Abstract
The p160 family of steroid receptor coactivators (SRCs) are pleiotropic transcription factor coactivators and “master regulators” of gene expression that promote cancer cell proliferation, survival, metabolism, migration, invasion, and metastasis. Cancers with high p160 SRC expression exhibit poor clinical outcomes and resistance to therapy, highlighting the SRCs as critical oncogenic drivers and, thus, therapeutic targets. microRNAs are important epigenetic regulators of protein expression. To examine the regulation of p160 SRCs by microRNAs, we used and combined 4 prediction algorithms to identify microRNAs that could target SRC1, SRC2, and SRC3 expression. For validation of these predictions, we assessed p160 SRC protein expression and cell viability after transfection of corresponding microRNA mimetics in breast cancer, uveal melanoma, and prostate cancer (PC) cell lines. Transfection of selected microRNA mimetics into breast cancer, uveal melanoma, and PC cells depleted SRC protein expression levels and exerted potent antiproliferative activity in these cell types. In particular, microRNA-137 (miR-137) depleted expression of SRC1, SRC2, and very potently, SRC3. The latter effect can be attributed to the presence of 3 miR-137 recognition sequences within the SRC3 3′-untranslated region. Using reverse phase protein array analysis, we identified a network of proteins, in addition to SRC3, that were modulated by miR-137 in PC cells. We also found that miR-137 and its host gene are epigenetically silenced in human cancer specimens and cell lines. These results support the development and testing of microRNA-based therapies (in particular based on restoring miR-137 levels) for targeting the oncogenic family of p160 SRCs in cancer.
The 3 steroid receptor coactivator (SRC) members of the p160 family: SRC1 (NCOA1), SRC2 (TIF2/GRIP1/NCOA2), and SRC3 (amplified in breast cancer [BC]1 [AIB1]/ACTR/NCOA3/pCIP/RAC3/TRAM1) are critical components of the transcriptional complexes of many nuclear receptors and other transcription factors (1–3). As a result, they are pleiotropic “master regulators” of steroid hormone receptor, including estrogen receptor (ER) and androgen receptor (AR), signaling and key drivers of cancer cell proliferation, survival, metabolism, metastasis, and resistance to therapy (3–23). Gene amplification, as well as overexpression at the mRNA and protein levels, have been reported for the p160 SRCs in numerous human malignancies, such as breast, prostate, endometrial, ovarian, lung, colon, esophageal, gastric and pancreatic carcinomas, and melanoma (2, 24–26). Notable examples include the observation that the SRC3/AIB1 gene is amplified in approximately 10% of BCs, leading to the name AIB1, and overexpressed at the mRNA level in more than 60% of primary BCs (24, 27); and the frequent gene amplification for SRC2 (NCOA2) in prostate cancer (PC) (11). This aberrant SRC overexpression is associated with poor clinical outcomes (2, 27), suggesting that targeting the SRC proteins represents an important and currently unused therapeutic opportunity in cancer. In experimental models, depletion of SRCs diminishes cell growth/proliferation through reduction of S phase in the cell cycle and suppresses key cancer pathways, including AKT/mTOR signaling and the antiapoptotic BCL2 protein (6, 7, 13, 14, 28).
Despite these critical roles of the p160 SRCs in cancer, they had previously received little attention as drug targets, because they had been considered “undruggable” due to the lack of a natural ligand-binding site that can be inhibited by small molecules. Recently, however, the natural compounds gossypol and bufalin were found to exert inhibitory effects on SRC1 and SRC3 (29, 30), suggesting that inhibition of at least some members of the family by small molecules may be feasible. However, due to their overlapping and complementary roles (31–33), it would be desirable to target all 3 p160 SRCs simultaneously. Because there is no clinically available modality to directly target the p160 SRCs for cancer treatment, there remains an unmet need for new therapeutic directions in this field.
microRNA are endogenous, small, nonprotein-coding, single-stranded RNAs of 17- to 22-nucleotide length (34). microRNAs are important epigenetic, posttranscriptional regulators of many normal cellular processes, including cell cycle control, cell proliferation, development, differentiation, and apoptosis. They control gene expression through imperfect pairing with target mRNAs of protein-coding genes, inducing direct mRNA degradation or translational repression (35–37). microRNAs can behave as potent oncogenes in the initiation and progression of cancer cells (38). In addition, microRNAs have also been demonstrated to act as tumor suppressors, serving a vital role in curbing the oncogenic potential of their target genes (36, 38). Advances in our understanding of the mechanisms of action of microRNAs and their regulation (or deregulation) in cancer cells has led to great interest in developing microRNAs and other noncoding RNAs as targeted therapies for treating cancer (35, 39). Using microRNAs to silence clinically relevant but otherwise undruggable oncogenes, such as the p160 SRCs, represents an innovative therapeutic strategy for treating a broad spectrum of cancers.
In the present study, we hypothesized that the protein expression of SRC1, SRC2, and SRC3 can be modulated by microRNAs and that mimetics of these microRNAs can serve as a therapeutic approach for cancer treatment. Towards this goal, we used and combined outputs from multiple computational algorithms to identify microRNAs predicted to bind to the 3′-untranslated region (UTR) of the p160 SRC genes. We then evaluated the molecular and cellular effects of transfecting mimetics of the microRNAs predicted to target SRC1, SRC2, and SRC3 into cancer cells. We identified several microRNAs that efficiently depleted the expression levels of SRCs. In particular, using SRC-dependent BC, PC, and uveal melanoma (UM) cell lines (29, 30, 33, 40), we found that microRNA-137 (miR-137) depleted the expression of SRC1, SRC2, and SRC3 and markedly inhibited proliferation and cell viability of a diverse group of human cancer cells. We also identified 3 putative miR-137 binding sites in the SRC3 3′-UTR as responsible for the capacity of miR-137 to potently deplete SRC3 expression. Using publicly available datasets from human BC and PC cell lines as well as human BC and PC patient specimens, we found that the miR-137 gene locus is epigenetically repressed in BC and PC cells vs normal cells and represents an interesting potential candidate for epigenetic targeted therapies focused on reexpressing silenced tumor suppressor genes.
Materials and Methods
Bioinformatics predictions of microRNAs targeting the p160 SRCs
An online search of Dianalab (41, 42), miRanda (43, 44), TargetScan (45–48), and PicTar (49, 50) was performed to predict microRNAs that can target the 3′-UTRs of SRC1, SRC2, and SRC3 genes. The predictions from each program were compared and rank-ordered to determine the strength of the prediction and to assist in the selection of microRNAs for further validation by in vitro cellular analyses. Because each algorithm uses different criteria to predict the strength of the binding and the potential effect of the microRNAs on the target mRNA sequence, we determined the microRNAs predicted to target the p160 SRC genes with a consensus (high) score across algorithms. To implement this consensus prediction evaluation, we converted each microRNA/mRNA binding prediction score into a rank, thus generating a ranked list of microRNAs for each algorithm. The individually ranked microRNA lists produced by each algorithm were then uniformly processed by assigning a normalized score to each microRNA based on its rank. For a particular microRNA list with ranks ranging from 1 to n, the score assigned to the i-th rank microRNA was (n+1-i)/n. Any microRNA that was not included in the list was given a score of zero. We computed a combined prediction score for a target gene by averaging the rank-normalized scores of all the prediction algorithms. From the combination of these algorithms, we identified and prioritized microRNAs predicted to target the p160 SRCs.
Reagents and antibodies
Mimetics for the individual microRNAs and nontarget (NT) control used in these studies were purchased from Life Technologies. Cells were transfected with RNAi Max transfection reagent (Life Technologies) according to the manufacturer's protocol. Anti-SRC1 and anti-SRC3 antibodies were obtained from Cell Signaling Technologies. Anti-SRC2 antibody was obtained from Bethyl Labs. Anti-β-actin antibody was obtained from Sigma-Aldrich.
Cell lines and cell culture
ER-positive MCF7, T47D, BT474, tamoxifen-resistant (TamR), and estrogen deprivation-resistant (EDR) MCF7 BC cells, as well as MCF10A and MCF12 breast cells were obtained from American Type Culture Collection via the Tissue and Cell Culture Core Laboratory at Baylor College of Medicine, where they are regularly submitted for cell line authentication and mycoplasma testing, and passaged for fewer than 6 months. Human PC cell lines LNCaP (expresses AR that harbors the T877A point mutation in its ligand-binding domain), VCaP (harbors amplification of the AR gene), 22Rv1 (AR-positive, including constitutively active variant AR), DU145 (AR-negative), and PC3 (AR-negative) were obtained from American Type Culture Collection or the Baylor College of Medicine Tissue and Cell Culture Core facility, cultured in the appropriate media and passaged as previously described (33). Prostate epithelial cells (PrECs) and human mammary epithelial cells (HMECs) were obtained from Lonza, Inc. Human embryonic kidney 293T cells were cultured in DMEM high glucose (Invitrogen) with 10% fetal bovine serum (FBS) (Invitrogen) in a 5% CO2 incubator at 37°C. The genotype of the OMM1.3, Mel202, and 92.1 (GNAQmt, GNA11wt, and BRAFwt) and the OMM1 (GNA11mt, GNAQwt, and BRAFwt) UM cell lines has been previously reported (51, 52). The GNAQwt/GNA11wt and BRAFV600E melanoma cell lines OCM1 and OCM3 were also used in these studies (51, 53). All cell lines used were confirmed by Sanger sequencing to harbor the genotypes described above and were passaged for less than 6 months afterwards. Logarithmically growing cells were used in all experiments described below.
Transfection of microRNA mimetics and silencer RNA (siRNA) against SRC2 and SRC3 into cancer cells
Cells were plated in 6-well plates (for immunoblot analyses) or 24-well plates (for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide [MTT] assay) in medium containing 10% FBS and allowed to adhere for 24 hours. The next day, the cells were transfected with 30nM each of the microRNA mimetics (miRvana; Life Technologies) or the NT microRNA (miR-NT) control or 50nM Silencer RNAi siRNA Duplex oligoribonucleotides for SRC2 and/or SRC3 (2 siRNAs per target protein) (Life Technologies) or the corresponding NT siRNA (si-NT) control using Lipofectamine RNAi MAX (Life Technologies) according to the instructions provided by the manufacturer. The microRNA mimetics, SRC2 and SRC3 siRNAs, and Lipofectamine solutions were prepared in Opti-MEM I Reduced Serum Medium (Invitrogen), mixed gently, and incubated 5–10 minutes at room temperature. The lipid-complexed microRNA mimetic was drop wise added to the 6- or 24-well plates and incubated for 24–96 hours as indicated in each experiment.
MTT assay
Cells were plated in 24-well plates in medium containing 10% FBS and allowed to adhere for 24 hours. Then, microRNA mimetics or NT control were transfected, and the cells were incubated for 96 more hours. Cell viability was quantified by MTT (obtained from Sigma-Aldrich) and expressed as a percentage of the miR-NT-transfected or si-NT-transfected wells. All experiments were repeated at least twice, with each experimental condition repeated at least in triplicate per experiment.
SDS-PAGE and immunoblot analysis
Total cell lysates were prepared from cells transfected with microRNA mimetics using cell lysis buffer (150mM NaCl, 0.5% NP-40, 50mM Tris-HCl [pH 7.5]) containing a cocktail of Protease and phosphatase inhibitors (1.0mM Na3VO4, 1.0mM dithiothreitol, 1.0mM phenylmethanesulfonyl fluoride, and 50mM NaF). Alternatively, radioimmunoprecipitation assay buffer was used with protease and phosphatase inhibitors. Total cell lysates from cells transfected with NT or specific microRNA mimetics were quantified with a BCA protein assay kit (Pierce). Total cell lysates were separated by SDS-PAGE, and immunoblot analyses were conducted with specific antisera or monoclonal antibodies. Signals were visualized after incubation with secondary mouse or rabbit antibody (1:5000) conjugated to horseradish peroxidase, and the signal was developed using Pierce enhanced chemiluminescence Western blotting substrate (Thermo Fisher Scientific). Immunoblots were performed at least twice, and representative immunoblots are shown.
Analysis of the proteomic footprint of miR-137 in PC cells via reverse phase protein array (RPPA)
LNCaP PC cells were transfected with microRNA mimics for 24 hours, respectively, and assayed by RPPA using a panel of 157 validated antibodies (set 062012–0055, 41 against phosphorylated epitopes, 112 against total proteins, and 4 against cleaved epitopes) with the help of the Functional Proteomics/RPPA Core Facility (The University of Texas M.D. Anderson Cancer Center, Houston, TX; http://www.mdanderson.org/education-and-research/resources-for-professionals/scientific-resources/core-facilities-and-services/functional-proteomics-rppa-core/rppa-process/index.html) and according to standardized protocols (54–57). Antibodies had been previously tested to detect the specific protein by immunoblot analysis by the RPPA Core before being considered validated for use on the RPPA. Protein expression was quantified by MicroVigene and normalized using the R package SuperCurve (58). We determined a proteomics signature of the microRNAs using the Student's t test (P < .05 was considered significant) and fold change exceeding 1.25×. Pathway analysis of corresponding genes for recurrently changed antibodies was performed using the ConsensusPathDB (59) software package. Hierarchical clustering and statistical analysis was performed using the R statistical analysis system. Network analysis for miR-137 altered proteins was performed with Netwalker software (60).
RNA isolation and reverse transcription
Cells were harvested into TRIzol and frozen at −80°C or immediately processed. RNA was isolated by the addition of chloroform to the TRIzol lysate, vigorously shaking the tube and centrifuging the tubes at 13 000 rpm (16 100g) for 3 minutes to separate the layers. The top, aqueous layer was pipetted into a clean 1.5-mL tube, and 1 mL of 100% isopropanol was added to precipitate the RNA. Tubes were centrifuged at 13 000 rpm for 10 minutes at 4°C. The isopropanol was decanted and the RNA was washed with 70% molecular grade ethanol for 5 minutes. The ethanol was decanted and the RNA was air dried. Ribonuclease-free water was used to resuspend the RNA pellet. The RNA was quantified and reverse transcribed using a high capacity reverse transcription kit (Life Technologies) and random hexamers according to the manufacturer's protocol. The resulting cDNA was used for quantitative RT-PCR analysis of KLK3/PSA gene expression in PC and GREB1 and TFF1 in BC cells, performed with SYBR Green PCR master mix and gene-specific primers using a StepOne Plus Real-Time PCR system.
Cloning and mutation of the SRC3 3′-UTR
Bioinformatics analysis with the 4 prediction algorithms above revealed that the 3′-UTR of SRC3 contain 3 putative miR-137 recognition sequences (MRSs). To analyze the effects of miR-137 directly on the SRC3 3′-UTR, we first amplified by PCR the 3′-UTR region from a human PC cell line (LNCaP). The SRC3 3′-UTR was amplified using 200nM each oligo (forward and reverse) (Sigma-Aldrich) (Supplemental Table 1). PCR was carried out using Phusion polymerase (New England BioLabs) after the cycling conditions recommended by the manufacturer using 2.5 minutes of extension time for 35 cycles. The resulting PCR product was digested with AsiSI/SpeI overnight at 37°C, then gel purified and ligated to pEZX-MT01 (a dual firefly/Renilla luciferase reporter mammalian expression vector; GeneCopoeia) also digested with AsiSI and SpeI. Escherichia coli cells were transformed and kanamycin (25 μg/mL) was used for the bacterial clonal selection. The vector pEZX-MT01 is a dual luciferase-expressing system (Supplemental Figure 1A), where we inserted the SRC3 3′-UTR sequence downstream of the firefly luciferase reporter gene. A chimeric mRNA is transcribed consisting of the firefly luciferase and the 3′-UTR target sequence and serves as a target to study the regulatory effects of microRNA. A tracking reporter gene, Renilla luciferase, is also present in the vector and serves as internal control of successful transfection and expression in cells. The dual-reporter vector system enables transfection-normalization for accurate across-sample comparison, as detailed on the manufacturer's web site.
The SRC3 3′-UTR was subsequently mutated by deleting the sequence for each one of the 3 MRSs (Supplemental Figure 1B). PCRs were carried out with oligos in Supplemental Table 1. The deletion of the first miR-137 MRS (MRS1), located at nucleotides 869–876 (agcaataa) within the SRC3 3′-UTR; the second miR-137 MRS (MRS2) located at nucleotide position 1933–1940 (agcaataa); and the third miR-137 MRS (MRS3) located at nucleotide position 3245–3251 (agcaata) was generated independently with a Site-Directed Mutagenesis kit (Takara Biologicals), to yield the corresponding single-deletion plasmids. Additionally, to generate a triple-deletion mutant plasmid, we used the MRS1-deleted SRC3 3′-UTR construct as a template and performed sequentially deletions in the MRS2, followed by deletion of the MRS3 as above. After each step, the plasmids were sequenced to confirm the sequence and orientation of inserts. The resultant PCR products were treated with 10–20 U of Dpn1 enzyme (New England BioLabs) at 37°C for 4 hours. After DpnI digestion, the PCR product was purified using a QIAGEN PCR purification kit. Next, 5 μL of each purified pEZX-MT01 SRC3 3′-UTR (mutated) plasmid were transformed to chemically competent DH5α E. coli, and clones were selected on LB plates with 25 μg/mL of kanamycin.
Luciferase reporter assay
HEK293T cells were seeded in a 6-well plate with regular growth medium without antibiotics for 24 hours before transfection. The next day, cells were cotransfected with 1.0 μg of reporter plasmid containing the wild-type SRC3 3′-UTR or the SRC3 3′-UTR containing mutations in the miR-137 MRS site and 30nM miR-137 mimetic or its NT mimetic using Lipofectamine 2000 (Life Technologies). Twenty-four hours after transfection, cell lysates were collected and luciferase activity was measured by Dual-Luciferase Reporter System (Promega) using a Biotek microplate reader. The intensity of firefly luciferase expression was normalized to that of Renilla luciferase and is expressed relative to the miR-NT-transfected cells.
Analysis of miR-137 expression in human tissues
To evaluate expression of the miR-137 microRNA in normal tissues, we used small-RNA-Seq data collected as part of the NIH Epigenome Roadmap (61, 62). The Illumina adapters were removed from the reads, and tags of length 10–30 nucleotides were selected for further analysis. The reads were mapped to the reference human genome build University of California, Santa Cruz (UCSC) hg19/NCBI 37 using PASH 3.0 (63), allowing up to 1000 matches per read. microRNA expression was evaluated using the miRNA database miRBase (64, 65); for sequencing reads with multiple mappings, the coverage was split equally among all the genome mapping locations. microRNAs expression was reported normalized to 1 million mapped reads; quantile normalization was applied across all the microRNA profiles using the R statistical system. We selected for visualization microRNAs with at least 50 reads per million mapped reads; visualizations were generated using the GraphPad statistical analysis system.
Analysis of H3K4me3 and H3K27me3 epigenetic marks in prostate and breast cells
We analyzed publicly available chromatin immunoprecipitation and sequencing (ChIP-Seq) data of H3K4me3 and H3K27me3 in PrEC and LNCaP (GSE38685) (66) prostate cells, in HMEC (61, 62) and MCF7 (GSE23701) (67, 68) breast cells. Sequenced reads were mapped to the human genome UCSC build hg19/NCBI build 37 using BWA (69). High-resolution genome-wide maps were derived and visualized in the UCSC Genome Browser (http://genome.ucsc.edu/) and using the IGV software (70, 71). Read coverage at selected genomic loci was computed using BEDTools (72). To determine enrichment of a signal between PrEC and LNCaP, or between HMEC and MCF7, at specific genomic loci, we first used a 100-kbp window around the locus of interest to establish the background signal, then we compared the ChIP-Seq signal by Fisher's exact test using the SciPy (73) Python library.
Analysis of DNA methylation in human PC and BC specimens
We analyzed DNA methylation differences at the level of individual CpG probes between 4 normal prostate samples and 8 metastatic PC samples assayed using the Illumina Infinium Human Methylation 450K BeadChip kit and reported in the GSE38240 dataset (74). Statistical significance was assessed using the Student's t test (P < .05) implemented in the R statistical system. We also analyzed DNA methylation data from 285 breast tissue samples (representing progression from ductal carcinoma in situ [DCIS] to invasive cancer), which were assayed using an Illumina Infinium Human Methylation 450K BeadChip kit and reported in the GSE60185 dataset (75). The dataset includes 46 normal breast biopsy samples, 22 samples from patients with DCIS, 31 samples from patients with mixed lesions containing both DCIS and invasive breast carcinoma, and 186 samples from patients with invasive BC.
Statistical analysis
Differences between cells transfected with the microRNA mimetics vs the NT control (miR-NT) were calculated using Student's t test. P < .05 was considered significant.
Results
Prediction of microRNAs targeting p160 SRCs
Accurate prediction of miRNA to mRNA binding is still an evolving science. To mitigate this, we used 4 computational algorithms in an integrative fashion and combined their outputs to predict microRNAs that could target the SRC1, SRC2, and SRC3 genes, respectively. Supplemental Tables 2–4 list microRNAs predicted to target SRC1, SRC2, and SRC3, respectively. The rank normalized scores were generated from averaging the score across the 4 prediction algorithms. To determine microRNAs that can target multiple coactivator genes, we averaged the individual rank-normalized prediction scores for SRC1, SRC2, and SRC3 and obtained a global prediction score for the p160 SRCs. From this analysis, miR-137 was predicted to target all 3 p160 SRCs. We used a rank-normalized prediction score of at least 0.5 across all algorithms as the main criterion for selecting a microRNA for further evaluation studies.
miR-137 depletes p160 SRC proteins in BC cells
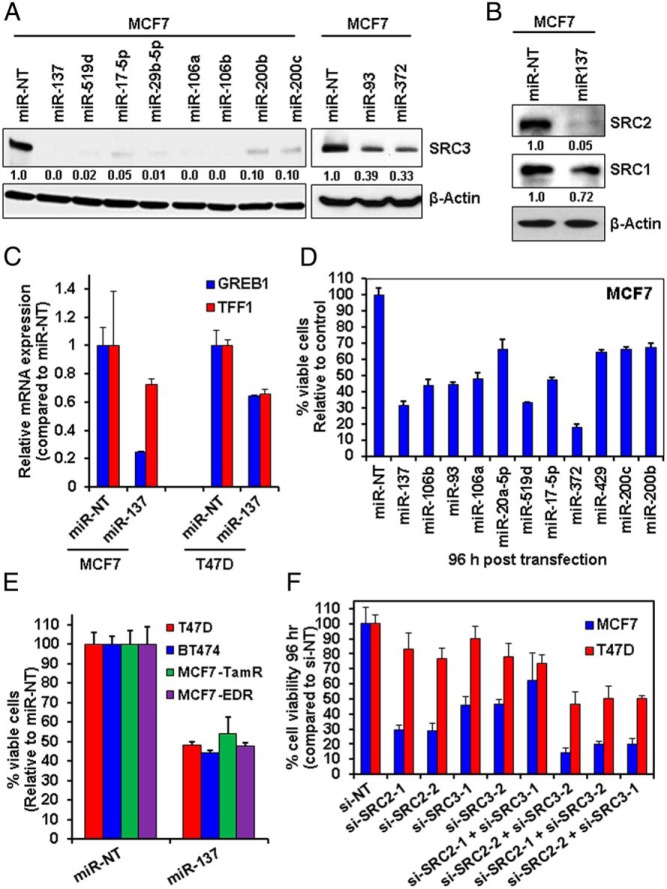
We used mimetics corresponding to the microRNAs with high prediction scores, to examine their effects on the protein expression of SRC1, SRC2, and SRC3 in MCF7 BC cells. As shown in Figure 1A, transfection of MCF7 cells with mimetics for miR-137, miR-519d, miR-17–5p, miR-29b-5p, miR-106a, miR-106b, miR-200b, miR-200c, miR-93, and miR-372 resulted in substantial depletion of SRC3 protein at 24 hours after transfection. Based on our algorithm results that miR-137 was also predicted to target the other 2 p160 SRC family members, we also examined the effects of the miR-137 mimetic on the expression of SRC1 and SRC2 in BC cells. Figure 1B shows that transfection of the miR-137 mimetic in MCF7 cells depleted the expression of SRC2 and (to a lesser degree) SRC1.
Figure 1.
microRNAs targeting p160 SRC3 exert significant antiproliferative activity against BC cells. A, MCF7 cells were transfected for 24 hours with microRNA mimetics predicted to target SRC3, then harvested, and total cell lysates were prepared. Immunoblot analyses were conducted for p160 SRC3 and β-actin in the lysates. The numbers beneath the bands represent densitometry analysis and are normalized to β-actin expression. B, MCF7 cells were transfected with microRNA mimetics as in A, then harvested, and total cell lysates were prepared. Immunoblot analyses were conducted for SRC1, SRC2, and β-actin in the cell lysates. The numbers beneath the bands represent densitometry analysis and are normalized to β-actin expression. C, MCF7 and T47D cells were transfected with miR-NT or miR-137 for 48 hours. At the end of treatment, total RNA was harvested and reverse transcribed. The resulting cDNA was used for qPCR of ER-target genes GREB1 and TFF1 (PS2). Relative expression of each mRNA was normalized to the expression of β-actin in the cDNA. D, MCF7 cells were transfected for 96 hours with microRNA mimetics or SRC3 siRNA or respective NT controls, followed by MTT assay. Values are reported as viable cells (% of control transfected cells) ± SD. E, T47D, BT474, MCF7-TamR, and MDF7-EDR cells were transfected with miR-NT or miR-137 mimetic for 96 hours, and MTT was performed as in C. Values are reported as viable cells (% of control, miR-NT-transfected cells) ± SD. F, MCF7 and T47D cells were transfected with si-NT, or 2 different siRNAs for SRC2 (1 and 2) and SRC3 (1 and 2) along with combinations of the siRNAs for 96 hours. At the end of treatment, MTT assay was performed. The percentage of viable cells in each transfected condition was compared with its own si-NT control.
We also examined the impact of other microRNAs with high prediction scores for targeting SRC2 and SRC1. In the case of SRC2, reexpression of miR-590–5p, miR-21, and miR-200c (as well as miR-137) resulted in substantial repression of SRC2 expression in MCF7 cells (whereas miR-429 did not cause depletion) (Supplemental Figure 2A). Thus, for both SRC3 and SRC2, our highest ranked bioinformatics predictions were experimentally validated. However, transfection of MCF7 cells with the top predicted microRNAs for SRC1 (miR219–5p and miR129–5p) did not result in depletion of SRC1 protein (Supplemental Figure 2A), a finding that agrees with the overall weak prediction scores obtained for targeting SRC1 compared with the other 2 p160 SRCs. Thus, our bioinformatics predictions and experimental validation identified miR-137 as a microRNA that can target p160 SRCs in BC cells.
miR-137 suppresses ER signaling and exerts antiproliferative effects on BC cells, including those resistant to hormonal therapy
We next examined whether miR-137 can inhibit the coactivator function of p160 SRCs on ER signaling. In particular, SRC3 cooperates with ER to mediate transcription of ER-target genes (23). Consequently, silencing SRC3 in BC cells attenuates the expression of ER-target genes, such as GREB1 and TFF1 (23). Figure 1C shows that after transfection of miR-137 mimetic, the expression of GREB1 and TFF1 is lowered in ER-positive MCF7 and T47D cells, suggesting that miR-137 suppresses ER signaling.
We next determined the effects of the microRNA mimetics on the viability of the ER-positive, SRC3-dependent MCF7 cells. Transfection of select, highly ranked SRC3-targeting microRNA mimetics exerted antiproliferative activity on MCF7 cells (Figure 1D). From these results, we elected to further study the effects of the miR-137 mimetic in a larger cohort of BC cells. ER-positive T47D, BT474, TamR, and EDR MCF7 cells were transfected with miR-137 mimetic and the miR-NT control. As shown in Figure 1E, transfection of miR-137 exerted significant antiproliferative activity (P < .05) in all the cell lines used. To determine the contribution of SRC2 and SRC3 to the loss of proliferation in the BC cells, we next determined the effects of depleting SRC2 and SRC3 alone and simultaneously in BC cells. Cells were transfected with 2 different siRNAs for SRC2 and SRC3, as well as combinations of the 2 siRNAs together. Figure 1F shows that in 3 out of the 4 combinations, silencing of SRC2 and SRC3 produces similar effects on proliferation/cell viability as the transfection of miR-137 in the BC cells.
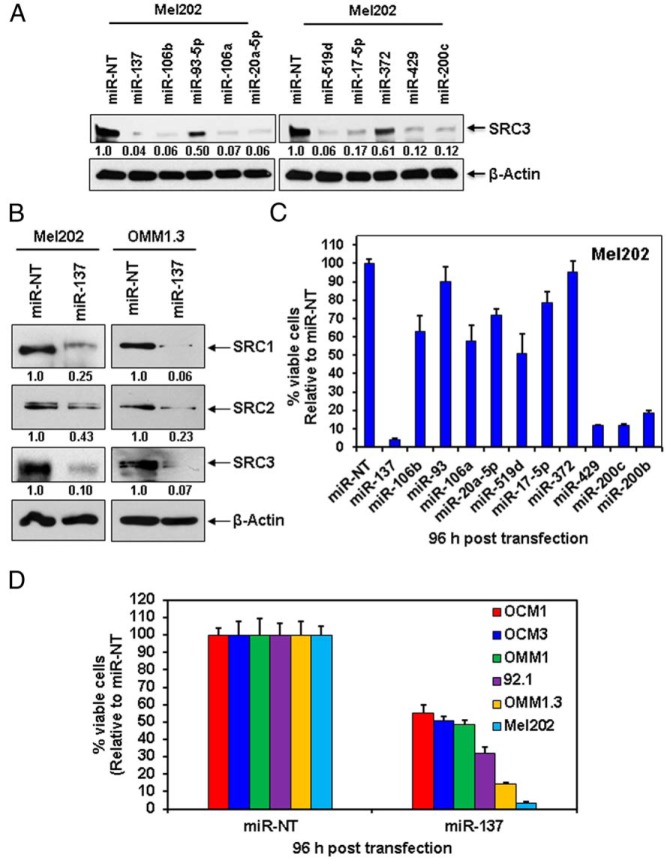
Transfection with microRNA mimetics depletes p160 SRC proteins and exerts antiproliferative activity against UM cells
We next determined the effects of mimetics corresponding to the microRNAs predicted to target SRC3 on the expression of SRC3 in Mel202 UM cells. Transfection of Mel202 cells with mimetics of miR-137, miR-106b, miR-106a, miR-20a-5p, miR-519d, miR-17–5p, miR-429, and miR-200c depleted SRC3 levels at 24 hours after transfection (Figure 2A). miR-93–5p and miR-372 had less pronounced but still substantial suppressive effects, a finding that is similar to our results in MCF7 cells. In agreement with our results above for BC cells, transfection of miR-137 into Mel202 and another UM cell line, OMM1.3, markedly reduced expression of the 3 p160 SRCs in both cell lines (Figure 2B). We next determined the effects of the SRC3-targeting microRNA mimetics on the SRC3-dependent Mel202 cells via MTT assay. Figure 2C shows that transfection of most microRNAs inhibited proliferation of Mel202 cells (to varying degrees), with the exception of miR-93 and miR-372 (in agreement with our previous finding that they exerted weaker effect on SRC3 protein levels). Supported by these data, we next expanded our study to a larger cohort of GNAQmt UM (92.1 and OMM1.3, in addition to the Mel202), GNA11mt UM (OMM1) and BRAFV600E melanoma (OCM1 and OCM3, which are wild type for GNAQ and GNA11) cells and tested the effects of miR-137 mimetic on cell viability. Figure 2D shows that although the GNAQmt melanoma cells were the most sensitive to the antiproliferative activity of miR-137 reexpression, the BRAFV600E and GNA11mt melanoma cells also exhibited approximately 50% loss of cell viability after 96 hours of exposure.
Figure 2.
miR-137 depletes the p160 SRCs and exerts significant antiproliferative activity against melanoma cells. A, Mel202 cells were transfected for 24 hours with microRNA mimetics against miR-137 and other microRNAs highly predicted to target SRC3, then harvested, and cell lysates were prepared. Immunoblot analyses were conducted for SRC3 and β-actin in the cell lysates. The numbers beneath the bands represent densitometry analysis and are normalized to β-actin expression. B, Mel202 and OMM1.3 cells were transfected for 24 hours with miR-137 mimetic, harvested, and total cell lysates were prepared. Immunoblot analyses were conducted for the expression levels of SRC1, SRC2, SRC3, and β-actin in the cell lysates. The numbers beneath the bands represent densitometry analysis and are normalized to β-actin expression. C, Mel202 cells were transfected for 96 hours with the indicated microRNA mimetics. Then, MTT assays were performed. Values are reported as viable cells (% of control, miR-NT-transfected cells) ± SD. D, BRAF-mutant melanoma (OCM1 and OCM3), GNA11-mutant (OMM1), and GNAQ-mutant (92.1, OMM1.3, and Mel202) UM cells were transfected for 96 hours with miR-137 mimetic or miR-NT control, followed by MTT assay. Values are reported as viable cells (% of control, miR-NT-transfected cells) ± SD.
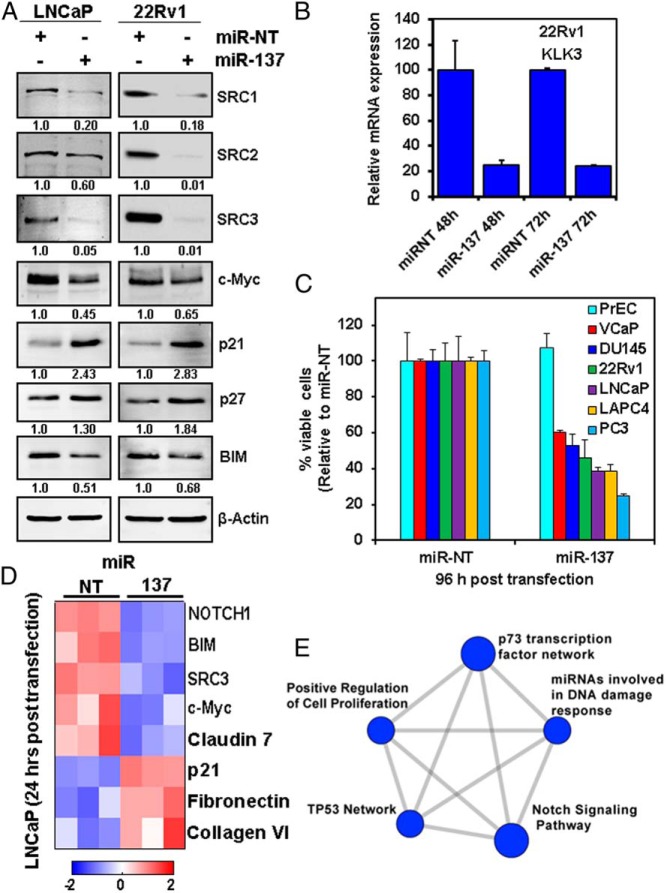
miR-137 depletes p160 SRC proteins and exerts antiproliferative activity against PC cells
In agreement with our observations in other tumor models, miR-137 significantly depleted the expression of the 3 p160 SRCs in the PC LNCaP and 22Rv1 cells (Figure 3A). Because the p160 SRCs are known to cooperate with AR to drive AR-dependent gene transcription (33), we next examined whether miR-137 can inhibit the coactivator function of SRC1, SRC2, and SRC3 on AR signaling. Specifically, because the p160 SRCs bind to the promoter/enhancer region of the AR-target gene KLK3 (PSA) gene in PC cells to drive transcription (33), we quantified the impact of miR-137 on the expression of KLK3. 22Rv1 cells transfected with miR-137 mimetic for 48 and 72 hours displayed a dramatic decrease in the expression of the AR-target gene KLK3 (Figure 3B), indicating inhibition of AR transcriptional activity. Transfection of miR-137 was also associated with marked inhibition of cell viability in a broad array of PC cells lines (Figure 3C) but not in normal PrEC cells.
Figure 3.
miR-137 depletes p160 SRCs and exerts significant antiproliferative activity against PC cells. A, PC LNCaP and 22Rv1 cells were transfected for 24 hours with miR-NT or miR-137 mimetic, then harvested, and total cell lysates were prepared. Immunoblot analyses were conducted for SRC1, SRC2, SRC3, and β-actin in the lysates. The numbers beneath the bands represent densitometry analysis and are normalized to β-actin expression in the cell lysates. B, 22Rv1 cells were transfected with miR-NT or miR-137 mimetic for 48 or 72 hours. At the end of exposure, total RNA was harvested from the cells, and q-RT-PCR was performed for KLK3 mRNA. Values were normalized to the expression of β-actin and expressed as a % of the value of the miR-NT-transfected cells of the corresponding time point. The expression of the AR-target gene KLK3 (PSA) was significantly depleted by miR-137, suggesting inhibition of AR axis activity. C, PrEC, VCaP, DU145, 22Rv1, LNCaP, LAPC4, and PC3 cells were transfected with miR-NT or miR-137 mimetic for 96 hours, followed by MTT assay. Values are reported as viable cells (% of control, miR-NT-transfected cells) ± SD. D, LNCaP cells were transfected with miR-NT and miR-137 mimetic for 24 hours in triplicate wells. At the end of treatment, cells were harvested, lysed, and RPPA assay was performed on the lysates. Shown are the proteins that exhibited a greater than or equal to 1.25-fold change in cells transfected with miR-137 mimetic compared with those transfected with miR-NT. SRC3 and c-Myc were the most down-regulated targets in the RPPA dataset. E, Pathway analysis of the RPPA results indicates that many of the targets altered by miR-137 reexpression are involved in Notch signaling, response to DNA damage, p53 pathway, and regulation of cell proliferation.
We next used RPPA to define a wider proteomic impact of miR-137 in LNCaP PC cells. Cells were transfected with miR-NT or miR-137 for 24 hours, then harvested, and lysed for RPPA analysis. Transfection of miR-137 depleted SRC3 as well as c-Myc and BIM protein expression, whereas it induced the cyclin-dependent kinase inhibitor CDKN1A (p21) and p27, which are known to induce/mediate cell cycle G1 phase growth arrest and cellular senescence (Figure 3, A and D). To further characterize the effects of miR-137, for each protein/target, we set a 25% increase or decrease as the threshold for a significant change and then performed pathway enrichment analysis using the ConsensusPathDB software. Figure 3E details the effects on p73 transcription factor network, positive regulation of cell proliferation, Notch signaling, and p53 signaling. In addition, we determined the functional network of the proteins whose expression was altered by miR-137 in LNCaP cells. Supplemental Figure 3 shows that SRC3 (NCOA3), NOTCH, and MYC are central mediators in the network of proteins affected by miR-137 in PC cells.
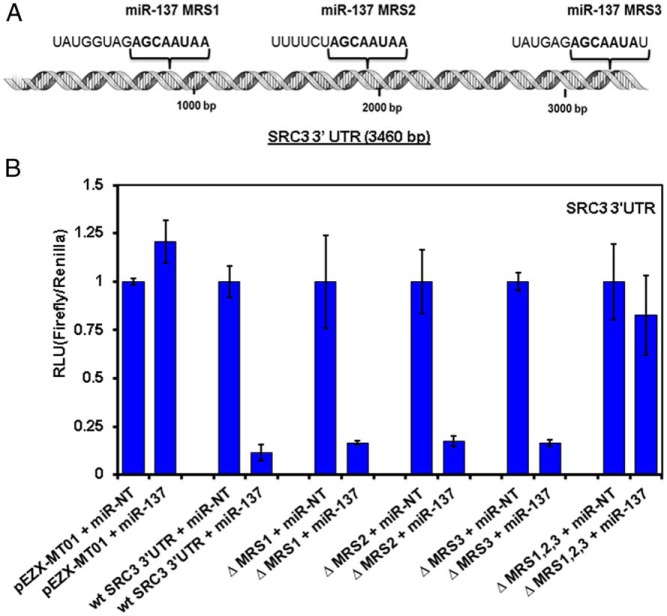
miR-137 down-regulates the SRC3 gene through interaction with its 3′-UTR
Bioinformatics analyses using Target Scan and DianaLab predicted that SRC3 was a potential target of miR-137 through putative microRNA recognition sequences (MRSs) in the SRC3 3′-UTR. Figure 4A shows a schematic of the SRC3 3′-UTR with the 3 predicted miR-137 MRSs, each containing 7–8 base pairs of complementary binding to the SRC3 3′-UTR sequence. We cloned the wild-type SRC3 3′-UTR into the pEZX-MT01 vector, and we then deleted each of the 3 putative miR-137 MRSs in the SRC3′-UTR clone individually as well as all 3 in 1 construct. Next, the empty pEZX-MT01 vector, the wild-type SRC3 3′-UTR construct, and the MRS-deleted constructs were transfected into 293T cells, together with miR-NT or miR-137 mimetic for 24 hours and then analyzed for reporter activity. As shown in Figure 4B, miR-137, but not the miR-NT, markedly suppressed the reporter activity of the wild-type SRC3 3′-UTR construct, proving that miR-137 can regulate SRC3 expression via its 3′-UTR. Deletion of any single MRS failed to attenuate this effect. However, no suppression in reporter activity was observed in cells transfected with the empty vector (no SRC3 3′-UTR insert) or in cells transfected with the SRC3 3′-UTR construct in which all 3 MRSs had been deleted (Figure 4C). These data support the presence of 3 functional MRSs within the SRC3 3′-UTR that can bind miR-137 and explain why miR-137 can so potently deplete SRC3 expression in all experimental models that we have tested.
Figure 4.
miR-137 directly binds to the SRC3 3′-UTR. A, Schematic model of the SRC3 3′-UTR (3460 bp), indicating the position of the 3 MRSs. B, HEK293 cells were cotransfected with the pEZX-MT01 dual luciferase-expressing vector carrying the SRC3 3′-UTR with no MRS mutations, mutations in 1 of the 3 individual MRSs (MRS1, MRS2, or MRS3) or simultaneous mutation in all 3 MRSs, as well as with miR-137 or NT mimetic. Twenty-four hours after transfection, cells were harvested, and luciferase activity assays were performed and analyzed using a plate reader. The intensity of firefly luciferase expression was normalized to that of Renilla luciferase and expressed relative to the miR-NT-transfected cells. Mutation of any single MRS was not sufficient to inhibit the activity of miR-137 for the 3′-UTR of SRC3. However, the impact of miR-137 was significantly abrogated by mutating all 3 MRSs.
miR-137 is epigenetically silenced in PC and BC cells
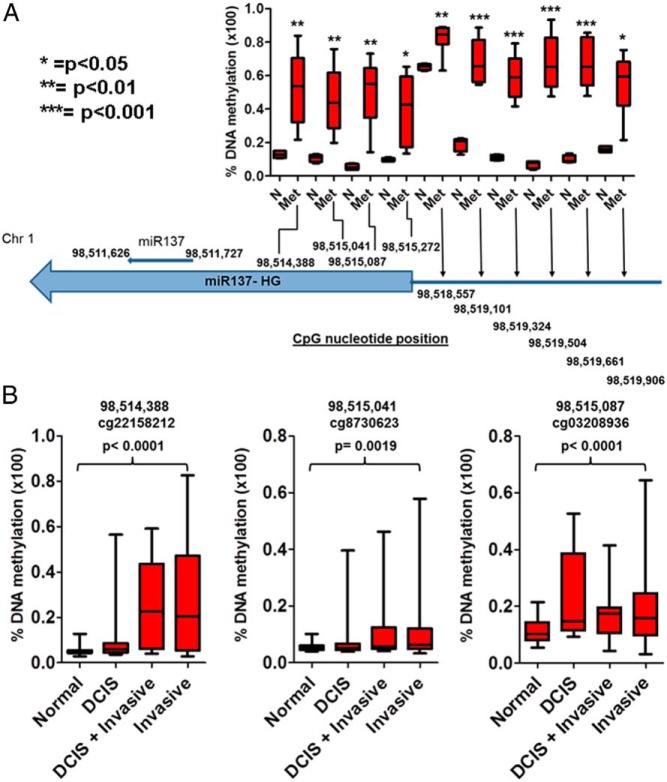
Our data indicate that miR-137 exerts potent tumor suppressor properties and is particularly toxic to cancer cells from many tissues. However, miR-137 was detected in multiple normal human tissues, including brain, HMECs, and fibroblasts (Supplemental Figure 4A). We next determined the miR-137 expression levels in normal breast and normal prostate cells compared with breast and PC cells. Supplemental Figure 4, B and C, shows that although the expression of miR-137 is high in HMEC and PrEC cells, the miR-137 expression levels are very low to nearly undetectable in the breast and PC cells. We next examined the regulation of miR-137 expression in PC and BC cells. We employed ChIP-Seq data for the distribution of the active transcription mark H3K4Me3 and the transcription repression mark H3K27Me3 (GSE38685) in PrEC cells and in the PC LNCaP cells. We observed that the sequence tag density of H3K4Me3 at the miR-137 host gene locus (MIR137HG) is significantly reduced in LNCaP cells compared with PrEC cells (Fisher's exact test, P = 1.4485 × 10−11). Conversely, the signal tag density for H3K27Me3 was significantly increased at the same locus in LNCaP compared with PrEC cells (Fisher's exact test, P = .0034) (Supplemental Figure 5, A and B). Similar observations were also noted in MCF7 BC cells compared with HMEC cells at the MIR137HG locus: specifically, the density of H3K4Me3 signal tags is significantly reduced (Fisher's exact test, P = 9.9411 × 10−31), whereas signal tag density for H3K27Me3 is significantly increased in the MCF7 cells compared with HMEC cells (Fisher's exact test, P = .0198) (Supplemental Figure 6, A and B). Next, we examined DNA methylation differences between normal prostate tissue and metastatic PC specimens at the MIR137HG locus and upstream promoter region. We observed that the genomic loci corresponding to miR-137 and its host gene is significantly hypermethylated in the metastatic PC samples compared with the normal prostate specimens (t test, P < .05) (Figure 5A). We also examined DNA methylation differences between normal breast, DCIS, and invasive BC specimens. We chose the 3 CpG loci closest to the miR-137 gene for further analysis in this dataset. We observed that in the 3 loci upstream of MIR137HG, there was a significant increase in DNA methylation between normal and invasive BC (P < .01) (Figure 5B). All these findings suggest epigenetic repression of miR-137 expression in PC and BC compared with respective normal tissues.
Figure 5.
DNA CpG methylation profiles of the miR-137 gene locus in human PC and BC specimens. A, Analysis of DNA methylation within the promoter and gene body of the miR-137 host gene (the microRNA gene locus) in metastatic PC samples compared with normal prostate samples in the GSE38240 dataset, which contains 4 normal (N) and 8 metastatic PC (Met) patient samples assayed with the Illumina Infinium Human Methylation 450K BeadChip kit. Our results include 10 CpG clusters. DNA methylation levels are significantly increased (P < .05) in metastatic PC compared with normal prostate tissue. B, Analysis of DNA methylation within the miR-137 host gene (the microRNA gene locus) in breast tissue samples representing progression of cancer from DCIS to BC and compared with normal breast tissue samples in the GSE60185 dataset, which includes 46 normal breast biopsy samples, 22 samples from patients with DCIS, 31 samples from patients with mixed lesions (containing both DCIS and invasive BC), and 186 samples from patients with invasive BC assayed with the Illumina Infinium Human Methylation 450K BeadChip kit. DNA methylation levels within the miR-137 host gene locus are significantly higher (P < .05) in BC compared with normal breast tissue.
Discussion
The p160 SRCs mediate the transcriptional functions of nuclear receptors and other transcription factors. They are overexpressed in a broad spectrum of cancers, including those of the breast and prostate, and play crucial roles in steroid hormone signaling, cell proliferation, metastasis, disease progression, and resistance to chemotherapy, supporting their roles as important oncogenes (2, 7). In addition to gene amplifications reported in a subset of cancers (11, 24), overexpression at the mRNA and protein levels is much more widely present (2, 11, 24, 76–78), suggesting that additional mechanisms are responsible for this overexpression. Several posttranslational mechanisms have been reported to control the turnover of p160 SRC proteins and may be dysregulated in cancer (79–82). In the present study, we hypothesized that the expression of the p160 SRCs could also be regulated by microRNAs. We performed an in silico search, using 4 microRNA prediction algorithms, for microRNAs that could target p160 SRCs. Accurate prediction of microRNA to mRNA binding is still an unsolved problem. The existing algorithms implement different heuristic strategies, such as matching of the microRNA seed to the mRNA target sequence, estimated free energy of the microRNA/mRNA duplex, conservation of the mRNA target sequence, and advanced modeling of microRNA/mRNA interactions using techniques such as hidden Markov models, maximum-likelihood estimation, or supervised machine learning (83–85). We integrated the results of all 4 algorithms in an unbiased fashion using rank normalization. We further used BC, UM, and PC cells with known dependence on SRC expression (7, 30, 40, 86, 87) to validate select, highly ranked microRNAs predicted to target p160 SRCs. One of the microRNAs identified by the in silico analysis was miR-17–5p, which has been previously reported to target SRC3 (86) and, thus, served as a positive control for our studies.
Several of the microRNAs that were predicted in silico to target SRC3 and SRC2, in particular miR-137, exhibited robust “on-target” activity and dramatically reduced the corresponding protein levels in BC, PC, and UM cells. The microRNA-mediated depletion of p160 SRC expression was strongly associated with antiproliferative activity in vitro in SRC-dependent BC (including those resistant to hormonal therapy), PC (including those that are AR-negative), and UM cells. We also noted that the antiproliferative effect of miR-137 in BC cells is quantitatively comparable with that achieved by combined silencing of SRC2 and SRC3. We found that miR-137 is expressed in normal prostate and breast epithelial cells but significantly silenced in PC and BC cell lines. Of note, normal prostate cells were refractory to the antiproliferative effect of transfecting additional miR-137.
In the case of SRC1, the bioinformatics algorithms gave only weak predictions, which not surprisingly were not validated in our in vitro studies, and we did not pursue them for further analysis. We did note, however, that miR-137, in addition to its potent effect on SRC3 and SRC2, also had a detectable effect on SRC1 protein expression (the latter validated a weak in silico prediction). Thus, we found that miR-137 can target the 3 members of the p160 family of SRCs. This is particularly significant, because SRCs frequently exhibit overlapping and complementary functions in transcriptional regulation (31–33, 88), thus necessitating targeting all 3 family members to achieve adequate pathway inhibition and clinical efficacy. Of note, the natural compounds gossypol and bufalin were recently found to preferentially target SRC1 and SRC3 over SRC2 (29, 30). Thus, miR-137 has the potential to target the 3 p160 SRCs, including SRC2, and represents an interesting modality to be used as monotherapy or in combination with gossypol, bufalin or other therapies to inhibit proliferation and growth of malignant cells.
Reintroduction of miR-137 into PC and BC cell lines led to decreases in expression of genes previously reported to be p160 SRC and ER dependent, such as GREB1 and TFF1 (PS2) in BC cells, and AR dependent, such as PSA/KLK3, in PC cells. These data provide evidence that miR-137 suppresses both ER- and AR-mediated signaling. However, although the p160 SRCs were originally discovered for and named after their capacity to cooperate with and mediate the transcriptional activity of steroid receptors in endocrine tissues, it has since been shown extensively that the p160 SRCs are also overexpressed in nonendocrine-related malignancies (lung, colon, esophageal, gastric and pancreatic carcinomas, and melanoma) and also participate in transcription triggered by numerous other, nonsteroid receptor, transcription factors (reviewed in Ref. 2). In agreement, we found significant antiproliferative activity of miR-137 even against the AR-negative PC3 and DU145 PC cells and against UM cell lines that are not AR/ER dependent. Thus, the role of the p160 SRCs and the potential benefits of miR-137 treatment extend beyond steroid receptor signaling.
We next proceeded to define a comprehensive profile of the proteomic footprint of miR-137 in LNCaP PC cells. RPPA analysis of LNCaP cells transfected with miR-137 mimetic revealed that SRC3 was the most significantly depleted target protein and p21 (CDKN1A) was the most up-regulated protein on the array. Interestingly, miR-137 also caused significant reduction of the expression levels of c-MYC (second most depleted protein target of miR-137 in LNCaP cells), which is known to be regulated by SRC3 (4, 89). In addition, our analysis identified that pathways altered by miR-137 in PC cells included those involved in p73, p53, and NOTCH signaling as well as regulation of cell proliferation and cellular response to DNA damage. Specifically, SRC3 (NCOA3), NOTCH, and MYC served as major hubs within this network of miR-137-affected proteins. These findings are in agreement with previous studies that have shown that depletion of SRC3 by siRNA leads to decreased cell proliferation as well as inhibition of cell cycle progression and induction of apoptosis in PC cells (7). Although not on the RPPA, our immunoblotting studies confirmed that miR-137 also significantly depleted SRC1 and SRC2 in LNCaP and 22Rv1 cells.
Analysis of publicly available datasets indicated that miR-137 is detected in multiple normal tissues, including HMECs, fibroblasts, and fetal brain. However, miR-137 is frequently down-regulated in cancers (90–93), which agrees with the potent antiproliferative activity that we have observed during reexpression of miR-137 in these cancer cells. We investigated for epigenetic mechanisms that could account for dysregulation of miR-137 expression in BC and PC cells. We noted significant increases in CpG methylation at, or within, the miR-137 promoter and its host gene in BC and PC specimens, compared with normal breast and prostate tissues, respectively. In agreement, we also found that cultured PC LNCaP cells exhibit decreased levels of H3K4Me3 (a mark of active transcription) and increased H3K27Me3 (a mark of transcriptional repression) at the miR-137 gene locus compared with PrEC cells. Moreover, BC MCF7 cells exhibit decreased levels of H3K4Me3 and increased H3K27Me3 at the miR-137 gene locus compared with HMEC cells. Thus, in agreement with its potent role as a tumor suppressor, miR-137 is epigenetically silenced in BC and PC.
In summary, miR-137 is a potent suppressor of the p160 SRC family of transcriptional coactivators, which can inhibit SRC-mediated, steroid receptor dependent and independent transcription, and can exert significant antiproliferative activity in BC, PC, and UM cells. Not surprisingly, it is the target of epigenetic silencing in cancer cells, which would contribute to the frequent overexpression of p160 SRCs, especially in the setting of resistance to endocrine therapies. As previously proposed for miR-31, another tumor suppressor microRNA that can target the AR axis and is silenced in PC (94), these findings further support the use of miR-137, or epigenetic therapies to reactivate miR-137 expression, to complement existing hormonal agents for the treatment of cancers such as BC and PC.
Acknowledgments
The authors acknowledge the joint participation by Adrienne Helis Malvin Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine. The authors thank Dr Preethi Gunaratne and Dr Arun Sreekumar for their advice and kind assistance in analysis of normal human breast cells.
This work was supported by the American Cancer Society Grant RSG-14-218-01-TBG (to N.M.), the Prostate Cancer Foundation (B.W.O. and N.M.), the Melanoma Research Alliance (N.M.), the Conquer Cancer Foundation of the American Society of Clinical Oncology Young Investigator and Career Development Awards (N.M.), the Translational Research in Breast Cancer Specialized Programs of Research Excellence Grant P50CA186784, The Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 8818 and Department of Defense Breast Cancer Research Program Innovator Award (B.W.O.), the Pilot/Feasibility Program of the Diabetes and Endocrinology Research Center Grant P30-DK079638 at Baylor College of Medicine (to N.M.), and an Alkek Foundation for Molecular Discovery Pilot grant (C.C.). N.M. is a Dan L. Duncan Scholar, a Caroline Wiess Law Scholar, and a member of the Dan L. Duncan Cancer Center (supported by the National Cancer Institute Cancer Center Support Grant P30CA125123) and the Center for Drug Discovery at Baylor College of Medicine. Assistance was also provided by the Shared Resources of the Dan L. Duncan Cancer Center (supported by the NCI Cancer Center Support Grant P30CA125123) and the Functional Proteomics RPPA Core Facility (The University of Texas M.D. Anderson Cancer Center, Houston, TX).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIB1
- amplified in breast cancer BC1
- AR
- androgen receptor
- BC
- breast cancer
- ChIP-Seq
- chromatin immunoprecipitation and sequencing
- DCIS
- ductal carcinoma in situ
- EDR
- estrogen deprivation resistant
- ER
- estrogen receptor
- FBS
- fetal bovine serum
- HMEC
- human mammary epithelial cell
- miR-137
- microRNA-137
- miR-NT
- NT microRNA
- MRS
- miR-137 recognition sequence
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NT
- nontarget
- PC
- prostate cancer
- PrEC
- prostate epithelial cell
- RPPA
- reverse phase protein array
- si-NT
- NT siRNA
- siRNA
- silencer RNA
- SRC
- steroid receptor coactivator
- TamR
- tamoxifen resistant
- UM
- uveal melanoma
- UTR
- untranslated region.
References
- 1. Lonard DM, O'Malley BW. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu J, Wu RC, O'Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jung SY, Malovannaya A, Wei J, O'Malley BW, Qin J. Proteomic analysis of steady-state nuclear hormone receptor coactivator complexes. Mol Endocrinol. 2005;19:2451–2465. [DOI] [PubMed] [Google Scholar]
- 4. Tien JC, Liu Z, Liao L, et al. The steroid receptor coactivator-3 is required for the development of castration-resistant prostate cancer. Cancer Res. 2013;73:3997–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yan J, Erdem H, Li R, et al. Steroid receptor coactivator-3/AIB1 promotes cell migration and invasiveness through focal adhesion turnover and matrix metalloproteinase expression. Cancer Res. 2008;68:5460–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou G, Hashimoto Y, Kwak I, Tsai SY, Tsai MJ. Role of the steroid receptor coactivator SRC-3 in cell growth. Mol Cell Biol. 2003;23:7742–7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou HJ, Yan J, Luo W, et al. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005;65:7976–7983. [DOI] [PubMed] [Google Scholar]
- 8. Yan J, Yu CT, Ozen M, Ittmann M, Tsai SY, Tsai MJ. Steroid receptor coactivator-3 and activator protein-1 coordinately regulate the transcription of components of the insulin-like growth factor/AKT signaling pathway. Cancer Res. 2006;66:11039–11046. [DOI] [PubMed] [Google Scholar]
- 9. Agoulnik IU, Vaid A, Nakka M, et al. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66:10594–10602. [DOI] [PubMed] [Google Scholar]
- 10. Agoulnik IU, Vaid A, Bingman WE, 3rd, et al. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res. 2005;65:7959–7967. [DOI] [PubMed] [Google Scholar]
- 11. Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qin J, Lee HJ, Wu SP, et al. Androgen deprivation-induced NCoA2 promotes metastatic and castration-resistant prostate cancer. J Clin Invest. 2014;124:5013–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torres-Arzayus MI, Font de Mora J, Yuan J, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6:263–274. [DOI] [PubMed] [Google Scholar]
- 14. Torres-Arzayus MI, Yuan J, DellaGatta JL, Lane H, Kung AL, Brown M. Targeting the AIB1 oncogene through mammalian target of rapamycin inhibition in the mammary gland. Cancer Res. 2006;66:11381–11388. [DOI] [PubMed] [Google Scholar]
- 15. Torres-Arzayus MI, Zhao J, Bronson R, Brown M. Estrogen-dependent and estrogen-independent mechanisms contribute to AIB1-mediated tumor formation. Cancer Res. 2010;70:4102–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cavarretta IT, Mukopadhyay R, Lonard DM, et al. Reduction of coactivator expression by antisense oligodeoxynucleotides inhibits ERalpha transcriptional activity and MCF-7 proliferation. Mol Endocrinol. 2002;16:253–270. [DOI] [PubMed] [Google Scholar]
- 17. Rollins DA, Coppo M, Rogatsky I. Minireview: nuclear receptor coregulators of the p160 family: insights into inflammation and metabolism. Mol Endocrinol. 2015;29:502–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. York B, Sagen JV, Tsimelzon A, et al. Research resource: tissue- and pathway-specific metabolomic profiles of the steroid receptor coactivator (SRC) family. Mol Endocrinol. 2013;27:366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Motamed M, Rajapakshe KI, Hartig SM, et al. Steroid receptor coactivator 1 is an integrator of glucose and NAD+/NADH homeostasis. Mol Endocrinol. 2014;28:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lonard DM, Kumar R, O'Malley BW. Minireview: the SRC family of coactivators: an entrée to understanding a subset of polygenic diseases? Mol Endocrinol. 2010;24:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han SJ, DeMayo FJ, Xu J, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol. 2006;20:45–55. [DOI] [PubMed] [Google Scholar]
- 22. Heemers HV, Regan KM, Schmidt LJ, Anderson SK, Ballman KV, Tindall DJ. Androgen modulation of coregulator expression in prostate cancer cells. Mol Endocrinol. 2009;23:572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lanz RB, Bulynko Y, Malovannaya A, et al. Global characterization of transcriptional impact of the SRC-3 coregulator. Mol Endocrinol. 2010;24:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anzick SL, Kononen J, Walker RL, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. [DOI] [PubMed] [Google Scholar]
- 25. Kashani-Sabet M, Venna S, Nosrati M, et al. A multimarker prognostic assay for primary cutaneous melanoma. Clin Cancer Res. 2009;15:6987–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rangel J, Torabian S, Shaikh L, et al. Prognostic significance of nuclear receptor coactivator-3 overexpression in primary cutaneous melanoma. J Clin Oncol. 2006;24:4565–4569. [DOI] [PubMed] [Google Scholar]
- 27. O'Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69:8217–8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li JV, Chien CD, Garee JP, Xu J, Wellstein A, Riegel AT. Transcriptional repression of AIB1 by FoxG1 leads to apoptosis in breast cancer cells. Mol Endocrinol. 2013;27:1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill TG, O'Malley BW. Small molecule inhibition of the steroid receptor coactivators, SRC-3 and SRC-1. Mol Endocrinol. 2011;25:2041–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Lonard DM, Yu Y, et al. Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1. Cancer Res. 2014;74:1506–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. [DOI] [PubMed] [Google Scholar]
- 32. Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell. 2005;19:631–642. [DOI] [PubMed] [Google Scholar]
- 33. He B, Lanz RB, Fiskus W, et al. GATA2 facilitates steroid receptor coactivator recruitment to the androgen receptor complex. Proc Natl Acad Sci USA. 2014;111:18261–18266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 35. Cheng CJ, Saltzman WM, Slack FJ. Canonical and non-canonical barriers facing antimiR cancer therapeutics. Curr Med Chem. 2013;20:3582–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slack FJ. MicroRNAs regulate expression of oncogenes. Clin Chem. 2013;59:325–326. [DOI] [PubMed] [Google Scholar]
- 37. Stahlhut C, Slack FJ. MicroRNAs and the cancer phenotype: profiling, signatures and clinical implications. Genome Med. 2013;5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thorsen SB, Obad S, Jensen NF, Stenvang J, Kauppinen S. The therapeutic potential of microRNAs in cancer. Cancer J. 2012;18:275–284. [DOI] [PubMed] [Google Scholar]
- 39. Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poulaki V, Chew SA, He B, et al. The protein kinase C (PKC)/protein kinase D (PKD)/steroid receptor coactivator (SRC)-3 pathway is an important therapeutic target in Gα-mutant uveal melanomas. ARVO Meeting Abstracts. 2012;53:Abstract 6871. [Google Scholar]
- 41. Kiriakidou M, Nelson PT, Kouranov A, et al. A combined computational-experimental approach predicts human microRNA targets. Genes Dev. 2004;18:1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paraskevopoulou MD, Georgakilas G, Kostoulas N, et al. DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41:W169–W173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human microRNA targets. PLoS Biol. 2004;2:e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–D153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- 46. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol. 2011;18:1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krek A, Grün D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. [DOI] [PubMed] [Google Scholar]
- 50. Chen K, Rajewsky N. Natural selection on human microRNA binding sites inferred from SNP data. Nat Genet. 2006;38:1452–1456. [DOI] [PubMed] [Google Scholar]
- 51. Mitsiades N, Chew SA, He B, et al. Genotype-dependent sensitivity of uveal melanoma cell lines to inhibition of B-Raf, MEK, and Akt kinases: rationale for personalized therapy. Invest Ophthalmol Vis Sci. 2011;52:7248–7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Griewank KG, Yu X, Khalili J, et al. Genetic and molecular characterization of uveal melanoma cell lines. Pigment Cell Melanoma Res. 2012;25:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lindholm EM, Krohn M, Iadevaia S, et al. Proteomic characterization of breast cancer xenografts identifies early and late bevacizumab-induced responses and predicts effective drug combinations. Clin Cancer Res. 2014;20:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu Y, Patel L, Mills GB, et al. Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lu Y, Muller M, Smith D, et al. Kinome siRNA-phosphoproteomic screen identifies networks regulating AKT signaling. Oncogene. 2011;30:4567–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gujral TS, Karp RL, Finski A, et al. Profiling phospho-signaling networks in breast cancer using reverse-phase protein arrays. Oncogene. 2013;32:3470–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Troncale S, Barbet A, Coulibaly L, et al. NormaCurve: a SuperCurve-based method that simultaneously quantifies and normalizes reverse phase protein array data. PLoS One. 2012;7:e38686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kamburov A, Wierling C, Lehrach H, Herwig R. ConsensusPathDB–a database for integrating human functional interaction networks. Nucleic Acids Res. 2009;37:D623–D628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Komurov K, Dursun S, Erdin S, Ram PT. NetWalker: a contextual network analysis tool for functional genomics. BMC Genomics. 2012;13:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, Ernst J, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bernstein BE, Stamatoyannopoulos JA, Costello JF, et al. The NIH roadmap epigenomics mapping consortium. Nat Biotechnol. 2010;28:1045–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coarfa C, Yu F, Miller CA, Chen Z, Harris RA, Milosavljevic A. Pash 3.0: a versatile software package for read mapping and integrative analysis of genomic and epigenomic variation using massively parallel DNA sequencing. BMC Bioinformatics. 2010;11:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Griffiths-Jones S. miRBase: the microRNA sequence database. Methods Mol Biol. 2006;342:129–138. [DOI] [PubMed] [Google Scholar]
- 65. Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bert SA, Robinson MD, Strbenac D, et al. Regional activation of the cancer genome by long-range epigenetic remodeling. Cancer Cell. 2013;23:9–22. [DOI] [PubMed] [Google Scholar]
- 67. Joseph R, Orlov YL, Huss M, et al. Integrative model of genomic factors for determining binding site selection by estrogen receptor-α. Mol Syst Biol. 2010;6:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kong SL, Li G, Loh SL, Sung WK, Liu ET. Cellular reprogramming by the conjoint action of ERα, FOXA1, and GATA3 to a ligand-inducible growth state. Mol Syst Biol. 2011;7:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Robinson JT, Thorvaldsdóttir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Olivier BG, Rohwer JM, Hofmeyr JH. Modelling cellular processes with Python and Scipy. Mol Biol Rep. 2002;29:249–254. [DOI] [PubMed] [Google Scholar]
- 74. Aryee MJ, Liu W, Engelmann JC, et al. DNA methylation alterations exhibit intraindividual stability and interindividual heterogeneity in prostate cancer metastases. Sci Transl Med. 2013;5:169ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fleischer T, Frigessi A, Johnson KC, et al. Genome-wide DNA methylation profiles in progression to in situ and invasive carcinoma of the breast with impact on gene transcription and prognosis. Genome Biol. 2014;15:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Byun B, Tak H, Joe CO. BTB/POZ domain of speckle-type POZ protein (SPOP) confers proapoptotic function in HeLa cells. Biofactors. 2007;31:165–169. [DOI] [PubMed] [Google Scholar]
- 77. Henke RT, Haddad BR, Kim SE, et al. Overexpression of the nuclear receptor coactivator AIB1 (SRC-3) during progression of pancreatic adenocarcinoma. Clin Cancer Res. 2004;10:6134–6142. [DOI] [PubMed] [Google Scholar]
- 78. Gnanapragasam VJ, Leung HY, Pulimood AS, Neal DE, Robson CN. Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer. Br J Cancer. 2001;85:1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu RC, Feng Q, Lonard DM, O'Malley BW. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell. 2007;129:1125–1140. [DOI] [PubMed] [Google Scholar]
- 80. Yi P, Feng Q, Amazit L, et al. Atypical protein kinase C regulates dual pathways for degradation of the oncogenic coactivator SRC-3/AIB1. Mol Cell. 2008;29:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li C, Ao J, Fu J, et al. Tumor-suppressor role for the SPOP ubiquitin ligase in signal-dependent proteolysis of the oncogenic co-activator SRC-3/AIB1. Oncogene. 2011;30:4350–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Geng C, He B, Xu L, et al. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc Natl Acad Sci USA. 2013;110:6997–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reyes-Herrera PH, Ficarra E. One decade of development and evolution of microRNA target prediction algorithms. Genomics Proteomics Bioinformatics. 2012;10:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Witkos TM, Koscianska E, Krzyzosiak WJ. Practical aspects of microRNA target prediction. Curr Mol Med. 2011;11:93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yue D, Liu H, Huang Y. Survey of computational algorithms for microRNA target prediction. Curr Genomics. 2009;10:478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hossain A, Kuo MT, Saunders GF. Mir-17–5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006;26:8191–8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nakka M, Agoulnik IU, Weigel NL. Targeted disruption of the p160 coactivator interface of androgen receptor (AR) selectively inhibits AR activity in both androgen-dependent and castration-resistant AR-expressing prostate cancer cells. Int J Biochem Cell Biol. 2013;45:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Winnay JN, Xu J, O'Malley BW, Hammer GD. Steroid receptor coactivator-1-deficient mice exhibit altered hypothalamic-pituitary-adrenal axis function. Endocrinology. 2006;147:1322–1332. [DOI] [PubMed] [Google Scholar]
- 89. Al-azawi D, Ilroy MM, Kelly G, et al. Ets-2 and p160 proteins collaborate to regulate c-Myc in endocrine resistant breast cancer. Oncogene. 2008;27:3021–3031. [DOI] [PubMed] [Google Scholar]
- 90. Silber J, Lim DA, Petritsch C, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Balaguer F, Link A, Lozano JJ, et al. Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis. Cancer Res. 2010;70:6609–6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen L, Wang X, Wang H, et al. miR-137 is frequently down-regulated in glioblastoma and is a negative regulator of Cox-2. Eur J Cancer. 2012;48:3104–3111. [DOI] [PubMed] [Google Scholar]
- 93. Dacic S, Kelly L, Shuai Y, Nikiforova MN. miRNA expression profiling of lung adenocarcinomas: correlation with mutational status. Mod Pathol. 2010;23:1577–1582. [DOI] [PubMed] [Google Scholar]
- 94. Lin PC, Chiu YL, Banerjee S, et al. Epigenetic repression of miR-31 disrupts androgen receptor homeostasis and contributes to prostate cancer progression. Cancer Res. 2013;73:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]