Abstract
The sigma-2 receptors are promising therapeutic targets because of their significant upregulation in tumor cells compared with normal tissue. Here, we characterize CM572 [3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)-6-isothiocyanatobenzo[d]oxazol-2(3H)-one] (sigma-1 Ki ≥ 10 µM, sigma-2 Ki = 14.6 ± 6.9 nM), a novel isothiocyanate derivative of the putative sigma-2 antagonist, SN79 [6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one]. CM572 bound irreversibly to sigma-2 receptors by virtue of the isothiocyanate moiety but not to sigma-1. Studies in human SK-N-SH neuroblastoma cells revealed that CM572 induced an immediate dose-dependent increase in cytosolic calcium concentration. A 24-hour treatment of SK-N-SH cells with CM572 induced dose-dependent cell death, with an EC50 = 7.6 ± 1.7 µM. This effect was sustained over 24 hours even after a 60-minute pretreatment with CM572, followed by extensive washing to remove ligand, indicating an irreversible effect consistent with the irreversible binding data. Western blot analysis revealed that CM572 also induced cleavage activation of proapoptotic BH3-interacting domain death agonist. These data suggest irreversible agonist-like activity. Low concentrations of CM572 that were minimally effective were able to attenuate significantly the calcium signal and cell death induced by the sigma-2 agonist CB-64D [(+)-1R,5R-(E)-8-benzylidene-5-(3-hydroxyphenyl)-2-methylmorphan-7-one]. CM572 was also cytotoxic against PANC-1 pancreatic and MCF-7 breast cancer cell lines. The cytotoxic activity of CM572 was selective for cancer cells over normal cells, being much less potent against primary human melanocytes and human mammary epithelial cells. Taken together, these data show that CM572 is a selective, irreversible sigma-2 receptor partial agonist. This novel irreversible ligand may further our understanding of the endogenous role of this receptor, in addition to having potential use in targeted cancer diagnosis and therapy.
Introduction
The sigma receptors are a class of membrane-bound receptors that have affinity for a wide variety of psychotropic agents. There are two subtypes of sigma receptors, denoted sigma-1 and sigma-2, which differ in their pharmacologic profile (Hellewell and Bowen, 1990; Hellewell et al., 1994). Both subtypes are found in the central nervous system and in peripheral tissues throughout the body. The sigma-1 receptor has been well characterized and functions as a ligand-regulated chaperone protein that modulates the function of other receptors, ion channels, and transporters, and plays a role in the promotion of cell survival (Hayashi and Su, 2007; Maurice and Su, 2009).
The sigma-2 receptor is highly expressed in a variety of cancer cell lines (Vilner et al., 1995b) and becomes even more highly upregulated when cancer cells are in a state of rapid proliferation (Mach et al., 1997; Al-Nabulsi et al., 1999; Wheeler et al., 2000). Ligands presumed to activate sigma-2 receptors induce apoptotic cell death in cancer cell lines (Vilner et al., 1995a; Crawford and Bowen, 2002; Ostenfeld et al., 2005; Bowen, 2007; Abate et al., 2012; Zeng et al., 2012; Mach et al., 2013). Because of these aspects, the sigma-2 receptor has received growing attention as a potential target in cancer therapy, and efforts are in progress to elucidate the identity and function of this protein and to develop highly selective targeting ligands. Using a fluorescent photoaffinity probe, [125I]RHM-4 [N-(4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-2-(2-fluoroethoxy)-5-iodo-3-methoxybenzamide] as the sigma-2 radioligand, and proteomic analysis, Mach and colleagues have demonstrated a relationship of sigma-2 receptors to progesterone receptor membrane component-1, which is also highly expressed in cancer cells and regulates cell growth and survival (Xu et al., 2011). This work highlights the importance of the development of unique tools with which to further study this receptor.
Although antagonists for sigma-1 receptors are readily available, selective antagonists for sigma-2 receptors have not been well described, in part because of a paucity of selective sigma-2 receptor ligands and detailed knowledge about the functional roles of sigma-2 receptors. Sigma-2 receptor ligand classification has had several proposals for criteria defining an agonist, antagonist, or partial agonist. As mentioned, compounds presumed to be sigma-2 agonists induce apoptotic cell death. Several aspects of the purported sigma-2 signaling mechanism have been used in an attempt to define agonists. For example, Mach and colleagues have used the criteria of caspase-3 activation and loss of cell viability in EMT-6 mouse breast cancer and MDA-MB-435 human melanoma cells to define putative sigma-2 agonists and antagonists that correlated with comparisons to siramesine (1′-{4-[1-(4-fluorophenyl)-1H-indol-3-yl]butyl}-3H-spiro[2-benzofuran-1,4′-piperidine]), a presumed sigma-2 full agonist (Ostenfeld et al., 2005; Zeng et al., 2012, 2014). The sigma-2 receptors mediate a rapid and transient calcium release from the endoplasmic reticulum of SK-N-SH neuroblastoma cells (Vilner and Bowen, 2000; Cassano et al., 2009). We have characterized CB-64D [(+)-1R,5R-(E)-8-benzylidene-5-(3-hydroxyphenyl)-2-methylmorphan-7-one] and CB-184 [(+)-1R,5R-(E)-8-(3,4-dichlorobenzylidene)-5-(3-hydroxyphenyl)-2-methylmorphan-7-one] as sigma-2 agonists based on their ability to induce a calcium transient and apoptotic cell death in SK-N-SH neuroblastoma and breast tumor cell lines (Bowen et al., 1995; Crawford and Bowen, 2002). We have also proposed that caspase-10–induced cleavage and activation of the proapoptotic Bcl-2 family protein BH3-interacting domain death agonist (Bid), mitochondrial depolarization, and subsequent release of mitochondrial apoptogenic factors are important steps in sigma-2–induced apoptosis in some cells (Hazelwood and Bowen, 2006; Wang and Bowen, 2006). CB-64D induces rapid Bid cleavage and mitochondrial release of endonuclease G and apoptosis inducing factor in SK-N-SH neuroblastoma cells, and effects are attenuated by a broad-spectrum caspase inhibitor, as well as a caspase-10–specific inhibitor (Wang and Bowen, 2006).
Despite these advances in classifying sigma-2 agonists, little has been reported on the direct characterization of antagonists in these assays, that is, direct demonstration that the activity of putative agonists can be attenuated by other sigma-2 ligands that do not exert an effect. The moderately sigma-2–selective ligand SN79 [6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one] has been characterized as an antagonist of sigma-1/sigma-2 receptors (Kaushal et al., 2011, 2013). SN79 protects against neurotoxicity induced by high doses of methamphetamine, which binds to sigma receptors, whereas the sigma receptor agonist 1,3-di-o-tolylguanidine (DTG) enhanced the neurotoxic effect. In an approach to develop a functional antagonist of sigma-2 receptors, we characterized a series of isothiocyanate derivatives of SN79 (Comeau et al., 2012). The goal was to incorporate the isothiocyanate moiety to impart the ability to bind irreversibly to the receptor, thereby producing chronic, functional sigma-2 receptor blockade. One of these compounds, CM572 [3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)-6-isothiocyanatobenzo[d]oxazol-2(3H)-one], is further characterized here. Using effects on radioligand binding, calcium response, Bid cleavage, and cell viability, we characterize CM572 as a potent, irreversible, sigma-2–selective partial agonist.
Materials and Methods
Radioligand Binding Assay.
Receptor binding assays were performed using rat liver membrane homogenates, as previously described with minor modifications (Hellewell et al., 1994). Briefly, incubations were carried out in 20 mM HEPES, pH 7.4, for 120 minutes at 25°C using 150 μg of rat liver membrane protein in a final volume of 0.5 ml. HEPES buffer was substituted for the normally used 50 mM Tris-HCl, pH 8.0, buffer in incubations with CM572 to avoid reaction of the isothiocyanate group with the amino group of Tris. The sigma-1 receptors were labeled with 3 nM [3H](+)-pentazocine (PerkinElmer, Waltham, MA). The sigma-2 receptors were labeled with 5 nM [3H]DTG (PerkinElmer) in the presence of 100 nM (+)-pentazocine to block the sigma-1 receptors. Nonspecific binding was determined in the presence of 10 μM haloperidol. Assays were terminated by addition of 5 ml of ice-cold 10 mM Tris-HCl, pH 7.4 and filtration using a Brandel cell harvester (Brandel, Gaithersburg, MD). Filters were washed twice with 5 ml of ice-cold buffer. Filters were soaked in 0.5% polyethyleneimine for at least 30 minutes at 25°C before use. Ki values were determined by competition assays with data analysis using GraphPad Prism (GraphPad Software, La Jolla, CA).
Irreversible Binding Analysis.
Membranes were incubated with the indicated concentration of CM572 for 60 minutes at 25°C in 20 mM HEPES, pH 7.4, at a protein concentration of 0.30 mg/ml. The preparation was then diluted to a protein concentration of 0.018 mg/ml with ice-cold buffer and centrifuged at 37,000g for 10 minutes. The pellet was resuspended to the original volume with ice-cold buffer and centrifuged again. After resuspension to the original volume with 20 mM HEPES, pH 7.4, the preparation was allowed to incubate for 60 minutes at 25°C to allow for dissociation of any noncovalently bound isothiocyanate. The preparation was then subjected to centrifugation at 37,000g for 10 minutes and resuspended to a protein concentration of 0.6 mg/ml in 50 mM Tris, pH 8.0, and used directly in the radioligand assay as already described herein, using 0.3 mg protein/ml in a final volume of 0.5 ml. The control membranes were treated in the identical manner without exposure to CM572. This washout method was shown to effect complete dissociation of 500 nM SN79 from both sigma-1 and sigma-2 receptors (data not shown).
Cell Culture.
Human SK-N-SH neuroblastoma and MCF-7 breast adenocarcinoma (American Type Culture Collection, Manassas, VA) cells were cultured in minimal essential media (MEM; Gibco, Grand Island, NY) with 10 mg/l insulin and 10% fetal bovine serum (FBS) at 37°C and 5% CO2 in a humidified atmosphere. Human PANC-1 pancreatic epithelioid carcinoma (PANC-1; American Type Culture Collection) cells were cultured in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO) with 10% FBS and 2 mM l-glutamine at 37°C and 5% CO2 in a humidified atmosphere. Human epidermal melanocytes (HEMs) from neonatal foreskin were a generous gift from Dr. Elena Oancea, Brown University. HEM cells were cultured in Medium 254 (Life Technologies, Grand Island, NY) with 5% Human Melanocyte Growth Supplement 2 (Life Technologies) at 37°C and 5% CO2 in a humidified atmosphere. Normal prestasis human mammary epithelial cells (HMECs) were kindly provided by Dr. Martha Stampfer, Lawrence Berkeley National Laboratory. HMECs were cultured in M87A + CT + X medium at 37°C and 5% CO2 in a humidified atmosphere.
Cell Viability Assay.
Cytotoxicity was measured using MTT (3-[4,5 dimethylthiazol-2-y]-2,5 diphenyltetrazolium bromide) assays (Trevigen, Gaithersburg, MD). Assays were performed in 96-well plates with 15,000 cells/well (SK-N-SH, HEM) or 10,000 cells/well (MCF-7, PANC-1, HMECs). Cells were allowed to attach overnight before dosing. After 24 hours (SK-N-SH, HEM, PANC-1) or 48 hours (MCF-7, HMEC), incubation with ligand (total volume 100 μl/well), 10 μl MTT reagent was added and allowed to be metabolized for 3 hours. Detergent was then added (100 μl) and allowed to solubilize membranes and formazan crystals for 2 hours. Absorbance was then measured at 570 nm. Dose-response curves were analyzed using GraphPad Prism 6.
In experiments where the irreversible effect of ligands was examined, cells were exposed acutely to sigma ligand for the indicated time (30, 60, or 120 minutes) in Hank’s buffered salt solution (HBSS; Gibco). Wells were then washed twice with 100 μl of culture medium to remove any unbound ligand. After the addition of 100 μl fresh normal culture medium, cells were allowed to incubate for 24 hours, and viability was assessed by MTT assay.
Calcium Release Assay.
Calcium responses were measured using the fura-2 ratiometric assay. Cells were allowed to attach and proliferate for 48 hours after seeding at 20,000 cells/well. Media was then replaced with 2.47 μM fura-2 acetoxymethyl ester (fura-2AM; Invitrogen, Grand Island, NY) and 0.065% pluronic acid in HBSS and incubated for 60 minutes. Fura-2 solution was then removed, and cells were washed three times with 100 μl HBSS each before injection of ligand in HBSS for a final total volume of 100 μl (injection volumes did not exceed 30 μl). Calcium changes indicated by ratio of fura-2 fluorescence were measured with excitation at 340 and 380 nm with emissions measured at 510 nm using a PerkinElmer Victor V plate reader in a 96-well format.
Western Blot.
Cells were plated in 35-mm petri dishes at 500,000 cells/dish and allowed to attach overnight before treatment. Samples were treated with the indicated dose of CM572 for the indicated time before lysis with radioimmunoprecipitation assay buffer with protease and phosphatase inhibitor cocktail. Western blotting for Bid was performed using 15% acrylamide gels and transferred overnight at 40 V at 4°C onto nitrocellulose paper. Blot was then blocked for 1 hour at room temperature shaking in 10% milk/0.1% Tween/Tris-buffered saline (TBS) and washed three times with ∼15 ml 0.1% Tween/TBS per wash. Blot was then incubated overnight with shaking at 4°C with 1:200 Bid antibody (Santa Cruz Biotechnology, Dallas, TX) in 10% milk/0.1% Tween/TBS, followed by continued incubation shaking at room temperature for 1 hour. Blot was then washed three times with ∼15 ml 0.1% Tween/TBS per wash, incubated at room temperature with 1:5000 rabbit secondary antibody (Santa Cruz Biotechnology) in 10% milk/0.1% Tween/TBS for 1 hour, washed three times with ∼15 ml 0.1% Tween/TBS per wash, and developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Waltham, MA) with directions as specified by the manufacturer. Exposures were made using UltraCruz autoradiography film (Santa Cruz Biotechnology). For loading control, the same procedure was followed with anti α/β-tubulin primary antibody (Cell Signaling Technology, Danvers, MA) with incubation at 1:1000 in 5% bovine serum albumin/0.1% Tween/TBS.
Results
Radioligand Binding and Examination of Irreversibility in Rat Liver Membranes.
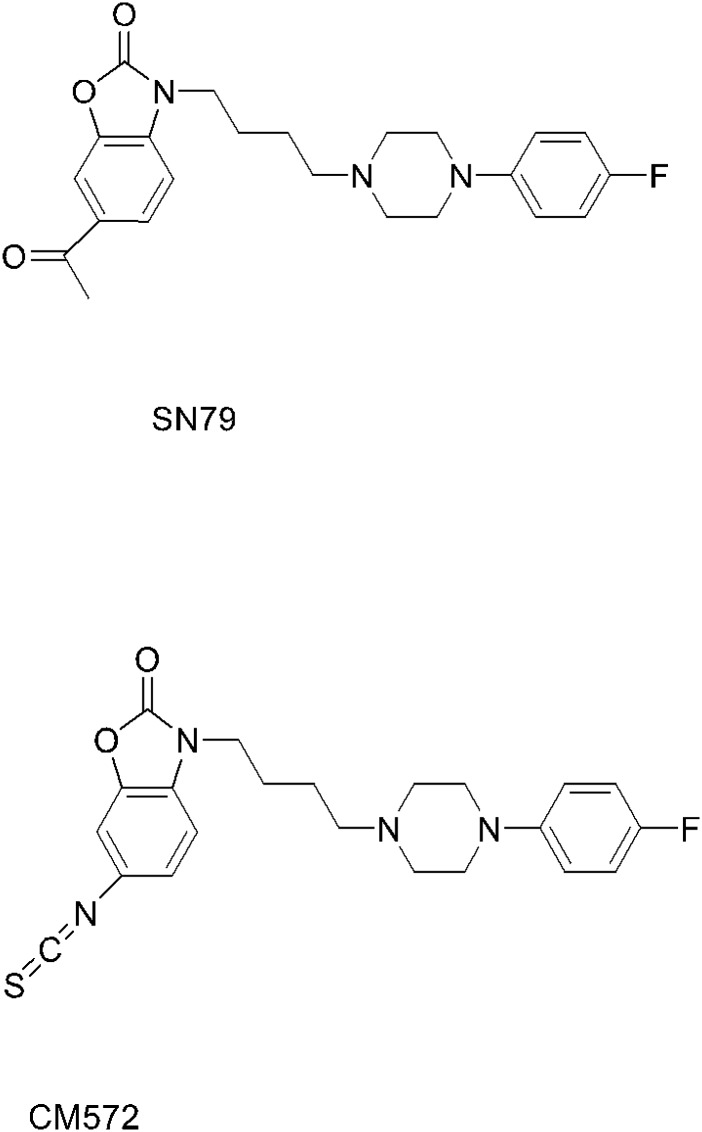
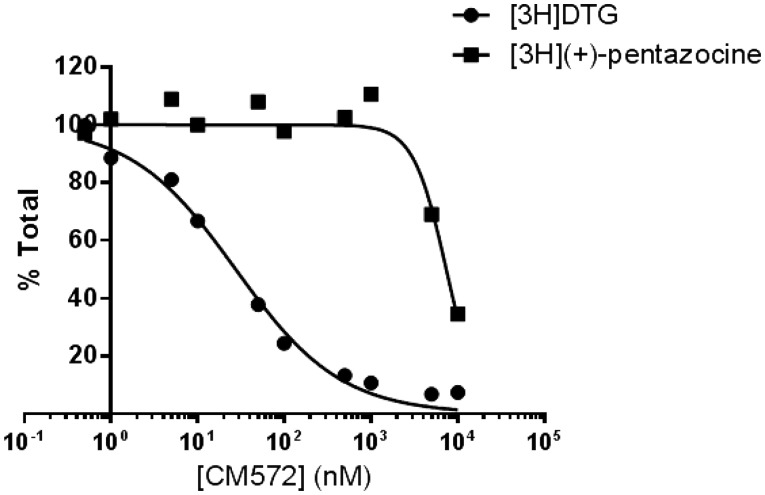
The structures of SN79 and CM572 are shown in Fig. 1. Substitution of the methyl ketone moiety of SN79 with an isothiocyanate group results in CM572 (synthesis described previously; McCurdy et al., 2014). Radioligand competition binding assays were performed in rat liver membranes to determine the affinity of CM572 for sigma-1 and sigma-2 receptors. These results are shown in Fig. 2. Ki values were determined to be ≥10 μM at sigma-1 and 14.6 ± 6.9 nM at sigma-2, demonstrating a ≥700-fold selectivity of the ligand for sigma-2 over sigma-1, compared with a ∼4-fold selectivity for SN79 (Ki = 7 nM and 27 nM at sigma-2 and sigma-1, respectively) (Kaushal et al., 2013). The increase in selectivity compared with the parent compound is due largely to the loss in sigma-1 receptor binding activity.
Fig. 1.
Structures of SN79 and CM572. Substitution of the methylketone moiety of SN79 with an isothiocyanate group results in CM572. Synthesis of CM572 was described previously (McCurdy et al., 2014).
Fig. 2.
Affinity of CM572 at sigma-1 and sigma-2 receptors. Affinity of CM572 for sigma-1 and sigma-2 receptors was determined by competition binding in rat liver membranes. Assays were carried out as described in Materials and Methods, using [3H](+)-pentazocine to label sigma-1 receptors and [3H]DTG in the presence of unlabeled (+)-pentazocine to label sigma-2 receptors. Ki values were determined by analysis of competition curves using GraphPad Prism 6 and previously determined radioligand Kd values ([3H](+)-pentazocine Kd, 7.5 nM; [3H]DTG Kd, 17.9 nM; Hellewell et al., 1994). The Ki value for CM572 at sigma-2 receptors was determined to be 14.6 ± 6.9 nM. At sigma-1 receptors, CM572 produced less than complete displacement, with 10 µM inhibiting radioligand binding by approximately 50% (Ki ≥ 10 µM). Values for sigma-2 are the average of four independent experiments ± S.D., and values for sigma-1 are results of two independent experiments. Each experiment was carried out in duplicate. Figure shown is a composite of the averaged experiments.
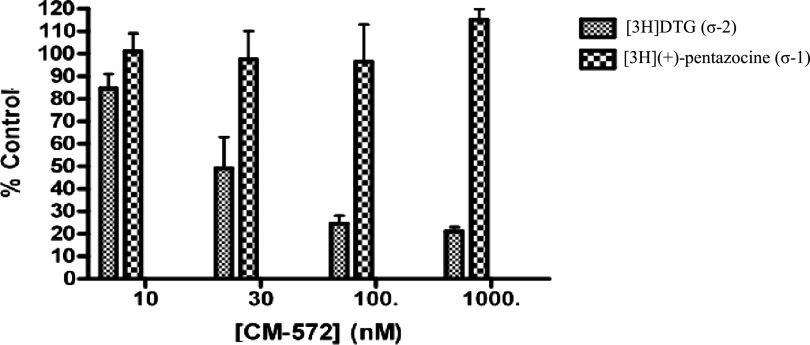
To examine whether introduction of the isothiocyanate moiety imparted irreversible binding capability, rat liver membranes were pretreated with various concentrations of CM572, followed by extensive washing to remove unbound compound. The sigma-1 and sigma-2 receptor binding activity (under standard conditions) was then assayed to determine the recovery of binding sites. Any loss of receptor binding activity is taken to be the result of residual occupation of the receptor by irreversibly bound CM572. The results are shown in Fig. 3. Pretreatment of membranes with CM572 for 60 minutes resulted in a dose-dependent loss of sigma-2 receptor binding, with an EC50 of approximately 30 nM. Less than 30% of sigma-2 binding sites remained at 100 nM, indicating that more than 70% of the available sigma-2 sites were irreversibly bound by CM572. By contrast, there was no loss of sigma-1 receptor binding, even at the highest dose of 1000 nM. When membranes were incubated with 500 nM SN79 and subjected to the same procedure nearly total recovery of both sigma-1 and sigma-2 binding was observed, showing that the conditions used are sufficient to remove a reversibly bound ligand of even higher binding affinity (data not shown). Thus, CM572 binds irreversibly to sigma-2 receptors in a highly selective manner.
Fig. 3.
Irreversible binding of CM572 at sigma-1 and sigma-2 receptors. Rat liver membranes were exposed to the indicated concentrations of CM572, subjected to extensive washing to remove any unbound compound, and then analyzed for recovery of sigma-1 and sigma-2 receptor binding, as described in Materials and Methods. Complete recovery of sigma-1 receptor binding activity (as measured by recovery of [3H](+)-pentazocine binding) was observed at all doses of CM572. By contrast, loss of sigma-2 receptor binding activity [as measured by recovery of [3H]DTG binding in the presence of (+)-pentazocine] was dose-dependent with CM572 pretreatment. Less than 30% of sigma-2 binding was recovered at and above 100 nM CM572, with an EC50 value of ∼30 nM. Results are expressed as percentage of total sigma-1 or sigma-2 binding recovered after pretreatment with CM572 at the indicated concentrations as compared with membranes that were not pretreated with CM572 but otherwise were treated identically. Data presented are an average of three independent experiments ± S.E.M. Each experiment was performed in duplicate.
CM572-Induced Calcium Response in SK-N-SH Neuroblastoma.
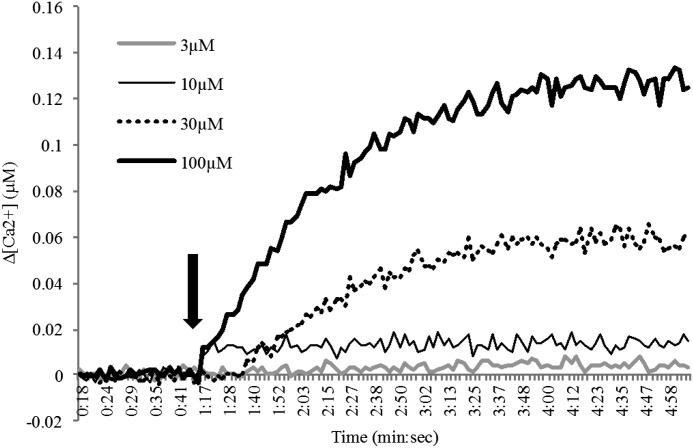
We next examined the effect of CM572 in cellular functional assays to determine the agonist or antagonist characteristics of the compound in human SK-N-SH neuroblastoma cells. To test for the immediate, transient calcium response characteristic of sigma-2 receptor activation, fura-2AM ratiometric calcium release assays were performed at varying concentrations of CM572. Results are shown in Fig. 4. At 3 μM CM572, no calcium response was elicited in SK-N-SH neuroblastoma and at 10 μM CM572 a small signal was observed, whereas higher concentrations (i.e., 30, 100 μM) produced a robust dose-dependent calcium response. These data suggest that CM572 has agonist properties.
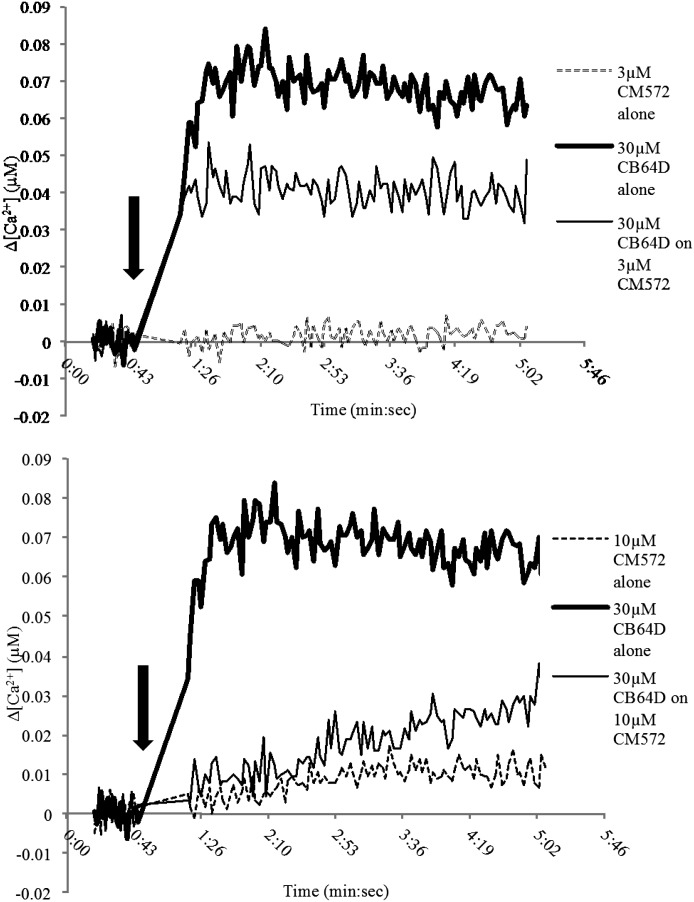
Fig. 4.
CM572-induced calcium response in SK-N-SH neuroblastoma. The effect of CM572 on calcium release was examined in fura-2–loaded SK-N-SH neuroblastoma cells. Cells were loaded with fura-2 and assays carried out as described in Materials and Methods. A 3 μM dose of CM572 did not elicit a calcium response, 10 μM CM572 elicited a very small calcium response, and concentrations at and above 30 μM induced calcium increases in a dose-dependent manner. Arrow indicates injection of CM572. The basal (preinjection) calcium concentration was determined for each trace, and that value was normalized to zero. Basal calcium concentrations ranged from 0.06–0.08 μM. Data are presented as change in calcium level above basal. Representative traces are shown from eight replicate experiments for 3 and 10 µM doses and three replicates for 30 and 100 µM doses. Experiments were performed using four wells per condition.
CM572 Attenuation of CB-64D–Induced Calcium Response.
CM572 is an analog of SN79, which is a sigma-2 receptor antagonist. Since low doses (i.e., 3 and 10 μM) of CM572 failed to produce a robust calcium response, we examined whether these doses might have antagonist actions against a known sigma-2 receptor agonist. CB-64D has previously been shown to produce a robust calcium signal in SK-N-SH neuroblastoma cells, consistent with its designation as a sigma-2 receptor agonist (Vilner and Bowen, 2000). CM572 was investigated for the ability to block the calcium signal produced by CB-64D. The results are shown in Fig. 5. Both 3 and 10 μM CM572 were able to attenuate significantly the calcium response induced by CB-64D when added simultaneously with 30 μM CB-64D. As shown previously, 3 μM CM572 alone showed no calcium response but was able to attenuate significantly the CB-64D–induced response. CM572 at 10 μM alone showed a small calcium response, but it was able to attenuate nearly completely the response produced by 30 μM CB-64D. Taken with the observation that higher concentrations of CM572 produce a robust calcium response, these data suggest that CM572 is a partial agonist at sigma-2 receptors.
Fig. 5.
CM572 attenuation of CB-64D–induced calcium response. Concentrations of CM572 that were unable to produce a strong calcium response alone, 3 μM (top trace) and 10 μM (bottom trace) were able to attenuate the robust calcium response induced by 30 µM CB-64D, with 10 μM CM572 exhibiting much stronger attenuation. Arrow indicates injection of CM572. Shown are representative traces from experiments that were repeated twice. Experiments were performed using four wells per condition.
CM572-Induced Cytotoxicity in SK-N-SH Neuroblastoma.
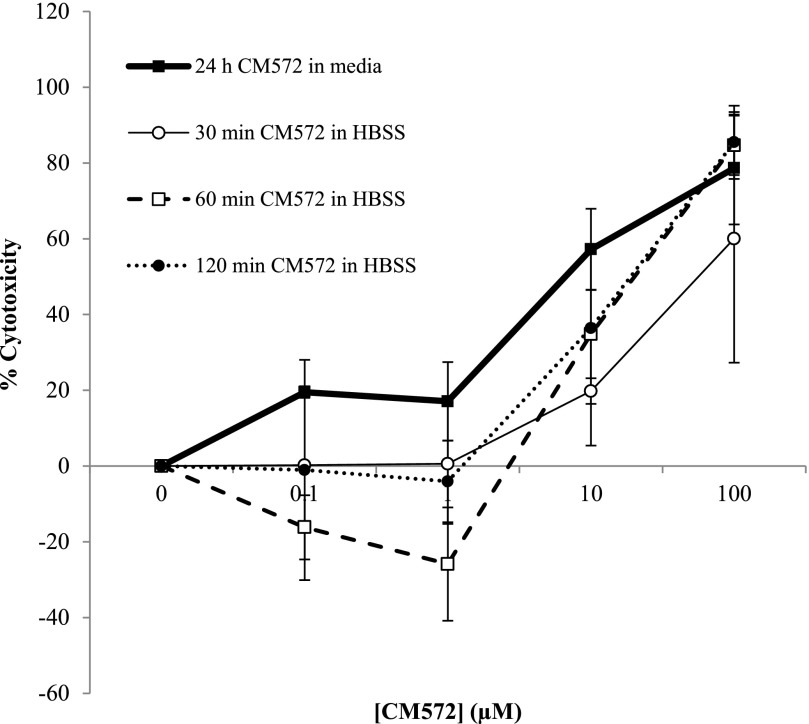
A second criterion for agonist characterization of a sigma-2 ligand in SK-N-SH neuroblastoma is the ability to induce apoptotic cell death. MTT assays were performed to measure cell viability in response to CM572 at different doses with differing incubation times and conditions. The results are shown in Fig. 6. The heavy solid line shows the effect of various doses of CM572 after a continuous 24-hour exposure of cells to the compound in normal culture medium. Concentrations of CM572 up to and including 1 μM did not induce cytotoxicity greater than 20%, whereas concentrations greater than 1 μM induced a robust loss of cell viability. The EC50 for CM572-induced cell death was 7.6 ± 1.7 μM.
Fig. 6.
CM572-induced cytotoxicity in SK-N-SH neuroblastoma. SK-N-SH neuroblastoma cells were exposed to various doses of CM572, and cell viability was examined by MTT assay, as described in Materials and Methods. Cells were either exposed to CM572 continuously for 24 hours (heavy solid line) or exposed acutely for 30, 60, or 120 minutes, unbound compound removed, and cells allowed to incubate for 24 hours in media without CM572 (light, dashed, and dotted lines) as described in Materials and Methods. Controls were treated similarly but without ligand. During the continuous 24-hour incubation, CM572 induced cell death in a dose-dependent manner. CM572 also induced dose-dependent cell death 24 hours after acute treatments followed by washing, with no statistically significant differences from 24-hour treatment effects (P > 0.05 for all acute treatments compared with continuous 24-hour exposure at same dose, t test). With acute CM572 treatment, no cell death was observed at or below 1 µM, resulting in a trend toward a rightward shift of the dose curve with comparable maximal effect compared with the continuous condition. Data shown for 24-hour continuous and 30-minute acute exposures are the average of two experiments ± S.D. Data for 60- and 120-minute acute treatment are the average of three experiments ± S.D. Acute exposures were performed in HBSS, although similar results were observed with media. Each experiment was performed using five wells for each condition.
Experiments were then carried out aimed at determining whether the irreversible binding demonstrated in rat liver membranes would manifest in the ability to induce cell death. Cells were treated for 30, 60, or 120 minutes with or without various concentrations of CM572 in HBSS, followed by extensive washing to remove any free compound from the system. Cells were then incubated for 24 hours in ligand-free culture media, and MTT assay was used to assess the viability of remaining cells. The results are shown in Fig. 6, indicated by the light solid line and broken lines. Washing out the CM572 after a brief exposure did not halt the cytotoxic effect of the compound. There was a general trend toward a rightward shift in the dose-response curve compared with the continuous exposure, but maximal effect was reached at the highest dose for all preincubation times, which indicates that CM572 is irreversibly bound during the preincubation and continues to activate the sigma-2 receptor after washout of unbound ligand to induce subsequent cell death. A preincubation time as short as 30 minutes was sufficient to produce this irreversible effect. Interestingly, in some experiments where cells were pretreated with concentrations of CM572 at or below 1 μM (e.g., 1 μM preincubated for 60 minutes) there appeared to be a slight stimulation of MTT reduction compared with control cells, resulting in negative “% cytotoxicity” values.
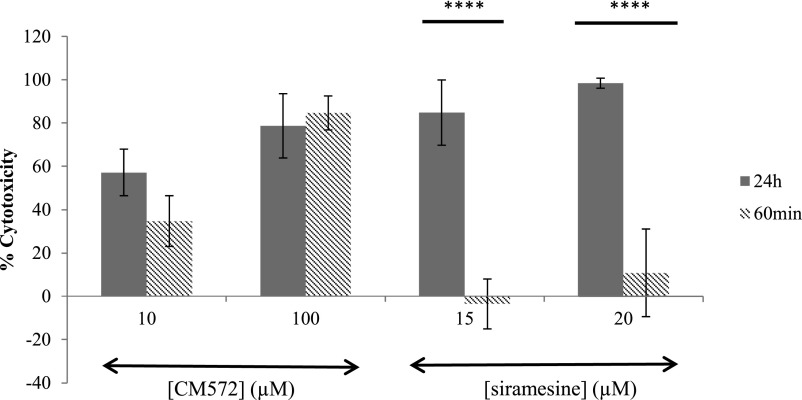
To address whether the cytotoxicity resultant from washout studies was due to irreversible binding of CM572 to the sigma-2 receptor or alternatively to a commitment of cells to proceed to apoptosis during the preincubation time frame, a comparative study was conducted with the sigma-2 agonist siramesine (MedChem Express, Princeton, NJ). Siramesine has high affinity and selectivity for sigma-2 receptors (Ki = 0.12 and 17 nM at sigma-2 and sigma-1, respectively) and has been demonstrated to induce apoptosis in tumor cell lines (Ostenfeld et al., 2005; Česen et al., 2013). Siramesine lacks an isothiocyanate group and thus cannot bind irreversibly to sigma receptors. Results are shown in Fig. 7. SK-N-SH neuroblastoma cells were treated with CM572 or siramesine for either 60 minutes, followed by washout and incubation for an additional 24 hours, or with continuous exposure to compound for 24 hours. With CM572, comparable levels of cell death were obtained with the pretreatment and washout as when the cells were exposed continuously for 24 hours. For 10 μM and 100 μM CM572, the cell death resulting 24 hours after the 60-minute pre-exposure and drug removal was ∼70% and ∼100%, respectively, of that observed with the corresponding continuous 24-hour exposure. By contrast, with siramesine, the 24-hour cell death was markedly reduced when the cells were only pre-exposed for 60 minutes compared with the continuous exposure. For both concentrations of siramesine (15 and 20 μM), the cell death resulting 24 hours after the 60-minute pre-exposure and drug removal was less than 15% of that observed with a continuous 24-hour exposure. This indicates that most of the response elicited from a 60-minute preincubation of cells with CM572 was likely due to irreversible ligand binding rather than to an irreversible commitment to apoptosis initiated within 60 minutes of ligand treatment since a 60-minute treatment with siramesine achieved far from comparable levels of cell death compared with 24-hour continuous exposure.
Fig. 7.
Comparison of effects of CM572 and siramesine upon acute or chronic exposure. The cytotoxic effects of irreversibly binding CM572 and reversibly binding siramesine were compared using acute and chronic treatment conditions. Treatment conditions were as described in Materials and Methods and in the Fig. 6 legend. Solid bars: Cells were exposed to the indicated concentration of sigma-2 ligand continuously for 24 hours. Hashed bars: Cells were exposed to the indicated sigma-2 ligand for 60 minutes, followed by extensive washing to remove unbound ligand, and then incubated for 24 hours. Cell viability was assessed by MTT assay at the end of the 24-hour period. As previously shown in Fig. 6, the cytotoxic effect of CM572 is more or less comparable when the 60-minute acute exposure is compared with the 24-hour continuous exposure (P = 0.06 and P = 0.3, t test for difference between acute and 24-hour treatment with 10 and 100 μM CM572, respectively). By contrast, at each dose of siramesine, only minimal cytotoxicity was observed after the 24-hour period when the 60-minute acute exposure was compared with the 24-hour continuous exposure (****P = 0.000003 and P = 0.000006, t test for difference between acute and 24-hour treatment with 15 and 20 μM siramesine, respectively). This finding indicates that the CM572-induced cytotoxicity after acute treatment is due to irreversible binding, not to irreversible commitment to apoptosis. Data are the averages of three experiments for CM572 and five experiments for siramesine, ± S.D. Each experiment was performed using five wells for each condition.
A concern with irreversibly binding compounds that have reactive electrophilic moieties, such as isothiocyanate, is stability in the culture medium. There is the possibility that components in the MEM culture medium or serum might degrade the compound by reacting with the isothiocyanate group; however, CM572 appears to be relatively stable in culture media. We observed that when the acute pretreatments described in Fig. 6 were carried out in normal culture medium instead of HBSS, the latent cytotoxicity was reduced by only about 25% (data not shown). Furthermore, for a 24-hour continuous incubation of cells with 1, 3, or 10 μM CM572, no significant difference was seen in the dose-dependent level of cell death, whether the media contained 0, 2, 5, or 10% FBS (three experiments, each with five wells per condition). When a full 24-hour dose-response curve was performed in media with 0% FBS, the EC50 value was 6.49 ± 1.17 μM (average of four experiments, each with five wells per condition). This is comparable to the EC50 of 7.6 μM observed in 10% FBS (Fig. 6). Thus, it appears that CM572 is neither bound to nor degraded by serum to any large extent.
CM572 Attenuation of CB-64D–Induced Cytotoxicity.
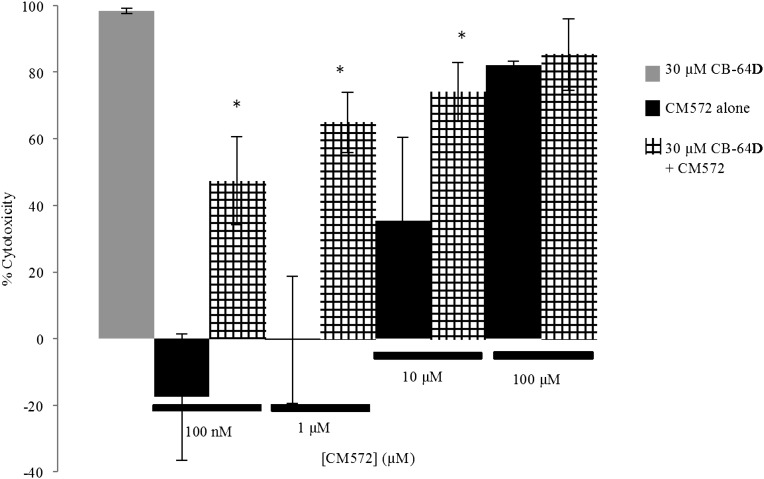
The results observed in the calcium release assay (Fig. 5) suggested that CM572 has antagonist properties (partial agonist) where minimally effective doses were able to attenuate the robust CB-64D–induced calcium response. To determine whether low-dose CM572 acts as a sigma-2 antagonist in cell viability assays, the ability to attenuate the apoptotic effect of CB-64D was examined. SK-N-SH neuroblastoma cells were pretreated for 60 minutes with or without various concentrations of CM572, followed by extensive washing to remove compound. Cells were then exposed to 30 μM CB-64D for 24 hours and remaining viable cells determined using the MTT assay. Results are shown in Fig. 8. All the doses of CM572 examined showed some ability to attenuate the cytotoxic effect of 30 μM CB-64D. The effect was most pronounced at the low concentrations of CM572 (100 nM and 1 μM). These concentrations alone had no detrimental effect on cell viability. Pretreatment of cells with both low doses of CM572 significantly attenuated the cytotoxic effect of 30 μM CB-64D. At 10 μM, CM572 pretreatment alone results in 36% cytotoxicity; however, when combined with 30 μM CB-64D, cytotoxicity reached 74%, significantly less than the 100% cell kill produced by 30 μM CB-64D alone, showing that there is a less than additive effect of the combination. This is also observed to some extent at 100 μM CM572, where alone it induces ∼80% cytotoxicity and nearly the same value on subsequent treatment with 30 μM CB-64D. Therefore, CM572 appeared to prevent the maximal 100% cytotoxicity induced by the 30 μM concentration of CB-64D alone, although the effect did not reach statistical significance. These results are consistent with CM572 acting as an irreversible partial agonist at sigma-2 receptors.
Fig. 8.
CM572 attenuation of CB-64D–induced cytotoxicity in SK-N-SH neuroblastoma. Cells were treated with the indicated concentration of CM572 for 60 minutes, followed by extensive washing to remove unbound ligand. Either vehicle (solid black bars) or 30 µM CB-64D (hatched bars) was then added to the wells and cells were incubated for an additional 24 hours. Results were compared with cells treated with 30 µM CB-64D alone (solid gray bar). The cell death induced by 30 µM CB-64D alone was set to 100% (maximal cytotoxicity), and all results were compared with this value and expressed as the percent of maximal cytotoxicity. CM572 (100 nM and 1 µM) did not induce cytotoxicity alone and were able to significantly attenuate cell death induced by CB-64D (*P = 0.017 and *P = 0.018, respectively, t test for CM572 with CB-64D versus effect of CB-64D alone), indicating antagonist properties. A higher dose of 10 µM CM572 induced significant cell death alone but still appeared able to attenuate cytotoxicity of CB-64D, suggesting partial agonist activity (*P = 0.032, t test for 10 µM CM572 with 30 µM CB-64D compared to 30 µM CB-64D alone). At 100 µM CM572, there was substantial cell death. When combined with 30 µM CB-64D, the maximal cell death induced by 30 µM CB-64D alone was unable to be achieved, indicating CM572 antagonism, although the effect did not achieve statistical significance (P = 0.12, t test). Data are the average of two experiments, ± S.D. Each experiment was performed using five wells for each condition.
CM572-Induced Bid Cleavage.
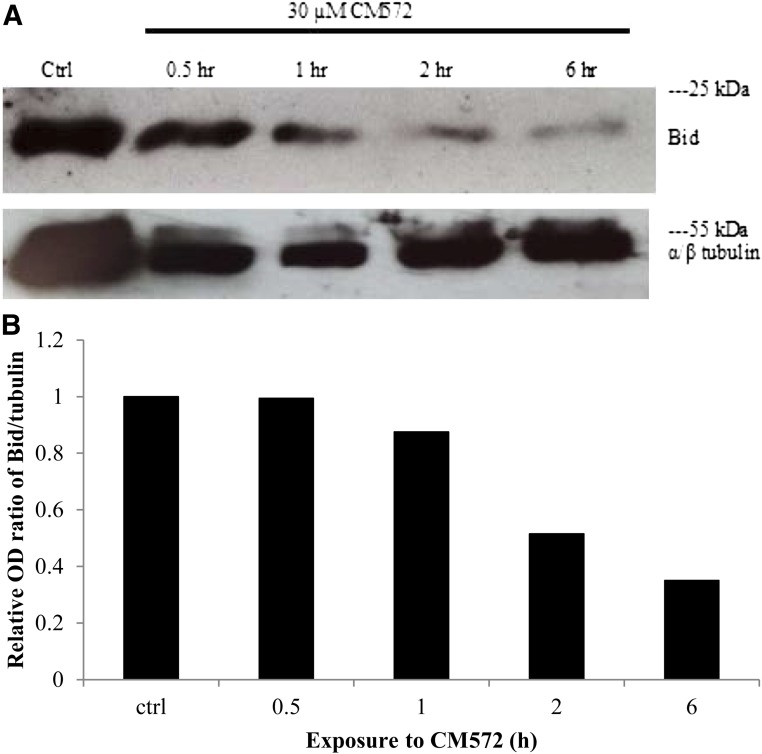
We have shown that sigma-2 agonists such as CB-64D induce caspase-10–dependent cleavage of the proapoptotic protein Bid, resulting in mitochondrial depolarization and release of mitochondrial proapoptotic factors (Wang and Bowen, 2006). Thus, a third criterion that was used to determine cellular function of CM572 in SK-N-SH neuroblastoma was the ability to induce cleavage of Bid. Cells were treated with 30 μM CM572 for various times up to 6 hours and cytosolic levels of Bid determined via Western blotting. The results are shown in Fig. 9. The level of full-length Bid (22 kDa) decreased with increasing exposure time to 30 µM CM572, indicating cleavage to an active fragment. The effect is quite prominent by 2 hours of treatment. This finding demonstrates that, like the apoptotic pathway induced by other sigma-2 agonists, Bid cleavage is a prominent element of CM572-induced apoptosis in SK-N-SH neuroblastoma. This result further supports agonist properties for CM572 in the higher dose range of the ligand.
Fig. 9.
Effect of CM572 on Bid cleavage in SK-N-SH neuroblastoma. SK-N-SH cells were treated with 30 µM CM572 for the indicated times. Cells were then extracted and subjected to Western blotting for Bid as described in Materials and Methods. (A) A 30-µM treatment of CM572 induced cleavage of Bid as indicated by decreased levels of full-length Bid (22 kDa) with increasing incubation times. α/β-Tubulin (55 kDa) was used as a loading control. Experiment was repeated twice with similar results. A representative Western blot is shown. (B) Quantitation of Western blot data. The optical density (OD) ratios of Bid to tubulin were determined for each time point, and the ratio in the control was set to 1. Ratios are expressed relative to control ratio. Data shown are the average of two experiments.
Effect of CM572 in Other Cancer Types and in Normal Cells.
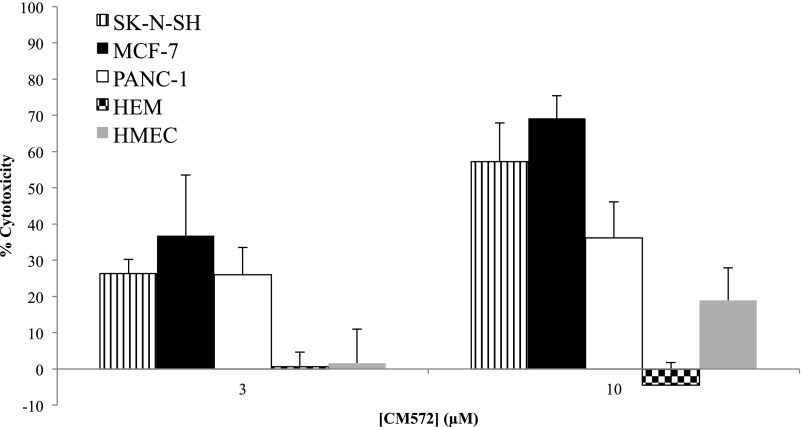
The effect of CM572 on cell viability was determined in two additional tumor cell lines, human PANC-1 pancreatic epithelioid carcinoma and human MCF-7 breast adenocarcinoma. CM572 was also examined in two types of normal cells from different organ systems, HEMs, and HMECs. Cells were exposed to various concentrations of CM572, and cell viability was examined. Figure 10 shows the results for 3 and 10 μM CM572. CM572 induced marked cell death in all three of the tumor cell lines. Considerably less cell death was observed with the two normal cell types over this concentration range compared with the tumor cells. CM572 showed high selectivity at 3 and 10 μM, where there was no effect on the viability of HEM cells and minimal effect on viability of HMECs compared with the tumor cell lines. For a same-tissue comparison of a full dose-response curve, the 48-hour EC50 for CM572 in MCF-7 breast tumor cells versus normal HMECs was 4.9 ± 1.17 μM versus 32.3 ± 1.7 μM, respectively, showing a 6.4-fold selectivity for the tumor cells (average of two experiments ± S.D., five wells for each point). Therefore, CM572 appears to have a wide-ranging effect against various cancer types known to express sigma-2 receptors and exhibits a selective cytotoxic effect against cancer cells over normal cells.
Fig. 10.
Effect of CM572 across human tumor cell lines from different organ sites and comparison with the effect in normal cell types. CM572 was incubated with SK-N-SH neuroblastoma, MCF-7 breast adenocarcinoma, PANC-1 pancreatic epithelioid carcinoma, normal HEM, and normal HMEC at the indicated concentrations of CM572 for 24 hours (SK-N-SH, PANC-1, HEM) or 48 hours (MCF-7, HMEC). Cell viability was determined after the incubation period using the MTT assay as described in Materials and Methods. Data are the averages of three or four experiments, ± S.D. Each experiment was performed using five wells for each condition.
Discussion
CM572 bound with a high affinity and selectivity to sigma-2 receptors, compared with sigma-1, exhibiting ≥700-fold selectivity (Fig. 2). Studies in rat liver membranes demonstrated that binding to sigma-2 receptors is irreversible, as indicated by failure to recover sigma-2 binding activity in pretreated membranes after extensive washing to remove any unbound ligand (Fig. 3). This effect was dose-dependent. The 30 nM EC50 for this effect is consistent with its affinity at sigma-2 receptors (Ki = 14.6 nM). This finding suggests that once bound in the sigma-2 receptor binding pocket, CM572 is oriented in such a manner that a suitable receptor amine or thiol group is in proper configuration to attack the isothiocyanate moiety resulting in covalent binding. The failure to bind irreversibly to sigma-1 receptors can be attributed to the low affinity (Ki > 10 µM).
CM572 induced a rise in cytosolic calcium levels in fura-2–loaded cells in a dose-dependent manner (Fig. 4). Calcium release was quite robust at 30 and 100 µM, indicating agonist-like properties. This result was surprising given that the parent compound SN79 has previously been shown to have only a small effect on calcium release even at high concentrations, consistent with its role as an antagonist (Garcia, 2012). Lower doses of CM572 (i.e., 3 and 10 µM) induced no or minimal release of calcium. These doses of CM572 that had minimal effect alone were able to attenuate the robust calcium signal induced by CB-64D, a compound characterized as a sigma-2 agonist (Fig. 5). It should be noted that SN79 also attenuates CB-64D–induced calcium release, consistent with its characterization as a sigma-2 antagonist (Garcia, 2012). Thus, unlike SN79, CM572 appears to behave as a partial agonist.
CM572 also exhibited the ability to induce dose-dependent cell death (Fig. 6), a characteristic of sigma-2 agonists. The EC50 value of 7.6 µM is comparable to that of CB-64D in these cells (EC50 = 5 µM). Again, this is in contrast to the actions of the parent compound, SN79, which has no effect on cell viability even at high concentrations (Garcia, 2012). The cytotoxic potency of CM572 was unaffected by the presence or absence of serum protein. Thus, CM572 appears to be relatively stable in culture medium despite the presence of the reactive isothiocyanate group.
Since CM572 becomes irreversibly bound to sigma-2 receptors (Fig. 3), we surmised that cells pretreated with the compound would continue to die even after any free, non–irreversibly-bound ligand was removed. Cells pretreated for 30, 60, or 120 minutes with various doses of CM572 continued to die over a period of 24 hours, even after the compound was washed free of the system after the acute exposure (Fig. 6). Although some loss of potency occurred, indicated by the trend toward a rightward shift in the dose curve for the pretreatment condition, the same maximal level of cell death could be achieved compared with the 24-hour continuous exposure condition.
This result strongly suggests that CM572 is binding covalently to sigma-2 receptors in live cells and continues to activate the receptor in an irreversible manner such that cell death occurs even though there is no free ligand in the system. However, an alternative interpretation could be that during the pretreatment period of 30–120 minutes, the cells become committed to apoptosis and then go on to die after the ligand is removed from the system. To test this, we compared the effect of CM572 with those of the potent putative sigma-2 receptor agonist siramesine, which lacks the ability to bind irreversibly (Fig. 7). Siramesine induced dose-dependent cell death in a continuous 24-hour exposure to cells; however, unlike cells pretreated acutely with CM572, cells pretreated with siramesine for 60 minutes and then washed were largely still viable 24 hours later (Fig. 7), showing that a 60-minute exposure of SK-N-SH cells to a potent sigma-2 agonist is not sufficient to induce commitment to apoptosis. A similar result was previously observed with a 3-hour treatment of C6 glioma cells with either reduced haloperidol or BD737, followed by washing. The treatment with 300 µM of either ligand resulted in extensive cell rounding, but when the ligand was removed, the cells survived and returned to near-normal morphology (Vilner et al., 1995a). These results support the notion that the effect observed with acute CM572 treatment is due to irreversible binding to and activation of sigma-2 receptors.
As low doses of CM572-induced minimal cytotoxic effect and given the observations on calcium release, we examined whether low doses of CM572 might block the cytotoxic effect of CB-64D (Fig. 8). In these experiments, cells were treated acutely for 60 minutes with various doses of CM572 and then washed to remove any unbound ligand. The effect of CB-64D was then observed over a 24-hour period. Concentrations of CM572 that were not cytotoxic alone (100 nM and 1 µM) significantly attenuated the effect of 30 µM CB-64D. At higher doses, CM572 showed significant cytotoxicity alone but was still able to attenuate the cytotoxic effect of CB-64D, not allowing it to induce maximal cell death. Thus, as with the results observed in calcium release assays, CM572 behaves as an irreversible partial agonist at sigma-2 receptors.
The final functional effect observed was on Bid cleavage. We have shown previously that sigma-2 receptor agonists, such as CB-64D and haloperidol, induce cleavage of the proapoptotic protein Bid in the cytosol (Wang and Bowen, 2006). As has been observed with CB-64D, 30 µM CM572 induced robust loss of intact Bid in the cytosol, beginning at 1 hour, with extensive loss by 6 hours (Fig. 9). This would thus classify CM572 as having agonist properties at this dose in this assay.
Because sigma-2 receptors are known to be upregulated in tumor cell lines from many different organ sites, we investigated the effect of CM572 on breast and pancreatic tumor cell lines in addition to the neuroblastoma line. CM572 induced cell death in both of these additional cell lines (Fig. 10). An important characteristic to note is the relative selectivity of the cytotoxic effect of CM572 for cancer cells over normal cells. CM572 exhibited much less cytotoxic activity in normal human melanocytes and normal mammary epithelial cells compared with the tumor cell lines (Fig. 10). When the sensitivity of normal breast cells to CM572 was compared with that of MCF-7 breast tumor cells over a 48-hour dose-response treatment period, the tumor cells were found to be 6.4 times more sensitive to the cytotoxic effect of CM572, which is consistent with a previous finding that breast tumor biopsy tissue expressed a much higher density of sigma receptors compared with surrounding normal tissue (John et al., 1996). This tumor selectivity likely also applies to melanoma versus normal melanocytes. CM572 had no effect on the viability of HEMs at concentrations up to 10 µM. Sigma-2 receptors are highly expressed in human melanoma cells (John et al., 1993; Vilner et al., 1995b). Although we have not investigated the effect of CM572 in a melanoma cell line, several studies by others have demonstrated cytotoxic effects of sigma-2 agonists on melanoma in vitro. The nonselective sigma-2 receptor agonist haloperidol induced apoptosis in mouse B16 and human SK-MEL-28 melanoma cells, and the highly selective sigma-2 agonists SV119, WC-26, RHM-138, and siramesine all induced apoptosis in human MDA-MB-435 melanoma cells (Nordenberg et al., 2005; Zeng et al., 2012, 2014). Thus, it is reasonable to assume that CM572 would also induce apoptosis in melanoma cells. The difference in efficacy between tumor cells and nontumor cells suggests potential therapeutic promise for CM572 or related ligands in treatment of cancers. The irreversible nature of the sigma-2 receptor interaction may allow novel treatment paradigms where less frequent dosing could be possible, reducing adverse side effects.
Taken together, these data show that CM572 is a potent and selective partial agonist at sigma-2 receptors. It binds irreversibly to the receptor via the isothiocyanate moiety and thus retains the ability to continuously activate the receptor even after free ligand is removed from the system. This finding was unexpected, given the properties of the parent compound, SN79, which lacks significant agonist activity (Garcia, 2012). Introduction of the isothiocyanate moiety in place of the methylketone in the heterocyclic ring of SN79 appears not only to impart irreversible binding activity, but also to shift the profile to impart more agonist activity. It is not clear at this time whether this change in efficacy is due to the irreversible binding component or to some change in the electronic properties of the heterocyclic ring due to the presence of the isothiocyanate moiety. Studies of reversibly binding SN79 analogs with modifications in this position may help to resolve this issue. The unique properties of CM572 give it potential as an agent for cancer therapy as well as a novel tool with which to study functional blockade of sigma-2 receptors.
Abbreviations
- Bid
BH3-interacting domain death agonist
- CB-64D
(+)-1R,5R-(E)-8-benzylidene-5-(3-hydroxyphenyl)-2-methylmorphan-7-one
- CB-184
(+)-1R,5R-(E)-8-(3,4-dichlorobenzylidene)-5-(3-hydroxyphenyl)-2-methylmorphan-7-one
- CM572
3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)-6-isothiocyanatobenzo[d]oxazol-2(3H)-one
- DTG
1,3-di-o-tolylguanidine
- FBS
fetal bovine serum
- HBSS
Hank's buffered salt solution
- HEM
human epidermal melanocyte
- HMEC
human mammary epithelial cell
- MEM
minimal essential media
- MTT
3-[4,5 dimethylthiazol-2-y]-2,5 diphenyltetrazolium bromide
- [125I]RHM-4
N-(4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-2-(2-fluoroethoxy)-5-iodo-3-methoxybenzamide
- SN79
(6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one)
- TBS
Tris-buffered saline
Authorship Contributions
Participated in research design: Nicholson, Bowen.
Conducted experiments: Nicholson, Comeau.
Contributed new reagents or analytic tools: Mesangeau, McCurdy.
Performed data analysis: Nicholson, Comeau.
Wrote or contributed to the writing of the manuscript: Nicholson, Bowen.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences T32 Predoctoral Pharmacology Training [Grant 1-T32-GM077995-01A2] (to H.N.); National Institutes of Health National Institute of General Medical Sciences R25 Initiative for Maximizing Student Development [Grant R25-GM083270] (to H.N.); Brown Pharmacia Predoctoral Fellowship (to H.N.); National Institutes of Health National Institute on Drug Abuse Postdoctoral T32 Training [Grant 5T32-DA016184-09] (to A.C.); National Institutes of Health National Institute on Drug Abuse [Grant R01-DA023205] (to C.M. and C.R.M.); National Institutes of Health National Institute of General Medical Sciences [Grant P20-GM104932] (to C.M. and C.R.M.); and the Upjohn Professorship in Pharmacology, Brown University (to W.D.B.).
This work has been previously presented in part in the following abstracts: Nicholson H, Comeau A, Mesangeau C, McCurdy CR, and Bowen WD (2013) Development of selective irreversible antagonists for sigma-2 receptors (Abstract 2242). American Association for Cancer Research Annual Meeting; 2013 Apr 6–10; Washington, DC; and Nicholson H, Comeau A, Mesangeau C, McCurdy CR, and Bowen WD (2013) Irreversible modulators of sigma-2 receptor function: Isothiocyanate derivatives of SN79 (Abstract 63.03). Society for Neuroscience Annual Meeting; 2013 Nov 9–13; San Diego, CA.
References
- Abate C, Perrone R, Berardi F. (2012) Classes of sigma2 (σ2) receptor ligands: structure affinity relationship (SAfiR) studies and antiproliferative activity. Curr Pharm Des 18:938–949. [DOI] [PubMed] [Google Scholar]
- Al-Nabulsi I, Mach RH, Wang LM, Wallen CA, Keng PC, Sten K, Childers SR, Wheeler KT. (1999) Effect of ploidy, recruitment, environmental factors, and tamoxifen treatment on the expression of sigma-2 receptors in proliferating and quiescent tumour cells. Br J Cancer 81:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WD. (2007) Sigma-2 receptors: regulation of cell growth and implications for cancer diagnosis and therapeutics, in Sigma Receptors: Chemistry, Cell Biology and Clinical Implications (Mastumoto R, Su TP, Bowen WD. eds) pp 215–235, Springer, New York. [Google Scholar]
- Bowen WD, Bertha CM, Vilner BJ, Rice KC. (1995) CB-64D and CB-184: ligands with high sigma 2 receptor affinity and subtype selectivity. Eur J Pharmacol 278:257–260. [DOI] [PubMed] [Google Scholar]
- Cassano G, Gasparre G, Niso M, Contino M, Scalera V, Colabufo NA. (2009) F281, synthetic agonist of the sigma-2 receptor, induces Ca2+ efflux from the endoplasmic reticulum and mitochondria in SK-N-SH cells. Cell Calcium 45:340–345. [DOI] [PubMed] [Google Scholar]
- Česen MH, Repnik U, Turk V, Turk B. (2013) Siramesine triggers cell death through destabilisation of mitochondria, but not lysosomes. Cell Death Dis 4:e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau A, Mesangeau C, McCurdy CR, and Bowen WD (2012) Development of selective irreversible affinity ligands for sigma-2 receptors. Neuroscience 2012; 2012 Oct 13–17; New Orleans, LA. [Google Scholar]
- Crawford KW, Bowen WD. (2002) Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res 62:313–322. [PubMed] [Google Scholar]
- Garcia DR (2012) Sigma-2 Receptor-Mediated Cytotoxicity and Calcium Signaling: Evidence for Bifurcating Pathways. Doctoral thesis, Brown University, Providence, RI. [Google Scholar]
- Hayashi T, Su TP. (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610. [DOI] [PubMed] [Google Scholar]
- Hazelwood S, Bowen WD. (2006) Sigma-2 receptor-mediated apoptosis in human SK-N-SH neuroblastoma cells: role of lipid rafts, caspases, and mitochondrial depolarization. Proc Am Assoc Cancer Res 47:1158. [Google Scholar]
- Hellewell SB, Bowen WD. (1990) A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res 527:244–253. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. (1994) Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol 268:9–18. [DOI] [PubMed] [Google Scholar]
- John CS, Bowen WD, Saga T, Kinuya S, Vilner BJ, Baumgold J, Paik CH, Reba RC, Neumann RD, Varma VM, et al. (1993) A malignant melanoma imaging agent: synthesis, characterization, in vitro binding and biodistribution of iodine-125-(2-piperidinylaminoethyl)4-iodobenzamide. J Nucl Med 34:2169–2175. [PubMed] [Google Scholar]
- John CS, Vilner BJ, Schwartz AM, Bowen WD. (1996) Characterization of sigma receptor binding sites in human biopsied solid breast tumors. J Nucl Med 37:267P.8667058 [Google Scholar]
- Kaushal N, Robson MJ, Vinnakota H, Narayanan S, Avery BA, McCurdy CR, Matsumoto RR. (2011) Synthesis and pharmacological evaluation of 6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (SN79), a cocaine antagonist, in rodents. AAPS J 13:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Seminerio MJ, Robson MJ, McCurdy CR, Matsumoto RR. (2013) Pharmacological evaluation of SN79, a sigma (σ) receptor ligand, against methamphetamine-induced neurotoxicity in vivo. Eur Neuropsychopharmacol 23:960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach RH, Smith CR, Al-Nabulsi I, Whirrett BR, Childers SR, Wheeler KT. (1997) Sigma-2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res 57:156–161. [PubMed] [Google Scholar]
- Mach RH, Zeng C, Hawkins WG. (2013) The σ2 receptor: a novel protein for the imaging and treatment of cancer. J Med Chem 56:7137–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Su TP. (2009) The pharmacology of sigma-1 receptors. Pharmacol Ther 124:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CR, Mesangeau C, Matsumoto RR, Poupaert JH, Avery BA, and Abdelazeem AHA (2014) inventors, University of Mississippi, assignee. Highly selective sigma receptor ligands. U.S. Patent US 8686008 B2. 2014 Apr 1.
- Nordenberg J, Perlmutter I, Lavie G, Beery E, Uziel O, Morgenstern C, Fenig E, Weizman A. (2005) Anti-proliferative activity of haloperidol in B16 mouse and human SK-MEL-28 melanoma cell lines. Int J Oncol 27:1097–1103. [PubMed] [Google Scholar]
- Ostenfeld MS, Fehrenbacher N, Høyer-Hansen M, Thomsen C, Farkas T, Jäättelä M. (2005) Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res 65:8975–8983. [DOI] [PubMed] [Google Scholar]
- Vilner BJ, Bowen WD. (2000) Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. J Pharmacol Exp Ther 292:900–911. [PubMed] [Google Scholar]
- Vilner BJ, de Costa BR, Bowen WD. (1995a) Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. J Neurosci 15:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilner BJ, John CS, Bowen WD. (1995b) Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res 55:408–413. [PubMed] [Google Scholar]
- Wang X and Bowen WD (2006) Sigma-2 receptors mediate apoptosis in SK-N-SH neuroblastoma cells via caspase-10-dependent Bid cleavage and mitochondrial release of endonuclease G and apoptosis-inducing factor. Neuroscience 2006; 2006 Oct 14–18; Atlanta, GA.
- Wheeler KT, Wang LM, Wallen CA, Childers SR, Cline JM, Keng PC, Mach RH. (2000) Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br J Cancer 82:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zeng C, Chu W, Pan F, Rothfuss JM, Zhang F, Tu Z, Zhou D, Zeng D, Vangveravong S, et al. (2011) Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat Commun 2:380 10.1038/ncomms1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Rothfuss J, Zhang J, Chu W, Vangveravong S, Tu Z, Pan F, Chang KC, Hotchkiss R, Mach RH. (2012) Sigma-2 ligands induce tumour cell death by multiple signalling pathways. Br J Cancer 106:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Rothfuss JM, Zhang J, Vangveravong S, Chu W, Li S, Tu Z, Xu J, Mach RH. (2014) Functional assays to define agonists and antagonists of the sigma-2 receptor. Anal Biochem 448:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]