Abstract
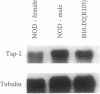
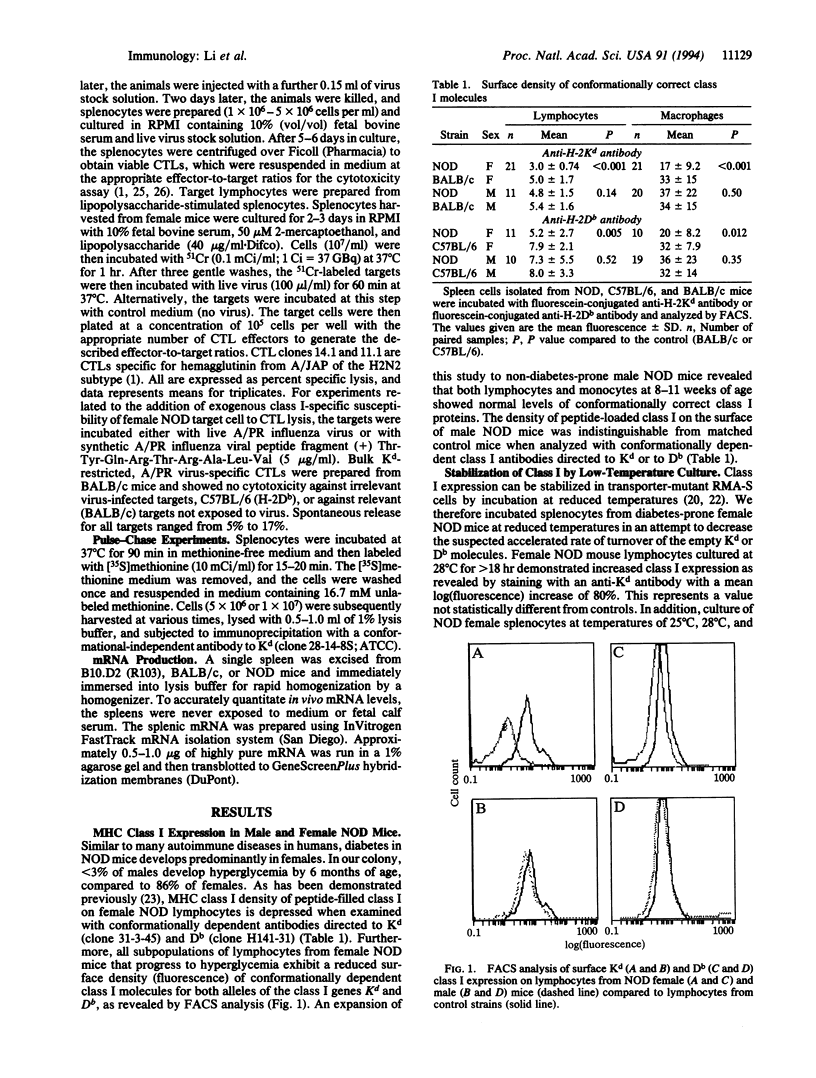
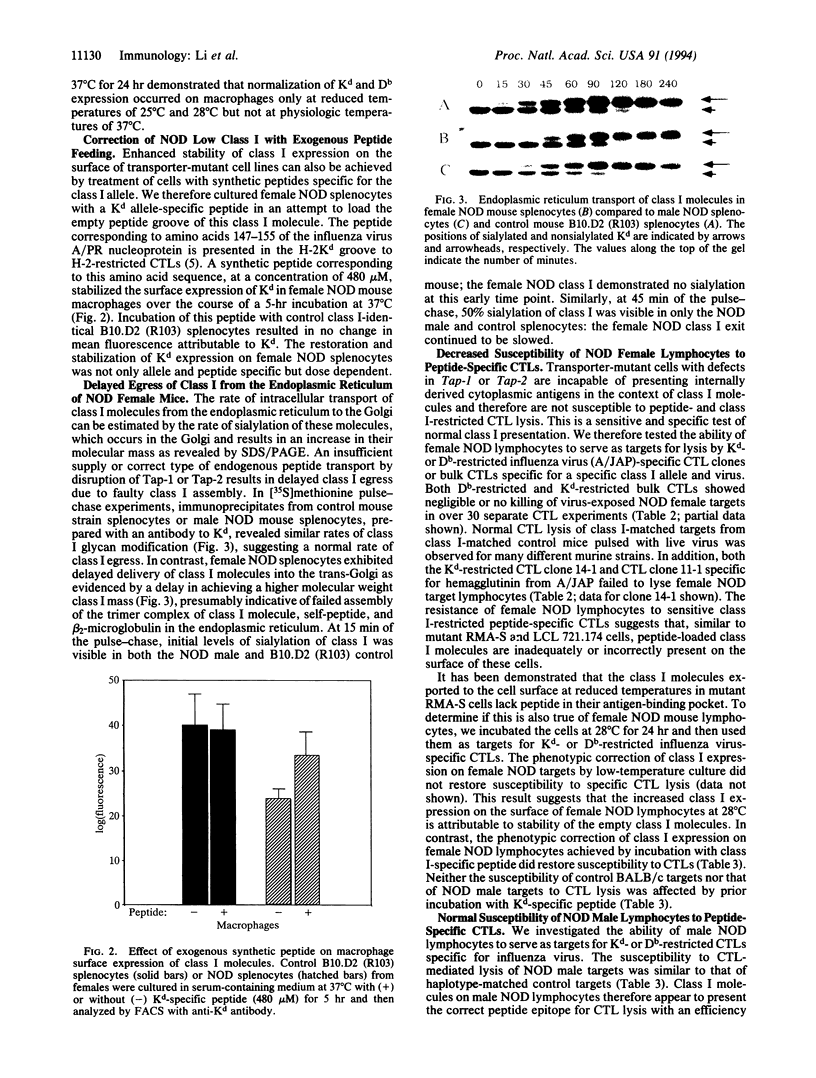
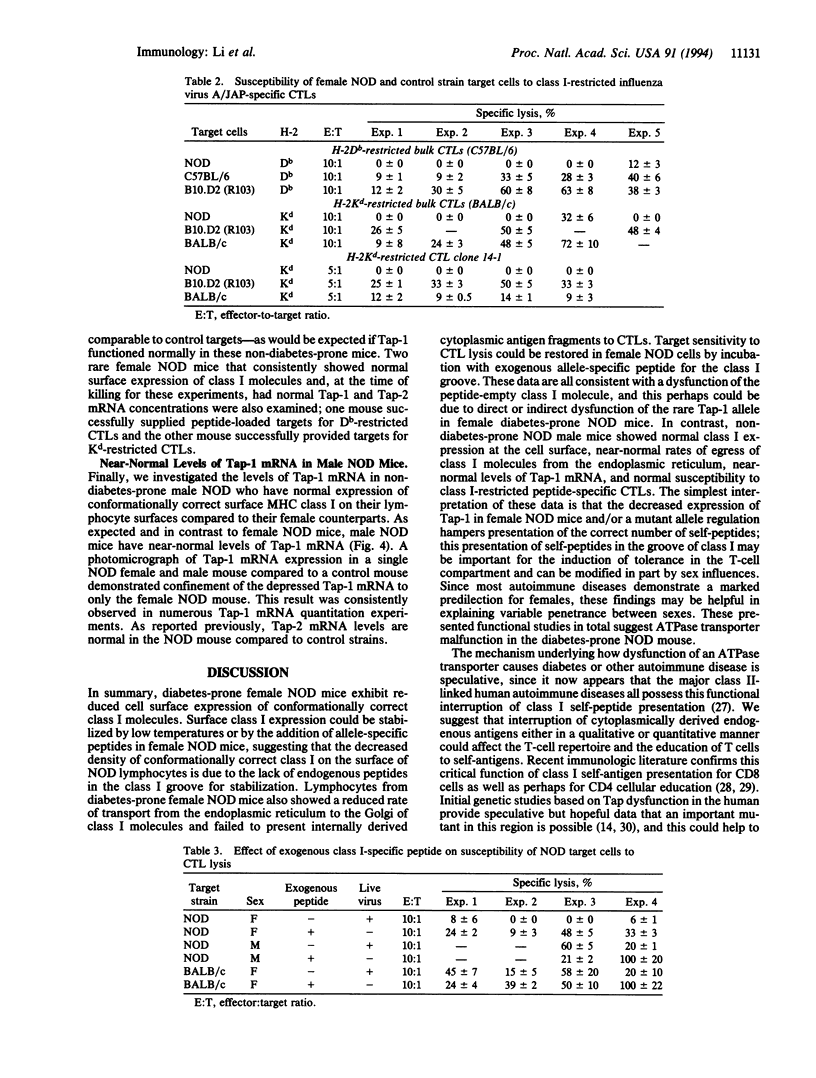
Presentation of self-antigens by major histocompatibility complex (MHC) class I molecules requires the function of the MHC class II-linked genes Tap-1 and Tap-2. Evidence suggests that interruption of self-peptide presentation results in reduced cell surface expression of MHC class I molecules and the interruption correlates with progression to diabetic autoimmunity in nonobese diabetic (NOD) mice and humans. NOD mice possess a rare Tap-1 allele (Tap-1b); this is associated with reduced Tap-1 mRNA abundance in lymphocytes from diabetes-prone females and decreased conformationally correct class I molecules on the cell surface. In this study, we demonstrate that, similar to lymphoma cell lines with mutations in Tap-1 or Tap-2, the reduced expression of class I molecules on the surface of lymphocytes from diabetes-prone female NOD mice was normalized by incubation at low temperatures or by exposure to class I allele-specific peptides. As would be expected for cells that express surface class I molecules not associated with peptide, female NOD lymphocytes were resistant to lysis by class I-restricted, peptide-specific cytotoxic T lymphocytes. Furthermore, the rate of class I exit from the endoplasmic reticulum of lymphocytes from female NOD mice was delayed as demonstrated by delayed glycosylation. Male NOD mice, which are not prone to diabetes, lacked these functional defects in class I assembly and had near-normal levels of Tap-1 mRNA and exhibited normal density of class I epitopes that were peptide filled. These results are consistent with the possibility that the rare Tap-1b allele is associated with a quantitative defect in Tap-1 expression that influences disease course.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton-Rickardt P. G., Van Kaer L., Schumacher T. N., Ploegh H. L., Tonegawa S. Peptide contributes to the specificity of positive selection of CD8+ T cells in the thymus. Cell. 1993 Jun 4;73(5):1041–1049. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- Attaya M., Jameson S., Martinez C. K., Hermel E., Aldrich C., Forman J., Lindahl K. F., Bevan M. J., Monaco J. J. Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature. 1992 Feb 13;355(6361):647–649. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- Caillat-Zucman S., Bertin E., Timsit J., Boitard C., Assan R., Bach J. F. Protection from insulin-dependent diabetes mellitus is linked to a peptide transporter gene. Eur J Immunol. 1993 Aug;23(8):1784–1788. doi: 10.1002/eji.1830230808. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Alexander J., Anderson K., Lamb C., Cresswell P., McMichael A., Gotch F., Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990 May 31;345(6274):449–452. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- Colonna M., Bresnahan M., Bahram S., Strominger J. L., Spies T. Allelic variants of the human putative peptide transporter involved in antigen processing. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3932–3936. doi: 10.1073/pnas.89.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMars R., Rudersdorf R., Chang C., Petersen J., Strandtmann J., Korn N., Sidwell B., Orr H. T. Mutations that impair a posttranscriptional step in expression of HLA-A and -B antigens. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8183–8187. doi: 10.1073/pnas.82.23.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverson E. V., Gow I. R., Coadwell W. J., Monaco J. J., Butcher G. W., Howard J. C. MHC class II region encoding proteins related to the multidrug resistance family of transmembrane transporters. Nature. 1990 Dec 20;348(6303):738–741. doi: 10.1038/348738a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Faustman D., Li X. P., Lin H. Y., Fu Y. E., Eisenbarth G., Avruch J., Guo J. Linkage of faulty major histocompatibility complex class I to autoimmune diabetes. Science. 1991 Dec 20;254(5039):1756–1761. doi: 10.1126/science.1763324. [DOI] [PubMed] [Google Scholar]
- Faustman D., Li X., Lin H. Y., Huang R., Guo J. Response. Science. 1992 Jun 26;256(5065):1830–1831. doi: 10.1126/science.256.5065.1830. [DOI] [PubMed] [Google Scholar]
- Fu Y., Nathan D. M., Li F., Li X., Faustman D. L. Defective major histocompatibility complex class I expression on lymphoid cells in autoimmunity. J Clin Invest. 1993 May;91(5):2301–2307. doi: 10.1172/JCI116459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotch F., Rothbard J., Howland K., Townsend A., McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. 1987 Apr 30-May 6Nature. 326(6116):881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- Hogquist K. A., Jameson S. C., Heath W. R., Howard J. L., Bevan M. J., Carbone F. R. T cell receptor antagonist peptides induce positive selection. Cell. 1994 Jan 14;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Kerr L. A., Mockridge I., Elliott T., Bastin J., Uchanska-Ziegler B., Ziegler A., Trowsdale J., Townsend A. Assembly and function of the two ABC transporter proteins encoded in the human major histocompatibility complex. Nature. 1992 Feb 13;355(6361):641–644. doi: 10.1038/355641a0. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Päbo S., Cochet M., Kling G., Kourilsky P., Kärre K. Molecular analysis of H-2-deficient lymphoma lines. Distinct defects in biosynthesis and association of MHC class I heavy chains and beta 2-microglobulin observed in cells with increased sensitivity to NK cell lysis. J Immunol. 1989 Apr 15;142(8):2911–2917. [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis S. H., Mockridge I., Kelly A., Kerr L. A., Glynne R., Gileadi U., Beck S., Trowsdale J. Polymorphism in a second ABC transporter gene located within the class II region of the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1463–1467. doi: 10.1073/pnas.89.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis S. J., Townsend A. R., Deverson E. V., Bastin J., Butcher G. W., Howard J. C. Restoration of antigen presentation to the mutant cell line RMA-S by an MHC-linked transporter. Nature. 1991 Dec 19;354(6354):528–531. doi: 10.1038/354528a0. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gramm C., Benacerraf B. Low temperature and peptides favor the formation of class I heterodimers on RMA-S cells at the cell surface. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4200–4204. doi: 10.1073/pnas.88.10.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Cresswell P. Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. EMBO J. 1986 May;5(5):943–949. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Spies T., DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature. 1991 May 23;351(6324):323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- Sweetser M. T., Braciale V. L., Braciale T. J. Class I MHC-restricted recognition of cells expressing a gene encoding a 41 amino acid product of the influenza hemagglutinin. J Immunol. 1988 Nov 15;141(10):3324–3328. [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Wei M. L., Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992 Apr 2;356(6368):443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- Yang Y., Früh K., Chambers J., Waters J. B., Wu L., Spies T., Peterson P. A. Major histocompatibility complex (MHC)-encoded HAM2 is necessary for antigenic peptide loading onto class I MHC molecules. J Biol Chem. 1992 Jun 15;267(17):11669–11672. [PubMed] [Google Scholar]