Abstract
The healthy immune system protects against infection and malignant transformation without causing significant damage to host tissues. Immune dysregulation results in diverse pathologies including autoimmune disease, chronic inflammatory disorders, allergies as well as immune deficiencies and cancer. Phosphoinositide 3-kinase (PI3K) signalling has been shown to be a key pathway in the regulation of the immune response and continues to be the focus of intense research. In recent years we have gained detailed understanding of PI3K signalling, and saw the development of potent and highly selective small molecule inhibitors, of which several are currently in clinical trials for the treatment of immune-related disorders and cancer. The role of PI3K signalling in the immune response has been the subject of detailed reviews; here we focus on relevant recent progress in pre-clinical and clinical development of PI3K inhibitors.
PI3K signalling
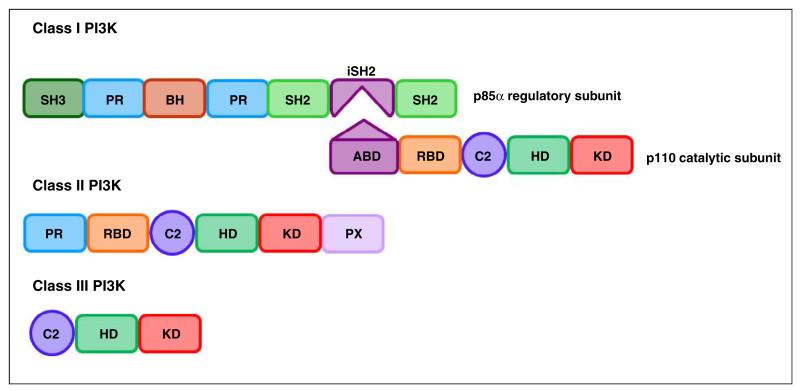
The PI3Ks are a family of lipid kinases that phosphorylate the 3rd hydroxyl on phosphoinositides in cell membranes. Structurally, these enzymes share a common PI3K core motif, consisting of a C2 domain, a helical domain and a catalytic (kinase) domain. PI3Ks are classified into three families based on structure and substrate specificity, with the class I PI3K being further subdivided into class IA and class IB, summarised in Table 1 and Figure 1 [1–3].
Table 1. Summary of PI3K classes.
| Isoforms | Tissue distribution | Substrate | Product | Adaptor molecules | |
|---|---|---|---|---|---|
| Class IA | p110α | Ubiquitous | PI(4,5)P2 | PI(3,4,5)P3 aka PIP3 |
p85α(p50α,p55α) p85β; p55γ |
| p110β | Ubiquitous | ||||
| p110† | Leukocytes, neurons | ||||
| Class IB | p110γ | Leukocytes, cardiac myocytes | PI(4,5)P2 | PI(3,4,5)P3 | p101/p84/p87 |
| Class II | C2α | Epithelium, endothelium | PI, PI4P | PI3P, PI(3,4)P2 | |
| C2β | Ubiquitous | ||||
| C2γ | Hepatocytes | ||||
| Class III | VPS34 | Ubiquitous | PI | PI3P | VPS15(p150) |
Figure 1.
Schematic representation of class I-III PI3K structures ABD: adaptor binding domain; RBD: RAS binding domain; C2: C2 domain; HD: helical domain; KD: kinase domain; PR: proline rich domain; PX: phox homology domain; BH: breakpoint cluster region homology domain (Rho-Gap-like domain); iSH2: inter-SH2 domain (p110 binding domain). Complexes between p110α, p110β, p110δ and p110γ and their respective regulatory subunits are often referred to as PI3Kα, PI3Kβ, PI3Kδ and PI3Kγ.
Class I PI3K
Gene targeted mouse models exist for all the class I PI3K catalytic and regulatory subunits and, together with the availability of isoform specific inhibitors, have greatly enhanced our understanding of PI3K signalling. Class I PI3K function as heterodimers consisting of a regulatory subunit associated with a catalytic subunit and phosphorylate PI(4,5)P2 to form PI(3,4,5)P3 which recruits pleckstrin homology(PH)-domain containing effector proteins such as AKT (PKB) to the cell membrane [2]. Under most circumstances class IA enzymes are activated through receptor tyrosine kinases (RTK) and other tyrosine kinase coupled receptors, while the class IB isoform p110γ is activated through G-protein coupled receptors (GPCRs). However, this distinction is becoming increasingly unclear: p110β can be activated by GPCRs [1,2], and one study found that p110γ function downstream of RTK, TLR and type-I cytokine receptors [4].
Class I PI3Ks play an important role in immune regulation, and the four isoforms differ in terms of tissue distribution and function: PI3Kα is ubiquitously expressed and essential for angiogenesis and insulin signalling [5]. PI3Kα can also compensate for the loss of PI3Kδ during early B cell development [6]. Like PI3Kα, PI3Kβ is ubiquitously expressed, but plays a non-redundant role in Fcγ receptor-dependent phagocytosis and ROS production in macrophages and neutrophils [7,8]. PI3Kδ and PI3Kγ expression is mainly restricted to leukocytes, and their expression levels and function vary based on cell type and activation conditions. PI3Kδ function is critical for mature B cell development as well as effector T cell and regulatory T cell (Treg) differentiation and function [6,9–11]. PI3Kδ and PI3Kγ can act synergistically to modulate myeloid effector function: sequential PI3Kγ and PI3Kδ activation is required for effective ROS production in human, but not mouse neutrophils [12], and aberrant migration in aged neutrophils could be partially corrected by PI3Kδ (CAL-101) or PI3Kγ (AS252424) inhibitors [13•]. The relative contribution of PI3Kδ and PI3Kγ to mast cell function is still controversial: while some studies found PI3Kγ signalling to be critical for mast cell infiltration and degranulation, with transient inhibition of p110γ with NVS-PI-4 sufficient to prevent mast cell extravasation in a passive cutaneous anaphylaxis (PCA) model [14,15], another study showed an essential role for PI3Kδ, but not PI3Kγ, signalling in PCA induced mast cell extravasation [16]. PI3Kβ, PI3Kδ and PI3Kγ also contribute to optimal dendritic cell (DC) and macrophage function [1,17].
PI3K signalling can promote pro-inflammatory cytokine production through NFκB activation downstream of AKT and mediate IL-6 secretion in response to CD80/CD86 stimulation in DC [18]. However, PI3K also play a regulatory role in certain innate immune responses. Several studies identified an inhibitory role for PI3K signalling in TLR mediated inflammation: PI3Kδ activation downstream of TIRAP-MyD88 dependent (TLR2, TLR4) and TRAM-TRIF dependent (TLR4, TLR3) stimulation inhibits pro-inflammatory cytokine secretion while increasing the production of IL-10 in macrophages and DC [19–24]. Possible mechanisms are thought to be through AKT-dependent inhibition of GSK3β, leading to increased levels of CREB and competitive inhibition of NFκB-p65 and AKT dependent inhibition of FoxO1 [23,24]. TLR4 is unique in being activated via a TIRAP dependent mechanism on the cell membrane and also via TRAM following endocytosis. PI3Kδ can mediate a switch between TIRAP dependent pro-inflammatory cytokine secretion and TRAM-dependent IL-10 secretion, thereby limiting inflammation and protecting mice from LPS induced endotoxic shock [21]. PI3Kδ can also control type I IFN production by regulating IRF-7 nuclear translocation in human plasmacytoid DC [25]. PI3Kδ could therefore be a promising therapeutic target in diseases where this pro-inflammatory response is dysregulated. Physiologically, PI3K is regulated by phosphatase and tensin homolog (PTEN) which reverts PIP3 to PI(4,5)P2. Myeloid cell-specific PTEN deficiency leads to increased PIP3 levels, reduced inflammation, increased macrophage phagocytic ability and resistance to infection in mice [26]. Similarly, aged macrophages show increased expression of PI3Kδ with decreased pro-inflammatory cytokine production in response to TLR stimulation, which is partially reversed by the pan-class I PI3K inhibitor LY924002 [20]. Recently it was also shown that LY924002 can reduce TLR3 dependent IL-10 secretion in BCG infected macrophages [22].
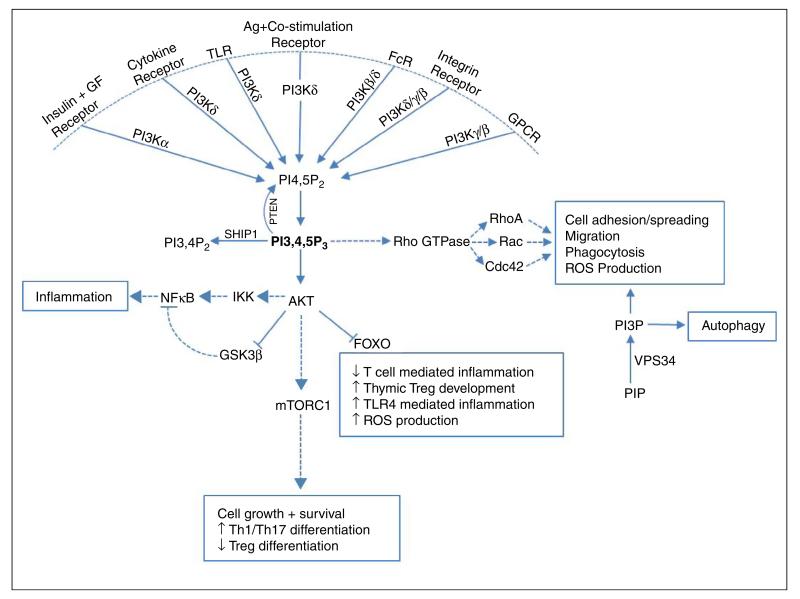
Together, these studies show that the PI3K/AKT signalling pathway plays a complex role in orchestrating both pro-inflammatory and anti-inflammatory pathways to maintain effective immunity while protecting host tissues (Figure 2).
Figure 2.
Summary of positive and negative immune regulation by PI3Ks. PI3K signalling play a role in positive and negative regulation of immune cell effector functions, and the outcome of inhibition will depend on inhibitor selectivity and disease context.
Targeting class I PI3K in autoimmune and inflammatory disorders
Autoimmune disease results from a breakdown in tolerance leading to an immune response directed against host cells, causing conditions such as multiple sclerosis (MS), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), psoriasis and autoimmune (type I) diabetes. Chronic inflammatory conditions such as chronic obstructive pulmonary disease (COPD), atherosclerosis and inflammatory bowel disease (IBD) arise from failure to resolve an ongoing immune response [1,27]. Several of the driving factors of COPD and atherosclerosis have been identified. In atherosclerosis patients oxidised LDL promotes arterial inflammation, while in a large number of COPD patients cigarette smoke contributes to the pathogenesis. However, it is clear that pathogen-driven responses trigger exacerbations in COPD patients which lead to worsened inflammation and a general decline in health status [28]. Allergic conditions such as asthma or anaphylaxis are caused by an inappropriate immune response directed against a normally harmless antigen [1]. Uncontrolled inflammation is also a risk factor for the development of cancer, and has been shown to contribute to tumour growth and metastasis [5].
PI3Kδ and PI3Kγ are extensively studied as potential targets for anti-inflammatory treatments and the fact that these isoforms have complementary roles in many aspects of immune function provides a clear rationale for the therapeutic use of PI3Kδ and/or PI3Kγ inhibitors (see Table 2 for inhibitors in clinical trials and Table 3 for inhibitors used in pre-clinical models). Indeed, inhibiting PI3Kδ and PI3Kγ in different mouse models of inflammatory disease produced promising results: the dual PI3Kδ/γ inhibitor TG100-115 reduced inflammatory cell infiltrates in an OVA-induced asthma model as well as in smoke-induced and LPS-induced models of airway inflammation when administered as an aerosol [29]. More recently another dual selective PI3Kδ/γ inhibitor, IP-145 (duvelisib), administered systemically also reduced eosinophil infiltration in an OVA-induced asthma model [30••]. Interestingly, these preclinical models show that both inhaled and systemic administration routes are effective. Selective PI3Kδ inhibition was found to restore glucocorticoid sensitivity in smoke-induced COPD models by preventing tyrosine nitration of HDAC-2 [31] and IC87114, a selective PI3Kδ inhibitor, reduced inflammatory cell infiltrates and IL-17 secretion in an OVA-induced asthma model [32]. PI3Kδ kinase dead mice are also protected against OVA-induced airway eosinophilia due to decreased Th2, but not Th1 mediated inflammation [33]. Collectively, these data show that class I PI3K signalling may play a key role in the pathogenesis of COPD and asthma [28,34]. This is strengthened by the observation that aberrant migration and decreased accuracy of human neutrophils derived from COPD patients is corrected by PI3Kδ inhibition [35]. However, increased neutrophil survival is also an important aspect of COPD and this was not influenced by isoform-selective PI3K inhibition [36]. GSK recently developed an inhaled p110δ inhibitor GSK2269557 which is currently in phase 2 clinical trials for COPD and asthma (NCT02294734). Another approach to PI3Kδ inhibition is being developed by Aquinox: their SHIP-1 activator AQX-1125 is being tested in a phase 2 study in exacerbating COPD patients (NCT01954628).
Table 2. Class I PI3K/mTOR inhibitors in clinical trials.
| Compound | Target | Indication | Clinical trial identifier |
|---|---|---|---|
| BYL-719 | p110α | Recurrent or Metastatic Squamous Cell Carcinoma |
NCT02145312, Phase 1/2 |
| MLN1117 (INK-1117) | p110α | Advanced Nonhaematologic Malignancies |
NCT01899053, Phase 1b |
| AZD6482 | p110β | Antiplatelet Effect | NCT00853450, Phase 1 |
| AMG 319 | p110δ | Haematologic Malignancies | NCT01300026, Phase 1 |
| GSK2269557 | p110δ | COPD | NCT02294734, Phase 2 |
| Idelalisib (CAL-101) | p110δ | Chronic Lymphocytic Leukaemia (CLL) Non-Hodgkin Lymphoma |
FDA and EMA approved, 2014 |
| INCB040093 | p110δ | B cell malignancies | NCT01905813, Phase 1 |
| TGR-1202 | p110δ | Cancer (CLL and B-cell lymphoma) | NCT01767766, Phase 1 |
| UCB-5857 | p110δ | Psoriasis | NCT02303509, Phase 1 |
| AZD8835 | p110α/p110δ | Advanced Solid Malignancies | NCT02260661, Phase 1 |
| BAY80-6946 (Copanlisib) | p110α/p110δ | Non-Hodgkin’s Lymphoma | NCT01660451, Phase 2 |
| GDC-0941 (Pictilisib) | p110α/p110δ | Breast Cancer | NCT01437566, Phase 2 |
| AZD8186 | p110β/p110δ | Prostate, Lung and Breast Cancer | NCT01884285, Phase 1 |
| GS-9820 (Acalisib) | p110β/p110δ | Lymphoid Malignancies | NCT01705847 Phase 1b |
| IPI-145 (Duvelisib) | p110δ/p110γ | Non-Hodgkin Lymphoma Small lymphocytic lymphoma; CLL |
NCT01882803, Phase 2 NCT02004522, Phase 3 |
| RP-6530 | p110δ/p110γ | Haematologic malignancies | NCT02017613, Phase 1 |
| RV-1729 | p110δ/p110γ | Asthma/COPD | NCT01813084, Phase 1 |
| BKM120 | pan-class I | Metastatic Breast Cancer | NCT01633060, Phase 3 |
| XL-147 (SAR245408) | pan-class I | Malignant neoplasm | NCT01587040, Phase 1 |
| ZSTK474 | pan-class I | Advanced Solid Malignancies | NCT01682473, Phase 1 |
| BEZ235 | pan-class I/mTOR | Renal Cancer | NCT01453595, Phase 1/2 |
| BGT226 | pan-class I/mTOR | Solid Tumours, Breast Cancer | NCT00600275, Phase 1/2 |
| GSK2126458 | pan-class I/mTOR | Solid Tumours Pulmonary Fibrosis |
NCT00972686, Phase 1 NCT01725139, Phase 1 |
| VS-5584 | pan-class I/mTOR | Non-haematologic metastatic cancer Lymphoma |
NCT01991938, Phase 1 |
| XL-765 (SAR245409) | pan-class I/mTOR (p110γ) | Malignant neoplasm | NCT01587040, Phase 2 |
| PX866 | pan PI3K | Metastatic prostate cancer | NCT01331083, Phase 2 |
| SF1126 | pan PI3K | Neuroblastoma | NCT02337309, Phase 1 |
| AQX-1125 | SHIP1 activator | COPD Atopic Dermatitis Interstitial Cystitis |
NCT01954628, Phase 2 NCT02324972, Phase 2 NCT01882543, Phase 2 |
Table 3. Some isoform-selective PI3K inhibitors used in pre-clinical studies: IC50 μM.
| Compound | Target | p110α | p110β | p110δ | p110γ | Vps34 | Ref. |
|---|---|---|---|---|---|---|---|
| A66 | p110α | 0.032 | 0.236 | 1.25 | 3.48 | [1] | |
| NVS-PI3-2 | p110α | 0.075 | 5.5 | 0.98 | 2.4 | [36] | |
| PW12 | p110α | 0.015 | 0.83 | 0.73 | 0.97 | [17] | |
| HBC-417 | p110β | 0.38 | 0.007 | 0.03 | 0.2 | [36] | |
| TGX-115 | p110β | 61 | 0.13 | 0.63 | >100 | [17] | |
| TGX-221 | p110β | 5 | 0.007 | 0.1 | 3.5 | [1] | |
| AS252424 | p110γ | 1.07 | >20 | >20 | 0.035 | [12] | |
| AS614006 | p110γ | 1.68 | 0.062 | 0.166 | 0.003 | [43] | |
| AS605240 | p110γ | 0.06 | 0.27 | 0.3 | 0.008 | [1] [52] | |
| CZC24832 | p110γ | >10 | 1.1 | 8.2 | 0.027 | [80] | |
| NVS-PI3-4 | p110γ | 1.8 | 0.25 | 0.75 | 0.09 | [36] | |
| TASP0415914a | p110γ | 0.029 | [51] | ||||
| GS-9820 | p110δ | 5.441 | 3.377 | 0.012 | 1.389 | 12.685 | [79] |
| GS-9829 | p110δ | >10 | >10 | 0.0703 | >10 | >10 | [48] |
| IC87114 | p110δ | >100 | ±5 | 0.1 | ±1 | [1] | |
| NVS-PI3-3 | p110δ | 0.18 | 0.6 | 0.003 | 0.31 | [36] | |
| PI-3065 | p110δ | 0.91 | 0.6 | 0.005 | >1000 | [41••] | |
| YM-024 | p110α/p110δ | 0.3 | 2.65 | 0.33 | 9.07 | [36] | |
| TG100-115 | p110δ/p110γ | 1300 | 1200 | 0.235 | 0.083 | [81] | |
| PI-103 | pan-class I | 0.0008 | 0.088 | 0.048 | 0.15 | [36] | |
| wortmannin | pan-PI3K/mTOR | 0.001 | 0.01 | 0.005 | 0.009 | [36] | |
| LY294002 | pan-PI3K/mTOR | 0.7 | 0.306 | 1.33 | 7.26 | [36] | |
| PIK-III | VPS34 | 3.96 | >9.1 | 1.2 | 3.04 | 0.018 | [75•] |
| SAR405 | VPS34 | >10 | >10 | >10 | >10 | 0.0012 | [74•] |
| VPS34-IN1 | VPS34 | 8.036 | 21.44 | 1.896 | 2 | 0.025 | [76•] |
No selectivity data published.
Experimental autoimmune encephalitis (EAE) is a model for multiple sclerosis. EAE progression is mainly driven by Th17 mediated inflammation of the CNS leading to the destruction of myelin, with antigen presenting cells (APC) playing a key role in the amplification of inflammation [37]. Genetic and pharmacological inhibition of PI3Kγ significantly reduced CNS inflammation and disease progression [38,39], while PI3Kδ kinase dead mice also showed reduced disease severity in conjunction with a defective Th17 response [40]. However, PI3Kδ signalling is also essential for the optimal development and function of Treg [10,11,41••]. In fact, our data indicate that despite reduced Th17 and Th1 responses, p110δ kinase dead mice are not protected against EAE progression, likely due to a concomitant reduction in Treg (A Stark, E Slack, K Okkenhaug, unpublished). Furthermore, PTEN deficient macrophages show increased expression/secretion of arginase I, which could inhibit the pro-inflammatory effects of DC and T cells and protect mice against EAE [42]. Psoriasis is also a Th17 driven disease and may benefit from PI3Kδ and/or PI3Kγ inhibition. Imiquimod-induced skin inflammation was reduced in PI3Kγ deficient and PI3Kδ kinase dead mice, while PI3Kδ (IC87114) and PI3Kγ (AS605240 and AS614006) inhibitors reduced pro-inflammatory cytokine secretion in human CD4+ memory T cells and PBMC from psoriasis patients [43]. Inhibiting PI3Kδ using IC87114 improved graft survival in a mouse heart transplant model [44] and delayed disease progression the NOD mouse model of diabetes [45].
PI3Kδ and PI3Kγ inhibition also attenuate disease progression in mouse models of SLE [46–50]. SLE is driven by autoreactive T cells and B cells, with renal immune complex deposition and macrophage driven inflammation key features of the disease. Treatment of MRL/lpr mice with the PI3Kδ selective inhibitor GS-9829 reduced kidney damage and prolonged life span. GS-9829 decreased effector-memory T cells and serum IL-6 and TNF-α levels, and also reduced macrophage infiltration in the kidneys [48]. These results were corroborated by another study reporting that the PI3Kδ selective inhibitor MSC2360844 can inhibit pro-inflammatory cytokine secretion by B cells, T cells and DC, and improve renal disease in a NZBW F1 mouse model [49]. Interestingly, haploinsufficient p110δWT/D910A showed resistance to an autoreactive B cell driven lupus-like syndrome when crossed to a Lyn−/− background, by a mechanism that appear to involve attenuated T cell function [50]. Treatment with the PI3Kδ inhibitor IC87114 also improved disease outcome in the BXSB model of SLE [46] and the PI3Kγ inhibitor AS605240 was effective in reducing disease severity and increasing life-span in MRL/lpr mice [47]. Furthermore the dual p110δ/p110γ inhibitor IP-145 inhibited disease progression the NZBWF1/J mouse model of SLE [30••].
Inhibitors of PI3Kδ, PI3Kγ and dual selective inhibition are also effective in alleviating the symptoms of RA in animal models. The PI3Kγ inhibitors AS605240, TASP0415914 and CZC24823 reduced the development of collagen induced arthritis (CIA) [39,51,52], and genetic as well as pharmacological inhibition improved symptoms in the effector phase K/BxN serum transfer and αCII models, mainly driven by neutrophilic inflammation [52,53]. Neutrophil migration to LTB4 is markedly reduced by dual PI3Kγ/δ inhibition compared to inhibition of either isoform alone [53]. However, while the dual PI3Kγ/δ inhibitor IP-145 could significantly reduce ankle swelling in a rat CIA model [30••], it did not improve RA scores in a recent phase 2 clinical trial, showing that animal models do not always predict clinical outcomes in patients. Using the K/BxN mouse model, a separate study show reduced disease development in PI3Kβ deficient mice at low, but not high doses of serum transfer, while additional PI3Kδ deficiency markedly reduced disease severity at high serum transfer doses, indicating a role for dual PI3Kδ/PI3Kβ inhibitors in this context [7].
ZSTK474 is a pan-class I PI3K inhibitor, and was also found to reduce inflammation and disease progression in RA and EAE mouse models [54,55]. However, there is a greater risk of adverse side effects when inhibiting PI3Kα and PI3Kβ in addition to PI3Kδ and/or PI3Kγ. Results from clinical trials show that pan-class I inhibitors are associated with hyperglycaemia, gastrointestinal and psychiatric effects [56]. Moreover, pan-class I inhibitors do not necessarily control inflammation better than dual PI3Kδ/PI3Kγ inhibitors [57].
PI3Kδ and PI3Kγ single and dual isoform selective inhibitors are generally well tolerated in mouse models, and mice deficient in p110δ or p110γ do not show overt clinical phenotypes despite established immunological defects. There is considerable redundancy among the PI3K isoforms and not all immune functions are PI3K dependent. Therefore, selective inhibition is likely to blunt, rather than completely ablate immune function. Mice are normally kept under specific pathogen free (SPF) conditions and are not exposed to common pathogens and co-morbidities; therefore potential increased susceptibility to infection needs to be considered in human trials [58]. Serious side effects were reported for patients treated with the PI3Kδ selective inhibitor idelalisib which included neutropenia, pneumonitis, colitis, diarrhoea and evidence of liver damage as indicated by the black box label attached to Zydelig (Idelalisib) [59••,60]. Among these, colitis appears to be the most common and it is worth noting that the kinase dead p110δD910A mice predicted PI3K inhibition can cause colitis [61]. The side effects associated with idelalisib suggest that transient, low dose, or local administration such as inhalation of PI3Kδ inhibitors should be considered to manage inflammatory conditions where possible.
Increased class I PI3K signalling is a cause of primary immunodeficiency
Recently, autosomal dominant gain of function mutations of PIK3CD (encoding p110δ) and PIK3R1 (encoding p85α) were described in individuals diagnosed with primary immune deficiencies [62••,63••,64–67]. These patients suffer from severe recurrent respiratory infections and have increased susceptibility to lymphoma. B cells from the patients were defective in immunoglobulin class switching. Many patients also presented with T cell lymphopenia associated with increased numbers of senescent T cells. Stimulation of patient T cells resulted in low cytokine production and increased activation-induced cell death, which could be partially rescued by the addition of IC87114 which also reduced PIP3 levels [62••,66]. These results indicate that idelalisib, or other PI3Kδ inhibitors under development, could significantly improve the outcome of immune-deficient patients with activating p110δ or p85α mutations. Also, in one patient, rapamycin treatment restored normal T cell populations [63••]. It remains to be determined whether an oral or inhaled route of administration would be preferable in these severely affected patients, and this is likely to depend on the disease profile of the individual patient and the specific side effects associated with each route.
Class I PI3K and cancer
The PI3K/AKT/mTOR pathway is of critical importance in tumour development and PIK3A (encoding p110α) as well as PTEN are among the most frequently mutated in human cancers. This provides a strong rationale for pan-class I as well as PI3Kα and PI3Kβ selective inhibition in treating solid cancers expressing these isoforms. Initially this strategy was met with limited success, mainly due to dose-limiting side effects and development of resistance due to negative feedback mechanisms activating alternative survival pathways. These issues can be addressed by combination-therapies inhibiting several signalling nodes at once, and current strategies for targeted inhibition of PI3Kα and PI3Kβ were recently reviewed [5,56,68]. PI3Kδ and PI3Kγ are potential targets in haematological cancers, and a notable success is the development of idelalisib which has shown remarkable efficacy in treating Chronic Lymphocytic Leukaemia (CLL) and non-Hodgkin’s lymphoma, and is now approved for clinical use [59••,60,69•].
In addition to targeting the PI3K pathway to inhibit tumour cell growth directly, PI3K inhibitors may also be used to improve anti-tumour immune responses. Genetic or pharmacological inhibition of PI3Kδ (PI-3065) reduced tumour burden and metastasis in a range of mouse cancer models including melanoma, thymoma, lung, breast and pancreatic cancer [41••]. In these models, PI3Kδ inhibition attenuated Treg function and tumour infiltration while leaving the cytotoxic T cell response relatively unscathed, resulting in enhanced anti-tumour immunity. PI3Kδ inhibition can also alleviate graft versus host disease while maintaining strong graft versus leukaemia effect [70].
Genetic or pharmacological inactivation of p110γ using TG100-115 and AS605240 was also found to reduce tumour growth and metastasis in melanoma, lung, pancreatic and breast cancer models. PI3Kγ signalling was required for myeloid cell recruitment to the tumour microenvironment through integrin α4β1 mediated adhesion, in response to growth factors and chemokines. Therefore, inhibition of p110γ signalling was effective in reducing general tumour associated inflammation and angiogenesis without affecting systemic numbers of myeloid cells [4].
Dual p110δ/p110γ inhibitors are already in clinical trials for haematological cancers, and are effective in controlling inflammation [30••]. It would therefore be interesting to evaluate the effect of these compounds on anti-tumour immune responses in solid cancer models.
Class II PI3K
Class II PI3K phosphorylate PIP and PI4P to form PI3P and PI(3,4)P2 respectively. Although the biology of class II PI3K signalling is still incompletely understood recent progress have indicated a role for PI3KC2 isoforms in immune cell signalling and tumour development [71]. However, because selective inhibitors against the class II PI3Ks have yet to be described, we do not consider this class further in this review.
Class III PI3K
Vps34 phosphorylate PIP to form PI3P at the pre-autophagosome or endosome leading to the recruitment of FYVE and PX domain containing proteins [1,2,72]. Vps34 associates with the protein kinase Vps15 in different protein complexes, and play an important role in membrane trafficking and protein sorting pathways. PI3P produced by Vps34 is critical for autophagosome and phagosome maturation as well as NOX2 mediated ROS production, thereby playing a key role in autophagy, as well as pathogen uptake and killing by innate immune cells.
Autophagy maintains normal cell function by removing misfolded proteins and damaged organelles, but also has specialised functions in the immune system. Autophagy mediates intracellular TLR activation by bringing cytoplasmic antigens in contact with TLR in the lysosome, and promotes cross-presentation of intracellular antigens on MHCII [2]. T cell-specific loss of Vps34 impairs invariant NKT cell development and peripheral T cell homeostasis, which ultimately lead to intestinal inflammation and wasting syndrome as a result of Treg dysfunction [73].
Recently three independent groups published selective Vps34 inhibitors: SAR405, PIK-III and Vps34-IN1 [74•,75•,76•,77]. These compounds will increase our understanding of the functions performed by Vps34 and opens up the possibility to target this kinase for therapeutic benefit. Already, SAR405 was found to act synergistically with the mTOR inhibitor everolimus to reduce proliferation in a renal tumour cell line [74•], while PIK-III was used to identify a novel autophagy substrate: NCOA4 binds ferritin and plays a role in recycling iron from red blood cells in the spleen [75•]. VPS34-IN1 revealed that class I and class II PI3K activity contribute to PIP3 mediated activation of SGK3 [76•]. This opens up the possibility that synergistic class I PI3K and Vps34 inhibitors could be used in the treatment of tumours with elevated SGK3 activity. Whether Vps34 inhibitors have potential for use in immune-mediated diseases remains to be explored: germ-line loss of Vps34 is embryonically lethal, and tissue specific deletion found a critical role for Vps34 in normal neuron, heart and liver function [78]. However, further study is required to establish if systemic Vps34 inhibition will be tolerated.
Conclusion
Although much progress has been made in understanding the role of PI3K signalling in inflammation and cancer, many questions still remain. PI3K signalling plays a complex and often opposing role in the regulation of immune responses and the effect of inhibiting PI3K is dependent on the context of activation. The factors modulating opposing functions of PI3K signalling are not yet clearly understood and warrant further investigation. This complexity of PI3K pathway regulation poses an interesting challenge for the therapeutic application of PI3K inhibitors: a better understanding of which isoforms are critical in different disease mechanisms and to what extent inhibition is favourable or not is essential. Animal models and early clinical trials show great potential in therapeutic targeting of this pathway in immune-related disorders and cancer, but do not always predict clinical efficacy.
Acknowledgements
Research in the Okkenhaug laboratory was funded by BBSRC (BBS/E/B/000C0409 and BBS/E/B/000C0407), the Wellcome Trust (095691) and GlaxoSmithKline.
Footnotes
Conflict of interest statement
AS and KO receive research funding from GSK. KO has consultancy agreements with GSK, Merck and Karus. SS and EMH are employees of GSK.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Hawkins PT, Stephens LR. PI3K signalling in inflammation. Biochim Biophys Acta. 2015;1851:882–897. doi: 10.1016/j.bbalip.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol. 2013;31:675–704. doi: 10.1146/annurev-immunol-032712-095946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster JG, Blunt MD, Carter E, Ward SG. Inhibition of PI3K signaling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and hematological malignancies. Pharmacol Rev. 2012;64:1027–1054. doi: 10.1124/pr.110.004051. [DOI] [PubMed] [Google Scholar]
- 4.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, Acevedo LM, Manglicmot JR, Song X, Wrasidlo W, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19:715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramadani F, Bolland DJ, Garcon F, Emery JL, Vanhaesebroeck B, Corcoran AE, Okkenhaug K. The PI3K isoforms p110 alpha and p110 delta are essential for pre-B cell receptor signaling and B cell development. Sci Signal. 2010;3 doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkarni S, Sitaru C, Jakus Z, Anderson KE, Damoulakis G, Davidson K, Hirose M, Juss J, Oxley D, Chessa TA, et al. PI3Kbeta plays a critical role in neutrophil activation by immune complexes. Sci Signal. 2011;4:ra23. doi: 10.1126/scisignal.2001617. [DOI] [PubMed] [Google Scholar]
- 8.Leverrier Y, Okkenhaug K, Sawyer C, Bilancio A, Vanhaesebroeck B, Ridley AJ. Class I phosphoinositide 3-kinase p110beta is required for apoptotic cell and Fcgamma receptor-mediated phagocytosis by macrophages. J Biol Chem. 2003;278:38437–38442. doi: 10.1074/jbc.M306649200. [DOI] [PubMed] [Google Scholar]
- 9.Soond DR, Bjorgo E, Moltu K, Dale VQ, Patton DT, Torgersen KM, Galleway F, Twomey B, Clark J, Gaston JS, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115:2203–2213. doi: 10.1182/blood-2009-07-232330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patton DT, Emery JL, Rowan WC, Okkenhaug K. PI3K p110 delta controls the differentiation and function of regulatory T cells. Immunology. 2008;125:129. [Google Scholar]
- 11.Patton DT, Wilson MD, Rowan WC, Soond DR, Okkenhaug K. The PI3K p110 delta regulates expression of CD38 on regulatory T cells. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condliffe AM, Davidson K, Anderson KE, Ellson CD, Crabbe T, Okkenhaug K, Vanhaesebroeck B, Turner M, Webb L, Wymann MP, et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106:1432–1440. doi: 10.1182/blood-2005-03-0944. [DOI] [PubMed] [Google Scholar]
- 13•.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports aberrant neutrophil migration and increased neutrophil proteinase activity in the elderly due to increased PI3K activity. PI3Kδ and PI3Kγ inhibitors improve neutrophil migration in cells from healthy donors aged 65 and above.
- 14.Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 15.Collmann E, Bohnacker T, Marone R, Dawson J, Rehberg M, Stringer R, Krombach F, Burkhart C, Hirsch E, Hollingworth GJ, et al. Transient targeting of phosphoinositide 3-kinase acts as a roadblock in mast cells’ route to allergy. J Allergy Clin Immunol. 2013;132:959–968. doi: 10.1016/j.jaci.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Ali K, Camps M, Pearce WP, Ji H, Ruckle T, Kuehn N, Pasquali C, Chabert C, Rommel C, Vanhaesebroeck B. Isoform-specific functions of phosphoinositide 3-kinases: p110 delta but not p110 gamma promotes optimal allergic responses in vivo. J Immunol. 2008;180:2538–2544. doi: 10.4049/jimmunol.180.4.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papakonstanti EA, Zwaenepoel O, Bilancio A, Burns E, Nock GE, Houseman B, Shokat K, Ridley AJ, Vanhaesebroeck B. Distinct roles of class IA PI3K isoforms in primary and immortalised macrophages. J Cell Sci. 2008;121:4124–4133. doi: 10.1242/jcs.032763. [DOI] [PubMed] [Google Scholar]
- 18.Koorella C, Nair JR, Murray ME, Carlson LM, Watkins SK, Lee KP. Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells. J Biol Chem. 2014;289:7747–7762. doi: 10.1074/jbc.M113.519686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, Vogel SN. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol. 2009;85:966–977. doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallah MP, Chelvarajan RL, Garvy BA, Bondada S. Role of phosphoinositide 3-kinase-Akt signaling pathway in the age-related cytokine dysregulation in splenic macrophages stimulated via TLR-2 or TLR-4 receptors. Mech Ageing Dev. 2011;132:274–286. doi: 10.1016/j.mad.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, Pearce WP, Berenjeno IM, Nock G, Filloux A, et al. The p110delta isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol. 2012;13:1045–1054. doi: 10.1038/ni.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai W, Liu H, Ji Q, Zhou Y, Liang L, Zheng R, Chen J, Liu Z, Yang H, Zhang P, et al. TLR3 regulates mycobacterial RNA-induced IL-10 production through the PI3K/AKT signaling pathway. Cell Signal. 2014;26:942–950. doi: 10.1016/j.cellsig.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–427. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan W, Morinaga H, Kim JJ, Bae E, Spann NJ, Heinz S, Glass CK, Olefsky JM. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 2010;29:4223–4236. doi: 10.1038/emboj.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schabbauer G, Matt U, Gunzl P, Warszawska J, Furtner T, Hainzl E, Elbau I, Mesteri I, Doninger B, Binder BR, et al. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol. 2010;185:468–476. doi: 10.4049/jimmunol.0902221. [DOI] [PubMed] [Google Scholar]
- 27.Banham-Hall E, Clatworthy MR, Okkenhaug K. The therapeutic potential for PI3K inhibitors in autoimmune rheumatic diseases. Open Rheumatol J. 2012;6:245–258. doi: 10.2174/1874312901206010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sriskantharajah S, Hamblin N, Worsley S, Calver AR, Hessel EM, Amour A. Targeting phosphoinositide 3-kinase delta for the treatment of respiratory diseases. Ann N Y Acad Sci. 2013;1280:35–39. doi: 10.1111/nyas.12039. [DOI] [PubMed] [Google Scholar]
- 29.Doukas J, Eide L, Stebbins K, Racanelli-Layton A, Dellamary L, Martin M, Dneprovskaia E, Noronha G, Soll R, Wrasidlo W, et al. Aerosolized phosphoinositide 3-kinase gamma/delta inhibitor TG100-115 [3-[2,4-diamino-6-(3-hydroxyphenyl)pteridin-7-yl]phenol] as a therapeutic candidate for asthma and chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2009;328:758–765. doi: 10.1124/jpet.108.144311. [DOI] [PubMed] [Google Scholar]
- 30••.Winkler DG, Faia KL, DiNitto JP, Ali JA, White KF, Brophy EE, Pink MM, Proctor JL, Lussier J, Martin CM, et al. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20:1364–1374. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]; Extensive pre-clinical evaluation of the dual PI3Kδ/PI3Kγ inhibitor IPI-145 (duvelisib) in murine models of asthma, arthritis and lupus.
- 31.Marwick JA, Caramori G, Stevenson CS, Casolari P, Jazrawi E, Barnes PJ, Ito K, Adcock IM, Kirkham PA, Papi A. Inhibition of PI3Kdelta restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med. 2009;179:542–548. doi: 10.1164/rccm.200810-1570OC. [DOI] [PubMed] [Google Scholar]
- 32.Park SJ, Lee KS, Kim SR, Min KH, Moon H, Lee MH, Chung CR, Han HJ, Puri KD, Lee YC. Phosphoinositide 3-kinase delta inhibitor suppresses interleukin-17 expression in a murine asthma model. Eur Respir J. 2010;36:1448–1459. doi: 10.1183/09031936.00106609. [DOI] [PubMed] [Google Scholar]
- 33.Nashed BF, Zhang T, Al-Alwan M, Srinivasan G, Halayko AJ, Okkenhaug K, Vanhaesebroeck B, Hayglass KT, Marshall AJ. Role of the phosphoinositide 3-kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol. 2007;37:416–424. doi: 10.1002/eji.200636401. [DOI] [PubMed] [Google Scholar]
- 34.Marwick JA, Chung KF, Adcock IM. Phosphatidylinositol 3-kinase isoforms as targets in respiratory disease. Ther Adv Respir Dis. 2010;4:19–34. doi: 10.1177/1753465809352792. [DOI] [PubMed] [Google Scholar]
- 35.Sapey E, Stockley JA, Greenwood H, Ahmad A, Bayley D, Lord JM, Insall RH, Stockley RA. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1176–1186. doi: 10.1164/rccm.201008-1285OC. [DOI] [PubMed] [Google Scholar]
- 36.Juss JK, Hayhoe RP, Owen CE, Bruce I, Walmsley SR, Cowburn AS, Kulkarni S, Boyle KB, Stephens L, Hawkins PT, et al. Functional redundancy of class I phosphoinositide 3-kinase (PI3K) isoforms in signaling growth factor-mediated human neutrophil survival. PLOS ONE. 2012;7:e45933. doi: 10.1371/journal.pone.0045933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Park D, Abdul-Muneer PM, Xu B, Wang H, Xing B, Wu D, Li S. PI3Kgamma inhibition alleviates symptoms and increases axon number in experimental autoimmune encephalomyelitis mice. Neuroscience. 2013;253:89–99. doi: 10.1016/j.neuroscience.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berod L, Heinemann C, Heink S, Escher A, Stadelmann C, Drube S, Wetzker R, Norgauer J, Kamradt T. PI3Kgamma deficiency delays the onset of experimental autoimmune encephalomyelitis and ameliorates its clinical outcome. Eur J Immunol. 2011;41:833–844. doi: 10.1002/eji.201040504. [DOI] [PubMed] [Google Scholar]
- 40.Haylock-Jacobs S, Comerford I, Bunting M, Kara E, Townley S, Klingler-Hoffmann M, Vanhaesebroeck B, Puri KD, McColl SR. PI3Kdelta drives the pathogenesis of experimental autoimmune encephalomyelitis by inhibiting effector T cell apoptosis and promoting Th17 differentiation. J Autoimmun. 2011;36:278–287. doi: 10.1016/j.jaut.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 41••.Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, Bouabe H, Scudamore CL, Hancox T, Maecker H, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]; PI3Kδ inhibition attenuate regulatory T cell function, thereby enabling T cell mediated anti-tumour immune responses. This study highlights that PI3Kδ inhibition can be effective against solid tumours in addition to haematologic malignancies.
- 42.Sahin E, Haubenwallner S, Kuttke M, Kollmann I, Halfmann A, Dohnal AB, Chen L, Cheng P, Hoesel B, Einwallner E, et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J Immunol. 2014;193:1717–1727. doi: 10.4049/jimmunol.1302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roller A, Perino A, Dapavo P, Soro E, Okkenhaug K, Hirsch E, Ji H. Blockade of phosphatidylinositol 3-kinase (PI3K)delta or PI3K gamma reduces IL-17 and ameliorates imiquimod-induced psoriasis-like dermatitis. J Immunol. 2012;189:4612–4620. doi: 10.4049/jimmunol.1103173. [DOI] [PubMed] [Google Scholar]
- 44.Ying H, Fu H, Rose ML, McCormack AM, Sarathchandra P, Okkenhaug K, Marelli-Berg FM. Genetic or pharmaceutical blockade of phosphoinositide 3-kinase p110delta prevents chronic rejection of heart allografts. PLOS ONE. 2012;7:e32892. doi: 10.1371/journal.pone.0032892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durand CA, Richer MJ, Brenker K, Graves M, Shanina I, Choi K, Horwitz MS, Puri KD, Gold MR. Selective pharmacological inhibition of phosphoinositide 3-kinase p110delta opposes the progression of autoimmune diabetes in non-obese diabetic (NOD) mice. Autoimmunity. 2013;46:62–73. doi: 10.3109/08916934.2012.732130. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Zhang L, Wei P, Zhang H, Liu C. Inhibition of PI3Kdelta improves systemic lupus in mice. Inflammation. 2014;37:978–983. doi: 10.1007/s10753-014-9818-0. [DOI] [PubMed] [Google Scholar]
- 47.Barber DF, Bartolome A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, Camps M, Ruckle T, Schwarz MK, Rodriguez S, et al. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 48.Suarez-Fueyo A, Rojas JM, Cariaga AE, Garcia E, Steiner BH, Barber DF, Puri KD, Carrera AC. Inhibition of PI3Kdelta reduces kidney infiltration by macrophages and ameliorates systemic lupus in the mouse. J Immunol. 2014;193:544–554. doi: 10.4049/jimmunol.1400350. [DOI] [PubMed] [Google Scholar]
- 49.Haselmayer P, Camps M, Muzerelle M, El Bawab S, Waltzinger C, Bruns L, Abla N, Polokoff MA, Jond-Necand C, Gaudet M, et al. Characterization of novel PI3Kdelta inhibitors as potential therapeutics for SLE and lupus nephritis in pre-clinical studies. Front Immunol. 2014;5:233. doi: 10.3389/fimmu.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maxwell MJ, Tsantikos E, Kong AM, Vanhaesebroeck B, Tarlinton DM, Hibbs ML. Attenuation of phosphoinositide 3-kinase delta signaling restrains autoimmune disease. J Autoimmun. 2012;38:381–391. doi: 10.1016/j.jaut.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Oka Y, Yabuuchi T, Oi T, Kuroda S, Fujii Y, Ohtake H, Inoue T, Wakahara S, Kimura K, Fujita K, et al. Discovery of N-{5-[3-(3-hydroxypiperidin-1-yl)-1,2,4-oxadiazol-5-yl]-4-methyl-1,3-thiazol-2-yl}acetamide (TASP0415914) as an orally potent phosphoinositide 3-kinase gamma inhibitor for the treatment of inflammatory diseases. Bioorg Med Chem. 2013;21:7578–7583. doi: 10.1016/j.bmc.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 52.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 53.Randis TM, Puri KD, Zhou H, Diacovo TG. Role of PI3Kdelta and PI3Kgamma in inflammatory arthritis and tissue localization of neutrophils. Eur J Immunol. 2008;38:1215–1224. doi: 10.1002/eji.200838266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haruta K, Mori S, Tamura N, Sasaki A, Nagamine M, Yaguchi S, Kamachi F, Enami J, Kobayashi S, Yamori T, et al. Inhibitory effects of ZSTK474, a phosphatidylinositol 3-kinase inhibitor, on adjuvant-induced arthritis in rats. Inflamm Res. 2012;61:551–562. doi: 10.1007/s00011-012-0444-8. [DOI] [PubMed] [Google Scholar]
- 55.Xue Z, Li W, Wang H, Huang B, Ge Z, Gu C, Liu Y, Zhang K, Yang J, Han R, et al. ZSTK474, a novel PI3K inhibitor, modulates human CD14+ monocyte-derived dendritic cell functions and suppresses experimental autoimmune encephalomyelitis. J Mol Med (Berl) 2014;92:1057–1068. doi: 10.1007/s00109-014-1158-x. [DOI] [PubMed] [Google Scholar]
- 56.Martini M, Ciraolo E, Gulluni F, Hirsch E. Targeting PI3K in cancer: any good news? Front Oncol. 2013;3:108. doi: 10.3389/fonc.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams O, Houseman BT, Kunkel EJ, Aizenstein B, Hoffman R, Knight ZA, Shokat KM. Discovery of dual inhibitors of the immune cell PI3Ks p110delta and p110gamma: a prototype for new anti-inflammatory drugs. Chem Biol. 2010;17:123–134. doi: 10.1016/j.chembiol.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maus UA, Backi M, Winter C, Srivastava M, Schwarz MK, Ruckle T, Paton JC, Briles D, Mack M, Welte T, et al. Importance of phosphoinositide 3-kinase gamma in the host defense against pneumococcal infection. Am J Respir Crit Care Med. 2007;175:958–966. doi: 10.1164/rccm.200610-1533OC. [DOI] [PubMed] [Google Scholar]
- 59••.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report results from a phase 3 clinical trial testing idelalisib and retuximab vs placebo and retuximab. This study is the first to report PI3Kδ inhibition greatly improve progression free survival rates in CLL patients with significant co-morbidities.
- 60.Miller BW, Przepiorka D, de Claro RA, Lee K, Nie L, Simpson N, Gudi R, Saber H, Shord S, Bullock J, et al. FDA approval: idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin Cancer Res. 2015;21:1525–1529. doi: 10.1158/1078-0432.CCR-14-2522. [DOI] [PubMed] [Google Scholar]
- 61.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 62••.Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of an autosomal dominant gain of function mutation in the PIK3D gene, causing primary immunodeficiency. T and B cell abnormalities are reported, and some could be reversed by treating patient cells with PI3Kδ inhibitors in vitro.
- 63••.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports three gain of function PIK3D mutations leading to immundeficiency associated with abnormal T cell and B cell responses. Treatment of one patient with rapamycin improved T cell abnormalities.
- 64.Crank MC, Grossman JK, Moir S, Pittaluga S, Buckner CM, Kardava L, Agharahimi A, Meuwissen H, Stoddard J, Niemela J, et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol. 2014;34:272–276. doi: 10.1007/s10875-014-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kracker S, Curtis J, Ibrahim MA, Sediva A, Salisbury J, Campr V, Debre M, Edgar JD, Imai K, Picard C, et al. Occurrence of B-cell lymphomas in patients with activated phosphoinositide 3-kinase delta syndrome. J Allergy Clin Immunol. 2014;134:233–236. doi: 10.1016/j.jaci.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deau MC, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, Cavazzana M, Picard C, Durandy A, Fischer A, et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest. 2014;124:3923–3928. doi: 10.1172/JCI75746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lucas CL, Zhang Y, Venida A, Wang Y, Hughes J, McElwee J, Butrick M, Matthews H, Price S, Biancalana M, et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med. 2014;211:2537–2547. doi: 10.1084/jem.20141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2014;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]; Results from a phase 2 clinical trial evaluating the efficacy of idelalisib in iNHL patients who did not respond to standard treatments show idelalisib has anti-tumour effects and an acceptable safety profile.
- 70.Doisne JM, Huber CM, Okkenhaug K, Colucci F. Immunomodulation of selective naive T cell functions by p110delta inactivation improves the outcome of mismatched cell transplantation. Cell Rep. 2015;10:702–710. doi: 10.1016/j.celrep.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maffucci T, Falasca M. New insight into the intracellular roles of class II phosphoinositide 3-kinases. Biochem Soc Trans. 2014;42:1378–1382. doi: 10.1042/BST20140140. [DOI] [PubMed] [Google Scholar]
- 72.Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 73.Parekh VV, Wu L, Boyd KL, Williams JA, Gaddy JA, Olivares-Villagomez D, Cover TL, Zong WX, Zhang J, Van Kaer L. Impaired autophagy, defective T cell homeostasis, and a wasting syndrome in mice with a T cell-specific deletion of Vps34. J Immunol. 2013;190:5086–5101. doi: 10.4049/jimmunol.1202071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Ronan B, Flamand O, Vescovi L, Dureuil C, Durand L, Fassy F, Bachelot MF, Lamberton A, Mathieu M, Bertrand T, et al. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat Chem Biol. 2014;10:1013–1019. doi: 10.1038/nchembio.1681. [DOI] [PubMed] [Google Scholar]; Report of a selective class III PI3K inhibitor, SAR405. The authors show that this compound inhibit proliferation in a renal tumour cell line in conjuction with mTOR inhibition.
- 75•.Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol. 2014;16:1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]; Report of a selective class III PI3K inhibitor, PIK-III. The authors used this compund to screen for new autophagy substrates, and identified a role for NCOA4 in iron homeostasis.
- 76•.Bago R, Malik N, Munson MJ, Prescott AR, Davies P, Sommer E, Shpiro N, Ward R, Cross D, Ganley IG, et al. Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem J. 2014;463:413–427. doi: 10.1042/BJ20140889. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of a selective class III PI3K inhibitor, VPS34-IN1. This compound was used to show that PI3P derived from vps34 and class I PI3K activity activate SGK3.
- 77.Marsh T, Debnath J. Ironing out VPS34 inhibition. Nat Cell Biol. 2014;17:1–3. doi: 10.1038/ncb3089. [DOI] [PubMed] [Google Scholar]
- 78.Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shugg RP, Thomson A, Tanabe N, Kashishian A, Steiner BH, Puri KD, Pereverzev A, Lannutti BJ, Jirik FR, Dixon SJ, et al. Effects of isoform-selective phosphatidylinositol 3-kinase inhibitors on osteoclasts: actions on cytoskeletal organization, survival, and resorption. J Biol Chem. 2013;288:35346–35357. doi: 10.1074/jbc.M113.507525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergamini G, Bell K, Shimamura S, Werner T, Cansfield A, Muller K, Perrin J, Rau C, Ellard K, Hopf C, et al. A selective inhibitor reveals PI3Kgamma dependence of T(H)17 cell differentiation. Nat Chem Biol. 2012;8:576–582. doi: 10.1038/nchembio.957. [DOI] [PubMed] [Google Scholar]
- 81.Doukas J, Wrasidlo W, Noronha G, Dneprovskaia E, Fine R, Weis S, Hood J, Demaria A, Soll R, Cheresh D. Phosphoinositide 3-kinase gamma/delta inhibition limits infarct size after myocardial ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:19866–19871. doi: 10.1073/pnas.0606956103. [DOI] [PMC free article] [PubMed] [Google Scholar]