Abstract
Synthetic cathinones, often sold as “bath salts,” are a popular class of recreational drugs used as quasi-legal alternatives to cocaine, methamphetamine, and methylenedioxymethamphetamine. The increased prevalence and health consequences of synthetic cathinone use has prompted regulatory agencies to control a number of these compounds; however, a broad class of analogous compounds known as the second-generation cathinones has been brought to the market to take the place of the banned synthetic cathinone derivatives. The current study aims to characterize the behavioral pharmacology of three pyrrolidinylated second-generation cathinones: 4-methyl-α-pyrrolidinopropiophenone (4ʹ-MePPP), α-pyrrolidinopropiobutiophenone (α-PBP), and α-pyrrolidinopentiophenone (α-PVP). Locomotor activity was tested in mice over an 8-hour period. The discriminative stimulus effects of these compounds were tested in rats trained to discriminate either cocaine or methamphetamine. The rewarding effects of these drugs were assessed in mice using conditioned place preference. Both α-PBP and α-PVP produced long-lasting increases in locomotor activity across a wide range of doses, whereas 4ʹ-MePPP produced locomotor stimulation only at 30 mg/kg. Both α-PBP and α-PVP fully substituted for the discriminative stimulus effects of both cocaine and methamphetamine, whereas 4ʹ-MePPP substituted fully for the discriminative stimulus effects of methamphetamine only. Both α-PBP and α-PVP produced conditioned place preference in an inverted U-shaped dose effect, whereas 4ʹ-MePPP did not produce conditioned place preference. These findings suggest that α-PBP and α-PVP are likely to be recreationally used and have potential for addiction and abuse, but 4ʹ-MePPP may not.
Introduction
Synthetic cathinones, which have become increasingly popular in the global drug market as quasi-legal alternatives to controlled stimulants, now comprise 25% of all novel psychoactive substances reported globally (UNODC, 2014). Cathinone derivatives are often sold online and in smoke shops in heterogeneous mixtures of compounds known as “bath salts” and have been found in many ecstasy formulations in lieu of MDMA (± methylenedioxymethamphetamine) (German et al., 2014; UNODC, 2014). These derivatives, of which there are dozens, are synthetic analogs of cathinone, a naturally occurring compound found in the khat plant, which is used for its stimulant properties in East African and Middle Eastern regions (De Felice et al., 2014).
The synthetic cathinones have largely been classified as psychomotor stimulants. Users have reported bath salts producing similar subjective effects as known stimulant drugs of abuse, and the clinical presentation is also similar to cocaine and amphetamine-like drugs (Prosser and Nelson, 2012). Our laboratory has previously demonstrated that a number of synthetic cathinones produce locomotor stimulation in mice and cocaine- and methamphetamine-like discriminative stimulus effects in rats (Gatch et al., 2013, 2015). The pharmacodynamic profile of synthetic cathinones is also similar to other psychomotor stimulants, with some derivatives producing amphetamine-like monoamine-releasing properties, and others producing cocaine-like blockade of monoamine uptake (Eshleman et al., 2013; Simmler et al., 2013).
Among the myriad synthetic congeners of cathinone, there are numerous structural analogs of pyrovalerone, such as 3,4-methylenedioxypyrovalerone (MDPV). Although MDPV has been the compound most frequently incorporated into bath salt mixtures, the pyrovalerone analogs β-naphyrone, α-pyrrolidinopentiophenone (α-PVP), 4-methyl-α-pyrrolidinopropiophenone (4ʹ-MePPP), and 3,4-methylenedioxy-α-pyrrolidinobutiophenone (MDPBP) also have been found in various bath salts (Leffler et al., 2014). Despite MDPV being permanently classified as a schedule I compound in 2012, use of synthetic cathinones has continued, prompting emergency scheduling of 10 more synthetic cathinones in 2014, including pyrovalerone analogs 4ʹ-MePPP, α-PVP, β-naphyrone, and α-pyrrolidinopropiobutiophenone (α-PBP) (DEA, 2014).
The molecular and behavioral aspects of MDPV have been characterized (Aarde et al., 2013; Cameron et al., 2013), but the other pyrovalerone analogs have not received the same attention and little is known about their mechanism and abuse liability. Naphyrone has been demonstrated to act as a monoamine uptake inhibitor (Eshleman et al., 2013; Simmler et al., 2013) that produces stimulation of locomotor activity and discriminative stimulus effects similar to cocaine and methamphetamine (Gatch et al., 2013). Similarly α-PVP, α-PBP, and α-pyrrolidinopropiophenone act as monoamine uptake inhibitors and produce locomotor effects similar to other known psychomotor stimulants (Marusich et al., 2014). A study using in vivo microdialysis demonstrated that α-PVP increased striatal dopamine levels to a lesser degree than methamphetamine (Kaizaki et al., 2014). 4ʹ-MePPP blocked the uptake of both dopamine and serotonin but was 40-fold selective for the dopamine transporter over the serotonin transporter and caused sharp increases in extracellular dopamine but not serotonin, which correlated with increases in locomotor activity (Saha et al., 2015).
Although data regarding the mechanism of these novel synthetic cathinones are accumulating, there remains a paucity of information regarding their abuse liability. We investigated the abuse liability profile of three pyrovalerone analogs that were recently emergency scheduled by the Drug Enforcement Administration: 4ʹ-MePPP, α-PBP, and α-PVP. Locomotor activity (test for psychostimulant effects), drug discrimination (test for discriminative stimulus effects), and conditioned place preference (test for reward-like effects) assays are commonly used for predicting the abuse potential of drugs (Carter and Griffiths, 2009; Horton et al., 2013).
Materials and Methods
Subjects.
Male Swiss Webster mice were obtained from Harlan (Indianapolis, IN) at 8 weeks of age and were tested at approximately 10 weeks of age. The mice were group housed in cages on a 12-hour light/dark cycle and were allowed free access to food and water. Male Sprague–Dawley rats were obtained from Harlan. All rats were housed individually and were maintained on a 12-hour light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 15 g/day, which included the food received during operant sessions. Water was freely available. Temperatures in the animal facility and testing rooms were maintained at 22 to 24°C. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Locomotor Activity.
Studies of locomotor activity were conducted using a Digiscan apparatus (model RXYZCM-16; Omnitech Electronics, Columbus, OH) and clear acrylic locomotor activity testing chambers (40.5 × 40.5 × 30.5 cm) housed in sets of two within sound-attenuating chambers. A panel of infrared beams (16 beams) and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination. Fans provided an 80-dB ambient noise level within the chamber.
Separate groups of eight mice were injected (i.p.) with either vehicle (0.9% saline) or a test compound immediately before locomotor activity testing: 4ʹ-MePPP (1, 3, 10, 30, 100 mg/kg), α-PBP (1, 2.5, 5, 10, 25 mg/kg), or α-PVP (1, 2.5, 5, 10, 25 mg/kg). In all studies, horizontal activity (interruption in photocell beams) was measured for 8 hours within 10-minute periods, beginning at 8:00 AM (1 hour after lights on).
Discrimination Training.
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC–compatible computers via interfaces (MED Associates, St. Albans, VT). The computers were programmed in MED-PC IV (MED Associates) for the operation of the chambers and collection of data. Rats were trained to discriminate cocaine (10 mg/kg i.p.) or methamphetamine (1 mg/kg i.p.) from vehicle (saline) using a two-lever choice methodology. Food (45-mg food pellets; Bio-Serv, Frenchtown, NJ) was available as a reinforcer under a fixed-ratio 10 schedule when responding occurred on the injection-appropriate lever. There was no consequence for responses on the incorrect lever. The rats received approximately 60 training sessions before they were used in substitution experiments.
Animals were selected for use in experiments when they had met the criteria of emitting 85% of responses on the injection-correct lever for both the first fixed ratio and for the remainder of the session during their last 10 training sessions. Training sessions occurred in a double alternating fashion (D-D-S-S-D, etc.), and tests were conducted between pairs of identical training sessions (i.e., between either two vehicle or two drug training sessions).
Before each session, the rats received an injection of either vehicle or drug. Ten minutes later, the rats were placed in an operant chamber. Each training session lasted a maximum of 10 minutes, and the rats could earn up to 20 food pellets. In contrast with training sessions, both levers were active during the discrimination test sessions such that 10 consecutive responses on either lever led to reinforcement. Data were collected until the first reinforcer was obtained or for a maximum of 20 minutes. Rats were tested only if they had achieved 85% drug-lever responding for both first fixed ratio and total session on the two prior training sessions. At least 3 days elapsed between test sessions.
4ʹ-MePPP, α-PBP, and α-PVP were tested in six rats trained to discriminate cocaine and six rats trained to discriminate methamphetamine. A repeated-measures design was used such that each rat was tested at all doses. During substitution experiments, intraperitoneal injections of saline (1 ml/kg), 4ʹ-MePPP (1, 2.5, 5, 10, 25, 50 mg/kg), α-PBP (1, 2.5, 3.2, 5, 10 mg/kg), or α-PVP (0.1, 0.25, 0.5, 1, 2.5, 5, 10 mg/kg) were administered 15 minutes before the start of the test session.
Conditioned Place Preference.
The place preference apparatus consisted of 16 acrylic test chambers (12 × 6 × 12-inch, length × width × height, respectively) (Model 71-CFCPP; Omnitech Electronics) with interchangeable grid (bar) and hole (perforated) floors (full, 12 × 6-inch, and split, 6 × 6-inch). The position of the mouse within the apparatus was recorded using a photocell-based system (Model 71-CPPX; Omnitech Electronics). The acrylic chambers were housed separately in sound-attenuating chambers (Model 71-ECC; Omnitech Electronics). Ambient noise within the chambers was 64 dB, and testing took place under dim illumination (31.8 ± 1.5 lux).
Place conditioning, using biased assignment, consisted of three phases: a pretest for initial floor bias, four place conditioning sessions, and a final preference test. The pretest was conducted on day 1, during which initial floor bias was examined by injecting mice intraperitoneally with 0.9% saline (10 ml/kg) then allowing them free access to both floor types for 30 minutes. The amount of time spent on either floor was measured and the floor on which less time was spent was designated the drug-paired floor. Positioning of the floors alternated between chambers.
On days 2 and 3, place conditioning occurred, wherein mice received one vehicle and one drug conditioning session on both days. In the mornings, mice were injected with saline and immediately placed in the chamber with the non–drug-paired floor for 30 minutes, then returned to their home cage. After 4 hours, mice were injected with α-PBP (1, 3, 10, 30 mg/kg), α-PVP (0.1, 0.3, 1, 3, 10, 30 mg/kg), 4ʹ-MePPP (3, 10, 30, 100 mg/kg) or saline and immediately placed in the chambers with the drug-paired floors for 30 minutes. The final preference test, occurring on day 4, was identical to the pretest. All subjects were administered 0.9% saline, and the time spent on the drug-paired floor was measured. Sixteen mice were tested at each dose.
Data Analysis.
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-minute period of testing. A 30-minute period, beginning when maximal stimulation of locomotor activity first appeared, as a function of dose, was used for analysis of dose–response data. A two-way repeated-measures analysis of variance (dose × 5-minute time bin) was performed on horizontal activity counts/10-minute interval. A two-way analysis of variance (dose × drug) was conducted on the area under the curve (sum of the effects for each 5-minute bin for the time course data in Fig. 1). A one-way analysis of variance was performed on horizontal activity counts for the 30-minute period of maximal effect, and planned comparisons were made between each dose and the vehicle (0.9% saline) control. A one-way analysis of variance was conducted on the peak effects for the three compounds.
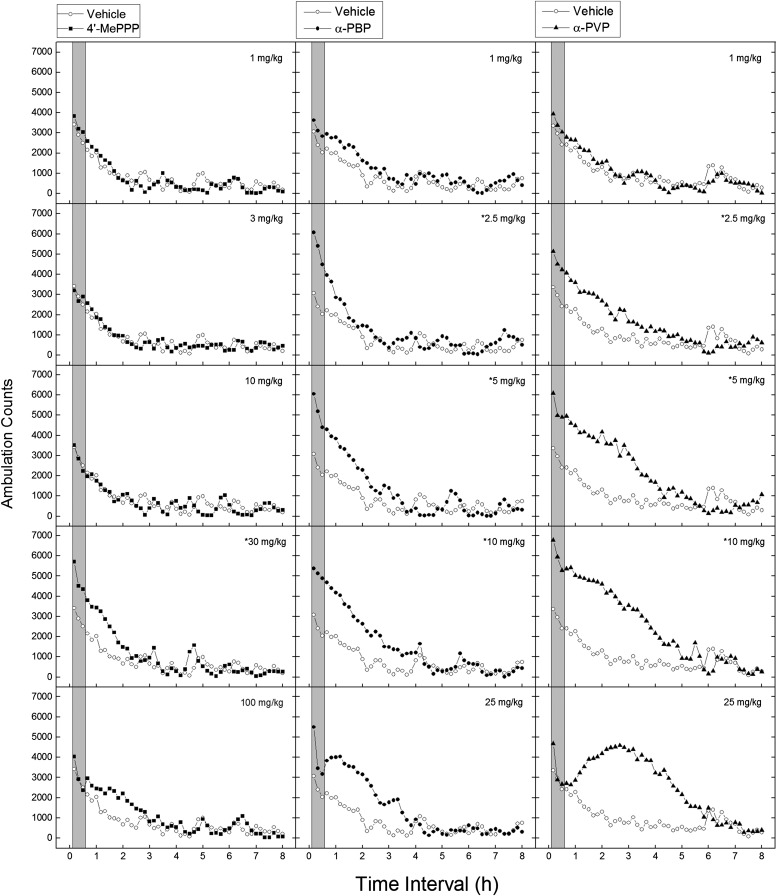
Fig. 1.
Time course of locomotor activity in mice. Data are represented as mean number of ambulation counts for each 10-minute period over 8 hours for each dose (n = 8) of 4ʹ-MePPP (left), α-PBP (middle), and α-PVP (right). The gray bar shows the time range of maximal effect used for analysis of dose effect (0–30 minutes). *Doses statistically significantly different from vehicle for the period of 0–30 minutes after injection (P < 0.05). Vehicle (0.9% saline) data were obtained from one group of mice (n = 8) and are displayed in each panel to indicate dose-dependent differences of drug-induced motor activity from vehicle-treated mice.
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses occurring in each test period. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Graphs for the percentage of drug-appropriate responding and response rate were plotted as a function of the dose of the test compound (log scale). Percentage of drug-appropriate responding was shown only if at least three rats completed the first fixed ratio. Full substitution was defined as ≥80% drug-appropriate responding. Data on response rate data were analyzed by one-way repeated-measures analysis of variance. Effects of individual doses were compared with those of the vehicle control value using a priori contrasts. P < 0.05 was considered statistically significant.
Conditioned place preference data were expressed as the mean time in seconds spent on the drug-paired floor over 30 minutes. These data were analyzed using a two-way analysis of variance to compare the difference in time spent on the drug-paired floor before and after conditioning with each test compound, with pretest/preference test time as a within-groups factor and dose as a between-groups factor. Effects of individual doses on the time spent on the drug-paired floor were determined using a one-way repeated-measures analysis of variance. P < 0.05 was considered statistically significant.
Drugs.
The National Institute on Drug Abuse Supply Program provided the (−)-cocaine hydrochloride, (+)-methamphetamine hydrochloride, α-PBP, α-PVP, and 4ʹ-MePPP. All drugs were dissolved in 0.9% saline.
Results
Locomotor Activity.
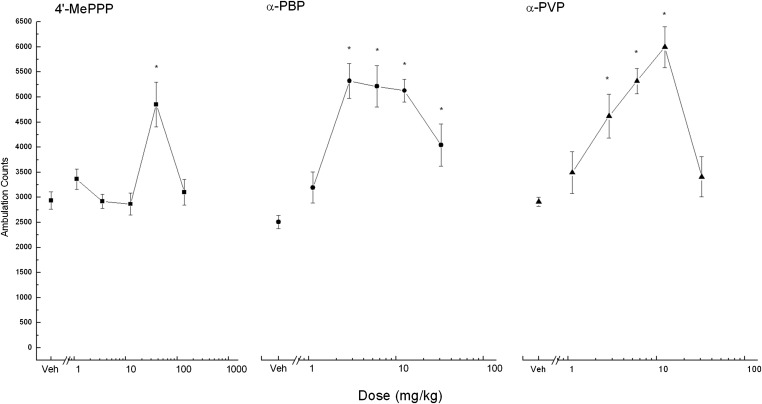
Figure 1 illustrates the average horizontal activity counts/10 minutes as a function of time (0–8 hours) and dose for each compound. The vehicle data are included in each panel for comparison. Figure 2 shows the dose-effect curves for each compound at their time of peak effect (0–30 minutes), which corresponds to the shaded areas in Fig. 1. Each compound produced increases in locomotor activity as dose increased up to a maximal effect, whereupon higher doses produced sharp decreases in locomotor activity during the time of peak effect. Rebound stimulation was seen after the top doses of α-PBP and α-PBP. The peak locomotor stimulant effects of α-PBP (5321 ± 344 counts) and α-PVP (6001 ± 408) were larger than those of 4ʹ-MePPP (4849 ± 446), (F2,21 = 9.926, P < 0.001) based on the period of maximal effect shown in Fig. 2. There was a statistically significant difference in the area under the curve calculated from Fig. 1 between compounds (F2,105 = 21.552, P < 0.001), between doses (F4,105 = 12.412, P < 0.001), and for the compound × dose interaction (F8,105 = 3.032, P = 0.004), with 4ʹ-MePPP producing a much smaller increase in locomotor activity at fewer doses and that lasted less time than either α-PBP or α-PBP.
Fig. 2.
Dose–response curve for the locomotor activity assay in mice. Data are represented as the mean number of activity counts (± S.E.M.) per 10 minutes for the first 30 minutes of testing (n = 8 per dose). The unconnected symbol (left) represents the activity counts after treatment with vehicle (vehicle, 0.9% saline), and the connected symbols represent activity counts after treatment with 4ʹ-MePPP (left, squares), α-PBP (middle, circles), and α-PVP (right, triangles). *Statistically significantly different doses from vehicle for the period of 0–30 minutes after injection (P < 0.05).
4ʹ-MePPP produced time- and dose-dependent stimulation of locomotor activity in doses from 30 to 100 mg/kg (Fig. 1, left column). Stimulant effects of 30 mg/kg occurred within 10 minutes after injection and lasted 130 minutes. After 100 mg/kg, stimulant effects did not occur until 40 minutes after injection and lasted 110 minutes. A two-way analysis of variance revealed a statistically significant main effect of time (F47,1645 = 60.83, P < 0.001) and a time × dose interaction (F235,1645 = 60.83, P < 0.001), but no main effect of dose. During the 30-minute time period in which maximal stimulant effects first appeared (0–30 minutes after injection; Fig. 2, left), significant stimulant effects occurred only after administration of 30 mg/kg (F4,35 = 8.82, P < 0.001).
α-PBP produced time- and dose-dependent stimulation of locomotor activity in doses from 2.5 to 25 mg/kg (Fig. 1, middle). Stimulant effects of 2.5, 5, and 10 mg/kg occurred within 10 minutes after injection and lasted 80–240 minutes. Stimulant effects of 25 mg/kg peaked 50–70 minutes after administration. A two-way analysis of variance revealed statistically significant main effects of dose (F5,42 = 4.546, P = 0.002) and time (F47,1974 = 124.929, P < 0.001), as well as a time × dose interaction (F235,1974 = 3.817, P < 0.001). During the 30-minute time period in which maximal stimulant effects first appeared (0–30 minutes after injection, Fig. 2, middle), significant stimulant effects occurred after administration of 2.5, 5, 10, and 25 mg/kg (F5,42 = 13.373, P < 0.001).
α-PVP produced time- and dose-dependent stimulation of locomotor activity in doses from 2.5 to 25 mg/kg (Fig. 1, right). Stimulant effects of 2.5, 5, and 10 mg/kg occurred within 10 minutes after injection and lasted 240–290 minutes. Stimulant effects of 25 mg/kg did not occur until 60 minutes after injection and lasted 280 minutes. A two-way analysis of variance revealed statistically significant main effects of dose (F5,42 = 11.24, P < 0.001) and time (F47,1974 = 97.69, P < 0.001), as well as a time × dose interaction (F235,1974 = 5.97, P < 0.001). During the 30-minute time period in which maximal stimulant effects first appeared (0–30 minutes after injection; Fig. 2, right), significant stimulant effects occurred after administration of 2.5, 5, and 10 mg/kg (F5,42 = 10.55, P < 0.001).
Drug Discrimination.
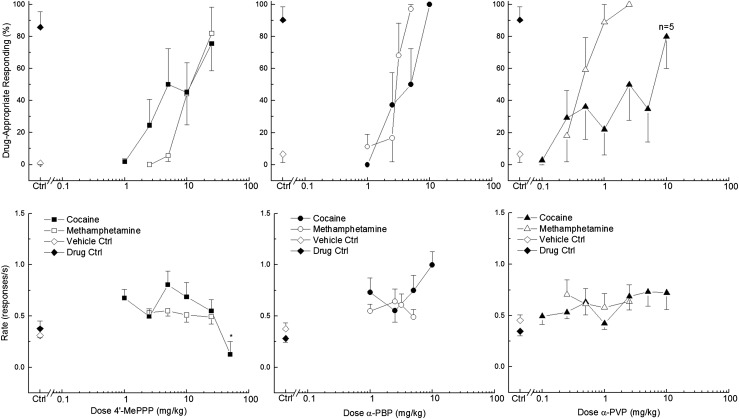
Both α-PBP and α-PVP fully substituted for the discriminative stimulus effects of cocaine, producing 100% and 80% ± 20% cocaine-appropriate responding, respectively (Fig. 3). Neither compound produced effects on rate of responding. In contrast, 4ʹ-MePPP (25 mg/kg) produced only 75% ± 17% cocaine-appropriate responding, and decreased the response rate in cocaine-trained rats to 19% of vehicle control after the 50 mg/kg dose (F6,24= 5.00, P < 0.01).
Fig. 3.
4ʹ-MePPP (left, squares), α-PBP (middle, circles), and α-PVP (right, triangles) substitution for the discriminative stimulus effects of cocaine or methamphetamine in rats. Upper panels show mean percentage of total responses (± S.E.M.) made on the drug-appropriate lever for doses with three or more rats completing the first fixed ratio. Bottom panels show rate of responding (± S.E.M.) in responses per second (r/s). Testing in cocaine-trained rats is shown in the filled symbols, and testing in methamphetamine-trained rats is shown in the open symbols. Ctrl indicates vehicle (0.9% saline) and drug control, and n = 6 except where shown.
4ʹ-MePPP, α-PBP, and α-PVP fully substituted for the discriminative stimulus effects of methamphetamine (Fig. 3). 4ʹ-MePPP produced 82% ± 16% methamphetamine-appropriate responding, α-PBP produced 97% ± 3%, and α-PVP 100%. None of the test compounds produced significant effect on response rate in methamphetamine-trained rats. The dose effect curves for α-PBP and 4ʹ-MePPP in cocaine- and methamphetamine-trained rats were very close. In contrast, the slope of the dose effect for α-PVP in cocaine-trained rats was substantially shallower than the dose-effect curve for α-PVP in the methamphetamine-trained animals.
Conditioned Place Preference.
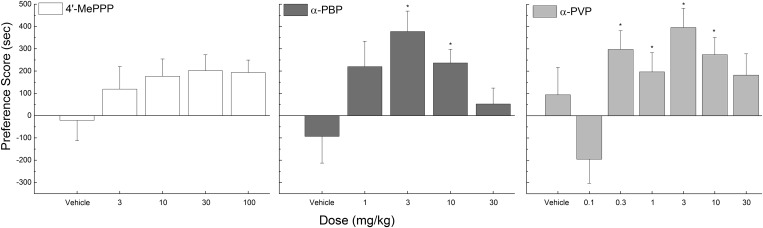
α-PBP and α-PVP induced conditioned place preference in a dose-dependent manner, producing inverted U-shaped dose effect curves (Fig. 4). 4ʹ-MePPP (Fig. 4, left) failed to increase the time spent on the drug-paired floor in doses from 10 to 100 mg/kg (F4,75 = 1.287, P = 0.283). In contrast, α-PBP produced an overall effect on conditioned place preference (F4,75 = 3.688, P < 0.009), increasing the time spent on the drug-paired floor at 3 and 10 mg/kg, but not 1 or 30 mg/kg (Fig. 4, middle). Similarly, α-PVP produced an overall effect (F6,104 = 3.936, P = 0.001, increasing time spent on the drug-paired floor in doses from 0.3 to 10 mg/kg, but not at 0.1 or 30 mg/kg (Fig. 4, right).
Fig. 4.
Preference scores (mean time spent on drug-paired floor during pretest subtracted from mean time spent on drug-paired floor during post-test) for 4ʹ-MePPP (left, white bars), α-PBP (middle, dark gray bars), and α-PVP (right, gray bars). Data are represented as mean ± S.E.M. (n = 16 per dose). The vehicle was 0.9% saline. *Statistically significantly different doses from baseline (P < 0.05).
Discussion
The synthetic cathinones α-PBP and α-PVP produced robust and long-lasting stimulation of locomotor activity, full substitution for the discriminative stimulus effects of cocaine and methamphetamine, and conditioned place preference. In contrast, 4ʹ-MePPP produced modest and relatively shorter acting locomotor stimulant effects, fully substituted for the discriminative stimulus effects of methamphetamine but not of cocaine, and failed to produce conditioned place preference across a range of behaviorally relevant doses.
A wide range of cathinone compounds, including those currently used recreationally, produce locomotor stimulant effects (see review in Glennon, 2014). It is of interest to note that when the time courses of the full range of doses from no effect to full effect are examined, there are commonalities among the cathinones and related psychostimulants, but some differences can also be observed. Most psychostimulant compounds produce inverted U-shaped dose-effect curves (e.g., Katz et al., 2001; Gatch et al., 2013, 2015) because all compounds will decrease locomotor activity at some dose. This is not always apparent in the published data because doses on the descending limb of the curve may not be tested if there are known toxicities or if higher doses are not relevant to the experimental questions being tested in the study.
Some compounds, including MDPV, naphyrone, pentylone, and methcathinone, produce large increases in locomotor activity, with peak effects observed during a time range consistent over doses on the ascending limb (Gatch et al., 2013, 2015). Large doses of these compounds produce marked suppression of locomotor activity at the time of peak effect, followed by a long-lasting rebound effect. In the present study, the locomotor stimulant effects of α-PBP and α-PVP followed this pattern.
A prior study reported that α-PBP and α-PVP increased locomotor activity, but because only the first 60 minutes was tested at doses lower than in our study, the rebound effect was not observed (Marusich et al., 2014). One explanation for the initial decrease in locomotor activity at higher doses is a potential increase in the incidence of stereotyped behavior at higher doses, which has been demonstrated to occur in α-PBP and α-PVP (Marusich et al., 2014) and 4ʹ-MePPP (Saha et al., 2015); this may have inhibited exploration of the testing arena during the time of peak effect. In contrast, 4ʹ-MePPP produced modest locomotor stimulant effects that peaked immediately and lasted about 2 hours. Similar time courses have been produced by the synthetic cathinones 3-fluoromethcathinone (3-FMC), 4-methylethcathinone (4-MEC), and mephedrone (Gatch et al., 2013, 2015).
α-PBP and α-PVP both produced full substitution for the discriminative stimulus effects of cocaine and methamphetamine. These findings agree with a recent study demonstrating that α-PVP fully substitutes for the discriminative stimulus effects of methamphetamine (Naylor et al., 2015) and previous reports that other cathinone compounds fully substitute for the discriminative stimulus effects of cocaine, amphetamine, and methamphetamine (Dal Cason et al., 1997; Schechter 1997; Bondareva et al., 2002; Gatch et al., 2013, 2015; Kohut et al., 2013). α-PVP produced a much flatter dose–effect curve in the cocaine-trained rats than in the methamphetamine-trained rats. Why this might be the case is not clear, as α-PBP and α-PVP produced similar profiles as inhibitors of monoamines (Marusich et al., 2014). Whether α-PVP has some different mechanism of action that might produce such a difference remains to be determined.
In contrast, 4ʹ-MePPP fully substituted for the discriminative stimulus effects of methamphetamine but narrowly missed the criterion for full substitution for cocaine, producing only 75% cocaine-appropriate responding. These findings in methamphetamine-trained rats are in accordance with a recent study indicating that 4ʹ-MePPP fully substitutes for the discriminative stimulus effects of methamphetamine (Naylor et al., 2015). A higher dose of 4ʹ-MePPP (50 mg/kg) suppressed responding in cocaine-trained rats so that discrimination performance could not be assessed. The peak methamphetamine-appropriate responding of 82% was not greatly different from the 75% seen in the cocaine-trained rats, so it is difficult to make strong conclusions about the relative efficacy of 4ʹ-MePPP at producing cocaine- or methamphetamine-like stimuli.
α-PBP and α-PVP produced conditioned place preference, but 4ʹ-MePPP failed to produce significant effects. The dose-effect curves for α-PBP and α-PVP were both inverted U-shaped functions, with the low and high doses not producing conditioned place preference. Similar inverted U dose–effect functions have been reported for conditioned place preference with other psychostimulants such as amphetamine, cocaine, and methamphetamine (Adriani and Laviola, 2003; Rodriguez-Alarcòn et al., 2007; Zakharova et al., 2009). Other cathinones also produce reward and reinforcement. Mephedrone, methylone, and MDPV produced conditioned place preference (Lisek et al., 2012; Karlsson et al., 2014), and mephedrone and MDPV were self-administered (Hadlock et al., 2011; Aarde et al., 2013; Watterson et al., 2014). In addition, methcathinone, mephedrone, methylone, and MDPV facilitated intracranial self-stimulation in rats (Bonano et al., 2014).
The behavioral findings from our study are in general accordance with in vitro data previously published regarding the pharmacodynamics of these compounds. 4ʹ-MePPP consistently produced fairly weak locomotor stimulation and failed to induce conditioned place preference, which may be explained by its fairly weak dopaminergic activity compared with α-PVP and α-PBP. In vivo microdialysis studies have demonstrated that 4ʹ-MePPP produces brief increases in extracellular dopamine lasting roughly 1 hour (Saha et al., 2015), whereas α-PVP increases dopamine concentrations up to 2 hours after injection (Kaizaki et al., 2014).
Furthermore, compounds with ring substitutions in the para position on the aromatic ring tend to increase activity at the serotonin transporter relative to the dopamine transporter and tend to be less robustly self-administered (Iversen et al., 2014). Although 4ʹ-MePPP does not produce changes in serotonin concentrations, it has higher affinity at the serotonin transporter and lower affinity for the dopamine transporter than its non–para-methyl-substituted analog α-pyrrolidinopropiophenone (Marusich et al., 2014; Saha et al., 2015). The decreased relative efficacy for increasing extracellular dopamine by 4ʹ-MePPP may explain the weaker locomotor stimulation and lack of conditioned place preference compared with the other compounds tested in this study.
In summary, all three of the pyrovalerone cathinone analogs were psychomotor stimulants and produced discriminative stimulus effects similar to the abused psychostimulants cocaine and methamphetamine. α-PBP and α-PVP produced reward-like effects, but 4ʹ-MePPP did not. None of the three compounds produced adverse effects at the doses tested, although α-PVP produced a long-lasting rebound effect similar to that of MDPV and naphyrone. These findings suggest that α-PBP and α-PVP are likely to be recreationally used and have potential for addiction and abuse. 4ʹ-MePPP may not produce as much interest for recreational use.
Acknowledgments
The authors thank Carla Elsken, Elva Flores, Margaret Rutledge, and Cynthia Taylor for excellent technical assistance.
Abbreviations
- α-PBP
α-pyrrolidinopropiobutiophenone
- α-PPP
α-pyrrolidinopropiophenone
- α-PVP
α-pyrrolidinopentiophenone
- 3-FMC
3-fluoromethcathinone
- MDMA
± methylenedioxymethamphetamine
- MDPBP
3,4-methylenedioxy-α-pyrrolidinobutiophenone
- MDPV
methylenedioxypyrovalerone
- 4-MEC
4-methylethcathinone
- 4ʹ-MePPP
4-methyl-α-pyrrolidinopropiophenone
Authorship Contributions
Participated in research design: Forster, Gatch, Dolan.
Conducted experiments: Dolan, Gatch.
Performed data analysis: Dolan, Gatch.
Wrote or contributed to the writing of the manuscript: Dolan, Gatch, Forster.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse Addiction Treatment Discovery Program [Grant N01-DA78872 (to M.J.F.)].
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. (2013) The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 71:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Laviola G. (2003) Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci 117:695–703. [DOI] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. (2014) Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 231:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareva TS, Young R, Glennon RA. (2002) Central stimulants as discriminative stimuli. Asymmetric generalization between (−)ephedrine and S(+)methamphetamine. Pharmacol Biochem Behav 74:157–162. [DOI] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. (2013) Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol 168:1750–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. (2009) Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend 105 (Suppl 1):S14–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cason TA, Young R, Glennon RA. (1997) Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem Behav 58:1109–1116. [DOI] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, Negus SS. (2014) Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci 97:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration (DEA) (2014) Schedules of controlled substances: temporary placement of 10 synthetic cathinones into Schedule I. Final order. Fed Regist 79:12938–12943. [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. (2013) Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol 85:1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Forster MJ. (2015) Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology (Berl) 232:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol 24:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. (2014) Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 97:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA. (2014) Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention. Adv Pharmacol 69:581–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, et al. (2011) 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther 339:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. (2013) A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol 24:410–436. [DOI] [PubMed] [Google Scholar]
- Iversen L, White M, Treble R. (2014) Designer psychostimulants: pharmacology and differences. Neuropharmacology 87:59–65. [DOI] [PubMed] [Google Scholar]
- Kaizaki A, Tanaka S, Numazawa S. (2014) New recreational drug 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (alpha-PVP) activates central nervous system via dopaminergic neuron. J Toxicol Sci 39:1–6. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. (2014) Mephedrone, methylone and 3,4-methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clin Pharmacol Toxicol 115:411–416. [DOI] [PubMed] [Google Scholar]
- Katz JL, Agoston GE, Alling KL, Kline RH, Forster MJ, Woolverton WL, Kopajtic TA, Newman AH. (2001) Dopamine transporter binding without cocaine-like behavioral effects: synthesis and evaluation of benztropine analogs alone and in combination with cocaine in rodents. Psychopharmacology (Berl) 154:362–374. [DOI] [PubMed] [Google Scholar]
- Kohut SJ, Fivel PA, Blough BE, Rothman RB, Mello NK. (2013) Effects of methcathinone and 3-Cl-methcathinone (PAL-434) in cocaine discrimination or self-administration in rhesus monkeys. Int J Neuropsychopharmacol 16:1985–1998. [DOI] [PubMed] [Google Scholar]
- Leffler AM, Smith PB, de Armas A, Dorman FL. (2014) The analytical investigation of synthetic street drugs containing cathinone analogs. Forensic Sci Int 234:50–56. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM. (2012) Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend 126:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. (2014) Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL. (2015) Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend 149:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. (2012) The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol 8:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Alarcón G, Canales JJ, Salvador A. (2007) Rewarding effects of 3,4-methylenedioxymethamphetamine (“Ecstasy”) in dominant and subordinate OF-1 mice in the place preference conditioning paradigm. Prog Neuropsychopharmacol Biol Psychiatry 31:191–199. [DOI] [PubMed] [Google Scholar]
- Saha K, Partilla JS, Lehner KR, Seddik A, Stockner T, Holy M, Sandtner W, Ecker GF, Sitte HH, Baumann MH. (2015) ‘Second-generation’ mephedrone analogs, 4-MEC and 4-MePPP, differentially affect monoamine transporter function. Neuropsychopharmacology 40:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD. (1997) Discriminative characteristics of high and low cocaine administration: effect of other psychostimulants. Pharmacol Biochem Behav 56:457–463. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC (2014) Global Synthetic Drugs Assessment: Amphetamine-Type Stimulants and New Psychoactive Substances, United Nations Office on Drugs and Crime, Vienna. [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. (2009) Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res 198:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]