Abstract
Introduction
Clostridium innocuum is an anaerobic Gram-positive bacterium, unable to produce toxins and rarely causes infections. We report the first case of C. innocuum osteomyelitis and bacteremia in a patient with acute lymphoblastic leukemia (ALL). Findings were compared with previously reported cases of C. innocuum infections in immunocompromised patients, e.g., patients with acquired immune deficiency syndrome, leukemia, and organ transplantation.
Case description
A 32-year-old Japanese male was admitted for persistent low-grade fever and purpura lasting for 1 month. Complete blood counts and cytogenetic analysis identified Ph1-positive ALL, which was successfully treated using chemotherapy. However, the patient developed high fever and lumbar pain during complete remission. Fluorodeoxyglucose-positron emission tomography and computed tomography demonstrated osteomyelitis. C. innocuum was identified as the causative agent and the patient was successfully treated using antibiotic therapy.
Discussion and evaluation
We performed a literature review revealing a number of common aspects to the clinical presentation of C. innocuum infection and an association with various comorbidities. Further, we highlight the most efficient diagnostic and treatment strategies for C. innocuum osteomyelitis.
Conclusions
Clostridium innocuum can be a causative pathogen of osteomyelitis and bacteremia in immunocompromised patients.
Electronic supplementary material
The online version of this article (doi:10.1186/s40064-015-1176-3) contains supplementary material, which is available to authorized users.
Keywords: Clostridium innocuum, Acute lymphoblastic leukemia, Osteomyelitis, Direct 16S-rRNA sequencing, Neutropenic enterocolitis
Introduction
Clostridial species are common anaerobic, spore-forming, Gram-positive bacteria found in the normal flora of the oropharynx and gastrointestinal tract. Among them, Clostridium innocuum is an unusual cause of infections in humans. A few reports have described bacteremia due to C. innocuum in immunocompromised patients, such as those with acquired immune deficiency syndrome (AIDS), leukemia, and organ transplantation. Because C. innocuum has intrinsic resistance to several common antibiotics, including vancomycin, it may cause intractable infections (Alexander et al. 1995; David et al. 2004). We report the first case of pelvic osteomyelitis and sepsis due to C. innocuum infection in a patient with acute lymphocytic leukemia (ALL). We performed a literature review of previous reports to determine the most appropriate diagnostic strategies and treatment regimens in cases of C. innocuum infection in patients with distinct comorbidities.
Review
Case description
A 32-year-old Japanese male with no previous medical history was admitted to our hospital for a persistent low-grade fever and purpura lasting for 1 month. A complete blood count (CBC) revealed marked anemia, thrombocytopenia, and hyperleukocytosis (136,130/µL; blasts 97%, neutrophils 0%, lymphocytes 2%, and eosinophils 1%). Bone marrow aspiration from iliac crest were hypercellular with 97.6% lymphoblasts. Cytogenetic analysis revealed that blasts were positive for the Philadelphia chromosome (Ph1) with minor BCR/ABL mRNA transcripts. Therefore, the patient was diagnosed with Ph1-positive ALL.
Combination chemotherapy with dasatinib was immediately initiated as remission induction. On treatment day 27, CBCs had normalized and bone marrow aspiration from iliac crest analysis confirmed complete remission. On treatment day 39, consolidation chemotherapy with daunorubicin, cyclophosphamide, vincristine, prednisolone, methotrexate, and dasatinib was initiated. On treatment day 51, the patient became pyrexial (39.2°C) and reported severe lower back pain. The pain rapidly worsened and radiated to the right axilla. WBC count was 430/mm3, with 8% segmented neutrophils, 2% bands, 80% lymphocytes, and 10% monocytes. We immediately collected blood culture and started empiric antibiotic therapy with meropenem, vancomycin, and liposomal amphotericin B. CT imaging revealed no apparent abnormal findings; however, fluorodeoxyglucose (18F)-positron emission tomography (FDG-PET) revealed increased uptake of FDG in the iliac bones and right side of the sacrum. These findings suggested osteomyelitis of the iliac bone and sacrum and Gram-positive bacteria were detected by needle aspiration biopsy of the iliac bone (Fig. 1). Gram-positive bacterium was also found in the blood culture; however, the bacteria could not be identified. For this reason, we tested the blood culture for direct 16S ribosomal RNA (16S rRNA) sequencing and identified C. innocuum 2 days later. The presence of this pathogen was also confirmed by bone marrow culture from iliac crest. As a result of these findings, the patient was treated with piperacillin/tazobactam, metronidazole, and clindamycin. In this case, the causative pathogen was sensitive to ampicillin, piperacillin/tazobactam, meropenem, clindamycin, and metronidazole. Intermediate sensitivity to penicillin G and cefmetazole was observed. Further, the isolated strain was resistant to vancomycin with a minimum inhibitory concentration (MIC) of 8 μg/mL.
Fig. 1.
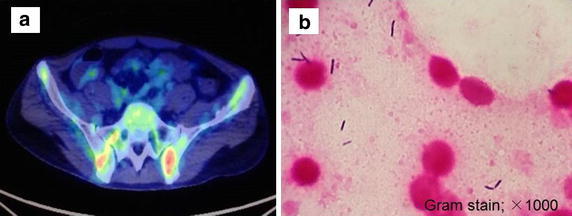
Diagnostic approach for a 32-year-old male with Clostridium innocuum osteomyelitis. a (18F)-Fluorodeoxyglucose positron emission tomography (FDG-PET) revealed marked uptake of FDG in the sacroiliac joint and iliac bone. b Bone marrow biopsy from iliac crest confirmed C. innocuum infection (Grambiopsy conf1000).
Fever gradually resolved over the next 3 weeks, but the lumbar pain persisted. CT imaging identified a small abscess in the iliacus muscle. Therefore, CT-guided drainage was performed. No pathogens were detected in cultures of the abscess fluid or blood. The previously administered antibiotic regimen was consequently deemed effective. After another 8 weeks of antibiotic therapy, the lumbar pain subsided and treatment was terminated. On treatment day 104, the patient was asymptomatic and chemotherapy was reinitiated.
Discussion and evaluation
We identified previously published cases of C. innocuum infection by conducting a PubMed search of the literature using the following keywords: Clostridium innocuum; ALL; osteomyelitis; and anaerobic bacteria. PubMed queries also included infections that developed in immunocompromised patients during chemotherapy.
The first human case of C. innocuum infection was reported in the 1960s (Smith and King 1962). The term “innocuum” is derived from “innocuous” (i.e., meaning innocent) as the organism lacks the ability to produce toxins. A PubMed search of the literature identified 16 previously reported cases of C. innocuum infection (Smith and King 1962; Castiglioni et al. 2003; Crum-Cianflone 2009; Hung et al. 2014; Bodey et al. 1991; Cutrona et al.1995). Details of these cases are summarized in Additional file 1: Table S1. Median age of patients was 38.0 years, and 66.7% were male. All but one patient had a comorbid disorder, namely acute leukemia, AIDS, chronic hepatitis, genitourinary malignancy, gastro-intestinal malignancy, or organ transplantation (Crum-Cianflone 2009; Hung et al. 2014; Bodey et al. 1991; Cutrona et al. 1995; Shah et al. 2009). The most common clinical symptom was fever of unknown origin followed by the gastrointestinal tract disorder, such as diarrhea or constipation, and/or respiratory disorder. Almost all patients developed bacteremia. Most commonly used agents were piperacillin/tazobactam, metronidazole, and clindamycin to which C. innocuum appeared susceptible. Nevertheless, the prognosis of these patients was poor, with a mortality rate of 33.3%.
The diagnosis of anaerobic infections is clinically challenging. Detection rates from blood cultures are extremely low (approximately 4.0%), mainly because of the slow growth of anaerobic bacteria. In addition, a number of anaerobic bacteria species are part of the normal microbiota in humans and concomitant infection with aerobic bacteria is common (Cockerill et al. 1997). One study in patients with blood cultures positive for anaerobic bacteria demonstrated patients who received appropriate anti-anaerobic antibiotics as the initial treatment, or who were immediately changed to appropriate antibiotics following identification of the causative pathogen, had markedly improved prognosis compared with patients who did not receive appropriate antibiotic treatment (Salonen et al. 1998).
Direct 16S ribosomal RNA gene sequencing has recently been developed, allowing the composition of microbial communities to be analyzed. In the present case, this approach identified C. innocuum in blood cultures within a few days of admission and that allowed the selection of an appropriate initial antibiotic regimen. Therefore, direct 16S ribosomal RNA gene sequencing may be useful in the early detection of Clostridium species in clinical samples (Drancourt et al. 2000; Mory et al. 1998). Unlike C. difficile, C. innocuum is susceptible to penicillin, clindamycin, and metronidazole but not to vancomycin (Ackermann et al. 2001; Goldstein et al. 2014). Our patient was successfully treated by a 8-week combination antibiotic therapy comprising piperacillin/tazobactam, clindamycin, and metronidazole. There is currently no standard duration of treatment for C. innocuum infections. However, 4–6 weeks with appropriate antibiotics and debridement is generally recommended for the treatment of osteomyelitis (Howard et al. 1994; Spellberg and Lipsky 2012).
Comprehensive blood and bone marrow aspiration analysis revealed Ph1-positive ALL in our patient warranting immediate initiation of systemic chemotherapy. The patient developed high fever and back pain, although complete remission of ALL was achieved. Therefore, we suspected that these symptoms were due to an infection rather than related to a lymphoproliferative disease.
Systemic chemotherapy has been reported to induce neutropenic enterocolitis (NEC), a common complication in neutropenic cancer patients (Nesher and Rolston 2013). Symptoms generally develop after the third week of chemotherapy and include neutropenic fever and abdominal pain (mainly in the right lower abdomen). The diagnostic criteria for NEC is neutropenia (absolute neutrophil count <500 × 106 cells/L), bowel wall thickening >4 mm, and high fever with the exclusion of other diagnoses. The mortality of NEC patients is relatively high (>60%). Risk factors for NEC include acute leukemia, lymphoma, solid tumor, and neutropenia in addition to cytotoxic chemotherapeutic agents such as cytosine arabinoside, anthracyclines, and taxanes. In this case, the patient complained of abdominal distention and constipation after consolidation chemotherapy was started. Computed tomography (CT) revealed marked constipation and edematous intestinal wall, findings that were compatible with NEC. Therefore, we believed that bacterial translocation of C. innocuum occurred from the damaged intestinal tract to the iliac bone.
According to identified case reports of C. innocuum, previous C. difficile associated diarrhea (CDAD) may predispose to C. innocuum bacteremia (Crum-Cianflone 2009); however, C. difficile infection was not detected in our case. NEC without CDAD may induce bacterial translocation of C. innocuum from the enteral flora. Osteomyelitis due to Clostridium species is quite rare. In the previously reported articles on osteomyelitis due to C. difficile, C. clostridioforme, C. celerecrescens, C. bifermentans, and C. septicum, several have reported an association with trauma (Mischnik et al. 2011; Al-Najjar et al. 2013; Scanlan et al. 1994). The treatment that was most commonly chosen was metronidazole, clindamycin, or β-lactam. Almost all anaerobic osteomyelitis occur by direct extension from an adjacent focus of infection and are rarely due to bacteremia; however, in the present case, the bacteria may have reached from the intestinal tract to the iliac bone via blood circulation.
Immunodeficiency may contribute to the progression of infection. As pathogens are difficult to identify, treatments generally include broad spectrum antibiotics covering rare anaerobic bacteria and antifungal agents. Surgical intervention is recommended only in cases of bowel perforation or necrosis. The recommended duration of antibiotic therapy is from 4 weeks to 6 months. For immunosuppressed patients, the recommended treatment is more than 8 weeks. Further, it is considered that less than 4 weeks of antibiotic therapy is a risk factor for recurrence in patients who have undergone surgical management (Pigrau et al. 2015; Lima et al. 2014).
Conclusions
In conclusion, we report a rare case of osteomyelitis and bacteremia due to C. innocuum. Although C innocuum lacks the ability to produce toxins and has weak pathogenicity, it may cause severe infections in immunocompromised patients, such as those with acute leukemia, chronic viral hepatitis, or HIV.
Consent
Informed consent for publication of this report and any accompanying images was obtained from the patient.
Authors’ contributions
YM drafted this manuscript. YM, RH, and AT treated patient as a member of attending physicians. TM, EM, and NO were consulted regarding appropriate treatment of this infection and commented on disease as consultants of infectious diseases. SK and MN analyzed this pathogen using direct 16S ribosomal RNA gene sequencing. SH helped to draft and commented on the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was supported by Grants from International Health Cooperation Research (25–114) from the Ministry of Health, Labor and Welfare of Japan.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Additional file
Reported cases of C. innocuum infection
Contributor Information
Yoshikazu Mutoh, Email: tsu_ka_sa31@yahoo.co.jp.
Risen Hirai, Email: r-hira@hosp.ncgm.go.jp.
Akira Tanimura, Email: atanimur@hosp.ncgm.go.jp.
Takashi Matono, Email: sincedec2012@gmail.com.
Eriko Morino, Email: eada-iws@cj8.so-net.ne.jp.
Satoshi Kutsuna, Email: sonare.since1192@gmail.com.
Maki Nagamatsu, Email: dfrmm217@yahoo.co.jp.
Norio Ohmagari, Email: lukenorioom@gmail.com.
Shotaro Hagiwara, Email: shagiwar@hosp.ncgm.go.jp.
References
- Ackermann G, Tang YJ, Jang SS, Silva J, Rodloff AC, Cohen SH. Isolation of Clostridium innocuum from cases of recurrent diarrhea in patients with prior Clostridium difficile associated diarrhea. Diagn Microbiol Infect Dis. 2001;40:103–106. doi: 10.1016/S0732-8893(01)00259-0. [DOI] [PubMed] [Google Scholar]
- Alexander CJ, Citron DM, Brazier JS, Goldstein EJ. Identification and antimicrobial resistance patterns of clinical isolates of Clostridium clostridioforme, Clostridium innocuum and Clostridium ramosen compared with these of clinical isolates of Clostridium perfringens. J Clin Microbiol. 1995;33:3209–3215. doi: 10.1128/jcm.33.12.3209-3215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Najjar A, Al-Rawahi GN, Hoang LM, Kollmann TR. Clostridium difficile vertebral osteomyelitis. Pediatr Infect Dis J. 2013;32:1030–1032. doi: 10.1097/INF.0b013e318296b424. [DOI] [PubMed] [Google Scholar]
- Bodey GP, Rodriguez S, Fainstein V, Elting LS. Clostridial bacteremia in cancer patients. A 12-year experience. Cancer. 1991;67:1928–1942. doi: 10.1002/1097-0142(19910401)67:7<1928::AID-CNCR2820670718>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Castiglioni B, Gautam A, Citron DM, Pasculle W, Goldstein EJ, Strollo D, et al. Clostridium innocuum bacteremia secondary to infected hematoma with gas formation in a kidney transplant recipient. Transpl Infect Dis. 2003;5:199–202. doi: 10.1111/j.1399-3062.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Cockerill FR, 3rd, Hughes JG, Vetter EA, Mueller RA, Weaver AL, Ilstrup DM, et al. Analysis of 281,797 consecutive blood cultures performed over an eight-year period: trends in microorganisms isolated and the value of anaerobic culture of blood. Clin Infect Dis. 1997;24:403–418. doi: 10.1093/clinids/24.3.403. [DOI] [PubMed] [Google Scholar]
- Crum-Cianflone N. Clostridium innocuum bacteremia in an AIDS patient: case report and review of the literature. Am J Med Sci. 2009;337:480–482. doi: 10.1097/MAJ.0b013e31819f1e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona AF, Watanakunakorn C, Schaub CR, Jagetia A. Clostridium innocuum endocarditis. Clin Infect Dis. 1995;21:1306–1307. doi: 10.1093/clinids/21.5.1306. [DOI] [PubMed] [Google Scholar]
- David V, Bozdogan B, Mainardi JL, Legrand R, Gutmann L, Leclercq R. Mechanism of intrinsic resistance to vancomycin in Clostridium innocuum NCIB 10674. J Bacteriol. 2004;186:3415–3422. doi: 10.1128/JB.186.11.3415-3422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drancourt M, Bollet C, Carlioz A, Martelin R, Gayral JP, Raoult D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol. 2000;38:3623–3630. doi: 10.1128/jcm.38.10.3623-3630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein EJ, Citron DM, Tyrrell KL. Comparative in vitro activities of SMT19969, a new antimicrobial agent, against 162 strains from 35 less frequently recovered intestinal Clostridium species: implications for Clostridium difficile recurrence. Antimicrob Agents Chemother. 2014;58:1187–1191. doi: 10.1128/AAC.02184-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CB, Einhorn M, Dagan R, Yagupski P, Porat S. Fine-needle bone biopsy to diagnose osteomyelitis. J Bone Joint Surg Br. 1994;76:311–314. [PubMed] [Google Scholar]
- Hung YP, Lin HJ, Wu CJ. Vancomycin-resistant Clostridium innocuum bacteremia following oral vancomycin for Clostridium difficile infection. Anaerobe. 2014;30C:24–26. doi: 10.1016/j.anaerobe.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Lima ALL, Oliveira PR, Carvalho VC, Cimerman S, Savio E, Diretrizes Panamericanas para el Tratamiento de las Osteomielitis e Infecciones de Tejidos Blandos Group Recommendations for the treatment of osteomyelitis. Braz J Infect Dis. 2014;18:526–534. doi: 10.1016/j.bjid.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischnik A, Zimmermann S, Bekeredjian-Ding I, Egermann M. Relapse of posttraumatic osteomyelitis due to Clostridium celerecrescens. Infection. 2011;39:491–494. doi: 10.1007/s15010-011-0125-5. [DOI] [PubMed] [Google Scholar]
- Mory F, Lozniewski A, David V, Carlier JP, Dubreuil L, Leclercq R. Low-level vancomycin resistance in Clostridium innocuum. J Clin Microbiol. 1998;36:1767–1768. doi: 10.1128/jcm.36.6.1767-1768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesher L, Rolston KV. Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. Clin Infect Dis. 2013;56:711–717. doi: 10.1093/cid/cis998. [DOI] [PubMed] [Google Scholar]
- Pigrau C, Rodríguez-Pardo Fernández-Hidalgo N, et al. Health care associated hematogenous pyogenic vertebral osteomyelitis: a severe and potentially preventable infectious disease. Medicine. 2015;94:e365. doi: 10.1097/MD.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26:1413–1417. doi: 10.1086/516355. [DOI] [PubMed] [Google Scholar]
- Scanlan DR, Smith MA, Isenberg HD, Engrassia S, Hilton E. Clostridium bifermentans bacteremia with metastatic osteomyelitis. J Clin Microbiol. 1994;32:2867–2868. doi: 10.1128/jcm.32.11.2867-2868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Bishburg E, Baran DA, Chan T. Epidemiology and outcomes of clostridial bacteremia at a tertiary-care institution. Sci World J. 2009;9:144–148. doi: 10.1100/tsw.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LD, King E. Clostridium innocuum, sp. n. a sporeforming anaerobe isolated from human infections. J Bacteriol. 1962;83:938–939. doi: 10.1128/jb.83.4.938-939.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54:393–407. doi: 10.1093/cid/cir842. [DOI] [PMC free article] [PubMed] [Google Scholar]


