106.9 (11.1) | 108.9 (11.1) | 107.6 (12.9) | 109.2 (13.2) | 108.4 (13.0) | 0.765 |
| WMI | 105.3 (10.7) | 105.8 (10.5) | 105.6 (10.5) | 102.8 (14.8) | 104.8 (14.3) | 103.8 (14.5) | 0.345 |
| PSI | 100.0 (12.5) | 105.04(13.6) | 102.4 (13.2) | 94.5 (11.0) | 99.8 (13.5) | 97.2 (12.5) | 0.007 |
| ADHD boys v girls p-value | |||||||
| ADHD subtype %COM:IA:HI | N/A | N/A | N/A | 33:8:0 | 24:14:2 | 57:22:2 | 0.080 |
| Comorbid ODD (%) | N/A | N/A | N/A | 49 % | 43 % | 46 % | 0.570 |
| Comorbid Simple phobia (%) | N/A | N/A | N/A | 14% | 15 % | 14% | 0.713 |
| DuPaul IA | 2.8 (3.1) | 1.4 (1.9) | 2.1 (2.7) | 20.9 (3.7) | 19.8 (5.2) | 20.4 (4.5) | 0.260 |
| DuPaul HI | 1.5(2.1) | 1.2 (1.8) | 1.4 (2.0) | 17.6 (5.6) | 12.7 (6.9) | 15.3 (6.7) | 0.001 |
| DuPaul total | 4.3 (4.7) | 2.6 (3.0) | 3.5 (4.1) | 38.5 (6.5) | 32.4 (9.8) | 35.6 (8.8) | 0.002 |
| Conners IA T | 43.8 (4.3) | 45.5 (4.3) | 44.6 (4.3) | 73.0 (6.2) | 78.8 (11.3) | 75.7 (9.3) | 0.006 |
| Conners HI T | 45.6 (4.6) | 46.4 (4.4) | 46.0 (4.5) | 76.0 (9.3) | 73.1 (15.4) | 74.7 (12.5) | 0.317 |
| Conners total T | 44.0 (4.4) | 45.5 (4.0) | 44.7 (4.3) | 76.4 (5.7) | 77.7 (12.4) | 77.0 (9.4) | 0.549 |
SES Socio-economic status from Hollingshead total score, FSIQ Wechsler Intelligence Scale for Children, Third (WISC-III) or Fourth Edition (WISC-IV) Full Scale Intelligence Quotient, VCI WISC-IV Verbal Comprehension Index, PRI WISC-IV Perceptual Reasoning Index, WMI WISC-IV Working Memory Index, PSI WISC-IV Processing Speed Index, Com Combined, IA Inattentive, HI Hyperactive/Impulsive, T T-score
The final sample of 181 participants used in data analysis included 81 children (40 girls) diagnosed with ADHD and 100 (47 girls) TD controls, and groups were balanced in terms of: participant age, sex, general intellectual ability, and within the ADHD groups, ADHD symptom severity and subtype, providing a strong test of ADHD-related impairments in response control among boys and girls. Participant demographic and clinical characteristics of the final balanced sample are included in Table 1.
Go/No-Go Task Performance
Descriptive statistics for all of the measures obtained from each of the GNG tasks are presented in Table 2. Results of the repeated measures ANOVA revealed a significant main effect of task across diagnostic groups for both commission error rate, F(1177)=27.55, p<0.001, d=0.41, and tau, F(1177)=11.83, p=0.001, d=0.24, such that participants displayed poorer response control on the complex compared to simple GNG task. There was also a significant main effect of diagnosis for commission error rate, F(1177)=16.24, p<0.001, d=0.60, and tau, F(1177)=9.1, p=0.003, d=0.45, such that children with ADHD made significantly more commission errors and displayed higher tau compared to TD children. Further, there was a main effect of sex for commission error rate, F(1177)=4.5, p=0.036, d=0.28, which was higher among boys than among girls, but not for tau, F(1177)=0.02, p=0.885, d=0.01. None of the two-way interactions (Diagnosis×Sex; Diagnosis×Task; Sex×Task) were significant for commission error rate or tau, Fs<2.5, ps>0.11.
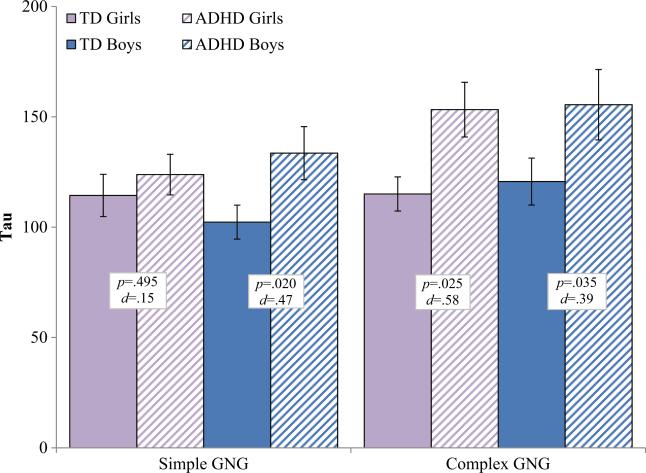
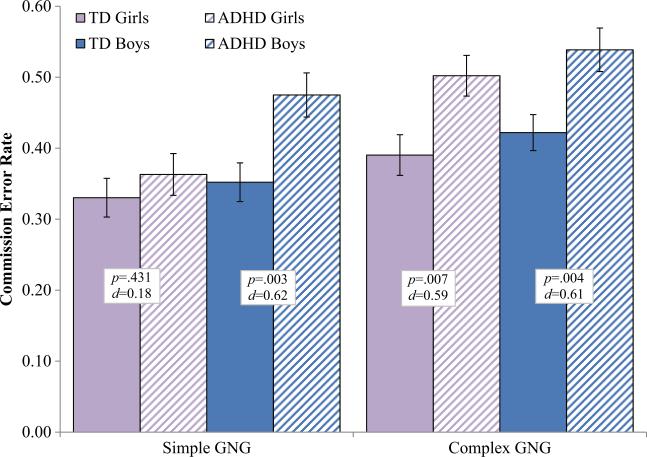
The Diagnosis×Sex×Task interaction was not significant for either commission error rate, F(1177)=1.8, p=0.180, d= 0.20, or tau, F(1177)=1.51, p=0.220, d=0.18, although power analysis suggests our power to detect an effect of this size was only 53 % and that we would have needed a larger sample to adequately test the three-way interaction of Diagnosis x Sex x Task. However, in order to further probe whether response control is differentially impaired in boys and girls with ADHD compared to same-sex TD children, we conducted separate 2 Diagnosis×2 Task repeated measures ANOVAs for boys and girls separately (see Figs. 1 and 2 for tau and commission error rate respectively). Among girls, there was a significant Diagnosis×Task interaction for tau, F(1,85)=6.4, p=0.013, d=0.54, and a marginal Diagnosis×Task interaction for commission error rate, F(1,85)=3.06, p=0.084, d=0.37. Post-hoc comparisons indicated that girls with ADHD displayed higher tau than TD girls on the complex GNG (p=0.025, d=0.58), but not on the simple GNG (p=0.495, d=0.15). Similarly, girls with ADHD made more commission errors than TD girls on the complex GNG (p=0.007, d=0.59), but not on the simple GNG (p=0.431, d=0.18). In contrast, among boys, there was no evidence of a Diagnosis×Task interaction for either tau, F(1, 92)=0.05, p=0.831, d=0.04, or commission error rate, F(1, 92)=0.02, p=0.887, d=0.03, suggesting that boys with ADHD displayed higher tau and made more commission errors than TD boys on both the simple and complex GNG.
Fig. 1.
Intrasubject variability (tau) for boys and girls with ADHD and typically developing (TD) children on the simple and complex go/no-go tasks. Boys with ADHD show increased tau on both the simple and complex GNG; Girls with ADHD show increased tau only on the complex GNG. Error bars represent standard error of the mean (SEM); p=p-value; d=Cohen's d effect size
Fig. 2.
Commission error rate (proportion of “no-go” trials) for boys and girls with ADHD and typically developing (TD) children on the simple and complex go/no-go tasks. Boys with ADHD show increased tau on both the simple and complex GNG; Girls with ADHD show increased tau only on the complex GNG. Error bars represent standard error of the mean (SEM); p=p-value; d=Cohen's d effect size
For both mu, the ex-Gaussian measures of response speed in the normal component of the RT distribution, and sigma, a measure of variability in the normal part of the RT distribution, the Diagnosis×Sex×Task interactions were not significant (respectively p=0.965 and p=0.578). However, to probe the effect of gender, we conducted separate 2 Diagnosis×2 Task repeated measures ANOVAs for boys and girls separately for each mu and sigma. For mu, among girls, there was no main effect of task, F(1,85)=0.33, p=0.570, or diagnosis, F(1, 85)=0.38, p=0.539, nor was there a Diagnosis×Task interaction, F(1,85)=0.01, p=0.935. For boys, there was no main effect of task, F(1,92)=3.18, p=0.078, or diagnosis, F(1, 92)=3.22, p=0.076, nor was there a Diagnosis×Task interaction, F(1,92)=0.001, p=0.980 for mu. Similarly for sigma, among girls, there was no main effect of task F(1,85)=1.79, p=0.185, or diagnosis, F(1,85)=0.98, p=0.326, and the Diagnosis×Task interaction, F(1,85)=0.08, p=0.779 was not significant. For boys, there was no main effect of task, F(1, 92)=0.006, p=0.937, or diagnosis, F(1,92)=0.88, p=0.350, nor was there a Diagnosis×Task interaction, F(1,92)=0.28, p=0.600 for sigma.
Discussion
The current study extends the literature on impaired response control in children with ADHD by examining the effect of increasing cognitive load on GNG task performance, with a particular focus on commission error rate and ISV (as assessed using the ex-Gaussian measure, tau). Furthermore, the present study examined whether both girls and boys with ADHD, relative to sex-matched TD peers, exhibit a different profile of impaired response control across GNG tasks that vary in cognitive load. Our results suggest that increasing cognitive load differentially impacts response control (i.e., commission error rate and tau) for girls, but not boys, with ADHD compared to same-sex TD children. Specifically, boys with ADHD demonstrated higher commission error rate and tau on both the simple and complex GNG tasks compared to TD boys. In contrast, girls with ADHD did not differ from TD girls on the simple GNG task, but showed higher commission error rate and tau on the complex GNG task. Taken together, our results not only extend the literature on the effect of increasing cognitive load on response control in children with ADHD, but also suggest that this effect may be different for boys versus girls with the ADHD.
Consistent with prior literature, our results show deficient response inhibition and increased ISV in children with ADHD compared to TD children (Epstein et al. 2011; Leth-Steensen et al. 2000; Vaurio et al. 2009; Wodka et al. 2007). Specifically, our results revealed that compared to TD children, children with ADHD (across sex) demonstrated greater commission error rates and increased tau across both the simple and complex GNG tasks. Prior literature has shown that individuals with ADHD demonstrate greater commission error rates, and moderate to large increases in ISV relative to TD individuals on response inhibition tasks (Castellanos and Tannock 2002; Epstein et al. 2011; Hervey et al. 2006; Leth-Steensen et al. 2000; Vaurio et al. 2009). Also in line with prior research, we found that compared to TD children, children with ADHD did not display greater response variability in the normal component of the RT distribution during the behavioral tasks (i.e., sigma), but rather had subsets of abnormally slow responding (i.e., tau) which may be the result of periodic lapses in attention or related to other neurocognitive deficits (Kofler et al. 2013).
While consistent with meta-analytic results indicating a moderate to large effect size (ES) of increased ISV in children with ADHD compared to TD children, our results are more modest (tau ES combined across tasks = 0.45) than previously reported (ES range 0.63 to 0.95) which may be the result of differences in sample characteristics (Epstein et al. 2011; Hervey et al. 2006; Kofler et al. 2013). For example, ADHD participants in the Epstein et al. (2011) sample were younger, had greater levels of comorbidity, and included fewer girls than our sample, which may have affected ISVas our findings suggest that elevated tau among girls with ADHD is task-dependent. In addition, our findings suggest that task characteristics may also influence effect size estimates, with smaller effects of diagnosis on tasks with minimal cognitive demands (simple GNG tau ES = 0.32) compared to tasks with greater cognitive demands (complex GNG tau ES = 0.46). Finally, reaction times were limited to a maximum of 2300 ms (the trial duration), which may have reduced estimates of tau compared to longer response windows used in previous studies (e.g.,5 s in Epstein et al. 2011). However, a study with a similar response window (e,g., 2250 ms trial duration in Hervey et al. 2006) but a greater percentage of boys in their sample (77 %) and a different task (Conners’ CPT) demonstrated larger diagnostic group differences (ES = 0.82) than in our study. In sum, characteristics of the sample and task appear to influence estimates of tau and diagnostic group differences and should be taken into consideration in future research.
Our results further revealed that increasing cognitive load, via increased WM demands during the complex GNG task, had a greater effect on impairments in response control (i.e., both commission error rate and tau) for girls, but not boys, with ADHD compared to same-sex TD children. Specifically, boys with ADHD demonstrated more commission errors and greater ISVon both simple and complex GNG task compared to TD boys, whereas girls with ADHD only exhibited increased commission errors and ISV compared to TD girls when cognitive load was increased on the complex GNG task, thereby driving the Diagnosis×Task interaction among girls. This pattern of findings is in contrast to those of previous studies (Vaurio et al. 2009; Wodka et al. 2007), which did not find a differential effect of task complexity on response control for children with ADHD compared to TD children, likely due to the inclusion of primarily male samples, as this effect only emerged among girls with ADHD in the current study. It is important to note that the non-significant effect of diagnosis for girls with ADHD on the simple GNG was not due to reduced power because of the smaller sample size (n= 87 girls, n=94 boys) or greater symptom severity in girls with ADHD as analyses were conducted in a sample of boys with ADHD that did not differ in symptom severity from the girls with ADHD.
Prior studies examining cognitive load on ISV in children with ADHD compared to TD controls have not reported ADHD-related sex differences, but this lack of findings may be the result of low power due to small samples of females with ADHD (Klein et al. 2006; Vaurio et al. 2009; Wodka et al. 2007). In addition, Epstein and colleagues (2011) reported no effect of sex, but their sample of girls was smaller (n= 45) and they did not directly compare girls with ADHD to TD girls and boys with ADHD to TD boys. In addition, their tasks varied in a number of ways, as did the task manipulations (i.e., event rate and incentives), potentially limiting their ability to detect sex differences in response control as a function of increasing working memory demands. Other studies have reported an overall effect of sex, such that boys demonstrated faster Go RT (Uebel et al. 2010) or more commission errors (Wodka et al. 2007) than girls, regardless of diagnosis. Thus, our large sample of girls with ADHD and our controlled manipulation of task complexity revealed novel, sexually dimorphic effects regarding impaired response control in children with ADHD.
Our findings suggest that boys with ADHD demonstrate impairments in response control even when cognitive load is minimized and that these impairments persist under conditions with greater cognitive load, in particular when WM is necessary to guide response selection/inhibition. In contrast, response control is intact among girls with ADHD when cognitive load is minimized with greater impairment emerging with increasing cognitive load. These sexually dimorphic effects are consistent with evidence from prior studies showing that, within this age range (8–12 years-old), motor control deficits are more prominent in boys than in girls with ADHD (Cole et al. 2008) whereas impairments in WM and related executive dysfunctions are present in both boys and girls with the disorder (Rucklidge 2010). In concert with prior literature showing how WM impairments underlie inhibitory control deficits (Alderson et al. 2010), our distinct pattern of results for boys compared to girls with ADHD may suggest that for girls an increase in cognitive load via increased WM demands results in greater inhibition difficulties whereas for boys with ADHD these deficits in response control may be rooted in more anatomical abnormalities regardless of cognitive load. Indeed, this pattern of behavioral findings, in concert with evidence from neuroimaging studies, suggests that ADHD in boys may be associated with neural dysfunction in both premotor (PM) and prefrontal (PF) systems; whereas in girls with ADHD, abnormalities may be more localized to PF circuits. Anatomic neuroimaging studies of ADHD, which include predominantly male samples, reveal structural abnormalities spanning both PM and PF circuits (Mostofsky et al. 2002; Shaw et al. 2006; Wolosin et al. 2009). Further, functional neuroimaging studies of response control highlight the critical role of PM motor regions, particularly the supplementary motor complex (SMC) under conditions in which cognitive demands are minimal (e.g., simple GNG) (Mostofsky et al. 2003; Simmonds et al. 2008). However, under conditions in which cognitive load is increased as in the complex GNG task, when WM is necessary to guide response selection, prefrontal regions particularly the dorsolateral prefrontal cortex are recruited (Mostofsky et al. 2003; Simmonds et al. 2013) These neuroimaging findings, taken together with our behavioral results, suggest that ADHD in boys may be associated with deficits spanning both PM and PF regions, whereas in girls with ADHD such deficits are localized to PF regions. Given that PM regions develop prior to PF regions, and that girls mature at a faster rate than boys (Gogtay et al. 2004; Lenroot et al. 2007; Sowell et al. 2004, 2006), our findings could be the results of typical neural maturational processes (i.e., passage of time) rather than sexually dimorphic effects. As such, longitudinal research following the behavioral and neural development of large samples of prepubescent girls and boys with ADHD into adolescent is required to parse apart these effects.
Our findings of impaired response control among boys and girls with ADHD under conditions in which WM is necessary to guide response selection is consistent with prior evidence showing that ADHD is associated with impairments in WM and related executive functions, and lends support to multiprocess models of ADHD which highlight the role of multiple cognitive deficits in ADHD (Coghill et al. 2013). WM has been conceptualized as a multi-component systems with a central executive which oversees the allocation of cognitive resources, and engages in the supervisory control and attention of WM in relation to other cognitive processes (Baddeley 2007). In relation to our study, our complex GNG increased cognitive load via increased WM demands of the central executive because it required participants to engage in ongoing processing, reordering and updating of information being held in short term memory (STM; i.e., counting green spaceships). Further, the task required higher-order cognitive processes including decision-making and response control (i.e., decision to respond or withhold response to red spaceship) within the context of these increased WM demands. In relation to response control, it has been suggested that deficits in the central executive (CE) may briefly disturb task performance resulting in atypically slow responses (e.g., tau) (Rapport et al. 2008). In fact, a recent study empirically testing the application of Baddeley's WM model to ADHD showed that the largest ADHD-related deficits in WM were found within the central executive which is critically important in directing and focusing attention (Kofler et al. 2014). As such, deficits in CE, may contribute to core difficulties with response control in individuals with ADHD as has been shown in prior research examining WM in the context of stop-signal task performance in children with ADHD (Alderson, et al. 2010; Kofler et al. 2014; Raiker et al. 2012). Indeed, research has shown a strong latent negative correlation between tau and established measures of WM, providing further support for this relationship (Schmiedek et al. 2007). Moving forward, additional research is needed to determine whether there is a linear effect of cognitive load on response control in children with ADHD by comparing high and low WM conditions. Furthermore, these studies should account for sex, as the impact of WM may differ for boys and girls with ADHD.
Our study has a number of significant clinical implications. First, our results suggest the importance of careful assessment of neurocognitive deficits in children with ADHD. Specifically, children with ADHD present with heterogeneous neurocognitive and behavioral profiles resulting in different strengths and weaknesses. With regards to our results, such assessment should examine response control and WM in boys and girls with ADHD as different neurocognitive profiles may emerge. As for treatment implications, in line with prior work that has suggested the benefits of cognitive training to address neuropsychological deficits in children with ADHD (Chacko et al. 2014; Gray et al. 2012; Green et al. 2012; Klingberg et al. 2005), our results support the use of cognitive remediation training with a focus on systematically increasing WM demands in children with ADHD, and while preliminary and in need of replication, our results may suggest such training could be particularly important for girls with ADHD. However, it will be important to replicate our findings in future studies adequately powered to detect sex differences. Given that laboratory behavioral tasks may not generalize to cognitive demands in the real world (i.e., real world WM demands or response control demands), it will be important to try and make tasks used for cognitive remediation training as generalizable to actual world demands as possible. For example, children with ADHD who demonstrated impairments in response control on time-limited behavioral tasks (e.g., 10 min) are likely to demonstrate even greater impairments in daily activities which often require response control efforts lasting for longer durations.
As with all studies, our results should be interpreted in light of some limitations. First, we assessed ISV during two response inhibition tasks, which may limit generalization of these findings to other tasks. For example, the RT distribution during a response-inhibition task may be influenced by the infrequent “no-go” trials or post-error slowing, a self-regulatory, adaptive response that refers to the slowing of response speed on a trial following an error (Rabbit 1997). Post-error slowing has been shown to be impaired in children with ADHD but also sensitive to task demands, such as tasks requiring infrequent response inhibition (e.g., GNG tasks) compared to those that consistently require responses to a “go” stimulus (e.g., attentional control tasks) (Schachar et al. 2004; Shiels et al. 2012; Wiersema et al. 2005). A second limitation is comorbidity, aside from ODD, was limited in our ADHD group due to the inclusion/exclusion criteria. As the aim of this study was to examine the effects of ADHD on cognitive control, the inclusion of multiple comorbid conditions would have required a substantially larger ADHD sample which was beyond the scope of this study. The lack of comorbidity may limit the generalizability of our findings to the broader population of children with ADHD; however, over 30 % of children with ADHD do not present with comorbidity. As a result of this limitation, we are unable to comment on the effect of comorbidity on ISV in children with ADHD. In light of this limitation, it will be important in future studies of sex differences in ISV in children with ADHD to examine the role of comorbidities especially for disorders that show sex-based differences in prevalence rates such as anxiety and/or depression. Finally, our groups of boys and girls with ADHD were balanced on ADHD sex-normed t-scores (i.e., Conners) rather than raw scores (DuPaul). As a result, it is possible that the groups differed in hyperactivity/impulsivity symptom severity (i.e., boys with ADHD had higher levels of hyperactivity/ impulsivity than girls) which may have affected the results. While inattentive symptoms compared to hyperactive/ impulsive symptoms have been more related to neuropsycho-logical impairments (Willcutt et al. 2012), future research examining the effects of sex on neurocognitive abilities should consider whether to balance groups on raw versus normed symptom ratings.
Despite these limitations, our results provide novel information on the differential impact of cognitive load on response control in children with ADHD and associated sex differences. These findings contribute to the large literature on response control in children with ADHD by elucidating factors that contribute to impaired response control associated with ADHD in the largest sample of children with ADHD, oversampled for girls, examined to date. Further our results contribute to the literature suggesting important sex differences in children with ADHD, which may have important implications for longitudinal functional outcomes.
Acknowledgments
This research was supported in part by NIH grants R01 MH078160, R01 MH085328, and K23 MH101322 and Johns Hopkins University School of Medicine Institute for Clinical and Translational Research (NIH/National Center for Research Resources Clinical and Translational Science Award program, UL1 RR025005).
Footnotes
To assess the impact of simple phobia on our results, we re-ran our primary analyses for tau and commission error rate excluding children diagnosed with specific phobia (ADHD=11; TD=6). Our results did not change for either tau or commission error rate.
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Karen E. Seymour, Division of Child and Adolescent Psychiatry, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Stewart H. Mostofsky, Departments of Neurology and of Psychiatry and Behavioral Sciences and Kennedy Krieger Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Keri S. Rosch, Department of Psychiatry and Behavioral Sciences and Kennedy Krieger Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA
References
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. doi:10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Hudec KL, Sarver DE, Kofler MJ. Competing core processes in attention-deficit/hyperactivity disorder (ADHD): Do working memory deficiencies underlie behavioral inhibition deficits? Journal of Abnormal Child Psychology. 2010;38:497–507. doi: 10.1007/s10802-010-9387-0. doi:10.1007/s10802-010-9387-0. [DOI] [PubMed] [Google Scholar]
- Almeida Montes LG, Prado Alcantara H, Martinez Garcia RB, De La Torre LB, Avila Acosta D, Duarte MG. Brain cortical thickness in ADHD: Age, sex, and clinical correlations. Journal of Attention Disorders. 2013;17:641–654. doi: 10.1177/1087054711434351. doi:10.1177/1087054711434351. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostics and statistics manual of mental disorders (DSM-5) American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- Antonini TN, Narad ME, Langberg JM, Epstein JN. Behavioral correlates of reaction time variability in children with and without ADHD. Neuropsychology. 2013;27:201–209. doi: 10.1037/a0032071. doi:10.1037/a0032071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory, thought and action. Oxford University Press; New York: 2007. [Google Scholar]
- Balint S, Czobor P, Komlosi S, Meszaros A, Simon V, Bitter I. Attention deficit hyperactivity disorder (ADHD): Gender- and age-related differences in neurocognition. Psychological Medicine. 2009;39:1337–1345. doi: 10.1017/S0033291708004236. doi:10.1017/S0033291708004236. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, Morgan CL, Faraone SV. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. Journal of Consulting and Clinical Psychology. 2004;72:757–766. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- Brown TE, Landgraf JM. Improvements in executive function correlate with enhanced performance and functioning and health-related quality of life: evidence from 2 large, double-blind, randomized, placebo-controlled trials in ADHD. Postgraduate Medicine. 2010;122:42–51. doi: 10.3810/pgm.2010.09.2200. doi:10.3810/pgm.2010.09.2200. [DOI] [PubMed] [Google Scholar]
- Buzy WM, Medoff DR, Schweitzer JB. Intra-individual variability among children with ADHD on a working memory task: an ex-Gaussian approach. Child Neuropsychology. 2009;15:441–459. doi: 10.1080/09297040802646991. doi:10.1080/09297040802646991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. doi:10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. doi:10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko A, Bedard AC, Marks DJ, Feirsen N, Uderman JZ, Chimiklis A, Rajwan E, Cornwell M, Anderson L, Zwilling A, Ramon M. A randomized clinical trial of cogmed working memory training in school-age children with ADHD: a replication in a diverse sample using a control condition. Journal of Child Psychology and Psychiatry. 2014;55:247–255. doi: 10.1111/jcpp.12146. doi:10.1111/jcpp.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Pedroso S, Usala T, Currie J, Gagliano A. Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biological Psychiatry. 2013;76:603–615. doi: 10.1016/j.biopsych.2013.10.005. doi:10.1016/j.biopsych.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Earlbaum Associates; Hillsdale: 1988. [Google Scholar]
- Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71:1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. doi:10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Conners’ rating scales- revised. Toronto: Multi-Health Systems. 2002 [Google Scholar]
- Diamantopoulou S, Rydell AM, Thorell LB, Bohlin G. Impact of executive functioning and symptoms of attention deficit hyperactivity disorder on children’s peer relations and school performance. Developmental Neuropsychology. 2007;32:521–542. doi: 10.1080/87565640701360981. doi:10.1080/87565640701360981. [DOI] [PubMed] [Google Scholar]
- Dirlikov B, Rosch KS, Crocetti D, Denckla MB, Mahone EM, Mostofsky SH. Distinct frontal lobe morphology in girls and boys with ADHD. Neuroimage: Clinical. 2013 doi: 10.1016/j.nicl.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale-IV. Guilford Press; New York: 1998. [Google Scholar]
- Epstein JN, Hwang ME, Antonini T, Langberg JM, Altaye M, Arnold LE. Examining predictors of reaction times in children with ADHD and normal controls. Journal of the International Neuropsychological Society. 2010;16:138–147. doi: 10.1017/S1355617709991111. doi:10.1017/S1355617709991111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Antonini TN, Brinkman WB, Froehlich T, Simon JO, Altaye M. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–441. doi: 10.1037/a0022155. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1036–1045. doi: 10.1097/00004583-199708000-00011. doi:10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders. 2002;5:143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Getahun D, Jacobsen SJ, Fassett MJ, Chen W, Demissie K, Rhoads GG. Recent trends in childhood attention-deficit/hyperactivity disorder. JAMA Pediatriatrics. 2013;167:282–288. doi: 10.1001/2013.jamapediatrics.401. doi:10.1001/2013.jamapediatrics.401. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SA, Chaban P, Martinussen R, Goldberg R, Gotlieb H, Kronitz R, Hockenberry M, Tannock R. Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: a randomized controlled trial. Journal of Child Psychology and Psychiatry. 2012;53:1277–1284. doi: 10.1111/j.1469-7610.2012.02592.x. doi:10.1111/j.1469-7610.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- Green CT, Long DL, Green D, Iosif AM, Dixon JF, Miller MR, Fassbender C, Schweitzer JB. Will working memory training generalize to improve off-task behavior in children with attention-deficit/hyperactivity disorder? Neurotherapeutics. 2012;9:639–648. doi: 10.1007/s13311-012-0124-y. doi:10.1007/s13311-012-0124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevremont D, Barkley RA. Attention deficit hyperactivity disorder in children. In: Hooper SR, Hynd GW, Mattison RF, editors. Child psychopathology: Diagnostic criteria and clinical assessment. Lawrence Erlbaum Associates; New Jersey: 1992. pp. 137–178. [Google Scholar]
- Hasson R, Fine JG. Gender differences among children with ADHD on continuous performance tests: a meta-analytic review. Journal of Attention Disorders. 2012;16:190–198. doi: 10.1177/1087054711427398. doi:10.1177/1087054711427398. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene Arnold L, Keith Conners C, Hinshaw SP, Swanson JM, Hechtman L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychology. 2006;12:125–140. doi: 10.1080/09297040500499081. doi:10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr., Howard J, McGrath L, Steele S, Frazier JA, Tager-Flusberg H, Harris GL. Cerebellum, language, and cognition in autism and specific language impairment. Journal of Autism and Developmental Disorders. 2010;40:300–316. doi: 10.1007/s10803-009-0872-7. doi:10.1007/s10803-009-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LA, Ryan M, Martin RB, Ewen J, Mostofsky SH, Denckla MB, Mahone EH. Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychology. 2011;17:209–224. doi: 10.1080/09297049.2010.532204. doi:10.1080/09297049.2010.532204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, March JS, Arnold LE, Cantwell DP, Conners CK, Elliot GR, Greenhill LL, Hechtman L, Hoza B, Pelham WE, Severe JB, Swanson JM, Wells KC, Wigal T, Vitello B. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:147–158. doi: 10.1097/00004583-200102000-00009. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. doi:10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Gillberg CG, Forssberg H, Westerberg H. Computerized training of working memory in children with ADHD– A randomized, controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. doi:10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS, Alderson RM. Working memory deficits and social problems in children with ADHD. Journal of Abnormal Child Psychology. 2011;39:805–817. doi: 10.1007/s10802-011-9492-8. doi:10.1007/s10802-011-9492-8. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, Kolomeyer EG. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clinical Psychology Review. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. doi:10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Alderson RM, Raiker JS, Bolden J, Sarver DE, Rapport MD. Working memory and intraindividual variability as neurocognitive indicators in ADHD: Examining competing model predictions. Neuropsychology. 2014;28:459–471. doi: 10.1037/neu0000050. doi:10.1037/neu0000050. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Arias-Vasquez A, Buitelaar JK, McLoughlin G, Rommelse NN, Sergeant JA, Sonuga-Barke EJ, Uebel H, van der Meere JJ, Banaschewski T, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Steinhausen HC, Faraone SV, Asherson P. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Archives of General Psychiatry. 2010;67:1159–1167. doi: 10.1001/archgenpsychiatry.2010.139. doi:10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture Y, Cousineau D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutorials in Quantitative Methods for Psychology. 2008;4:35–45. [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. doi:10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: Deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. doi:10.1037/0021-843x.114.2.216. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Ranta ME, Crocetti D, O’Brien J, Kaufmann WE, Denckla MB, Mostofsky SM. Comprehensive examination of frontal regions in boys and girls with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2011;17:1047–1057. doi: 10.1017/S1355617711001056. doi:10.1017/S1355617711001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, Pekar JJ. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Research. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. doi:10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Nikolas MA, Nigg JT. Neuropsychological performance and attention-deficit hyperactivity disorder subtypes and symptom dimensions. Neuropsychology. 2013;27:107–120. doi: 10.1037/a0030685. doi:10.1037/a0030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D, Jacobson LA, Crocetti D, Rosch KS, Mostofsky SH. Premotor white matter integrity correlates with response control in males, but not females with ADHD.. Poster presented at the 20th Annual Meeting of the Organization for Human Brain Mapping; Hamburg, Germany. 2014. [Google Scholar]
- Rabbit P. Methodology of frontal and executive function. Psychology Press; East Sussex: 1997. [Google Scholar]
- Raiker JS, Rapport MD, Kofler MJ, Sarver DE. Objectively-measured impulsivity and attention-deficit/hyperactivity disorder (ADHD): Testing competing predictions from the working memory and behavioral inhibition models of ADHD. Journal of Abnormal Child Psychology. 2012;40:699–713. doi: 10.1007/s10802-011-9607-2. doi:10.1007/s10802-011-9607-2. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, Sims V. Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): the contribution of central executive and subsystem processes. Journal of Abnormal Child Psychology. 2008;36:825–837. doi: 10.1007/s10802-008-9215-y. [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA). Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ. Impact of ADHD on the neurocognitive functioning of adolescents with bipolar disorder. Biological Psychiatry. 2006;60:921–928. doi: 10.1016/j.biopsych.2006.03.067. doi:10.1016/j.biopsych.2006.03.067. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ. Gender differences in ADHD: implications for psychosocial treatments. Expert Review of Neurotherapeutics. 2008;8:643–655. doi: 10.1586/14737175.8.4.643. doi:10.1586/14737175.8.4.643. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatric Clinics of North America. 2010;33:357–373. doi: 10.1016/j.psc.2010.01.006. doi:10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Chen S, Logan GD, Ornstein TJ, Crosbie J, Ickowicz A, Pakulak A. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2004;32:285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Oberauer K, Wilhelm O, Suss HM, Wittmann WW. Individual differences in components of reaction time distributions and their relations to working memory and intelligence. Journal of Experimental Psychology: General. 2007;136:414–429. doi: 10.1037/0096-3445.136.3.414. doi: 10.1037/0096-3445.136.3.414. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Vidair HB, Vogel LR, Muskin PR. Anxiety, obsessive-compulsive and stress disorders. In: Cutler JL, editor. Psychiatry. Oxford University Press; New York: 2014. pp. 168–203. [Google Scholar]
- Seidman LJ, Biederman J, Monuteaux MC, Valera E, Doyle AE, Faraone SV. Impact of gender and age on executive functioning: Do girls and boys with and without attention deficit hyperactivity disorder differ neuropsychologically in preteen and teenage years? Developmental Neuropsychology. 2005;27:79–105. doi: 10.1207/s15326942dn2701_4. doi: 10.1207/s15326942dn2701_4. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biological Psychiatry. 2005;57:1248–1255. doi: 10.1016/j.biopsych.2004.09.010. doi:10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Seymour KE, Tang X, Crocetti D, Nettles C, Miller MI, Mostofsky SH. Amygdala and Hippocampal Volumes in ADHD: The Importance of Sex.. Poster presented at the 20th Annual Meeting of the Organization for Human Brain Mapping; Hamburg, Germany. 2014. [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. doi:10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shiels Rosch K, Dirlikov B, Mostofsky SH. Increased intrasubject variability in boys with ADHD across tests of motor and cognitive control. Journal of Abnormal Child Psychology. 2013;41:485–495. doi: 10.1007/s10802-012-9690-z. doi:10.1007/s10802-012-9690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Tamm L, Epstein JN. Deficient post-error slowing in children with ADHD is limited to the inattentive subtype. Journal of the International Neuropsychological Society. 2012;18:612–617. doi: 10.1017/S1355617712000082. doi:10.1017/s1355617712000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. doi:10.1016/j.neuropsychologia. 2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. NeuroImage. 2013;92c:356–368. doi: 10.1016/j.neuroimage.2013.12.044. doi:10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjowall D, Roth L, Lindqvist S, Thorell LB. Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. Journal of Child Psychology and Psychiatry. 2013;54:619–627. doi: 10.1111/jcpp.12006. doi:10.1111/jcpp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD–A dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience & Biobehavioral Reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Halperin JM. Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: Potential targets for early intervention? Journal of Child Psychology and Psychiatry. 2010;51:368–389. doi: 10.1111/j.1469-7610.2009.02195.x. doi:10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Sergeant J. The neuroscience of ADHD: multidisciplinary perspectives on a complex developmental disorder. Developmental Science. 2005;8:103–104. doi: 10.1111/j.1467-7687.2005.00396.x. doi:10.1111/j.1467-7687.2005.00396.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of Age. Cerebral Cortex. 2006;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Narad ME, Antonini TN, O'Brien KM, Hawk LW, Jr., Epstein JN. Reaction time variability in ADHD: a review. Neurotherapeutics. 2012;9:500–508. doi: 10.1007/s13311-012-0138-5. doi:10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. Sex-dependent basal ganglia abnormalities in children with attention-deficit/hyperactivity disorder.. Poster presented at the 20th Annual Meeting of the Organization for Human Brain Mapping; Hamburg, Germany. 2014. [Google Scholar]
- Uebel H, Albrecht B, Asherson P, Borger NA, Butler L, Chen W, Christiansen H, Heise A, Schafer U, Andreou P, Manor I, Marco R, Miranda A, Mulligan A, Oades RD, van der Meere J, Faraone SV, Rothenberger A, Banaschewski T. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. Journal of Child Psychology and Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. doi:10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47:2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. doi:10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler individual achievement test-II. The Psychological Corporation; San Antonio: 2002. [Google Scholar]
- Wechsler DL. Wechsler intelligence scale for children. 4th ed. The Psychological Corporation; San Antonio: 2003. [Google Scholar]
- Wiersema JR, van der Meere JJ, Roeyers H. ERP correlates of impaired error monitoring in children with ADHD. Journal of Neural Transmission. 2005;112:1417–1430. doi: 10.1007/s00702-005-0276-6. doi:10.1007/s00702-005-0276-6. [DOI] [PubMed] [Google Scholar]
- Willcutt E, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. doi:10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt E, Sonuga-Barke E, Nigg J, Sergeant J. Recent developments in neuropsychological models of child psychiatric disorders. In: Banaschewski T, Rohde LA, editors. Biological child psychiatry: recent trends and developments. Vol. 24. Karger; Basel: 2008. pp. 194–226. [Google Scholar]
- Willcutt E, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology. 2012;121:991–1010. doi: 10.1037/a0027347. doi:10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Larson JC, Fotedar S, Denckla MB, et al. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. doi:10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Human Brain Mapping. 2009;30:175–184. doi: 10.1002/hbm.20496. doi:10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]