Abstract
Objective
Adenomatoid tumor is a benign neoplasm of mesothelial origin encountered most often in the male and female genital tracts. This tumor has a distinct morphology and is characterized by anastomosing and variably sized tubules lined by epithelioid and flattened cells. Only 4 cases of the extremely rare leiomyoadenomatoid variant are on record. We report 5 cases of adenomatoid tumor including 3 cases of leiomyoadenomatoid tumor of the uterus, which is an extremely rare variant of adenomatoid tumor, difficult to recognize on morphology.
Method
A detailed histopathological review of all the uterine tumor diagnosed as fibroid and adenomatoid tumor over the period of 4 years was done.
Results
A total of 5 cases of adenomatoid tumor were documented including 3 cases of leiomyoadenomatoid variant.
Conclusion
Leiomyoadenomatoid variant of adenomatoid tumor often missed both on imaging and histopathological examination and hence needs to be recognized as a distinct morphological entity.
Keywords: Adenomatoid tumor, Leiomyomata, Leiomyoadenomatoid variant, Uterus
Introduction
Adenomatoid tumor (AT) is a rare benign tumor of mesothelial origin, which predominantly arises in the male and female genital tracts [1]. The term adenomatoid tumor was coined by Golden and Ash et al. [2] and it was first described by Sakaguchi in 1916 as an adenomyomata [3]. Their mesothelial origin was described later by Masson and Evans in 1942 [4].
We report 5 cases of AT including 3 cases of the rare leiomyoadenomatoid variant in uterus, which can be easily mistaken as a leiomyoma.
Methods
All the cases diagnosed as adenomatoid tumor and leiomyoma over a period of 4 years (2008–2012) were retrieved from the archives of the Department of Pathology at our institution were studied and analyzed. Approximately 1,800 hysterectomies were received during this period. In all the leiomyomas where a component of vascular and cystic areas with hobnailed cell lining were identified, an immunohistochemical panel including Calretinin, WT 1, Cytokeratin, Estrogen receptor, smooth muscle actin (SMA), and CD 34 were done. A diagnosis of AT was entertained when mesothelial markers (Calretinin, WT 1, Cytokeratin) were positive and endothelial marker (CD 34) was negative. SMA was done to highlight the smooth muscle component.
Results
During the study period, a total of 5 cases of AT were identified. Of these 5 cases, 2 had classical morphology and 3 cases exhibited features of leiomyoadenomatoid variant. All these 3 cases were previously diagnosed as uterine leiomyoma. The mean age of our patients was 38 years (age range of 32–43 years). Four out of 5 patients presented with complaints of menorrhagia and dysmenorrhea, while one patient presented as secondary infertility. The duration of symptoms ranged from 1 to 7 years. Per vaginal examination revealed enlarged uterus of 8 and 14 weeks size in two cases, while it was normal in rest of the cases. Per speculum examination was normal in all the cases. Routine hematological and biochemical parameters were within normal limits. Ultrasonography showed the presence of fibroid in 4 cases, ranging in diameter from 10 to 30 mm. These fibroids were single in 3 cases and multiple in 1 of the case. Imaging in the fifth patient revealed a uterine surface cyst. While 2 patients underwent total hysterectomy, 2 were managed with hysterectomy and bilateral salpingectomy. The patient presenting with secondary infertility was offered a diagnostic “uterine surface lesion” biopsy.
Pathological Findings
Grossly, the lesions ranged from 0.5 to 3 cm. They were single, solid, gray–white fibroid-like lesion except in one of the case where the lesions are multiple (Fig. 1). The tumors were located intramurally in 3 cases and on the uterine surface in the rest of the 2 cases. No other abnormalities were noted grossly in rest of the specimen.
Fig. 1.

Gross photograph of a case of leiomyoadenomatoid tumor
Microscopically, H&E stained sections showed a distinct lesions in 3 cases. These lesions were circumscribed and predominantly composed of smooth muscle arranged in long fascicles. In addition, there were few small cystic spaces of variable caliber, lined by flattened to cuboidal and occasional hobnailed cells (Fig. 2). The other 2 cases had a classical AT morphology with cystic and tubular spaces lined by plump, hobnailed cells, and few solid areas in between. There was no smooth muscle component in these 2 cases. Immunochemistry was performed in all the cases for calretinin, WT1, and cytokeratin. IHC staining for calretinin, WT1, and cytokeratin was positive in all of the cases, proving the origin of the tumor as mesothelial (Fig. 3). The immunostaining for ER was done in all the cases to rule out entrapped endometrial glands, simulating AT. None of the cases was positive for ER immunostain. In addition, the 3 cases showing considerable smooth muscle component were immunopositive for SMA and CD34. The cystic areas were CD 34 negative in 2 cases, ruling out any entrapped blood vessels within a leiomyoma. In 1 case, we found focal CD34 positivity in addition to diffuse Calretinin positivity, leading to the conclusion that some spaces were indeed blood vessels (Fig. 4). In these 3 cases with the overall light microscopy and immunohistochemical features, a final diagnosis of leiomyoadenomatoid tumor of the uterus was offered.
Fig. 2.
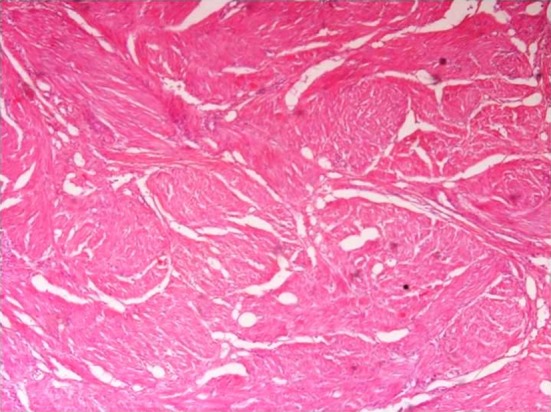
Photomicrograph (×10) showing cystic spaces and smooth muscle
Fig. 3.
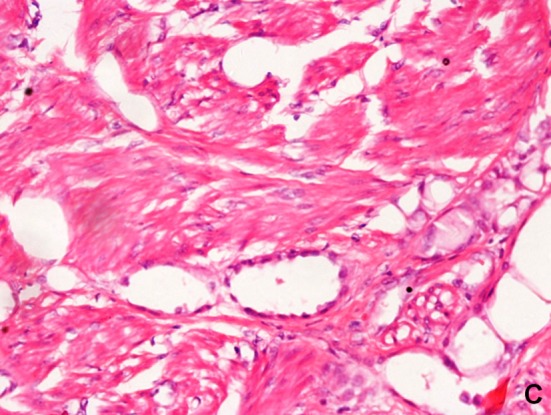
Photomicrograph (×40) showing cystic spaces lined by hobnailed cells
Fig. 4.

Photograph showing Immunopositivity for Calretinin
Discussion
AT is a tumor of mesothelial origin and hence they occur in organs close to mesothelium-lined surfaces most commonly in the genital tract followed by at extra genital sites, particularly in the vicinity of serosal membranes (pleura, peritoneum, and pericardium), the adrenal gland, and other visceral organs [5]. In the female genital tract, AT most commonly involves the uterus [1]. These tumors are often incidental findings in hysterectomy specimens of adult women, the incidence being approximately 1 % [6]. AT is typically solitary, subserosal, or myometrial masses, often located near the cornu and usually less than 4 cm in diameter [4]. Histologically, the tumor can have an adenoid, angiomatoid, solid, or cystic architecture or a combination of more than one type [1, 7, 8]. Some tumors, as in 3 of our cases, can have a prominent smooth muscle component [9]. Due to the many architectural variations, AT has an extensive differential diagnosis that includes hemangioma, angiosarcoma, lymphangioma, malignant mesothelioma, yolk sac tumor, and primary as well as metastatic adenocarcinoma.
AT can be difficult to diagnose, especially when it presents at sites other than the urogenital tract or when it closely mimics a leiomyoma. The term “leiomyoadenomatoid tumor” was first described in 1992 as a variant of adenomatoid tumor with a prominent smooth muscle component. Extensive smooth muscle overgrowth may sometimes obscure the adenomatoid tumor and result in a misdiagnosis of leiomyoma or malignant tumor infiltrating smooth muscle bundles. Various authors believe that these smooth muscle bundles either represent entrapped myometrium permeated by the AT or due to reactive hyperplasia of indigenous myometrial smooth muscle. Cases of AT in the uterus have been reported in the literature. However, an extensive literature search has revealed only 4 previously reported cases of leiomyoadenomatoid tumors of the uterus. [9] We report 3 additional case of this rare tumor and compare this with the 3 prior reported uterine AT (Table 1). The fourth leiomyoadenomatoid tumor was located in the epididymis.
Table 1.
Comparison of current cases with previous reported leiomyoadenomatoid tumors of female genital tract
| Sr No | Series | Age | Location | Size | Histological type |
|---|---|---|---|---|---|
| 1 | Hong R et al. | 24 | Intramural and subserosal | 4.5 cm | Angiomatoid with smooth muscle hypertrophy and presence of necrotic areas |
| 2 | Amre R et al. | 52 | Subserosal | 1 cm | Angiomatoid with smooth muscle hypertrophy |
| 3 | G Goel et al. | 51 | Intramural | 4 cm | Angiomatoid with smooth muscle hypertrophy |
| 4 | (Present study) | 40 | Intramural | 2 cm | Angiomatoid with smooth muscle hypertrophy |
| 5 | (Present study) | 36 | Intramural | Multiple 0.5–3.0 cm | Leiomyoadenomatoid variant along with some CD 34 positive vessels |
| 6 | (Present study) | 43 | Intramural | 1.2 cm | Leiomyoadenomatoid variant |
Another important feature brought out is that the incidence of uterine adenomatoid tumor worked out by Tiltman is 1 %. Ours is a large tertiary care institute and we receive around 800 hysterectomy specimens in a year. The database search showed that from 2009 to 2012, only 5 cases of uterine adenomatoid tumors were diagnosed. This points out that ATs are significantly under diagnosed, probably due to a lack of familiarity with these uncommon tumors and their relatively bland histology.
Conclusion
While AT of the female genital tract is comparatively common and is readily identifiable in its classical form, the leiomyoadenomatoid variant is rare with only 3 reported cases in the world literature. This entity is frequently missed due to its morphological overlap with leiomyoma and general lack of awareness among histopathologists. Further studies are needed to establish whether it is a distinct clinicopathological entity separate from classical AT.
Contributor Information
Richa Ranjan, Email: rrriiicha@yahoo.co.in.
Lavleen Singh, Email: singhlavleen04@yahoo.co.in.
Devajit Nath, Email: debojitnath@yahoo.com.
Mukund N. Sable, Email: mukundsable@yahoo.com
Siddhartha Datta Gupta, Email: sdattagupta@gmail.com.
References
- 1.David LW, Wünsch PH, Hartmann A, et al. Adenomatoid tumors of the female and male genital tract. A comparative clinicopathologic and immunohistochemical analysis of 47 cases emphasizing their site-specific morphologic diversity. Virchows Arch. 2011;458:593–602. doi: 10.1007/s00428-011-1054-5. [DOI] [PubMed] [Google Scholar]
- 2.Golden A, Ash JE. Adenomatoid tumors of the genital tract. Am J Pathol. 1945;21:63–79. [PMC free article] [PubMed] [Google Scholar]
- 3.Phillip AI, Gary LK, Sebo TJ, et al. Adenomatoid tumor of the adrenal gland A clinicopathologic study of five cases and review of the literature. Am J Surg Pathol. 2003;27:969–977. doi: 10.1097/00000478-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Evans N. Mesotheliomas of the uterine and tubal serosa and the tunica vaginalis testis: report of four cases. Am J Pathol. 1943;19:461–471. [PMC free article] [PubMed] [Google Scholar]
- 5.Glatz K, Wegmann W. Papillary adenomatoid tumor of the adrenal gland [Letter] Histopathology. 2000;37:376–382. doi: 10.1046/j.1365-2559.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 6.Tiltman AJ. Adenomatoid tumours of the uterus. Histopathology. 1980;4:437–443. doi: 10.1111/j.1365-2559.1980.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 7.Nogales FF, Isaac MA, Hardisson D, et al. Adenomatoid tumors of the uterus: an analysis of 60 cases. Int J Gynecol Pathol. 2002;21:34–40. doi: 10.1097/00004347-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Sangoi AR, McKenney JK, Schwartz EJ, et al. Adenomatoid tumors of the female and male genital tracts: a clinicopathological and immunohistochemical study of 44 cases. Mod Pathol. 2009;22:1228–1235. doi: 10.1038/modpathol.2009.90. [DOI] [PubMed] [Google Scholar]
- 9.Mattew M, Goel G. Leiomyoadenomatoid tumor of the uterus. Turk J Pathol. 2010;26(2):168–169. doi: 10.5146/tjpath.2010.01018. [DOI] [Google Scholar]


