Abstract
Motor system excitability is transiently inhibited during the preparation of responses. Previous studies have attributed this inhibition to the operation of two mechanisms, one hypothesized to help resolve competition between alternative response options, and the other to prevent premature response initiation. By this view, inhibition should be restricted to task-relevant muscles. Although this prediction is supported in one previous study (Duque et al., 2010), studies of stopping ongoing actions suggest that some forms of motor inhibition may be widespread (Badry et al., 2009). This motivated us to conduct a series of transcranial magnetic stimulation (TMS) experiments to examine in detail the specificity of preparatory inhibition in humans. Motor-evoked potentials were inhibited in task-irrelevant muscles during response preparation, even when the muscles were contralateral and not homologous to the responding effector. Inhibition was also observed in both choice and simple response task conditions, with and without a preparatory interval. Control experiments ruled out that this inhibition is due to expectancy of TMS or a possible need to cancel the prepared response. These findings suggest that motor inhibition during response preparation broadly influences the motor system and likely reflects a process that occurs whenever a response is selected. We propose a reinterpretation of the functional significance of preparatory inhibition, one by which inhibition reduces noise to enhance signal processing and modulates the gain of a selected response.
SIGNIFICANCE STATEMENT Motor preparation entails the recruitment of excitatory and inhibitory neural mechanisms. The current experiments address the specificity of inhibitory mechanisms, asking whether preparatory inhibition affects task-irrelevant muscles. Participants prepared a finger movement to be executed at the end of a short delay period. Transcranial magnetic stimulation over primary motor cortex provided an assay of corticospinal excitability. Consistent with earlier work, the agonist muscle for the forthcoming response was inhibited during the preparatory period. Moreover, this inhibition was evident in task-irrelevant muscles, although the magnitude of inhibition depended on whether the response was fixed or involved a choice. These results implicate a broadly tuned inhibitory mechanism that facilitates response preparation, perhaps by lowering background activity before response initiation.
Keywords: action selection, decision-making, gain modulation, inhibition, response preparation, transcranial magnetic stimulation
Introduction
Behavioral and electrophysiological studies have provided compelling evidence that the motor system is transiently inhibited during the preparation of volitional movements (Hasbroucq et al., 1997, 1999a,b; Touge et al., 1998; Davranche et al., 2007; van Elswijk et al., 2007; Duque and Ivry, 2009; Sinclair and Hammond, 2009; Duque et al., 2010, 2012; Soto et al., 2010). The functional significance of this transient inhibition has remained the subject of debate. One model proposes that this preparatory inhibition reflects the operation of two independent mechanisms (Duque et al., 2010, 2012). One mechanism is associated with the suppression of competing response representations to facilitate response selection. The other mechanism targets the selected response to prevent its premature execution.
This model makes specific predictions concerning the constraints on preparatory inhibition. In particular, the model predicts that inhibition should be restricted to task-relevant effectors. To test this prediction, Duque et al. (2010) used transcranial magnetic stimulation (TMS) to probe changes in corticospinal excitability while participants performed a delayed response task. They compared conditions in which the probed muscle was either task-relevant or task-irrelevant for a block of trials. Motor-evoked potentials (MEPs) were only attenuated in the former case, a finding that helped motivate the two-mechanism model.
The observation that inhibition during response preparation is limited to task-relevant muscles contrasts with studies showing inhibition in task-irrelevant muscles during response initiation or when people are required to abort a planned action. For example, when people make a unimanual response, inhibition is observed in the homologous resting muscles immediately before movement initiation, even though these muscles are task-irrelevant (Leocani et al., 2000; Liepert et al., 2001; Sohn et al., 2003; Weiss et al., 2003). However, this line of work did not include a preparatory delay, which helps separate signatures of brain mechanisms involved in response preparation from those involved in response execution. A separate line of research on reactive response inhibition using the stop signal task has found that signatures of inhibition are manifest in muscles completely irrelevant to the task (e.g., inhibition of leg muscles when aborting a finger response; Badry et al., 2009). Indeed, work on reactive inhibition suggests the recruitment of a global inhibitory process (Badry et al., 2009; Cai et al., 2012; Greenhouse et al., 2012; Majid et al., 2012; Wessel et al., 2013).
We set out to revisit the specificity of preparatory inhibition using modified versions of the delayed response task introduced by Duque et al. (2010, 2012). The first experiment included conditions in which there were multiple response options (choice RT) and conditions in which there was only one response option (simple RT). The choice RT condition provided a replication of prior studies in which the targeted muscle was always task relevant, either selected or not selected for the forthcoming response. The simple RT condition allowed us to evaluate the presence of inhibition in the absence of a choice. To further understand the constraints on preparatory inhibition, we also examined changes in corticospinal excitability when the preparatory cue was eliminated and the imperative served to indicate the response (no delay). In a second experiment, we tested whether inhibition is observable in resting effectors that are not homologous to a responding muscle, providing an assay of the anatomical specificity of preparatory inhibition. In a third experiment, we probed whether inhibition relates to anticipating the need to abort a prepared response.
Materials and Methods
Participants
All participants were screened for contraindications to TMS and provided informed consent following a protocol approved by the IRB of the University of California, Berkeley. A total of 38 participants (17 female, 21 male, 31 right-handed, 7 left-handed, 22.2 ± 2.5 years of age) were tested. Of these, 15 were tested in Experiment 1, 13 in Experiment 2, and 10 in Experiment 3. Two participants were tested in both Experiments 1 and 2. One participant was tested in both Experiments 1 and 3, and one participant was tested in both Experiments 2 and 3.
In all three experiments, participants were seated comfortably in front of a computer monitor with their hands palm-down on a pillow in their laps. The presentation of task stimuli was controlled with E-Prime (Psychology Software Tools) in Experiments 1 and 2, and was controlled with the Psychtoolbox within MATLAB (MathWorks) in Experiment 3.
Experiment 1
Experiment 1 was designed to explore two questions. First, we wanted to test whether motor inhibition was observable outside the context of a choice. To this end, we compared changes in motor excitability before response initiation during choice and simple reaction time tasks. The underlying assumption was that any inhibition observed during the preparation of simple responses could not be attributed to “competition” between candidate responses because there is only one response option in the simple RT task. Second, we asked whether inhibition attributed to response selection processes is only manifest when participants are provided with a preparatory interval. To answer this question, we compared conditions involving a delay period to conditions without a delay. In the latter, the cue also served as the imperative signal. Here we measured motor excitability after this stimulus, but before movement initiation.
Behavioral task.
Choice and simple response blocks were administered separately to test for inhibitory signatures in the presence and absence of a choice. Blocks were further divided into delay and no-delay conditions, creating a 2 × 2 factorial design. For all conditions, the response on each trial entailed a lateral flexion of either the right or left index finger (agonist muscle is the first dorsal interosseous; FDI).
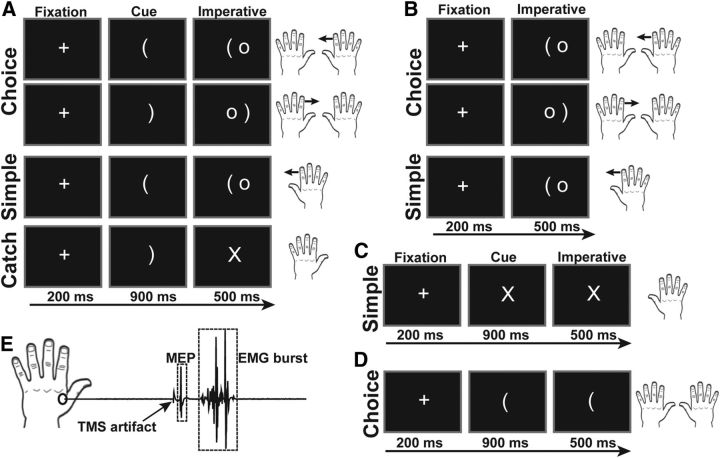
Example trials from the choice and simple delay conditions of the task are depicted in Figure 1A. Each trial began with a 4000–4500 ms (uniform distribution) intertrial interval, followed by the presentation of a fixation stimulus for 200 ms. In delay blocks, the fixation was replaced by a cue, a bracket opening to the left or to the right. The orientation of the cue indicated whether the participant should prepare a left or right index finger response and occurred with equal frequency for the two fingers. The cue remained on the screen throughout a 900 ms delay period and was followed by the presentation of a 500 ms imperative stimulus. Participants were instructed to respond quickly and accurately to the imperative stimulus by making a lateral flexion of the cued index finger. In no-delay blocks, the cue and imperative were presented simultaneously and remained visible for 500 ms (Fig. 1B); thus, there was no preparatory delay period.
Figure 1.
The delayed response task was administered in separate blocks of choice and simple trials. A cue indicated which response to prepare, and an imperative signaled to execute the prepared response. Responses were comprised of lateral flexions of the index finger or downward flexion of the pinky finger. Experiment 1 consisted of both choice and simple blocks of trials with a cue delay (A) and without a cue delay, i.e., only the imperative was presented (B). Experiment 2 included null response trials in which the cue was replaced by an X, and no response was prepared (C). Experiment 3 included three separate conditions with different types of catch trials: (1) no catch trials at all, (2) standard catch trials (A), and (3) no imperative trials (D) in which the cue remained on the screen throughout the trial. EMG was recorded from the left first dorsal interosseous muscle and used to measure MEP amplitudes and EMG burst onset times (E).
Task blocks consisted of 42 trials. To limit premature responding, an “X” was presented in place of the imperative stimulus on eight of the trials, and participants were instructed not to respond on these catch trials. In the delay condition, TMS (see below) was administered on four trials at fixation onset (baseline) and 800 ms into the delay period on 24 of the trials. No TMS was administered on the remaining 14 trials. In the no-delay condition, TMS was administered on four trials at fixation onset or 150 ms after the imperative on 24 trials.
Participants completed four blocks (2 delay, 2 no-delay) of the choice task, two blocks (1 delay, 1 no-delay) of the simple task in which only the left index finger was cued, and two blocks (1 delay, 1 no-delay) of the simple task in which only the right index finger was cued. We doubled the number of choice blocks relative to simple blocks to match the number of trials for each response. The order of the six conditions was randomized across participants, with the two choice delay blocks run consecutively and the two choice no-delay blocks run consecutively. Participants completed 10 trials of practice without TMS before the start of each new condition.
MEP measurements were always made from the left FDI muscle. Thus, during one simple delay block MEPs were recorded from the responding left hand (task-relevant), and in the other simple delay block, MEPs were recorded from the resting left hand (task-irrelevant).
Experiment 2
Experiment 2 was designed to address two questions. First, are signatures of motor suppression detectable in a task-irrelevant muscle that is not homologous to the responding effector? Second, is inhibition during delayed response tasks related to the preparation/anticipation of a movement, or might it also reflect anticipation of the TMS pulse?
Behavioral task.
Participants were only tested in the simple reaction time condition. In separate blocks, the responding effector was the left index finger (task-relevant), the right index finger (task-irrelevant, homologous), or the right pinky finger (task-irrelevant, nonhomologous). MEP measurements were always made from the left FDI. Each of these three conditions was tested in two 54-trial blocks in the delayed response condition (i.e., cue then imperative). In addition, participants completed two 38-trial blocks requiring right pinky responses in which the delay period was eliminated (i.e., simultaneous cue and imperative). The order of the four task conditions was randomized across participants.
In the delay condition blocks, we also included trials in which the cue was replaced by an X. Participants were instructed to not prepare a response on these trials (Fig. 1C). However, TMS was administered with the same likelihood as response trials. These no-response trials allowed us to ask whether delay period inhibition might be related to anticipation of the TMS pulse rather than processes related to response preparation.
In the six delay-condition blocks, there were 22 go trials, 8 catch trials, and 24 no-response trials. As in Experiment 1, TMS was administered at fixation onset (baseline) on six trials and 800 ms into the preparatory period on 34 trials of each block. There was no TMS pulse on the remaining 14 trials of each block. The proportion of TMS and no-TMS trials, along with the timing of the pulses, was matched between response and no-response trials. In the two no-delay blocks, nine trials were catch trials, and TMS was administered at fixation onset on six trials or 150 ms after the imperative on 24 trials.
In addition, 20 MEP measurements were obtained at rest, before and after the task. These measurements were made to assess the stability of our “baseline” MEP measurements during the task, and to detect general changes in motor excitability that might occur across the experimental session (Labruna et al., 2011).
Experiment 3
We examined the impact of the catch trials on preparatory inhibition in Experiment 3. In Experiments 1 and 2, as well as in our previous studies (Duque and Ivry, 2009; Duque et al., 2010, 2012; Labruna et al., 2014; Greenhouse et al., 2015), we included catch trials so that participants could not anticipate the imperative stimulus. However, the inclusion of these trials means that participants must discriminate between an imperative stimulus signaling a response and a catch stimulus indicating no response. We included conditions in Experiment 3 in which this discrimination was eliminated to examine whether anticipating the need to cancel a planned response might be a source of preparatory inhibition.
Behavioral task.
Three variants of the choice delayed response task were used in Experiment 3, each performed in two 54-trial blocks. The order of the three pairs of blocks was randomized across participants. The first condition was similar to the standard delayed response condition in which a preparatory cue was followed by either an imperative or a catch stimulus (Fig. 1A). The imperative stimulus appeared on 22 response trials, and the catch stimulus appeared on 32 no-response trials. These standard catch trials require participants to discriminate between these two possible stimuli. For the second condition, the cue remained visible for the 32 no-response trials (Fig. 1D); here, the “catch” was signaled by the absence of the appearance of the imperative. In the third condition, the imperative was presented on all 54 trials, i.e., there were no catch trials. Note that in the latter two conditions, participants do not need to discriminate between a go imperative and a catch stimulus. Thus, it was possible to test whether anticipation of stimulus discrimination might also account for motor inhibition.
As in Experiment 2, 20 MEP measurements from the left FDI were made at rest, before, and after the task.
TMS and EMG
TMS was administered using a Magstim 200-2 (Magstim) stimulator with a 7-cm-diameter figure-of-eight coil. In all of the experiments, the TMS coil was positioned over the right primary motor cortex to elicit MEPs in the left FDI muscle. Electromyography (EMG) was recorded using bipolar surface electrodes from both FDI muscles and the right abductor digiti minimi muscle in Experiment 2, sampled at 2000 Hz, amplified, and bandpass filtered (50–2000 Hz; Delsys).
To identify the optimal target for stimulation, the coil was first placed ∼5 cm lateral and 2 cm anterior to the vertex, oriented ∼45° off of the midline. During the hot-spotting procedure, TMS pulses were administered once every 3 s while the coil was repositioned and the intensity of stimulation was gradually increased. Once the optimal location was identified, the resting motor threshold (RMT) was determined by finding the intensity level that produced MEPs with peak-to-peak amplitude >50 μV on five of 10 pulses. The stimulation intensity during the experimental session was set at 115% of RMT. The average RMTs as a percentage of maximum stimulator output for Experiments 1, 2, and 3 were 43 ± 4%, 41 ± 7%, and 39 ± 6%, respectively.
MEP and EMG data analysis
The EMG data were analyzed offline using custom automated routines within MATLAB. Two dependent variables were measured: (1) the TMS-elicited MEP peak-to-peak amplitude, and (2) the onset time of the volitional response relative to the imperative stimulus (EMG-based RT). An example EMG trace with the MEP and EMG burst indicated is presented in Figure 1E. EMG events were identified whenever the EMG signal exceeded 3 SDs of the mean of the rectified signal for the entire trial epoch and was >0.1 mV. All data were visually inspected for the presence of artifacts. Trials in which EMG activity was detected within the 100 ms before the MEP, and trials in which the EMG burst onset preceded the imperative were excluded from the analysis. The EMG data were also used to calculate the number of failed catch trials, defined as trials in which EMG activity was detected following the presentation of the catch stimulus (or when the imperative would have occurred in Experiments 2 and 3).
Results
Experiment 1
MEP amplitudes
Experiment 1 was designed to examine how preparatory inhibition was modulated by the degree to which selection processes involve choice and anticipation. Participants performed a choice or simple RT task and initiated responses following an extended preparatory interval or immediately after a cue (i.e., delay vs no-delay). The simple versus choice contrast allowed us to ask whether inhibition reflects processes involved in making a choice. This question is especially relevant when considering inhibition of the left FDI when only the right hand is used to respond; if inhibition reflects competition between candidate response representations, then we would not expect to observe inhibition during response preparation in the simple RT task, based on the assumption that there is no competition. The delay versus no delay manipulation tested whether inhibition is observed in the absence of a preparatory delay.
The MEP data are presented in Figure 2A. Given that our prior work has focused on preparatory inhibition and that we used different TMS timings for the delay and no delay conditions, we opted to analyze the data separately for these conditions. We conducted two repeated-measures ANOVAs, with one factor being the type of RT task (simple vs choice) and the other being the response hand (left vs right). Note that because TMS was always applied over right motor cortex, the MEP data correspond to the selected hand when the left hand responded. For right-hand responses, the left hand was either task-relevant but not selected (choice) or task-irrelevant (simple).
Figure 2.
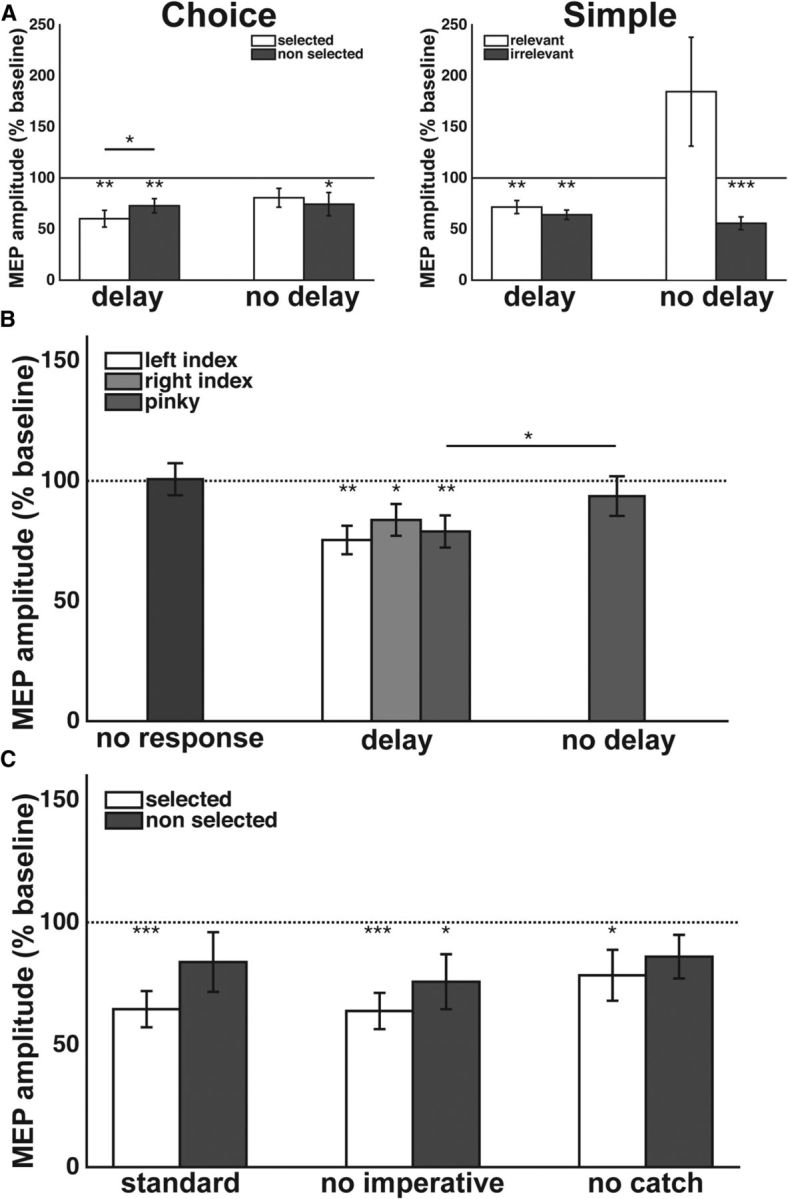
A, Experiment 1: Mean MEP amplitudes (±SE) in the left FDI muscle were reduced relative to baseline in all delay conditions (left), as well as 150 ms after a choice imperative or a simple imperative cuing the right finger, in the absence of a preparatory delay (right). B, Experiment 2: Mean left FDI MEP amplitudes (±SE) were also reduced during the delay period whenever a simple response was prepared but remained at baseline whenever a response was not being prepared and 150 ms after the right pinky was cued to respond in the absence of a preparatory delay. C, Experiment 3: the likelihood of a catch trial and the type of catch trial did not impact the observed reduction in mean MEP amplitudes (±SE). *p < 0.05, **p < 0.01, ***p < 0.001.
Given that the choice task is essentially identical to our prior work on preparatory inhibition, we used one-sample t tests to assess whether MEPs were attenuated in the delay period, relative to baseline. Replicating previous results (Duque and Ivry, 2009; Duque et al., 2010, 2012, 2014; Labruna et al., 2014; Greenhouse et al., 2015; Lebon et al., 2015), the MEPs were significantly inhibited in the choice task (both p < 0.005, two-tailed) and this effect was greater when the left hand was selected for the forthcoming response compared to when it was not selected (t(14) = 2.2, p < 0.05, two-tailed). When the same analysis was applied to the MEP data from the simple task, we also observed inhibition of left FDI (both p < 0.005, two-tailed), including when the right hand was used for the response. A comparison between the left (relevant) and right (irrelevant) simple responses was not significant (t(14) = 1.0, p = 0.3). Thus, preparatory inhibition is observed outside the context of a choice, and is of similar magnitude when the targeted muscle is task relevant or task-irrelevant. A repeated-measures ANOVA on the data from the delay conditions revealed no main effects (F(1,14) = 0.03, p = 0.9 and F(1,14) = 0.3, p = 0.6 for the effects of task and hand, respectively) indicating comparable levels of inhibition in all four conditions. A trend level interaction (F(1,14) = 3.7, p = 0.08) reflected the difference between the two hands in the choice but not the simple response task.
For the no-delay condition, MEP amplitudes were generally larger when the left (selected) hand was responding than when the right (nonselected) hand was responding (F(1,14) = 5.8, p < 0.05). This effect was more pronounced for simple than choice responses, resulting in a significant interaction (F(1,14) = 5.7, p < 0.05). The main effect of task (choice vs simple) was not significant (F(1,14) = 3.1, p = 0.1). As can be seen in Figure 2A (right), there was a marked increase, relative to baseline, in corticospinal excitability when the imperative signaled the participants to execute simple left-hand responses, although this effect was not statistically reliable (t(14) = 1.6, p = 0.13). Latencies were considerably shorter for simple responses (see below), and thus, the TMS probe at 150 ms occurred near the end of the RT interval. Moreover, MEP amplitudes tended to be inhibited relative to baseline when the left hand was selected in the choice task, although this effect was only marginally reliable (t(14) = 2.1, p = 0.06). Interestingly, left-hand MEPs were attenuated in the no-delay condition when the right hand was responding, regardless of whether the left hand was nonselected (choice: t(14) = 2.2, p < 0.05) or irrelevant to the task (simple: t(14) = 7.1, p < 0.001). The latter result is consistent with previous reports (Leocani et al., 2000; Liepert et al., 2001; Sohn et al., 2003; Weiss et al., 2003).
To directly compare the delay and no delay conditions, we conducted two additional ANOVAs, one for the choice response condition and another for the simple response condition. The level of inhibition did not differ between the delay and no delay conditions for choice responses (all p > 0.11). However, there was a significant interaction for simple responses (F(1,14) = 5.6, p < 0.05), driven by the greater difference between the delay and no delay conditions for the selected hand.
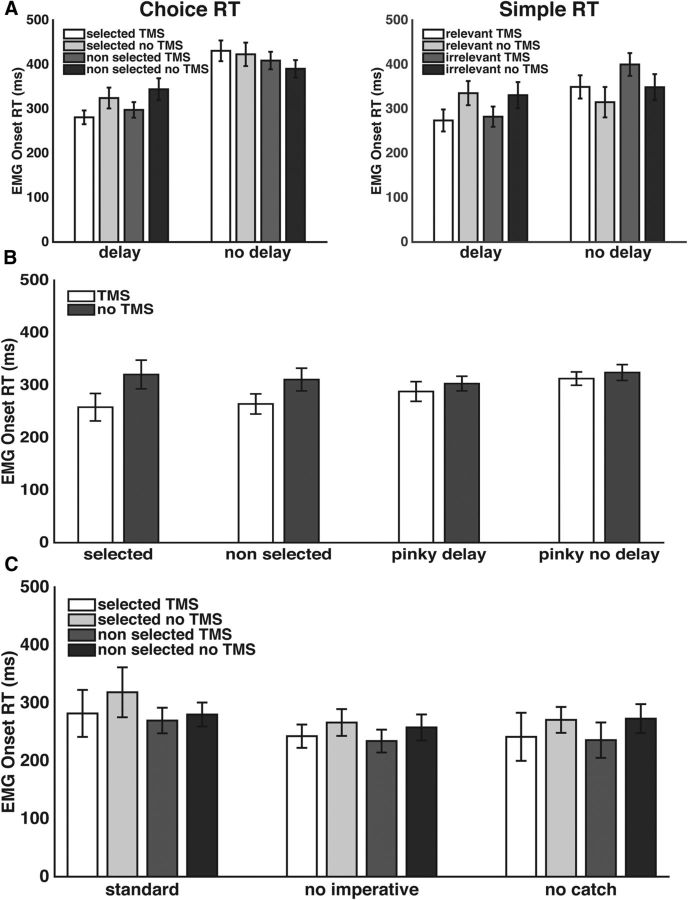
EMG onset times and failed catch trials
EMG-onset RTs are presented in Figure 3A. We analyzed these data with a repeated-measures ANOVA containing the factors condition (delay vs no-delay), response type (simple vs choice), hand (selected vs nonselected/irrelevant), and TMS (present vs absent). As expected, there was a main effect of condition, (F(1,14) = 10.3, p < 0.01), with faster RTs in the delay condition compared with the no-delay condition. This result indicates that, as instructed, participants used the cue to prepare their responses. This factor interacted with response type, (F(1,14) = 6.5, p < 0.05) and TMS, (F(1,14) = 17.5, p < 0.001). The first interaction reflects the fact that choice responses were slower than simple responses in the no-delay condition, but were similar in the delay condition. This interaction further indicates that participants used the delay period to prepare the forthcoming response and were able to negate the standard cost in RT observed for choice RT compared with simple RT.
Figure 3.
Mean EMG-based response times (±SE) are shown for all correct response trials in Experiment 1 (A), Experiment 2 (B), and Experiment 3 (C).
The second interaction is due to TMS facilitating RT in the delay condition and slowing RT in the no-delay condition. The facilitation of RT by TMS in the delay condition replicates earlier findings (Duque et al., 2012; Labruna et al., 2014; Greenhouse et al., 2015), as does the slowing of RT when TMS was administered close to movement onset in the no-delay condition (Weiss et al., 2003). In the delay condition where the response is likely prepared before the delivery of TMS, the TMS pulse may facilitate behavior either as an alerting signal and/or by further enhancing motor activation. In contrast, in the absence of a preparatory period, stimulus discrimination, and decision processes may still be operating at the time of the TMS pulse. TMS at this stage may interfere with these processes and consequently slow RT.
There was also a significant three-way interaction between response type, hand, and task condition (F(1,14) = 9.7, p < 0.01). Separate post hoc repeated-measures ANOVAs were conducted for the delay and no-delay conditions, collapsed across the TMS-present and TMS-absent trials. These post hoc tests revealed a significant interaction in the no-delay condition (F(1,14) = 11.1, p = 0.005) that was not present in the delay condition. In the former, left-hand RTs were faster for simple responses, but slower for choice responses.
We analyzed the number of failed catch trials with a repeated-measures ANOVA containing the factors condition (delay vs no-delay), response type (simple vs choice), and hand (selected vs nonselected/irrelevant). Participants generated an EMG burst on 0.05 ± 0.06% of the catch trials in the delay condition compared with 0.003 ± 0.009% of the catch trials in the no-delay condition (F(1,14) = 17.7, p < 0.001). In addition, EMG activity was detected on 0.03 ± 0.06% of the catch trials involving simple responses compared with 0.02 ± 0.04% of the catch trials involving choice responses (F(1,14) = 3.4, p = 0.09). There were no other main effects or interactions.
Experiment 2
In Experiment 1, we observed substantial preparatory inhibition in the absence of a choice. While we had predicted that we would see this inhibition when the left FDI was cued for the forthcoming response, we had not expected it when the left hand was irrelevant (i.e., simple right-hand response blocks). In Experiment 2, we repeated these two simple response tasks and also included blocks in which the responses were always made with the right pinky. This design allowed us to test whether inhibition is observed in task-irrelevant, nonhomologous muscles, or whether it is specific to homologous muscles. In addition, we assessed whether inhibition might arise from anticipation of the TMS pulse. To this end, we included no-response trials in which the cue indicated that no response should be prepared (Fig. 1C), even though TMS could still occur.
MEP amplitudes
The MEP data for Experiment 2 are depicted in Figure 2B. These data were analyzed with a repeated-measures ANOVA with the factors trial type (response vs no-response) and responding effector (left index, right index, or right pinky). MEP amplitudes were attenuated when participants were cued to prepare a response compared with when they were cued to not prepare a response (F(1,12) = 12.3, p < 0.005). The main effect of effector was not significant (F(2,24) = 1.0, p < 0.4) nor did the factors interact (F(2,24) = 0.6, p < 0.6).
Compared with baseline, left FDI MEPs were inhibited during the delay period regardless of whether the response was made with the left index finger, right index finger, or right pinky (independent t tests, all p < 0.05). The left and right index finger results replicate Experiment 1: inhibition in a simple RT task is not restricted to conditions in which the probed muscle is task relevant. In addition, the pinky results demonstrate that inhibition of a task-irrelevant muscle is observed even when it is not homologous to the responding effector. MEPs were not different from baseline in any of the conditions following the no-response cue (all p > 0.2). Thus, preparatory inhibition is not related to anticipation of the TMS pulse.
We included a fourth, no-delay block of trials in which all of the responses were made with the right pinky finger to examine the effect of homology on postimperative inhibition in a task-irrelevant muscle. MEPs were not significantly below baseline during these trials (t(12) = 0.8, p = 0.5). A planned comparison of the delay and no-delay conditions for right pinky blocks showed that task-irrelevant, nonhomologous inhibition was greater during the delay period compared with the postimperative period (t(12) = 2.2, p < 0.05, one-tailed). Thus, inhibition in the left hand was present during the delay period when the right pinky was prepared to respond but was absent in a postimperative window when the participants were initiating a right pinky response.
The results from Experiments 1 and 2 (see Fig. 2A,B) reveal inhibition in both homologous and nonhomologous task-irrelevant muscles during a preparatory delay period. In contrast, inhibition of the task-irrelevant hand following the imperative was only observed during right index finger responses (Experiment 1; Fig. 2A, right) and was not observed during pinky responses (Experiment 2; Fig. 2B). To directly compare these conditions, we conducted a between-subjects post hoc t test. Inhibition was greater in the task-irrelevant left index finger when the right index finger was responding compared with when the right pinky finger was responding (t(26) = 3.7, p < 0.001, two-tailed). This comparison reinforces the finding that inhibition observed in the task-irrelevant left hand was present during the preparation but not during the execution of right pinky responses and suggests that inhibition during response execution may be restricted to contralateral homologous muscles.
EMG onset times and failed catch trials
EMG onset times are presented in Figure 3B. A repeated-measures ANOVA for the delay condition data with the factors responding effector (left index, right index, right pinky) and TMS (present vs absent) showed that RTs were faster on TMS trials (F(1,12) = 23.6, p < 0.001). There was also a significant interaction (F(1,12) = 4.0, p < 0.05). The facilitatory effect of TMS on RT was smaller for the pinky responses compared with the two index finger responses. RTs did not differ between the pinky responses for the delay and no-delay conditions (t(12) = 1.2, p = 0.2). Participants responded on 0.04 ± 0.04% of catch trials in the delay conditions and on 0.03 ± 0.06% of catch trials in the no-delay pinky condition. The number of failed catch trials in the delay pinky and no-delay pinky conditions did not differ (t(12) = 1.5, p = 0.2).
Experiment 3
We included catch trials in Experiments 1 and 2 (Duque and Ivry, 2009; Duque et al., 2010, 2012; Labruna et al., 2014; Greenhouse et al., 2015; Lebon et al., 2015) so that participants would not anticipate the imperative stimulus. The catch trials require that participants discriminate between the go imperative and the no-go catch stimulus, a discrimination that is present even in the simple RT conditions. To assess whether preparatory inhibition is related to this discrimination, we compared, in separate test blocks, a condition with the standard version of catch trials, a condition with catch trials in which the cue was never replaced by the imperative stimulus (i.e., catch trials without a discrimination), and a condition with no catch trials. For this experiment, we returned to a choice task involving the left and right index fingers.
MEP amplitudes
The MEP data for Experiment 3 are depicted in Figure 2C. A repeated-measures ANOVA with the factors catch trial condition (standard catch, no imperative, and no catch) and hand (selected vs nonselected) indicated that MEP amplitudes measured during the delay period were smaller in the selected hand than in the nonselected hand (F(1,9) = 5.3, p < 0.05). There was no effect of the different types of catch trials (F(2,18) = 0.8, p = 0.5) and the factors did not interact (F(2,18) = 0.6, p = 0.6).
Relative to baseline, left FDI MEPs were inhibited during the delay period when the left hand was cued (selected MEP) in all three catch trial conditions (all p < 0.05, one-tailed). A similar pattern was observed when the right hand was cued (nonselected MEP), with significant inhibition in the no imperative condition (t(9) = 2.2, p < 0.05) and marginally reliable inhibition in the other two conditions (standard catch: t(9) = 1.3, p = 0.1; no catch: t(9) = 1.6, p = 0.07). In sum, preparatory inhibition does not appear to be related to the need to discriminate go and no-go stimuli, or the anticipation of catch trials.
EMG onset times and failed catch trials
The EMG RT data are presented in Figure 3C. RTs were faster for trials with TMS (F(1,9) = 7.3, p < 0.05). There was also a main effect of catch trial condition, (F(2,18) = 3.9, p < 0.05). As expected, RTs were slower in the standard catch trial condition compared to when there were no catch trials or when no-response trials were indicated by the absence of the imperative stimulus.
Participants responded on 0.03 ± 0.04% of the standard catch trials and 0.02 ± 0.05% of the no imperative catch trials. There were no significant differences between the types of catch trials and there was no interaction with the left or right hand.
Discussion
In three experiments, we assessed corticospinal excitability during the preparation and initiation of finger movements in the context of choice and simple response tasks. In agreement with previous studies (Duque and Ivry, 2009; Duque et al., 2010; Labruna et al., 2014; Greenhouse et al., 2015; Lebon et al., 2015), MEP amplitudes were reduced during the preparation of responses in the choice response context. Extending this line of research, we observed reduced MEPs during the preparation of simple responses, even when MEPs were recorded from a task-irrelevant muscle that was not homologous to the responding effector. Collectively, these experiments point to a nonspecific inhibitory process involved in response preparation. Although we only measured MEPs from the left FDI muscle here, previous studies suggest the results would generalize to other muscles (Hasbroucq et al., 1999b).
Novel control experiments helped to rule out the possibility that the corticospinal inhibition was due to factors other than response preparation. When no response was prepared, MEPs did not differ from baseline (Experiment 2), indicating that inhibition was not due to anticipation of the TMS pulse. Additionally, the presence or absence of no-go catch trials did not impact the level of MEP suppression (Experiment 3). These results support the idea that this signature of corticospinal inhibition reflects a response preparation process.
Broad inhibition of motor system excitability during response preparation
Many human electrophysiological studies have observed transient suppression of motor excitability during response preparation (Hasbroucq et al., 1997, 1999a,b; Touge et al., 1998; Davranche et al., 2007; van Elswijk et al., 2007; Duque and Ivry, 2009; Sinclair and Hammond, 2009; Duque et al., 2010, 2012; Soto et al., 2010). However, the underlying mechanisms and functional implications remain unclear. Previous work led to the hypothesis that two independent inhibitory mechanisms operate in concert. Inhibition of nonselected responses was attributed to a mechanism for resolving the competition between candidate responses, labeled competition resolution (Coles et al., 1985; Usher and McClelland, 2001, 2004; Duque et al., 2010). Inhibition of the selected response was proposed to prevent premature response execution, a process-labeled impulse control (Duque et al., 2010). Additional support for this two-process model was obtained in a study in which single-pulse TMS over M1 was preceded by repetitive TMS (rTMS) over frontal cortex. rTMS over lateral prefrontal cortex attenuated the signature of the competition resolution process, whereas rTMS over dorsal premotor cortex selectively attenuated the signature of the impulse control process (Duque et al., 2012). This result implicated independent prefrontal cortical projections to M1 in the operation of these two inhibitory mechanisms.
Neither the competition resolution nor the impulse control mechanism can account for the current observation of inhibition in a task-irrelevant muscle; these mechanisms, as proposed, only influence the task-relevant response options. We suggest an alternative framework by which the motor system is broadly suppressed as part of the response preparation process. This could represent a more general instantiation of “competition resolution,” one that facilitates response selection by broadly inhibiting motor representations, including those outside the task set. The fact that we observed inhibition in the task-irrelevant hand during the preparation of simple contralateral responses indicates that this broad inhibition does not require a specified competition, at least not in the manner of choosing between preassigned response options.
There is strong precedent for a broadly tuned inhibitory mechanism in the motor system. Studies of basal ganglia function in nonhuman primates have led to the suggestion of widespread inhibition of motor excitability during the preparation of voluntary movement (Mink and Thach, 1993; Berns and Sejnowski, 1996; Mink, 1996; Nambu et al., 2002). According to this framework, selection and preparation entail two stages. First, widespread output from the basal ganglia inhibits thalamocortical excitatory projections to M1, producing broad and transient motor suppression. Second, projections within the basal ganglia (striato-pallidal pathway) inhibit a subset of the output cells in a focused manner to disinhibit a specific thalamocortical motor channel, initiating a chosen action. Such a subcortical mechanism differs from the corticocortical mechanism proposed by Duque et al. (2012). Nevertheless, the prefrontal regions identified by Duque et al. could mediate basal ganglia-thalamocortical circuits.
Additional precedent for a broadly tuned motor inhibition mechanism derives from the observation that task-irrelevant muscles are suppressed when ongoing responses are stopped (Badry et al., 2009; Cai et al., 2012; Greenhouse et al., 2012; Majid et al., 2012; Wessel et al., 2013). These studies implicate a prefrontal-subthalamic network for global motor suppression (Aron et al., 2007). This same pathway has also been implicated in the prevention of impulsive responses (Frank et al., 2007). Notably, we did not observe a difference in MEP amplitudes between conditions with and without catch trials, despite significantly slower RTs when catch trials were present (Experiment 3). The lack of a difference in MEP amplitudes despite differences in RTs across conditions challenges the idea that preparatory inhibition reflects a mechanism involved in preventing premature responses (Boulinguez et al., 2009).
Moreover, preparatory inhibition does not appear to reflect the setting of a response decision threshold (Ratcliff and Frank, 2012). If greater preparatory inhibition corresponded to an increased decision threshold, we would have expected to observe a difference in the level of inhibition between choice and simple RT tasks, as well as a relationship between inhibition and RT. Neither pattern was observed. As such, we propose that preparatory inhibition reflects the operation of a mechanism involved in implementing the response after a decision threshold has been reached. Nevertheless, further experiments may identify a relationship between preparatory inhibition and impulse control.
Preparatory inhibition as a form of gain modulation
Surprisingly, the level of inhibition did not differ between the selected/relevant and task-irrelevant hands in the simple RT context of Experiment 1. This result contrasts with the observation that the selected hand is more inhibited than the nonselected hand in a choice context (Duque et al., 2010; Labruna et al., 2014; Greenhouse et al., 2015; Lebon et al., 2015), a pattern we replicated here.
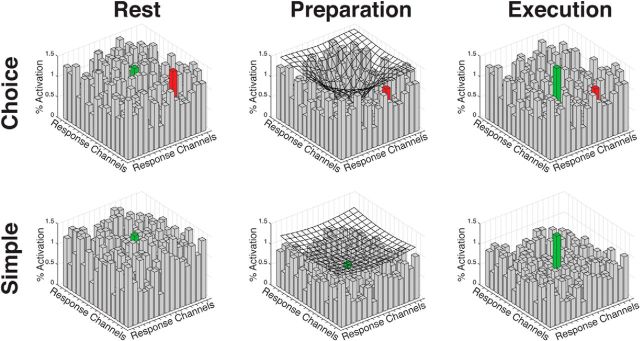
One way to conceptualize this result is that inhibition functions as a spotlight, centered at the selected response representation. Importantly, the aperture, or focus of the spotlight, is assumed to be context-dependent (Fig. 4). We propose that inhibition is narrowly focused at the selected response representation in the choice context and broadly focused in the simple context. This spotlight model is reminiscent of psychological and physiological models of response selection in which the extent of attentional focus is assumed to reflect task demands. For example, tuning functions may be sharpened when there is competition from conflicting inputs (Spitzer et al., 1988; Lavie et al., 2004; Çukur et al., 2013). The notion of a spotlight has also emerged in response selection models of basal ganglia function (Mink and Thach, 1993; Berns and Sejnowski, 1996; Nambu et al., 2002). Here we propose that the aperture of inhibition is narrower when the participant must select between potential responses.
Figure 4.
The inhibitory spotlight (mesh overlay) may be centered over the selected (green bar) response representation. In the context of a choice task (top row), the tuning of the spotlight is sharper, increasing separation between the selected and nonselected (red bar) response representations. Given the lack of competition, the tuning of the spotlight can be broader in the absence of a choice (simple RT, bottom row).
Preparatory inhibition has been principally implicated in preventing movement (e.g., competition resolution and impulse control). For example, during the course of response selection, greater control may be required to prevent selection of erroneous responses. Although the spotlight model is consistent with the idea that inhibition serves to prevent premature execution, it may be appropriate to consider how inhibition might facilitate response selection and initiation. Preparatory inhibition has been hypothesized to increase the signal-to-noise associate with a selected action (Hasbroucq et al., 1997). Consider the analogy of a noisy classroom in which a chosen student attempts to answer a question. If the teacher quiets the entire class (including the designated student), then the student will be more easily heard when she starts talking. Similarly, a response channel may be activated but will fail to elicit movement until motor noise has been sufficiently suppressed (Churchland et al., 2006). The narrower focus in the choice condition would target this inhibition at response options with a higher probability of being selected.
Physiologically, inhibition has been hypothesized to modulate the gain of individual neurons by having a divisive influence on a cell's sensitivity to excitatory drive (Chance et al., 2002). Greater inhibition of the corticospinal representation of the selected response could increase its sensitivity to excitatory input. Interestingly, a centrally controlled and widespread feedforward GABAergic inhibitory mechanism for this type of gain control on motor output has been observed in the leech (Baca et al., 2008). More generally, gain modulation is recognized as a fundamental computational principle of the nervous system (Salinas and Thier, 2000; Chance et al., 2002).
Together, broadly tuned inhibition could decrease background activity (i.e., general noise suppression) and also increase neuronal sensitivity to excitatory drive (i.e., gain modulation). Thus, following a burst of broad inhibition, the persistent activation of a selected response channel could rapidly drive response execution. The MEP measure of preparatory inhibition may be a signature of such a process in the selection and initiation of goal-driven actions.
Conclusion
A signature of motor inhibition, observed during response preparation, is evident in representations outside the set of candidate response options. This suppression is not an artifact of TMS expectancy and is not related to anticipating the need to abort a response. All of these properties suggest that the underlying mechanism is not under direct cognitive control, but rather operates automatically whenever a voluntary action is prepared. We hypothesize that the widespread suppression of motor excitability reflects a gain modulation mechanism that facilitates response selection. Further work is needed to identify the mechanisms—cortical, subcortical, and/or spinal—that underlie this inhibition and how they shape the output of the motor system.
Footnotes
This work was supported by the National Institute of Health (NS074917 and NS0085570).
The authors declare no competing financial interests.
References
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca SM, Marin-Burgin A, Wagenaar DA, Kristan WB., Jr Widespread inhibition proportional to excitation controls the gain of a leech behavioral circuit. Neuron. 2008;57:276–289. doi: 10.1016/j.neuron.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, Foly N, Nagiub H, Nagamine T, Fukuyama H. Suppression of human cortico-motoneuronal excitability during the stop-signal task. Clin Neurophysiol. 2009;120:1717–1723. doi: 10.1016/j.clinph.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Berns GS, Sejnowski TJ. How the basal ganglia make decisions. In: Damasio AR, Damasio H, Christen Y, editors. Neurobiology of decision-making. Berlin, Heidelberg: Springer; 1996. pp. 101–113. [Google Scholar]
- Boulinguez P, Ballanger B, Granjon L, Benraiss A. The paradoxical effect of warning on reaction time: demonstrating proactive response inhibition with event-related potentials. Clin Neurophysiol. 2009;120:730–737. doi: 10.1016/j.clinph.2009.02.167. [DOI] [PubMed] [Google Scholar]
- Cai W, Oldenkamp CL, Aron AR. Stopping speech suppresses the task-irrelevant hand. Brain Lang. 2012;120:412–415. doi: 10.1016/j.bandl.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/S0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV. Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci. 2006;26:3697–3712. doi: 10.1523/JNEUROSCI.3762-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MG, Gratton G, Bashore TR, Eriksen CW, Donchin E. A psychophysiological investigation of the continuous flow model of human information processing. J Exp Psychol Hum Percept Perform. 1985;11:529–553. doi: 10.1037/0096-1523.11.5.529. [DOI] [PubMed] [Google Scholar]
- çukur T, Nishimoto S, Huth AG, Gallant JL. Attention during natural vision warps semantic representation across the human brain. Nat Neurosci. 2013;16:763–770. doi: 10.1038/nn.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25:3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 2010;30:3793–3802. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 2012;32:806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Cazares C, Ivry RB. Dissociating the influence of response selection and task anticipation on corticospinal suppression during response preparation. Neuropsychologia. 2014;65:287–296. doi: 10.1016/j.neuropsychologia.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Oldenkamp CL, Aron AR. Stopping a response has global or nonglobal effects on the motor system depending on preparation. J Neurophysiol. 2012;107:384–392. doi: 10.1152/jn.00704.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Saks D, Hoang T, Ivry RB. Inhibition during movement preparation is sensitive to response difficulty. J Neurophysiol. 2015;113:2792–2800. doi: 10.1152/jn.00999.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamaï CA. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 1997;5:185–192. doi: 10.1016/S0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamaï CA. The time-course of preparatory spinal and cortico-spinal inhibition: an H-reflex and transcranial magnetic stimulation study in man. Exp Brain Res. 1999a;124:33–41. doi: 10.1007/s002210050597. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Osman A, Possamaï CA, Burle B, Carron S, Dépy D, Latour S, Mouret I. Cortico-spinal inhibition reflects time but not event preparation: neural mechanisms of preparation dissociated by transcranial magnetic stimulation. Acta Psychologica. 1999b;101:243–266. doi: 10.1016/S0001-6918(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Labruna L, Fernández-del-Olmo M, Ivry RB. Comparison of different baseline conditions in evaluating factors that influence motor cortex excitability. Brain Stimul. 2011;4:152–155. doi: 10.1016/j.brs.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Labruna L, Lebon F, Duque J, Klein PA, Cazares C, Ivry RB. Generic inhibition of the selected movement and constrained inhibition of nonselected movements during response preparation. J Cogn Neurosci. 2014;26:269–278. doi: 10.1162/jocn_a_00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. J Exp Psychol Gen. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lebon F, Greenhouse I, Labruna L, Vanderschelden B, Papaxanthis C, Ivry RB. Influence of delay period duration on inhibitory processes for response preparation. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv069. doi: 10.1093/cercor/bhv069. Advance online publication. Retrieved April 16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol. 2001;112:114–121. doi: 10.1016/S1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- Majid DS, Cai W, George JS, Verbruggen F, Aron AR. Transcranial magnetic stimulation reveals dissociable mechanisms for global versus selective corticomotor suppression underlying the stopping of action. Cereb Cortex. 2012;22:363–371. doi: 10.1093/cercor/bhr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/S0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia intrinsic circuits and their role in behavior. Curr Opin Neurobiol. 1993;3:950–957. doi: 10.1016/0959-4388(93)90167-W. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci Res. 2002;43:111–117. doi: 10.1016/S0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Frank MJ. Reinforcement-based decision making in corticostriatal circuits: mutual constraints by neurocomputational and diffusion models. Neural Comput. 2012;24:1186–1229. doi: 10.1162/NECO_a_00270. [DOI] [PubMed] [Google Scholar]
- Salinas E, Thier P. Gain modulation: a major computational principle of the central nervous system. Neuron. 2000;27:15–21. doi: 10.1016/S0896-6273(00)00004-0. [DOI] [PubMed] [Google Scholar]
- Sinclair C, Hammond GR. Excitatory and inhibitory processes in primary motor cortex during the foreperiod of a warned reaction time task are unrelated to response expectancy. Exp Brain Res. 2009;194:103–113. doi: 10.1007/s00221-008-1684-2. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Jung HY, Kaelin-Lang A, Hallett M. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Exp Brain Res. 2003;148:176–185. doi: 10.1007/s00221-002-1292-5. [DOI] [PubMed] [Google Scholar]
- Soto O, Valls-Solé J, Kumru H. Paired-pulse transcranial magnetic stimulation during preparation for simple and choice reaction time tasks. J Neurophysiol. 2010;104:1392–1400. doi: 10.1152/jn.00620.2009. [DOI] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109:489–495. doi: 10.1016/S0924-980X(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Usher M, McClelland JL. The time course of perceptual choice: the leaky, competing accumulator model. Psychol Rev. 2001;108:550–592. doi: 10.1037/0033-295X.108.3.550. [DOI] [PubMed] [Google Scholar]
- Usher M, McClelland JL. Loss aversion and inhibition in dynamical models of multialternative choice. Psychol Rev. 2004;111:757–769. doi: 10.1037/0033-295X.111.3.757. [DOI] [PubMed] [Google Scholar]
- van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci. 2007;19:121–131. doi: 10.1162/jocn.2007.19.1.121. [DOI] [PubMed] [Google Scholar]
- Weiss AC, Weiller C, Liepert J. Pre-movement motor excitability is reduced ipsilateral to low force pinch grips. J Neural Transm. 2003;110:201–208. doi: 10.1007/s00702-002-0780-x. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Reynoso HS, Aron AR. Saccade suppression exerts global effects on the motor system. J Neurophysiol. 2013;110:883–890. doi: 10.1152/jn.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]