Summary
Small RNAs are used to silence transposable elements (TEs) in many eukaryotes, which use diverse evolutionary solutions to identify TEs. In ciliated protozoans, small-RNA-mediated comparison of the germline and somatic genomes underlies identification of TE-related sequences, which are then eliminated from the soma. Here, we describe an additional mechanism of small-RNA-mediated identification of TE-related sequences in the ciliate Tetrahymena. We show that a limited set of internal eliminated sequences (IESs) containing potentially active TEs produces a class of small RNAs that recognize not only the IESs from which they are derived, but also other IESs in trans. This trans recognition triggers the expression of yet another class of small RNAs that identify other IESs. Therefore, TE-related sequences in Tetrahymena are robustly targeted for elimination by a genome-wide trans-recognition network accompanied by a chain reaction of small RNA production.
Graphical Abstract
Highlights
-
•
Two types of siRNAs (scnRNAs) are expressed in Tetrahymena sexual reproduction
-
•
Early-scnRNAs are produced from Type-A IESs containing potentially active transposons
-
•
Early-scnRNAs trans-recognize Type-B IESs and trigger late-scnRNA production in cis
-
•
Late-scnRNA production forms trans-recognition network for robust IES elimination
Noto et al. show that a class of Tetrahymena small RNAs from internal eliminated sequences (IESs) containing potentially active transposons recognize not only the IESs from which they are derived but also other IESs in trans, triggering the expression of another class of small RNAs and ensuring robust DNA elimination.
Introduction
Transposable elements (TEs) are threats to host genome integrity in addition to being drivers of host genome evolution. Hosts have therefore developed defense mechanisms to convert TEs into silent residents (Levin and Moran, 2011). Different eukaryotes use a variety of RNAi-related mechanisms to distinguish TEs from the host genome and to downregulate them. In mammals and flies, TE silencing is mediated by Piwi-interacting RNAs (piRNAs) that are produced from genomic loci, called piRNA clusters, where accumulation of TE remnants creating a genetic memory of TEs (Guzzardo et al., 2013; Siomi et al., 2011). In nematodes, however, piRNAs have enormous targeting capacity beyond TEs and are counteracted by CSR-1-bound 22G-RNAs that hold an epigenetic memory of previous gene expression (Seth et al., 2013; Wedeles et al., 2013). In contrast to these memory-based TE identification systems in metazoans, plants and yeasts utilize intrinsic features of TEs for their identification. In Arabidopsis pollen formation, the active de-silencing of TEs in somatic cells causes accumulation of TE-derived aberrant RNAs, producing small interfering RNAs (siRNAs), which then move into an adjacent germline cell to reinforce TE silencing (Slotkin et al., 2009). The yeasts Cryptococcus neoformans and Schizosaccharomyces pombe use transcripts with suboptimal RNA structure in the host-splicing machinery as a sign of non-self genetic material and compose siRNAs from these transcripts to silence TEs (Dumesic et al., 2013; Lee et al., 2013).
Nuclear dimorphism allows ciliated protozoans to identify TEs in a unique way (Chalker et al., 2013). Most ciliates contain two types of nuclei in a single cell: the transcriptionally silent germline micronucleus (MIC) and the transcriptionally active somatic macronucleus (MAC). During sexual reproduction (conjugation, Figure 1A), the MIC produces new MICs and MACs, while the parental MAC is discarded. TEs and their remnants are mostly removed from the new MAC by programmed DNA elimination (Arnaiz et al., 2012; Chen et al., 2014; Coyne et al., 2012). In the two closely related oligohymenophorean ciliates Paramecium and Tetrahymena, genome-wide production of siRNAs (called scnRNAs) occurs in the MIC upon sexual reproduction. These scnRNAs then move into the parental MAC, where scnRNAs complementary to its genome are degraded. The remaining MIC-specific scnRNAs are then transferred into the new MAC, where they target complementary DNA for elimination (Mochizuki et al., 2002; Sandoval et al., 2014; Schoeberl et al., 2012). In a conceptually similar strategy, the more distantly related spirotrichous ciliates (e.g., Oxytricha) produce small RNAs from the parental MAC that then protect their complementary DNA in the new MAC from elimination (Fang et al., 2012). Both mechanisms identify TEs and their remnants as sequences present in the MIC, but not in the MAC.
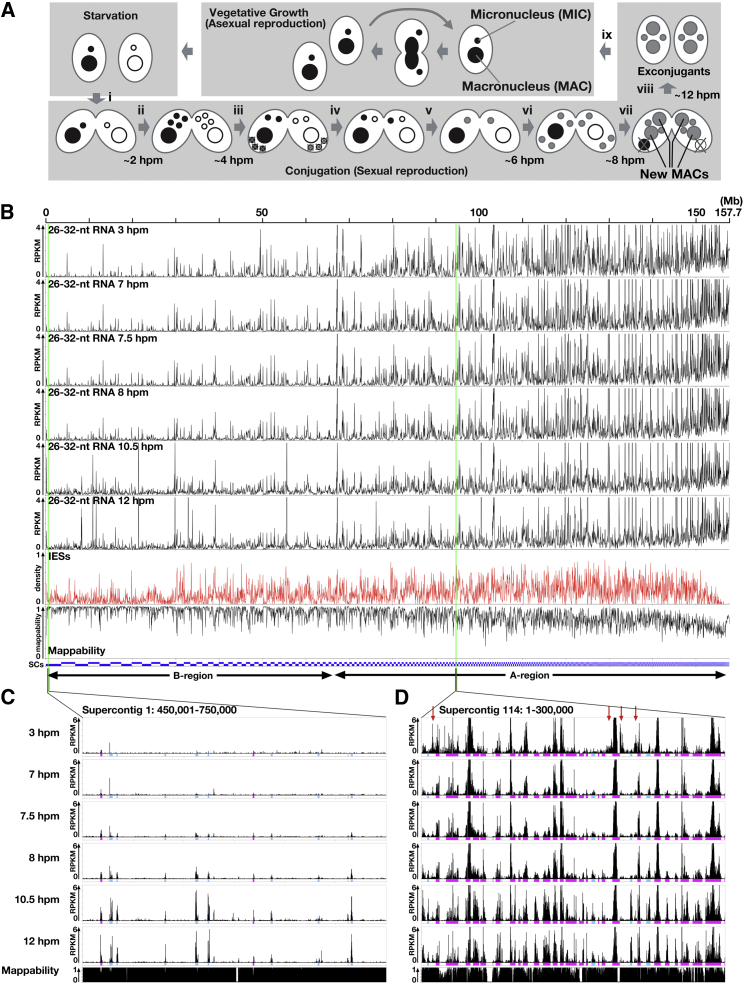
Figure 1.
Two Types of scnRNAs
(A) A Tetrahymena cell contains a macronucleus (MAC) and a micronucleus (MIC). During vegetative growth, both the MAC and the MIC divide and segregate to daughter cells. Mixing starved cells of different mating types induces conjugation (i). The MICs undergo meiosis (ii), and one of the selected products divides mitotically to form two pronuclei (iii). One of the pronuclei crosses the conjugation bridge (iv) and fuses with the stationary pronucleus to produce the zygotic nucleus (v), which then divides twice (vi) to form two new MACs and two MICs (vii). The parental MAC is degraded, and the pair is dissolved (viii). The exconjugants resume vegetative growth when the nutrient supply is restored (ix). The approximate time when each event occurs is indicated (hpm, hours post-mixing).
(B) 1,464 MIC genome supercontigs (SCs, blue bars) were ordered by their lengths (longest to shortest) and concatenated. Normalized numbers (reads per kilobase per million reads [RPKM]) of sequenced 26- to 32-nt RNAs from WT cells at the indicated time points that map uniquely to the MIC genome are shown as histograms with 50-kb bins. The densities of IESs and mappable (unique) sequences are also shown. The drop in IES density in the region containing very short SCs is probably because these SCs are shorter than most of the IESs, and the prediction of IESs from them failed. The regions enlarged in (C and D) are marked with green lines. Longer (1–50) and shorter (51–1,464) MIC SCs represent B- and A-regions of the MIC genome, respectively.
(C and D) Small RNA expression from the indicated 300-kb windows (shown and analyzed as in B, except with 100-nt bins). Colored boxes indicate the positions of IESs (magenta, type A; sky blue, type B; see Figure 3 for the IES classification). In (D), the arrows mark MAC-destined regions that are the origins of Early-scnRNAs that accumulated prominently at early stages (3 hpm) but were degraded later.
In Tetrahymena, DNA elimination reproducibly removes >8,000 DNA segments, called internal eliminated sequences (IESs), that comprise ∼30% of the MIC genome (Coyne et al., 2012). IESs include all known TEs and certain unique sequences that are potentially remnants of TEs (Eisen et al., 2006; Yao et al., 2003). Tetrahymena scnRNAs (∼26- to 32-nt [mainly 28- to 30-nt] siRNAs) are produced in the MIC by the Dicer protein Dcl1p (Malone et al., 2005; Mochizuki and Gorovsky, 2005) and are loaded into the Argonaute protein Twi1p (Noto et al., 2010; Woehrer et al., 2015). The absence of Dcl1p or Twi1p completely blocks DNA elimination, indicating that scnRNAs are necessary for the elimination of all IESs.
It has been suggested that IESs in Tetrahymena are identified solely by scnRNA-mediated subtraction of the MAC genome sequence from the MIC genome (Mochizuki et al., 2002). However, in such a genome-subtraction system, any errors in DNA elimination can be transgenerationally inherited. Therefore, there could be additional mechanisms ensuring the accuracy of DNA elimination. Furthermore, our previous report that the production of scnRNAs from the MIC genome is non-uniform (Schoeberl et al., 2012) indicates that an additional mechanism beyond simple genome-subtraction is involved in DNA elimination. In this study, we describe a small RNA-mediated regulation of DNA elimination in Tetrahymena, in which a genome-wide trans-recognition network functions in a piRNA pathway-like memory-based genome surveillance and information amplification system to ensure the robust transgenerational transmission of TE memory.
Results
Late-scnRNAs Are Expressed at Late Conjugation Stages
We previously reported that scnRNAs in Tetrahymena are produced non-uniformly from the MIC genome at two levels (Schoeberl et al., 2012): (1) although scnRNAs are produced from a variety of regions in the MIC genome, many >100-kb stretches of MIC regions are scnRNA deserts (referred to as global bias); and (2) within scnRNA-producing regions, scnRNAs are preferentially produced from IESs (referred to as local bias). We confirmed these observations by analyzing the production of scnRNAs throughout the MIC genome (Figure 1B). Currently available sequences of the MIC genome, which were derived from five MIC chromosomes, were assembled into 1,464 supercontigs (SCs). These SCs were ordered by length (longest to shortest; see Figure 1B, “SCs”) and concatenated as 158 Mb of linear DNA, which was used as the MIC genome for this study. The first 25 nt of scnRNA sequences (26–32 nt) from wild-type (WT) cells were then mapped to the MIC genome, and the number of scnRNAs mapping uniquely to the MIC genome was determined.
As shown in Figures 1B–1D, at early- (3 hr post-mixing [hpm]) to mid-conjugation (7 hpm), scnRNAs were mainly produced from regions of the MIC genome represented by shorter SCs (for example, SC114; Figure 1D), which are referred to as A-regions. In contrast, scnRNAs were rarely produced from regions represented by longer SCs (for example, SC1; Figure 1C), which are referred to as B-regions. This pattern is partially caused by the local bias because IESs are concentrated in A-regions (Figure 1B, IESs). However, local bias does not fully explain the scnRNA production pattern; although IESs are also present in B-regions, they are not the origin of scnRNAs (Figure 1C, 3 hpm and 7 hpm, where magenta and sky-blue boxes represent IESs). Therefore, we conclude that the global bias is due to a lack of scnRNA production from the B-regions of MIC chromosomes.
In contrast to early conjugation, 26- to 32-nt small RNAs were expressed from B-regions at late conjugation stages (7.5–12 hpm, Figures 1B and 1C) and were produced almost exclusively from IESs (Figure 1C). We refer to small RNAs expressed only at late conjugation stages as “Late-scnRNAs” and scnRNAs expressed in the MIC during early conjugation as “Early-scnRNAs.” Henceforth, the generic term scnRNA includes both Early- and Late-scnRNAs. Because DNA-dependent RNA polymerase proteins were detected only in the parental and new MACs at late conjugation (Mochizuki and Gorovsky, 2004) and the parental MACs lack IESs, Late-scnRNAs must be expressed in the new MAC. Late-scnRNAs from most of the B-region IESs were detected as early as 7.5 hpm, immediately after the formation of the new MACs (see Figure 1A) and before the earliest detectable IES excisions (10 hpm) (Austerberry et al., 1984; Saveliev and Cox, 1994), indicating that Late-scnRNAs are produced before the IESs from which they are derived are excised from new MAC chromosomes.
Similar IES-derived siRNAs (iesRNAs) are expressed in Paramecium during late stages of sexual reproduction (Sandoval et al., 2014). However, iesRNAs are distinct from Tetrahymena Late-scnRNAs in several respects: (1) Late-scnRNAs are produced from IESs prior to their excision, but Paramecium iesRNAs are made from excised IESs; (2) some IESs express only Late-scnRNAs in Tetrahymena, but most, if not all, Paramecium IESs express both scnRNAs (probably the equivalent of Early-scnRNAs) and iesRNAs; (3) Early- and Late-scnRNAs are probably produced by the same Dicer enzyme (see below), but Paramecium scnRNAs and iesRNAs are processed by distinct Dicers. The potential functional relevance of these IES-derived small RNAs will be discussed in the Discussion section.
Late-scnRNAs Are Loaded into Two Argonautes
Three Argonaute-encoding genes, TWI1, TWI2, and TWI11, are highly expressed at late conjugation stages (Figure 2A). To identify the Argonaute protein(s) loaded by Late-scnRNAs, we focused on Twi1p and Twi11p, because Twi2p is known to interact with another class of (23- to 24-nt) siRNAs (Couvillion et al., 2009). Consistent with the mRNA expression patterns, Twi1p is accumulated throughout conjugation, whereas Twi11p is only expressed during the late conjugation stages (Figure 2B).
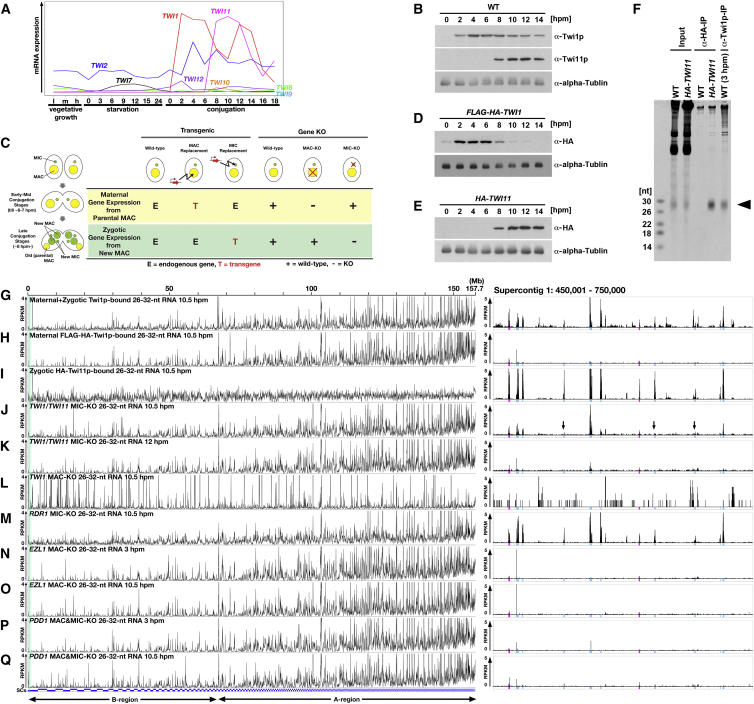
Figure 2.
Late-scnRNAs Interact with Twi1p and Twi11p
(A) Expression of the Argonaute genes in vegetatively growing (l, m, and h indicate low, medium, and high density, respectively), starved (numbers indicate hours after removal of nutrients), and conjugating (numbers indicate hpm) cells based on a microarray analysis.
(B) Twi1p and Twi11p from wild-type cells at the indicated time points of conjugation were detected by western blotting using the indicated antibodies.
(C) Different transgenic and gene-knockout strategies. In the vegetative and early- to mid-stage conjugating cells, the parental MACs (yellow) contribute all (maternal) gene expression, whereas the new MACs, which are formed from the MIC (green), provide zygotic gene expression at late conjugation.
(D and E) Proteins from cells expressing FLAG-HA-Twi1p from the maternal TWI1 loci by MAC replacement (D) or cells expressing HA-Twi11p from the zygotic TWI11 loci by MIC replacement (E) at the indicated time points of conjugation were detected by western blotting.
(F) HA-Twi11p was immunopurified using an anti-HA antibody (α-HA-IP) from the HA-TWI11 conjugating culture at 10.5 hpm. As a negative control, wild-type cells (WT) were used for similar immunopurification. Early-scnRNAs were immunopurified with Twi1p by an anti-Twi1p antibody (α-Twi1p-IP) from the wild-type conjugating culture at 3 hpm. RNAs were separated on denaturing gels and stained using a nucleic-acid-specific dye. Arrowhead indicates scnRNAs.
(G–Q) 26- to 32-nt RNAs from the indicated immunopurified RNAs or strains were analyzed as in Figure 1B. The 300-kb window on the right is marked with a green line.
See also Figure S1.
Small RNAs co-precipitated with Twi1p by an anti-Twi1p antibody at 10.5 hpm were analyzed. A fraction of Twi1p-bound 26- to 32-nt small RNAs were derived from B-regions (Figure 2G), indicating that maternal and/or zygotic Twi1p binds to Late-scnRNAs. Next, to distinguish maternal from zygotic Twi1p, FLAG-HA-tagged Twi1p (FLAG-HA-Twi1p) was expressed from a transgene inserted only into the maternal MAC in place of the endogenous TWI1 gene (MAC replacement, see Figure 2C). The accumulation of maternally expressed FLAG-HA-Twi1p peaked at 2–6 hpm and persisted until 12 hpm (Figure 2D). In contrast, total Twi1p detected by an anti-Twi1p antibody accumulated stably until 14 hpm in the WT cells (Figure 2B). These results indicate that both maternal and zygotic Twi1p are accumulated at late conjugation stages (8–12 hpm); however, B-region-derived 26- to 32-nt small RNAs did not co-precipitate with FLAG-HA-Twi1p at 10.5 hpm (Figure 2H). We suggest that this result was not caused by inhibition of Late-scnRNA loading by the FLAG-HA tag, because we found that B-region-derived 26- to 32-nt small RNAs were co-precipitated with FLAG-HA-Twi1p at 10.5 hpm when FLAG-HA-Twi1p was ectopically expressed by the inducible MTT1 promoter from 5 hpm (Figure S1L). Therefore, we conclude that maternal Twi1p binds only to Early-scnRNAs, whereas zygotic Twi1p interacts with Late-scnRNAs.
To analyze Twi11p-associated small RNAs, the TWI11 locus in the MIC was replaced with an HA-TWI11 gene (Figures S1A and S1B), resulting in zygotic expression of HA-Twi11p from the new MAC at late conjugation (MIC replacement; see Figure 2C). The temporal expression patterns of HA-Twi11p and endogenous Twi11p in WT cells were indistinguishable (compare Figure 2B, α-Twi11p and Figure 2E, α-HA), indicating that Twi11p is mainly zygotically expressed. Small RNAs that co-precipitated with HA-Twi11p shared a length distribution (Figures 2F and S1C) and base composition, including the Dicer-product signature (5′ U bias and A bias for the third base from the 3′ end) (Mochizuki and Kurth, 2013), with Twi1p-bound Early-scnRNAs (Figures S1D–S1G). Therefore, they were most likely produced by the same Dicer, Dcl1p. Twi11p-bound small RNAs were resistant to periodate oxidation (Figure S1H), indicating that, similar to Early-scnRNAs (Kurth and Mochizuki, 2009), they were modified at their 3′ ends, presumably by 2′-O-methylation.
The origins of HA-Twi11p-bound small RNAs included IESs in the B-regions (Figure 2I), indicating that Twi11p binds to Late-scnRNAs. Twi11p may predominantly interact with Late-scnRNAs, although we cannot exclude the possibility that certain Early-scnRNAs dissociate from Twi1p and are reloaded into Twi11p. HA-Twi11p-bound small RNAs mapped relatively homogeneously to the entire MIC genome (Figure 2I, left). This result is in clear contrast to the total (Figure 1B) and Twi1p-bound (Figure 2G, left) scnRNAs at the same stage (10.5 hpm), which mainly mapped to A-regions of the MIC genome. Because the density of IESs is higher in A-regions than B-regions, the homogeneous distribution of the origins of Twi11p-bound small RNAs indicates that Late-scnRNAs are preferentially produced from IESs in B-regions.
To understand the importance of Twi1p and Twi11p in the accumulation of Late-scnRNAs, we produced TWI1 and TWI11 double-MIC-knockout cells (TWI1/TWI11 MIC-KO), in which both copies of TWI1 and TWI11 in the MIC were replaced with a drug-resistance gene (Figures S1I and S1J). A MIC-KO disrupts the zygotic expression of a gene from the new MAC without eliminating its maternal gene expression from the parental MAC (see Figure 2C, MIC-KO). In TWI1/TWI11 MIC-KO cells, Late-scnRNAs from B-region IESs initially accumulated (Figure 2J) but were then largely lost by 12 hpm (Figure 2K), indicating that zygotically expressed Twi1p and Twi11p are dispensable for the biogenesis of Late-scnRNAs but are necessary for their stable accumulation. Therefore, we conclude that Late-scnRNAs are mainly loaded into the zygotically expressed Twi1p and Twi11p.
scnRNA Expression Defines Three Types of IESs
The results above indicate that there are two types of IESs: one that produces Early-scnRNAs and another that only produces Late-scnRNAs. To rigorously classify IESs by these criteria, we calculated Late-scnRNA/Early-scnRNA indexes (LEIs) as ratios of normalized, mapped Twi11p-bound scnRNAs at 10.5 hpm (representing Late-scnRNAs) to mapped Twi1p-bound scnRNAs at 3 hpm (representing Early-scnRNAs) (Figure 3A). Among the 8,105 IESs to which we can reliably map scnRNA data, 4,695 IESs showed LEI ≤ 10 (i.e., both types of scnRNAs were expressed) and were defined as Type-A IESs. Type-A IESs were subdivided into three groups: A1 (LEI ≤ 1), A2 (1 < LEI ≤ 4), and A3 (LEI > 4). The other 3,293 IESs showed LEI > 10 (i.e., Late-scnRNAs were predominantly expressed) and were defined as Type-B IESs. Type-B IESs were subdivided into two groups: B1 (LEI ≤ 40) and B2 (LEI > 40). The remaining 117 IESs, which we call type-C IESs, showed very little Twi11p-bound scnRNA expression: <1 reads mapped per kilobase per million reads (RPKM).
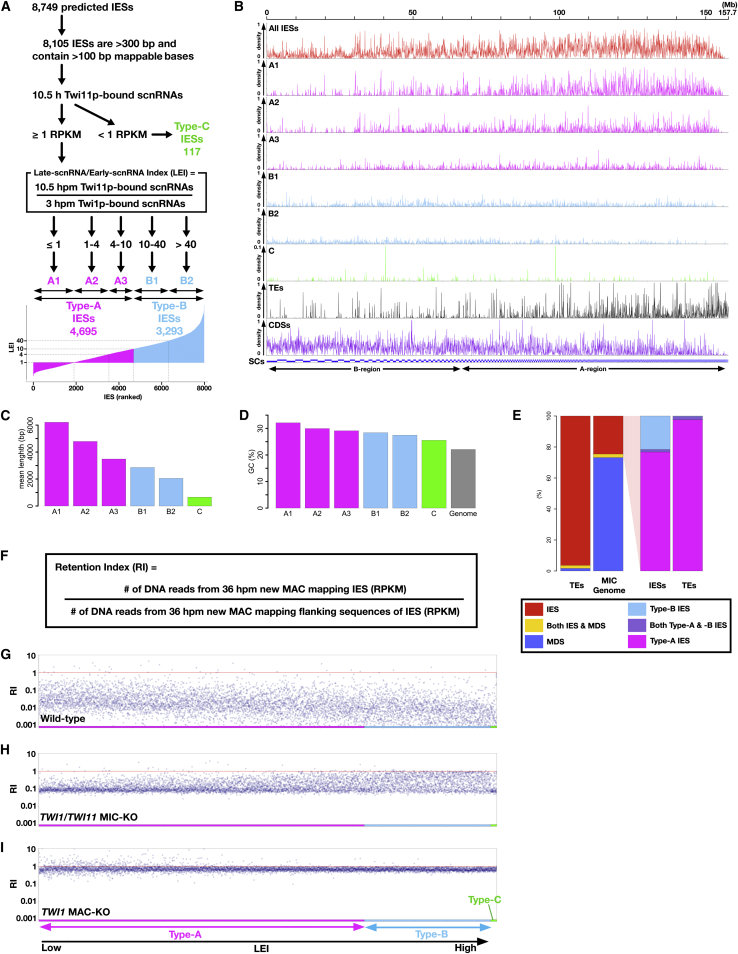
Figure 3.
Three Types of IESs
(A) Classification of IESs according to the expression of Early- and Late-scnRNAs.
(B) Localization of different types of IESs, transposons (TEs), and coding sequences (CDSs) in the MIC genome are shown in a histogram with 50-kb bins.
(C and D) Mean lengths (C) and GC contents (D) of IESs in different IES classes.
(E) Distributions of TE-related sequences among MIC genome components (left) and IES types (right). All possible 25-mers from the MIC genome sequences were classified as sequences that were complementary to only IESs (red), only MAC-destined sequences (MDSs, blue), or both (yellow). All possible 25-mers from the total IESs were classified as sequences that were complementary to only Type-A IESs (magenta), only Type-B IESs (sky blue), or both (purple). The fraction of TE-derived 25-nt sequences complementary to these DNA classes was calculated.
(F–I) Analysis of DNA elimination efficiency. The retention indexes (RIs) of individual IESs in the purified new MACs of the indicated strains at 36 hpm were plotted. IESs are ordered according to their LEIs (on the x axis). The red lines indicate RI = 1 (no DNA elimination).
LEIs were found to be correlated with the chromosomal locations, lengths, and GC contents of IESs: the lower the LEI of an IES group is, the greater its preference for A-regions (Figure 3B), the longer its mean length (Figure 3C), and the higher its GC contents (Figure 3D). These correlations are likely associated with the “age” of the IESs. We analyzed the localization of sequences related to Tlr1 DNA transposons and REP family retrotransposons (Fillingham et al., 2004; Wuitschick et al., 2002), the known potentially active TE families in Tetrahymena. All possible 25-mers were extracted from the previously reported DNA sequences of Tlr1- and REP-family TEs, and their occurrences in the MIC genome were calculated. We found that 98% of the sequences complementary to these two TEs are found in IESs (Figure 3E, left, TEs), which occupy only 23% of the MIC genome sequence we used for the analysis (Figure 3E, left, MIC genome). Within the IESs, the TE-derived sequences are mostly (99.5%) in Type-A IESs (Figure 3E right, TEs), which represent 77% of IESs (Figure 3E, right, IESs). Moreover, these TE-derived sequences co-localize with Type-A1/A2 IESs in the MIC genome (Figure 3B, TEs). Active TE-containing (i.e., young) IESs might have been constrained by natural selection to localize at A-regions, where the production of Early-scnRNAs (Figures 1B and 1D) ensures their DNA elimination from the new MAC. As TE sequences degenerate (i.e., become older), IESs may become shorter, more “tetrahymenanized” by reducing their GC contents in the AT-rich Tetrahymena genome (Figure 3D) and tolerated as residents of gene-rich B-regions (see CDSs in Figure 3B), where their DNA elimination occurs independently of their own Early-scnRNA expression (see below).
Late-scnRNAs Are Important for Elimination of Some IESs
To understand the importance of Early- and Late-scnRNAs in eliminating the different types of IESs, new MACs of exconjugants were isolated from different mutants at 36 hpm, and the genomic DNA was analyzed by high-throughput sequencing. As measure for DNA elimination, a retention index (RI; Figure 3F) was calculated for each IES by dividing normalized read numbers (RPM) mapping to an IES by the ones mapping to its flanking MAC-destined sequences. Although in theory, all IESs should show RI = 0 in WT cells, the indexes were typically 0.01-0.1 (Figure 3G), probably due to MIC contamination (∼2%–10%) in our new MAC preparations. The variability of RIs between IESs might be due to different endo-replication levels of different MAC chromosomes.
In TWI1/TWI11 MIC-KO cells, in which Late-scnRNAs are unstable (Figures 2J and 2I), RIs of some of Type-B IESs were ∼1 (not eliminated), whereas those of most Type-A IESs were ∼0.1 (eliminated) (Figure 3H). This result was in sharp contrast to TWI1 MAC-KO cells, in which both Early- and Late-scnRNAs were lost (Figure 2L), and the RIs of most IESs were ∼1 regardless of type (Figure 3I). These findings suggest that Early-scnRNAs are sufficient to induce DNA elimination for a majority of IESs, whereas Late-scnRNAs are important for DNA elimination of some, mainly Type-B, IESs. Elimination of type-C IESs, which lack their own scnRNA production (Figure 3A), was also affected in TWI1 MAC-KO cells (Figure 3I) and, to a lesser extent, in TWI1/TWI11 MIC-KO cells (Figure 3H), indicating that scnRNAs also play a role in the elimination of Type-C IESs.
trans-Recognition network for IES identification
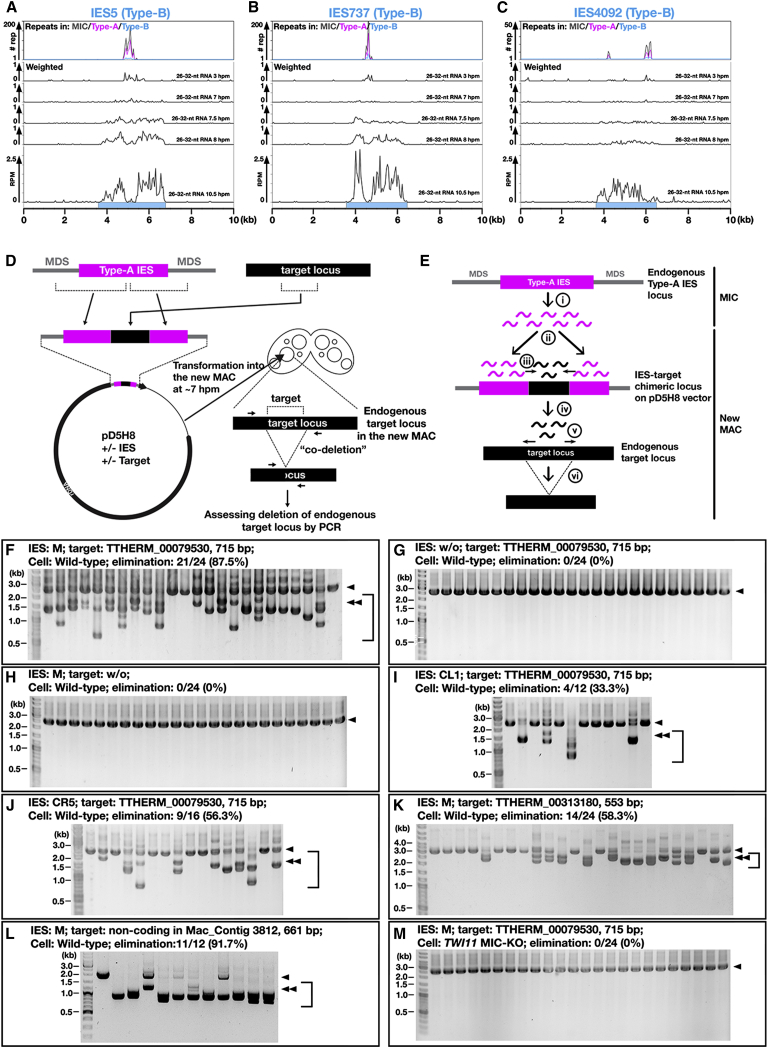
The sufficiency of Early-scnRNAs for the elimination of many Type-B IESs was puzzling, because Early-scnRNAs from WT cells at 3 hpm rarely map to any Type-B IESs (Figures 1C and 4A–4C, unique mappers). However, in all of the above scnRNA analyses (except for the IES classification in Figure 3A), we only counted sequence reads uniquely mapping to the MIC genome. If we instead allow multiple mapping and normalize for the effect of sequence repetition in the MIC genome (weighted mapping), more Early-scnRNAs mapped to the Type-B IESs (Figures 4A–4C, weighted, marked with arrows), because these Type-B IESs contain some repetitive sequences (Figures 4A–4C, repeats, gray lines) that mostly originate from Type-A IESs (magenta lines). We have termed these repetitive sequences “A-repeats.” Although a small fraction of Type-B IESs lack detectable A-repeats (Figure S2), some repetitive sequences sufficient for recognition by Early-scnRNAs might be overlooked because we only considered perfectly complementary 25-nt stretches to identify repetitive sequences.
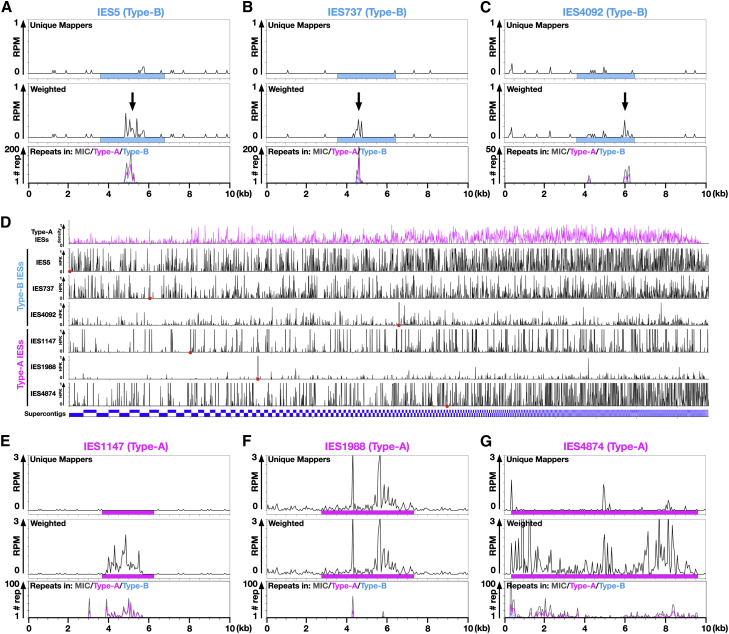
Figure 4.
trans Recognition of IESs
(A–C) Top two panels: normalized numbers (RPM) of sequenced 26- to 32-nt RNAs from wild-type cells at 3 hpm mapping to the three representative Type-B IES loci (10 kb) are shown as histograms with 50-nt bins. For the top histograms, only the numbers of sequences uniquely mapping to the MIC genome are shown (unique mappers). The middle histograms show the numbers of sequence reads mapping to each position within the loci divided by the total numbers of sites in the entire MIC genome to which the sequence reads map (weighted). Arrows indicate regions to which Early-scnRNAs map. Sky-blue boxes represent the IESs. Bottom panels (repeats): all possible 25-mers from the entire MIC genome sequence (gray), from Type-A IESs (magenta), or from Type-B IESs (sky blue) were mapped to the three Type-B IES regions, and their frequencies of occurrence are shown as histograms with 50-nt bins.
(D) All possible 25-mers were extracted from the indicated IESs, and their frequencies of occurrence (hits per kilobase [HPK]) on the MIC genome are shown as histograms with 50-kb bins. The locations of the IESs are marked with red dots. The density of the Type-A IESs is shown at the top.
(E–G) Three representative Type-A IES (magenta boxes) loci were analyzed as in (A)–(C).
See also Figure S2.
To understand the origins of A-repeats in Type-B IESs, all possible 25-mers were extracted from each Type-B IES, and their occurrence in the MIC genome was analyzed. We found that, for all analyzed Type-B IESs, sequences complementary to each Type-B IES were distributed throughout the MIC genome (Figure 4D), indicating that A-repeats in a Type-B IES are complementary to many different Type-A IESs in the MIC genome. These observations suggest that Early-scnRNAs from multiple Type-A IESs could target a Type-B IES for DNA elimination through A-repeats. Because most Type-A IESs also share certain A-repeats with other Type-A IESs (Figures 4D–4G), they may also be identified by Early-scnRNAs in trans.
A-Repeats Are Important for Type-B IES Elimination
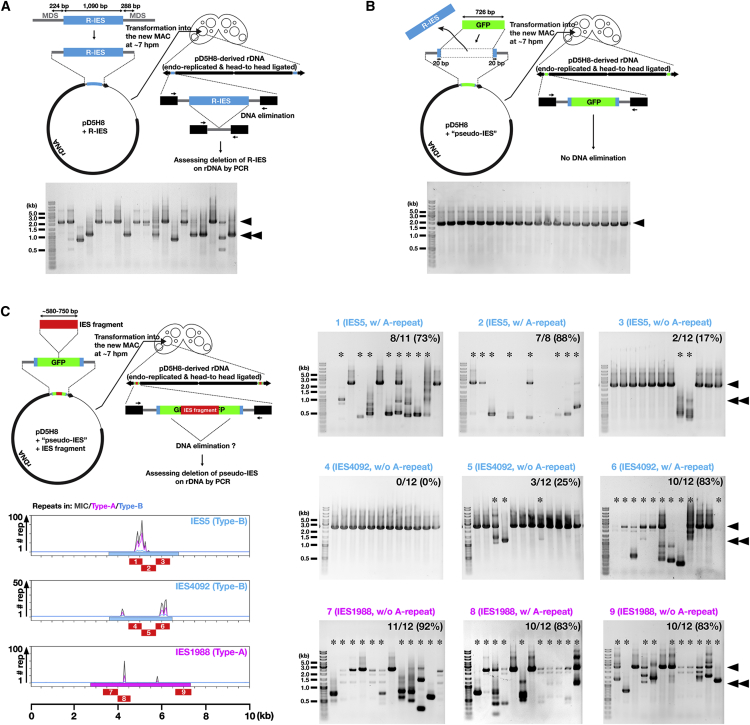
The importance of A-repeats for Type-B IES elimination was analyzed by an artificial DNA elimination assay (Figure 5). First, the previously characterized R-IES (a Type-B IES), including its flanking sequences, was inserted into an extra-chromosomal vector. As previously reported (Godiska and Yao, 1990), this IES was frequently removed from the vector, similar to its endogenous counterpart, upon introduction into the developing MAC (Figure 5A), although elimination did not occur in all progeny cell lines in this artificial assay. Next, most of the R-IES in the vector was replaced by a GFP-encoding sequence to generate a “pseudo-IES” that could not be eliminated (Figure 5B). Fragments of interest within IESs (red numbered boxes in Figure 5C, left bottom) were then inserted into the middle of the pseudo-IES (Figure 5C, left top). Among the fragments from the two representative Type-B IESs (IES5 and IES4092), we found that only DNA fragments containing A-repeats (fragments 1, 2, and 6 in Figure 5C) had strong activity in restoring DNA elimination of the pseudo-IES, whereas most of the fragments of a Type-A IES (IES1988) enabled the pseudo-IES to be eliminated (fragments 7–9 in Figure 5C). Some restoration of elimination by Type-B IES fragments without A-repeats might be due to “2° recognition” by Late-scnRNAs produced by the endogenous counterparts of the Type-B IESs (see below). Restored DNA elimination mostly did not share boundaries with the original R-IES (Figure 5C), indicating that the elimination boundaries are determined not only by information near the normal IES boundaries but also by the internal IES sequence. Nonetheless, all of the results above indicate that most, if not all, Type-B IESs possess A-repeats through which they can be targeted for DNA elimination in trans by Type-A IES-derived Early-scnRNAs.
Figure 5.
Role of A-Repeats in DNA Elimination
(A) DNA elimination assay of R-IES. R-IES (a Type-B IES) and its flanking MDS regions was inserted into the extra-chromosomal vector pD5H8 and introduced into the developing MAC. Progeny lines were established and elimination of R-IES on the vector was analyzed by PCR using primers complementary to the vector (arrows). The PCR results are shown on the bottom. The arrowhead and double arrowhead indicate the expected position of the PCR products from R-IES loci on the vector without or with R-IES elimination, respectively.
(B) DNA elimination assay of a pseudo-IES. Most of the R-IES in pD5H8 shown in (A) was replaced by a GFP-coding sequence and analyzed as in (A).
(C) DNA elimination assays assessing the DNA elimination-inducing activities of IES fragments. A fragment of an IES of interest (red boxes with numbers) was inserted into the middle of the pseudo-IES shown in (B) and tested to see if the fragment restored DNA elimination of the pseudo-IES. The progeny cell lines showing any deletion at the pseudo-IES loci (marked with asterisks) were counted. The arrowheads indicate PCR products from the pseudo-IES loci on the vector without any DNA elimination. The double arrowheads show the expected positions of PCR products from the pseudo-IES loci on the vector following DNA elimination exactly at the borders of the original R-IES.
cis Spreading of Late-scnRNA Production
Further examination of Late-scnRNAs revealed that they are produced from Type-B IESs outside the A-repeats (Figures 6A–6C, 7.5–10.5 hpm), suggesting that Early-scnRNAs recognize A-repeats in trans, which could trigger the expression of Late-scnRNAs from cis regions adjacent to A-repeats. Consistent with the idea of Early-scnRNA-triggered Late-scnRNA expression, TWI1 MAC-KO cells, which lack Early-scnRNAs because of the loss of maternally expressed Twi1p (Mochizuki et al., 2002), also failed to express Late-scnRNAs (Figure 2L).
Figure 6.
Co-deletion
(A–C) Normalized and weighted numbers of sequenced total 26- to 32-nt small RNAs from wild-type cells at indicated time points mapping to the representative Type-B IES loci are shown as histograms with 50-nt bins. Sky-blue boxes indicate IESs.
(D and E) Schematic drawings of the co-deletion (coDel) experiment (D) and of the hypothetical actions of scnRNAs in coDel (E). See the text for details.
(F–M) Indicated IESs and target loci were cloned into the extra-chromosomal vector pD5H8 and introduced into the new MACs of conjugating wild-type (F–L) or TWI11 MIC-KO (M) cells. Deletions at the endogenous target loci in progeny cells were analyzed by PCR as in (D). For the “w/o target” experiment (H), the TTHERM_00079350 locus was analyzed. The number of progeny lines showing any deletions at the endogenous target loci was determined. The arrowheads and brackets indicate PCR products from the target loci without or with deletions, respectively. The double arrowheads show the expected positions of PCR products from the target loci with deletions exactly corresponding to the target sequences.
See also Figure S3.
To validate the cis triggering of Late-scnRNA production by Early-scnRNAs, we designed another artificial DNA elimination assay (Figure 6D). A Type-A IES (Figure 6, magenta box) was inserted into an extra-chromosomal vector (pD5H8), and a non-IES target sequence (Figure 6, black box) was inserted into the middle of the IES. This chimeric construct was then introduced into the developing MAC. We hypothesized (Figure 6E) that Early-scnRNAs produced from the endogenous Type-A IES in the MIC (i) recognize the Type-A IES on the vector in the new MAC (ii) and trigger Late-scnRNA production from the adjacent target sequence in cis (iii), which then recognize the endogenous target locus in trans (iv) and induce ectopic DNA elimination (vi).
The introduction of a chimeric construct containing the well-studied M-IES (a Type-A IES) and part of a coding gene efficiently induced ectopic DNA elimination in trans at the endogenous chromosomal locus of the gene (Figure 6F), whereas the IES alone (Figure 6G) or the target DNA alone (Figure 6H) in the vector did not. Analogous ectopic DNA elimination was induced with combinations of different Type-A IESs (Figures 6I and 6J) and different coding (Figure 6K) as well as non-coding (Figure 6L) sequences. We refer to this ectopic DNA elimination phenomenon as co-deletion (coDel). Because coDel often removed regions wider than the target, Late-scnRNAs most likely further induce Late-scnRNA production at the target loci in cis, as predicted in Figure 6E (“v”). Because the assay relies on transformation of Tetrahymena cells, which occurs inefficiently (roughly 1 out of 10,000 cells), we lack the sensitivity to detect Late-scnRNAs from a target sequence during a coDel process. However, the fact that coDel was suppressed in TWI11 MIC-KO cells (Figure 6M) argues that coDel is mediated by Late-scnRNAs.
The coDel phenomenon not only supports the cis triggering of Late-scnRNA production by Early-scnRNAs but also indicates that (1) Late-scnRNAs can further target their complementary sequences in trans (2° recognition) for DNA elimination (because some Type-B IESs do not express Late-scnRNAs in TWI1/TWI11 MIC-KO [Figure 2J, arrows], expression of Late-scnRNAs from these Type-B IESs may be triggered only by 2° recognition with Late-scnRNAs produced from other Type-B IESs); (2) the prerequisite for becoming the target of 2° recognition is either highly relaxed or nonexistent because three randomly chosen target sequences induced ectopic DNA elimination (Figures 6F, 6K, and 6L); and (3) unlike Early-scnRNAs (Aronica et al., 2008; Schoeberl et al., 2012), Late-scnRNAs complementary to the parental MAC genome escape degradation, most likely because Twi1p/Twi11p-Late-scnRNA complexes do not localize to the parental MAC (see Figure S3 for Twi11p and Noto et al., 2010 for Twi1p).
Heterochromatin Is Required for Late-scnRNA Production
We assumed that the cis triggering of Late-scnRNA production by Early-scnRNAs might be mediated by the production of double-stranded RNAs through RNA-dependent RNA polymerase (RdRP) or by heterochromatin, which spreads along chromatin in many eukaryotes.
Rdr1p, the single RdRP in Tetrahymena, is essential for vegetative growth (Lee et al., 2009), and thus, the RDR1 MAC&MIC-KO is lethal. We instead analyzed the accumulation of scnRNAs in RDR1 MIC-KO cells and found that Late-scnRNAs were accumulated normally in the absence of zygotic expression of Rdr1p (Figure 2M). Therefore, although we cannot exclude the possibility that maternally expressed Rdr1p is sufficient to produce normal amounts of Late-scnRNAs, it is likely that Rdr1p is dispensable for the Late-scnRNA biogenesis.
The interaction of Early-scnRNA with new MAC chromatin induces heterochromatin formation, including the accumulation of H3K9/K27me and its interacting HP1-like protein Pdd1p (Liu et al., 2007; Taverna et al., 2002). We found that EZL1, which encodes H3K9/K27 methyltransferase, and PDD1 were necessary for the production of Late-scnRNAs (Figures 2O and 2Q), although they were not required for the accumulation of Early-scnRNAs (Figures 2N and 2P). Therefore, we conclude that heterochromatin components are required for Late-scnRNA production, and cis triggering of Late-scnRNA production is most likely mediated by the cis spreading of heterochromatin on IESs.
trans Recognition and cis Spreading Provide Robustness in DNA Elimination
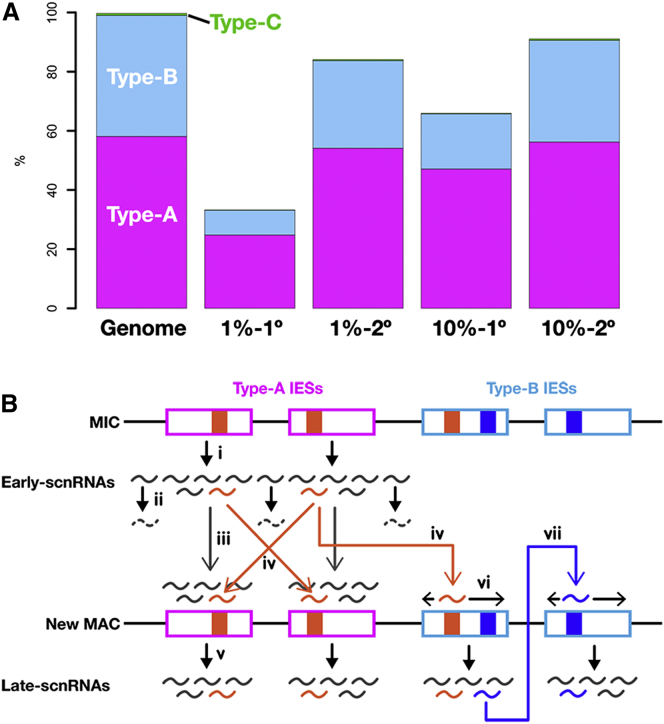
We tested the robustness of the DNA elimination system by simulating the accidental loss of Early-scnRNA expression from a majority of Type-A IESs. We randomly chose 47 (1%) or 469 (10%) non-redundant Type-A IESs, and all possible 20-mers were extracted from the chosen Type-A IESs. Then, IESs in which there are more than 250 complementary bases to the 20-mers in one of the possible 500-nt windows were identified as recognized IESs (1° IESs). Next, all possible 20-mers were extracted from the 1° IESs, and the same calculation was repeated to identify 2° IESs. The determination of the threshold for IES recognition in the simulation is based on two previous observations: (1) an ∼300-nt segment of the M-IES (a Type-A IES) is sufficient to induce its DNA elimination (Kowalczyk et al., 2006), and (2) for many small RNA-target interactions, the seed sequence, which typically spans positions 2–8 from the 5′ end, plays a more important role than the rest of the small RNA sequences. Therefore, we believe the target recognition criteria above gives a conservative prediction of target genomic loci.
The simulation of accidental loss of Early-scnRNA expression (Figure 7A) suggests that even if Early-scnRNAs are expressed only from 1% of Type-A IESs, they could recognize 33% of all IESs (1% to 1°), and if Late-scnRNAs derived from these 1° IESs also function in trans recognition, they could identify up to 84% of total IESs (1% to 2°). If 10% of Type-A IESs express Early-scnRNAs, such 1° and 2° IESs could increase coverage to 66% (10% to 1°) and 91% (10% to 2°), respectively. These results indicate that Early-scnRNAs from a small set of Type-A IESs are sufficient to identify most of the other IESs, demonstrating strong robustness of the DNA elimination mechanism.
Figure 7.
A trans-Recognition Network in IES Recognition
(A) Results of a simulation of accidental loss of Early-scnRNA expression. See the text for details. The average fractions of 1° and 2° IESs in each condition, with three different sets of randomly chosen seed Type-A IESs (47 and 469 Type-A IESs for 1% and 10% seeds, respectively) are shown.
(B) A model for IES recognition. Early-scnRNAs are expressed from Type-A IESs (magenta boxes) and their flanking sequences in the MIC (i), and the latter are degraded in the parental MAC (ii). In the new MAC, Early-scnRNAs recognize the Type-A IESs from which they are derived (iii) as well as other Type-A and Type-B (sky-blue boxes) IESs in trans (iv) through A-repeats (filled orange boxes) to trigger Late-scnRNA production (v). In an IES, regions producing Late-scnRNAs spread in cis (vi). Late-scnRNAs further trans-recognize other IESs (vii).
Next, to test the specificity of the trans-recognition system, we queried whether genic sequences are targeted by IES-derived scnRNAs in trans. By applying the target recognition criteria used above, we determined how many gene-coding sequences can be targeted if all of the IESs express scnRNAs homogeneously and if all of the expressed scnRNAs are involved in trans recognition. Out of 20,491 predicted gene-coding sequences used for the simulation, we found that only 312 (1.3%) were predicted to be trans-targeted by IES-derived scnRNAs. Therefore, gene-coding sequences are generally devoid of a trans-recognition system.
Discussion
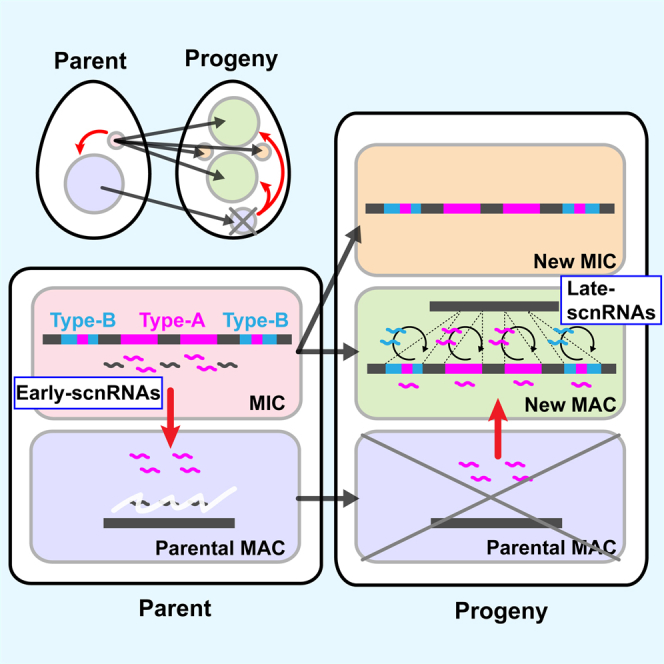
In this study, we demonstrated that there is an unanticipated mechanism regulating programmed DNA elimination: a genome-wide trans-recognition network for IES identification. In this mechanism (Figure 7B), Early-scnRNAs produced from Type-A IESs in the MIC (i) identify not only the IESs from which they are derived (iii) but also other IESs in trans (iv) to trigger the cis spreading of Late-scnRNA production in the IESs (vi). This cis spreading of Late-scnRNA production requires heterochromatin formation (Figures 2O and 2Q). Furthermore, these Late-scnRNAs can recognize other IESs in trans (vii). This “chain reaction” of Late-scnRNA production by the trans-recognition network most likely provides strong robustness in DNA elimination by buffering cell-to-cell variability in the initial Early-scnRNA populations (Figure 7A).
Conceptually, the trans-recognition network for IES identification in Tetrahymena and the piRNA pathway in flies and mammals are strategically similar: Type-A IESs may be the functional equivalents of piRNA clusters, as both contain remnants of TEs and produce small RNAs for the trans recognition of TE-related sequences across the genome (Aravin et al., 2007; Brennecke et al., 2007). Furthermore, similar to “ping-pong” amplification of piRNAs by the feed-forward endonucleolytic cycle (Brennecke et al., 2007; Gunawardane et al., 2007), Early-scnRNAs trigger a chain reaction of Late-scnRNA production by trans recognition and cis spreading, and this reaction is likely mediated by heterochromatin. Because the pattern of DNA elimination in the parent is the basis of DNA elimination in ciliate progeny, robust DNA elimination by the trans-recognition network would reinforce faithful transgenerational transmission of TE memory, just as piRNA amplification fuels the maternal transmission of piRNAs to silence TEs (Brennecke et al., 2008). Because DNA elimination in Tetrahymena occurs within a Dicer-dependent siRNA pathway, the two conceptually similar trans-recognition network-type TE surveillance systems, the piRNA pathway in metazoans and DNA elimination in Tetrahymena, are products of evolutionary convergence. It is very interesting that such TE surveillance can be achieved by two very distinct small RNA mechanisms. Because TEs are found in almost all eukaryotes, such systems might have evolved multiple times in different eukaryotic linages.
A similar small RNA amplification cycle has been proposed for iesRNAs in Paramecium (Sandoval et al., 2014). However, Paramecium IESs are mostly unique sequences (Arnaiz et al., 2012), and therefore, iesRNAs are most likely not involved in trans-recognition between different IESs. Instead, iesRNAs from one genome in the polycopy developing MAC probably help to recognize another copy of the same IESs to ensure complete DNA elimination (Sandoval et al., 2014). Recognition of the same IESs by Late-scnRNAs might serve a similar function in Tetrahymena, as DNA elimination of some Type-A IESs was mildly affected in TWI1/TWI11 MIC-KO cells (Figure 3H), in which Late-scnRNAs are unstable (Figures 2J and 2K).
Misregulation of the trans-recognition network accompanied by a chain reaction of Late-scnRNA production could potentially cause elimination of important genomic regions adjacent to IESs and lead to runaway Late-scnRNA amplification, resulting in the elimination of regions distal to IESs. Therefore, the cis triggering of Late-scnRNA production must occur with high precision within the IES borders. The previously characterized “cis-acting sequences” (Chalker et al., 1999; Godiska et al., 1993) might supply this boundary information.
Regardless of the nature of boundary information, it is difficult to imagine that newly invaded transposons are always associated with such regulatory sequences. During the coDel processes, DNA elimination boundaries occur mostly within ∼0.5–1 kb from target sequences (Figure 6), indicating that a system is likely in place to limit cis spreading, even at non-IES loci. Certain DNA sequences that insulate cis spreading may frequently occur throughout the genome, and cis spreading might always be limited within two such “insulator” sequences. Alternatively, because Type-A IESs, which we believe are “young” IESs, have markedly higher GC content than the other IESs as well as the entire MIC genome (Figure 3D), the difference in base compositions between TEs and the host genome may provide an insulator function. Nonetheless, because coDel occurs with heterogeneous boundaries (Figure 6), only authentic IESs or their flanking sequences should contain information that tightly restricts the cis triggering of Late-scnRNA production within IESs and thus maintains precise DNA elimination boundaries. It is possible that only the sequences associated with such boundary information have been selected through evolution to be IESs. Future studies should be designed to identify the molecular nature of the essential boundary information that allows the trans-recognition network to strictly distinguish TE-related sequences from the rest of the genome.
Experimental Procedures
Materials and Bioinformatic Analyses
Tetrahymena strains, antibodies, oligonucleotides, and bioinformatic analyses (distributions of IESs, CDSs, TEs and repeats, IES classification, and simulation of accidental loss of Early-scnRNA expression and targeting of protein coding sequences) are described in Supplemental Experimental Procedures. The draft MIC genome sequence (version 2) was obtained from the Tetrahymena Comparative Sequencing Project (Broad Institute of Harvard and MIT).
Small RNA Analyses
Small RNA purification, co-immunoprecipitation, high-throughput sequencing, and analyses by gel electrophoresis were performed as previously described (Noto et al., 2010; Schoeberl et al., 2012).
Purification of the New MAC and DNA Elimination Analysis
New MACs from exconjugants at 36 hpm were purified by fluorescence-activated cell sorting, and genomic DNA libraries were generated and sequenced using a HiSeq2000 platform with 50-nt single reads. To obtain RIs, the number of normalized sequence reads from purified new MACs mapping to each IES was calculated and divided by the number of reads that map the flanking MAC-destined sequences of the IES (1-kb sequences located 500 bp upstream and downstream of the IES). Detailed procedures are described in Supplemental Experimental Procedures.
DNA Elimination Analysis with Pseudo-IES
Fragments of IESs were cloned into the NotI site of pD5H8-pseudo-IES. The pD5H8-R-IES, pD5H8-pseudo-IES and pD5H8-pseudo-IES containing the IES fragments were introduced into conjugating cells (7 hpm) and cultured in 10 mM Tris (pH 7.5) overnight, and cells were selected with paromomycin. DNA elimination in the rDNA vector was analyzed by genomic DNA PCR. Detailed procedures are described in Supplemental Experimental Procedures.
coDel
The vectors pMcoDel, pCL1coDel, and pCR5coDel were digested with NotI, and target sequences were inserted. The vectors were introduced into WT or TWI11 MIC-KO cells as described above, and DNA eliminations at the endogenous target loci were analyzed by genomic DNA PCR. Detailed procedures are described in Supplemental Experimental Procedures.
Author Contributions
T.N., K.K., J.H.S., M.A.G., and K.M. designed the experiments; T.N., K.K., J.H.S., A.H., K.J.W., and K.M. performed the experiments; and T.N., K.K., and K.M. prepared the manuscript.
Acknowledgments
We wish to thank Eduardo Orias for his comments. This work was supported by the Austrian Academy of Sciences, the European Community’s 7th Framework Program (ERC Starting Grant 204986), and the Austrian Science Fund (P26032-B22; F4307-B09). K.K. was supported by a Naito Memorial Grant for Research Abroad from the Naito Foundation. K.J.W. was supported by an EMBO Long-Term Fellowship.
Published: June 18, 2015
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, three figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2015.05.024.
Accession Numbers
The accession numbers for the datasets reported in this paper are GEO: GSE68455, GSE68457.
Supplemental Information
References
- Aravin A.A., Sachidanandam R., Girard A., Fejes-Toth K., Hannon G.J. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–747. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- Arnaiz O., Mathy N., Baudry C., Malinsky S., Aury J.M., Denby Wilkes C., Garnier O., Labadie K., Lauderdale B.E., Le Mouël A. The Paramecium germline genome provides a niche for intragenic parasitic DNA: evolutionary dynamics of internal eliminated sequences. PLoS Genet. 2012;8:e1002984. doi: 10.1371/journal.pgen.1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica L., Bednenko J., Noto T., DeSouza L.V., Siu K.W., Loidl J., Pearlman R.E., Gorovsky M.A., Mochizuki K. Study of an RNA helicase implicates small RNA-noncoding RNA interactions in programmed DNA elimination in Tetrahymena. Genes Dev. 2008;22:2228–2241. doi: 10.1101/gad.481908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austerberry C.F., Allis C.D., Yao M.C. Specific DNA rearrangements in synchronously developing nuclei of Tetrahymena. Proc. Natl. Acad. Sci. USA. 1984;81:7383–7387. doi: 10.1073/pnas.81.23.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J., Aravin A.A., Stark A., Dus M., Kellis M., Sachidanandam R., Hannon G.J. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Brennecke J., Malone C.D., Aravin A.A., Sachidanandam R., Stark A., Hannon G.J. An epigenetic role for maternally inherited piRNAs in transposon silencing. Science. 2008;322:1387–1392. doi: 10.1126/science.1165171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker D.L., La Terza A., Wilson A., Kroenke C.D., Yao M.C. Flanking regulatory sequences of the Tetrahymena R deletion element determine the boundaries of DNA rearrangement. Mol. Cell. Biol. 1999;19:5631–5641. doi: 10.1128/mcb.19.8.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker D.L., Meyer E., Mochizuki K. Epigenetics of ciliates. Cold Spring Harb. Perspect. Biol. 2013;5:a017764. doi: 10.1101/cshperspect.a017764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Bracht J.R., Goldman A.D., Dolzhenko E., Clay D.M., Swart E.C., Perlman D.H., Doak T.G., Stuart A., Amemiya C.T. The architecture of a scrambled genome reveals massive levels of genomic rearrangement during development. Cell. 2014;158:1187–1198. doi: 10.1016/j.cell.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvillion M.T., Lee S.R., Hogstad B., Malone C.D., Tonkin L.A., Sachidanandam R., Hannon G.J., Collins K. Sequence, biogenesis, and function of diverse small RNA classes bound to the Piwi family proteins of Tetrahymena thermophila. Genes Dev. 2009;23:2016–2032. doi: 10.1101/gad.1821209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne R.S., Stover N.A., Miao W. Whole genome studies of Tetrahymena. Methods Cell Biol. 2012;109:53–81. doi: 10.1016/B978-0-12-385967-9.00004-9. [DOI] [PubMed] [Google Scholar]
- Dumesic P.A., Natarajan P., Chen C., Drinnenberg I.A., Schiller B.J., Thompson J., Moresco J.J., Yates J.R., 3rd, Bartel D.P., Madhani H.D. Stalled spliceosomes are a signal for RNAi-mediated genome defense. Cell. 2013;152:957–968. doi: 10.1016/j.cell.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen J.A., Coyne R.S., Wu M., Wu D., Thiagarajan M., Wortman J.R., Badger J.H., Ren Q., Amedeo P., Jones K.M. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 2006;4:e286. doi: 10.1371/journal.pbio.0040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W., Wang X., Bracht J.R., Nowacki M., Landweber L.F. Piwi-interacting RNAs protect DNA against loss during Oxytricha genome rearrangement. Cell. 2012;151:1243–1255. doi: 10.1016/j.cell.2012.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingham J.S., Thing T.A., Vythilingum N., Keuroghlian A., Bruno D., Golding G.B., Pearlman R.E. A non-long terminal repeat retrotransposon family is restricted to the germ line micronucleus of the ciliated protozoan Tetrahymena thermophila. Eukaryot. Cell. 2004;3:157–169. doi: 10.1128/EC.3.1.157-169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiska R., Yao M.C. A programmed site-specific DNA rearrangement in Tetrahymena thermophila requires flanking polypurine tracts. Cell. 1990;61:1237–1246. doi: 10.1016/0092-8674(90)90688-b. [DOI] [PubMed] [Google Scholar]
- Godiska R., James C., Yao M.C. A distant 10-bp sequence specifies the boundaries of a programmed DNA deletion in Tetrahymena. Genes Dev. 1993;7(12A):2357–2365. doi: 10.1101/gad.7.12a.2357. [DOI] [PubMed] [Google Scholar]
- Gunawardane L.S., Saito K., Nishida K.M., Miyoshi K., Kawamura Y., Nagami T., Siomi H., Siomi M.C. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science. 2007;315:1587–1590. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- Guzzardo P.M., Muerdter F., Hannon G.J. The piRNA pathway in flies: highlights and future directions. Curr. Opin. Genet. Dev. 2013;23:44–52. doi: 10.1016/j.gde.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk C.A., Anderson A.M., Arce-Larreta M., Chalker D.L. The germ line limited M element of Tetrahymena is targeted for elimination from the somatic genome by a homology-dependent mechanism. Nucleic Acids Res. 2006;34:5778–5789. doi: 10.1093/nar/gkl699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth H.M., Mochizuki K. 2′-O-methylation stabilizes Piwi-associated small RNAs and ensures DNA elimination in Tetrahymena. RNA. 2009;15:675–685. doi: 10.1261/rna.1455509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.R., Talsky K.B., Collins K. A single RNA-dependent RNA polymerase assembles with mutually exclusive nucleotidyl transferase subunits to direct different pathways of small RNA biogenesis. RNA. 2009;15:1363–1374. doi: 10.1261/rna.1630309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.N., Chalamcharla V.R., Reyes-Turcu F., Mehta S., Zofall M., Balachandran V., Dhakshnamoorthy J., Taneja N., Yamanaka S., Zhou M., Grewal S.I. Mtr4-like protein coordinates nuclear RNA processing for heterochromatin assembly and for telomere maintenance. Cell. 2013;155:1061–1074. doi: 10.1016/j.cell.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H.L., Moran J.V. Dynamic interactions between transposable elements and their hosts. Nat. Rev. Genet. 2011;12:615–627. doi: 10.1038/nrg3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Taverna S.D., Muratore T.L., Shabanowitz J., Hunt D.F., Allis C.D. RNAi-dependent H3K27 methylation is required for heterochromatin formation and DNA elimination in Tetrahymena. Genes Dev. 2007;21:1530–1545. doi: 10.1101/gad.1544207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone C.D., Anderson A.M., Motl J.A., Rexer C.H., Chalker D.L. Germ line transcripts are processed by a Dicer-like protein that is essential for developmentally programmed genome rearrangements of Tetrahymena thermophila. Mol. Cell. Biol. 2005;25:9151–9164. doi: 10.1128/MCB.25.20.9151-9164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K., Gorovsky M.A. RNA polymerase II localizes in Tetrahymena thermophila meiotic micronuclei when micronuclear transcription associated with genome rearrangement occurs. Eukaryot. Cell. 2004;3:1233–1240. doi: 10.1128/EC.3.5.1233-1240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K., Gorovsky M.A. A Dicer-like protein in Tetrahymena has distinct functions in genome rearrangement, chromosome segregation, and meiotic prophase. Genes Dev. 2005;19:77–89. doi: 10.1101/gad.1265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K., Kurth H.M. Loading and pre-loading processes generate a distinct siRNA population in Tetrahymena. Biochem. Biophys. Res. Commun. 2013;436:497–502. doi: 10.1016/j.bbrc.2013.05.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K., Fine N.A., Fujisawa T., Gorovsky M.A. Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in tetrahymena. Cell. 2002;110:689–699. doi: 10.1016/s0092-8674(02)00909-1. [DOI] [PubMed] [Google Scholar]
- Noto T., Kurth H.M., Kataoka K., Aronica L., DeSouza L.V., Siu K.W., Pearlman R.E., Gorovsky M.A., Mochizuki K. The Tetrahymena argonaute-binding protein Giw1p directs a mature argonaute-siRNA complex to the nucleus. Cell. 2010;140:692–703. doi: 10.1016/j.cell.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval P.Y., Swart E.C., Arambasic M., Nowacki M. Functional diversification of Dicer-like proteins and small RNAs required for genome sculpting. Dev. Cell. 2014;28:174–188. doi: 10.1016/j.devcel.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Saveliev S.V., Cox M.M. The fate of deleted DNA produced during programmed genomic deletion events in Tetrahymena thermophila. Nucleic Acids Res. 1994;22:5695–5701. doi: 10.1093/nar/22.25.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeberl U.E., Kurth H.M., Noto T., Mochizuki K. Biased transcription and selective degradation of small RNAs shape the pattern of DNA elimination in Tetrahymena. Genes Dev. 2012;26:1729–1742. doi: 10.1101/gad.196493.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth M., Shirayama M., Gu W., Ishidate T., Conte D., Jr., Mello C.C. The C. elegans CSR-1 argonaute pathway counteracts epigenetic silencing to promote germline gene expression. Dev. Cell. 2013;27:656–663. doi: 10.1016/j.devcel.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi M.C., Sato K., Pezic D., Aravin A.A. PIWI-interacting small RNAs: the vanguard of genome defence. Nat. Rev. Mol. Cell Biol. 2011;12:246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- Slotkin R.K., Vaughn M., Borges F., Tanurdzić M., Becker J.D., Feijó J.A., Martienssen R.A. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell. 2009;136:461–472. doi: 10.1016/j.cell.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S.D., Coyne R.S., Allis C.D. Methylation of histone h3 at lysine 9 targets programmed DNA elimination in tetrahymena. Cell. 2002;110:701–711. doi: 10.1016/s0092-8674(02)00941-8. [DOI] [PubMed] [Google Scholar]
- Wedeles C.J., Wu M.Z., Claycomb J.M. Protection of germline gene expression by the C. elegans Argonaute CSR-1. Dev. Cell. 2013;27:664–671. doi: 10.1016/j.devcel.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Woehrer S.L., Aronica L., Suhren J.H., Busch C.J., Noto T., Mochizuki K. A Tetrahymena Hsp90 co-chaperone promotes siRNA loading by ATP-dependent and ATP-independent mechanisms. EMBO J. 2015;34:559–577. doi: 10.15252/embj.201490062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuitschick J.D., Gershan J.A., Lochowicz A.J., Li S., Karrer K.M. A novel family of mobile genetic elements is limited to the germline genome in Tetrahymena thermophila. Nucleic Acids Res. 2002;30:2524–2537. doi: 10.1093/nar/30.11.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M.C., Fuller P., Xi X. Programmed DNA deletion as an RNA-guided system of genome defense. Science. 2003;300:1581–1584. doi: 10.1126/science.1084737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.