Abstract
Increasing evidence demonstrates that the antiretroviral drugs (ARVds) used for human immunodeficiency virus (HIV) treatment have toxic effects that result in various cellular and tissue pathologies; however, their impact on the cells composing the blood-brain barrier is poorly understood. The current study focused on ARVds, used either in combination or alone, on the induction of endoplasmic reticulum (ER) stress responses in human brain endothelial cells. Among studied drugs (efavirenz, tenofovir, emtricitabine, lamivudine, and indinavir), only efavirenz increased ER stress via upregulation and activation of protein kinase-like ER kinase PERK and inositol requiring kinase 1α (IRE1α). At the same time, efavirenz diminished autophagic activity, a surprising result because typically the induction of ER stress is linked to enhanced autophagy. These results were confirmed in microvessels of HIV transgenic mice chronically administered with efavirenz. In a series of further experiments, we identified that efavirenz dysregulated ER stress and autophagy by blocking the activity of the Beclin-1/Atg14/PI3KIII complex in regard to synthesis of phosphatidylinositol 3-phosphate, a process that is linked to the formation of autophagosomes. Because autophagy is a protective mechanism involved in the removal of dysfunctional proteins and organelles, its inhibition can contribute to the toxicity of efavirenz or the development of neurodegenerative disease in HIV patients treated with this drug.
Introduction
The impact of a dysfunctional blood-brain barrier (BBB) on the development of neurologic diseases has been highlighted in several studies (Zlokovic, 2008; Grammas et al., 2011). Alterations of BBB integrity have been implicated in multiple sclerosis (Larochelle et al., 2011), amyotrophic lateral sclerosis (Rodrigues et al., 2012), Parkinson's disease (Kortekaas et al., 2005), Alzheimer's disease (Erickson and Banks, 2013; Kelleher and Soiza, 2013), and brain infection by human immunodeficiency virus (HIV) (Nakagawa et al., 2012; Mishra and Singh, 2014). BBB alterations in HIV patients include alterations of tight junction protein expression and integrity (Dallasta et al., 1999; Boven et al., 2000; Ivey et al., 2009; Strazza et al., 2011), increased permeability (Boven et al., 2000), enhanced expression of extracellular matrix degrading enzymes such as matrix metalloproteinase-2 and -9 (Eugenin et al., 2006; Louboutin et al., 2010), and increased inflammatory cell migration (Hurwitz et al., 1994). In addition, dysfunctional BBB was proposed to participate in the accumulation of amyloid-β in HIV-infected brains (Andras and Toborek, 2013).
The introduction of combined antiretroviral therapy (cART) has changed the outcome and prognosis of HIV infection. What was once a fatal disease is now controlled, and infected patients can survive. With increased survival, it is estimated that patients aged 50 years and older represent approximately 50% of all HIV-infected individuals in the United States (Vance, 2010). In these long-term survivors, the infection itself is controlled, but several pathologies are observed, such as cardiovascular, lipid, metabolic, and neurologic disorders (Clifford and Ances, 2013; Deeks et al., 2013; Galescu et al., 2013; Kebodeaux et al., 2013; Lake and Currier, 2013). Before the advent of cART, neurologic disorders in HIV patients were often associated with severe cognitive dysfunction, such as HIV-associated dementia. Currently, neurologic disorders are rather associated with mild and slow progressive degeneration of cognitive and motor functions (Clifford and Ances, 2013); this susceptibility is correlated with age (Becker et al., 2004).
Whereas persistent (albeit at low rates) HIV replication in the brain may be responsible for neurocognitive alterations observed in infected individuals, the toxicity of antiretroviral drugs (ARVds) is also likely to contribute to neurodegenerative disorders in HIV patients. Indeed, ARVds have been described to disrupt the mechanisms of phagocytosis and production of amyloid-β (Giunta et al., 2011), impact mitochondrial function and DNA replication (Brinkman et al., 1999; Blas-Garcia et al., 2010; Apostolova et al., 2011; Bollmann, 2013), induce oxidative stress (Manda et al., 2011), and stimulate cellular stress responses (Apostolova et al., 2013). Protease inhibitors used in HIV treatment have been associated with the development of dyslipidemia (Overton et al., 2012) and inhibition of normal proteasome function (Piccinini et al., 2005). Several studies have linked the use of ARVds, in particular, efavirenz, to hepatotoxicity via multiple mechanisms, including alterations of calcium homeostasis, mitochondrial damage, enhanced proinflammatory cytokine levels, and interference with the cannabinoid receptor CB1 (Blas-Garcia et al., 2010; Gallego-Escuredo et al., 2010; Apostolova et al., 2011, 2013; Hecht et al., 2013); however, the toxicity and impact of those drugs have not been extensively studied in the context of the BBB.
The unfolded protein response/endoplasmic reticulum (ER) stress and autophagy are the major pathways of cellular response to a variety of stressors. For example, induction of ER stress is an important mechanism to remove misfolded proteins, cope with calcium imbalance, or cope with alterations of redox potential and glucose deprivation. Autophagy is closely linked to ER stress and serves multiple purposes in the cell, including degradation of aggregated proteins, recycling of organelles, and destroying intracellular pathogens (Criollo et al., 2010; Qin et al., 2010; Nardacci et al., 2014). Dysregulation of these responses can have a drastic impact on cellular homeostasis and, owing to their link to the apoptosis pathway, can result in cell death.
The goal of the present study was to identify the impact of ARVds, used in combination or alone, on induction of ER stress and autophagy in brain microvasculature. Our results demonstrate that efavirenz alone, or in combination with other ARVds, induces ER stress via stimulation of inositol requiring kinase 1 α (IRE1α) and protein kinase-like ER kinase (PERK) signaling. Importantly, we also demonstrate that efavirenz dysregulates autophagy by affecting the ability of the Beclin-1/Atg14/PI3KIII complex activity to synthesize phosphatidylinositol 3-phosphate (PI3P), a process that is linked to the formation of autophagosomes.
Materials and Methods
Cell Culture and Treatment with ARVds.
Brain endothelial cells used in this study are of the hCMEC/D3 cell line, which represent well characterized BBB endothelial cells (Weksler et al., 2013). They were cultured in EBM-2 media (Lonza Group, Basel, Switzerland) supplemented with vascular endothelial growth factor, insulin-like growth factor-1 epidermal growth factor, basic fibroblast growth factor, hydrocortisone, ascorbate, gentamycin, and 0.5% fetal bovine serum (FBS) (Lonza) on cell culture dishes coated with rat-tail collagen I (Becton-Dickinson Biosciences, Franklin Lakes, NJ) in 5% CO2 humid incubator at 37°C. Cells were exposed to the following combinations of ARVds: cART1 (10 μM efavirenz, 5 μM emtricitabine, and 1 μM tenofovir) or cART2 (1 μM atazanavir, 60 nM ritonavir, 5 μM emtricitabine, and 1 μM tenofovir) in serum-free and antibiotic-free EBM-2 basal media. These concentrations reflect the physiologic level of the drugs (Marzolini et al., 2001; Stahle et al., 2004; Droste et al., 2005; Boffito et al., 2011; Valade et al., 2014). For example, successful therapy was observed in patients with plasma concentrations of efavirenz between 1000 and 4000 µg/l, which translates to 3.17 and 12.67 µM. Higher rates of treatment failure were associated with plasma concentrations below this level, and central nervous system toxicity was linked to levels above this range (Marzolini et al., 2001). ARVds were acquired from the National Institutes of Health AIDS Reagent Program. Cultures were also exposed to tunicamycin (0.1 μM, a positive control for induction of ER stress), rapamycin (0.5 μM, a positive control for induction of ER stress), or vehicle [dimethylsulfoxide (DMSO) 0.1%]. Primary human brain endothelial cells [Cell Systems Corporation (CSC), Kirkland, WA] were cultured in the CSC complete media on dishes coated with CSC attachment factor (all from Cell Systems), followed by treatment with ARVds in serum-free CSC medium without antibiotics. SVG P12 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS (Gibco, Waltham, MA) and exposed to ARVds in Dulbecco’s modified Eagle’s medium without serum and antibiotic.
Secreted Embryonic Alkaline Phosphatase Assay.
Cells were seeded at a density of 6 × 104 cells per well on a 96-well plate and incubated for 24 hours. Plasmid pSELECT-Zeo–secreted embryonic alkaline phosphatase (SEAP; InvivoGen, San Diego, CA) encoding for SEAP was amplified in Escherichia coli and purified using Midi Plasmid kit (Sigma-Aldrich, St. Louis, MO). Transfections were performed for 6 hours using LipofectAMINE 2000 (Invitrogen, Carlsbad, CA) in a 3:1 ratio with 0.1 µg of plasmid diluted in OPTI/MEM medium (Gibco) per well. Then cells were washed with EBM-2, incubated overnight in complete media, and exposed to ARVds in EBM-2 without serum and phenol red for 48 hours. Supernatants were transferred to a 96-well plate (Thermo/Nunc, Somerset, NJ) and assayed for SEAP activity using a SEAP reported assay kit (InvivoGen) according to the manufacturer’s instructions. Data were normalized by staining cells with DRAQ-5 (Abcam, Cambridge, UK).
Autophagy Activity Assay.
Cells were seeded at 6 × 104 cells/well on 96-well white plates (Thermo/Nunc) coated with collagen for 24 hours, followed by treatment with ARVds for 48 hours. Then cells were washed twice with warmed phosphate-buffered saline and incubated for 1 hour with Cyto-ID detection dye and Hoechst 33342 (Enzo Life Sciences, Inc., Farmingdale, NY). Cells were subsequently washed twice in assay buffer, and the absorbance was read on a Spectramax Gemini EM microplate reader (Molecular Devices, Sunnyvale, CA) (excitation: 480 nm; cutoff: 495 nm; emission: 510–565 nm). Cyto-ID signal was normalized to account for cell number using Hoechst 33342 signal (excitation: 350 nm; cutoff: 420 nm; emission: 450–475 nm).
Immunoblotting, Immunoprecipitation, and Immunostaining.
Cells were exposed to ARVds in 100- or 150-mm dishes, washed, and lysed in RIPA buffer (Santa Cruz Biochemical, Inc., Dallas, TX) supplemented with protease/phosphatase inhibitors (Cell Signaling Technology, Beverly, MA). Protein concentration was assessed using bicinchoninic acid protein assay kit (Pierce/Thermo Fisher Scientific, Waltham, MA), and 30 μg of proteins was loaded per each lane on precast TGX 4–20% gradient gels (Bio-Rad, Hercules, CA). Transfer was performed using Trans-blot Turbo bovine serum albumin with nitrocellulose transfer packs (Bio-Rad). Membranes were blocked by 4% solution in TBS with 0.1% Tween-20 and then incubated with primary antibodies in 4% bovine serum albumin blocking solution. The antibody dilutions were anti-Atg2a (1:100); anti-CaMKKβ, anti-ATF6a, and anti–p-CaMKKβ (all 1:500); anti–p-PERK (1:600); anti-CHOP, anti–p-PKCɵ, and anti-ATF4 (all 1:750), anti-tubulin and anti-actin (Sigma-Aldrich) (both 1:10,000); and all remaining antibodies (1:1000). Signals were detected using Licor imaging system (Licor, Lincoln, NE). For two-color imaging, membranes were incubated using anti-rabbit 800CW and anti-mouse 680LT antibodies (Licor) (1:30,000), washed with Tris-buffered saline/Tween, and imaged on an Odyssey CLx scanner (Licor). Electrochemoluminescence detection was performed with anti-rabbit light chain horseradish peroxidase antibodies (1:10,000) (Jackson ImmunoResearch) and ECL reagent (GE Healthcare, Little Chalfont, UK). Proteins G Magnetic Beads (Cell Signaling Technology) were used for immunoprecipitation. Immunostaining was performed on cells grown on collagen-covered round coverslips (Thermo Fisher Scientific) or on isolated microvessels heat-fixed on slides. Samples were fixed using 4% paraformaldehyde (Santa Cruz Biochemical), permeabilized using 0.1% Triton X-100 solution, and blocked using NATS solution (20% FBS and 0.5% Tween-20 in phosphate-buffered saline). Samples were then exposed to primary antibodies at 37°C in a humidified chamber and washed. Alexa-488 and -594 secondary antibodies (Invitrogen) and DRAQ-5 (Cell Signaling Technology) were used to visualize the signals. Imaging was performed on an Olympus Fluoview 1200 microscope using a 60× oil immersion lens. Immunofluorescence and ECL quantification were performed using ImageJ software (National Institutes of Health, Bethesda, MD). Image Studio 4.0 (Licor) was used for Odyssey CLx acquisition quantification.
XBP-1 mRNA Analysis.
mRNA was harvested using RNA isolation kit (Qiagen, Hilden, Germany) and reverse-transcribed by a reverse transcriptase system (Promega, Madison, WI) with random primers. Then a XBP-1 fragment was amplified using primers 5′-TTACGAGAGAAAACTCATGGCC-3′ and 5′-GGGTCCAAGTTGTCCAGAATGC-3′. The polymerase chain reaction product was resolved on a 6% acrylamide gel (19:1 ratio; BioRad) for the XBP-1 full-length product (289 bp) and spliced product (263 bp).
Quantification of Calcium and PI3P.
Cells were seeded on a 96-well plate and exposed to ARVds for 48 hours in serum-free media. After three washes with Hanks’ balanced salt solution buffer without calcium, cells were incubated with Fluo-4-AM (Invitrogen) at 10 µM for 40 minutes. Then they were washed twice with Hanks’ balanced salt solution buffer, and fluorescence was read on a Spectramax Gemini EM microplate reader (excitation: 480 nm, cutoff: 495 nm, emission: 520 nm).
PI3P concentration was determined using a PI3P Assay Detection kit (Echelon Biosciences, Salt Lake City, UT). Cells were incubated with ARVds on 150-mm plates for 48 hours, and PI3P was isolated according to the supplied protocol. Enzyme-linked immunosorbent assay was then performed according to manufacturer’s instruction and the signals were read on a Spectramax 190 microplate reader (Molecular Devices).
Animal Studies.
All animal procedures have been carried out in accordance with National Institutes of Health guidelines in the Public Health Service animal welfare approved facilities. They were approved by the University of Miami Animal Care and use Committee. Tg26 HIV transgenic mice on a C57BL/6 background were acquired from Dr. Roy L. Sutliff (Emory University, Atlanta, GA). Males, 10–12 weeks old, were administered with efavirenz (10 mg/kg) or etravirine (6.6 mg/kg) via gavage for 30 days. The dosing of both drugs is consistent with therapeutic dosing in humans (Intelence, 2014; Sustiva, 2015). Etravirine is a new generation of non-nucleoside reverse transcriptase inhibitors (NNRTI), and it has demonstrated far less toxicity in patients than efavirenz (Nguyen et al., 2011). Control mice received vehicle. Drugs were dissolved in DMSO and mixed with saline at a final ratio of 25% DMSO and 75% saline. After the treatment period, mice were euthanized and perfused with saline. Brains were harvested and frozen in liquid nitrogen. Brain microvessels were isolated using a technique previously described by our laboratory (Seelbach et al., 2010; Park et al., 2013), lysed in RIPA buffer, and homogenized using a handheld homogenizer (Kontes/Kimble Chase, Vineland, NJ) or analyzed for expression of ER stress and autophagy markers by immunoblotting or spread on a slide and fixed for immunostaining.
Statistical Analysis.
Data were analyzed using GraphPad Prism software (GraphPad, San Diego, CA), and experimental treatments were compared pairwise with control treatments using either one-way or two-way analysis of variance, followed by Turkey’s multiple comparisons test, Fisher LSD, or student’s t test. Value of P < 0.05 was considered significant.
Results
Induction of ER Stress in Cells Exposed to ARVds.
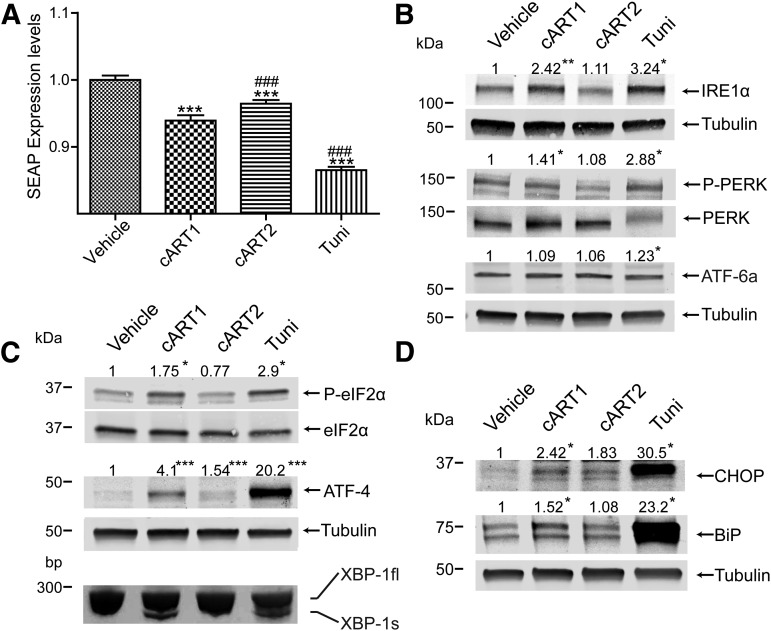
Toxicity of ARVds to human brain endothelial cells may result in stimulation of ER stress. Because induction of ER stress reduces the overall protein synthesis, this hypothesis was addressed by using an assay based on the expression of a secreted form of alkaline phosphatase (SEAP) (Kitamura and Hiramatsu, 2011). Cells were transfected using an expression plasmid for SEAP and incubated with a combination of ARVds, namely, efavirenz, tenofovir, and emtricitabine (abbreviated cART1 herein) and atazanavir, ritonavir, emtricitabine, and tenofovir (abbreviated cART2) in basal media. After 48 hours, supernatants were analyzed for SEAP by detecting alkaline phosphatase activity. As indicated in Fig. 1A, exposure to cART1 and tunicamycin (positive control) resulted in a significant reduction of SEAP activity compared with vehicle or cART2. Whereas cART2 also induced ER stress in brain endothelial cells, this effect was not so pronounced as in cART1-treated cells.
Fig. 1.
cART1 induces ER stress. (A) Brain endothelial cells transfected with a plasmid coding for SEAP were incubated with vehicle (DMSO, control), cART1, cART2, or tunicamycin (positive control) for 48 hours in basal media. Alkaline phosphatase levels in supernatant were detected. (B) Expression and phosphorylation of ER stress inducers detected in cultures exposed to the same treatment as in (A). Cells were lysed and analyzed by immunoblotting for IRE1α, ATF-6, phosphorylated PERK (P-PERK), and total PERK. (C) Expression of ER stress-related signaling molecules eIF2α, phosphorylated eIF2α (P-eIF2α), and ATF-4 evaluated by immunoblotting. In addition, splicing of XBP-1 mRNA was analyzed using reverse transcriptase and polymerase chain reaction amplification. Cultures were treated as in (A). (D) Immunoblot for ER stress-related chaperones CHOP and BiP in cultures treated as in (A). The numbers above the images indicate densitometric measurements of specific band intensity versus vehicle-treated cells. The values were normalized to housekeeping protein tubulin or to total (i.e., nonphosphorylated) expression of individual proteins. Data are mean ± S.E.M., n = 3–16. *P < 0.05 versus DMSO; **P < 0.01 versus DMSO; ***P < 0.001 versus DMSO; ###P < 0.001 versus cART1. Tuni, tunicamycin.
In the next series of experiments, we investigated the impact of cART1 on expression or activation of ER resident proteins (IRE1α, PERK, and ATF-6), which initiate the induction of ER stress. Exposure to cART1 for 48 hours enhanced the expression of IRE1α and PERK phosphorylation compared with cART2 or DMSO (Fig. 1B). Tunicamycin produced similar responses; however, it also resulted in activation of ATF-6. These results were then confirmed by analysis of the expression of downstream mediators implicated in the communication between the ER and the nucleus. Indeed, increased phosphorylation of eIF2α, splicing of XBP-1 mRNA, and increased levels of ATF-4 were observed in cultures exposed to cART1 or tunicamycin (Fig. 1C). In addition, the expression of ER stress effectors CHOP and BiP was elevated in response to cART1 and highly enhanced in tunicamycin-treated cultures (Fig. 1D). Taken together, these results indicate that exposure of brain endothelial cells to cART1 induces ER stress and implicates at least two pathways of ER stress activation: IRE1α and PERK.
Exposure to cART1 Reduces Autophagic Activity.
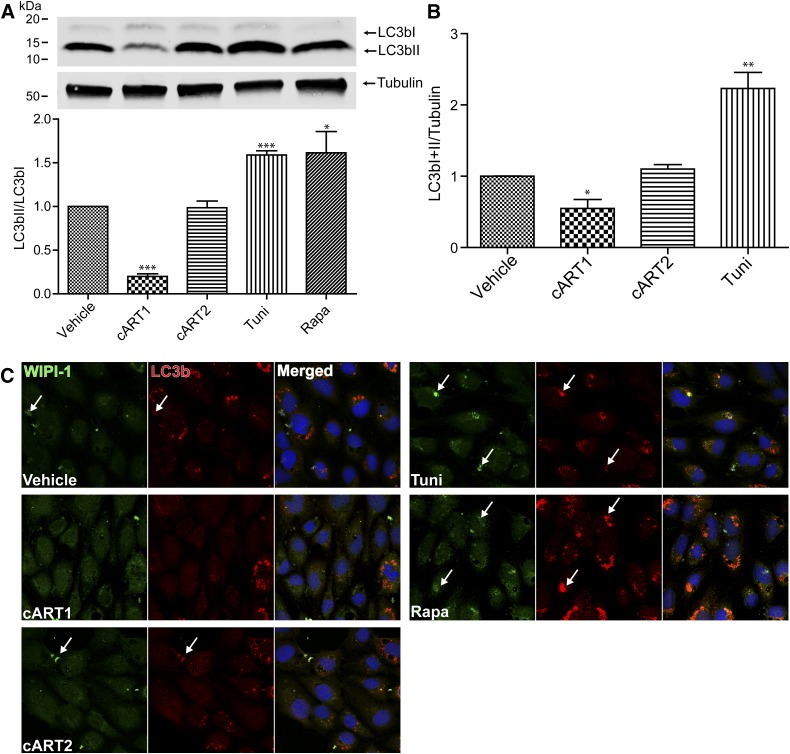
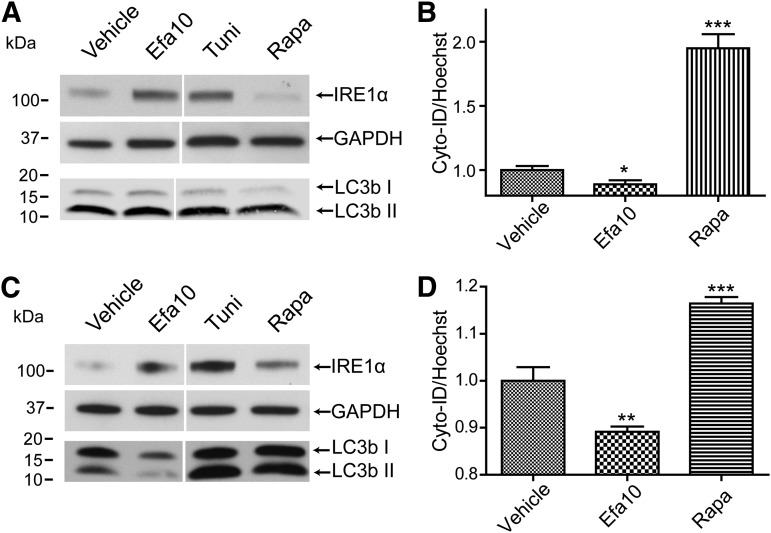
Both ER stress and autophagic pathways are linked by several signaling branches; therefore, induction of ER stress often leads to the activation of autophagy. We therefore evaluated autophagic activity in our experimental system. Cells were exposed to ARVds in basal media as in experiments presented in Fig. 1, followed by determination of the activation of a widely used autophagic marker, LC3b, which is processed from a 16 kDa (LC3bI) to a 14 kDa (LC3bII) migrating isoform as a result of autophagic activation (Karim et al., 2007; Zhou et al., 2011). Contrary to the expected results, a significant decrease in processing of LC3bI to LC3bII was observed in cells exposed to cART1 compared with vehicle, indicating a decrease in autophagic activity by this ARVd combination (Fig. 2A). We also detected a decrease in total LC3b (LC3bI plus LC3bII) level normalized to tubulin (Fig. 2B) compared with vehicle (DMSO)-treated controls. To further examine the autophagy response, we evaluated the impact of ARVds on the subcellular localization of LC3b and wipi-1, a protein that is recruited during autophagosome maturation (Proikas-Cezanne and Pfisterer, 2009). Cells were transfected with a plasmid encoding for a GFP-tagged wipi-1, recovered for 24 hours, and exposed to ARVds in basal media for 48 hours. Although a drastic increase in the formation of wipi-1 foci was observed in tunicamycin- and rapamycin-treated cells (Fig. 2C, arrows), such effects were not observed in cART1-exposed cells. In these cells, a weak and uniform wipi-1–positive immunostaining was detected. A concurrent increase in LC3b foci was also observed in cultures exposed to the positive controls (rapamycin and tunicamycin) but not in cells exposed to cART1 (Fig. 2C, arrows). Taken together, these results demonstrate that cART1 leads to a decrease in autophagy in brain endothelial cells.
Fig. 2.
cART1 reduces autophagy activity. Brain endothelial cells were treated as in Fig. 1. (A) Immunoblotting detection of autophagy marker LC3b. Graph reflects the ratio of LC3bII to LC3bI for four independent assays. (B) LC3b total expression levels in the same cultures as in (A) normalized to tubulin expression. (C) Representative confocal images of immunostaining for wipi-1 and LC3b. Cells were transfected with an expression vector for a green fluorescent protein–tagged wipi-1, immunostained for LC3b, and nuclei were labeled with DRAQ-5. The data are mean ± S.E.M.; n = 4. *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle. Tuni, tunicamycin; Rapa, rapamycin.
Efavirenz Is the Component of cART1 Responsible for the Dysregulation of ER Stress and Autophagy Pathways.
We next focused on identifying whether a single or multiple components of cART1 are responsible for the apparent dysregulation of ER stress and autophagy. Previous publications indicated that efavirenz could induce ER stress and autophagy in other models (Apostolova et al., 2011, 2013; Purnell and Fox, 2014); however, our observations indicated a reduction in autophagy upon ARVd treatment.
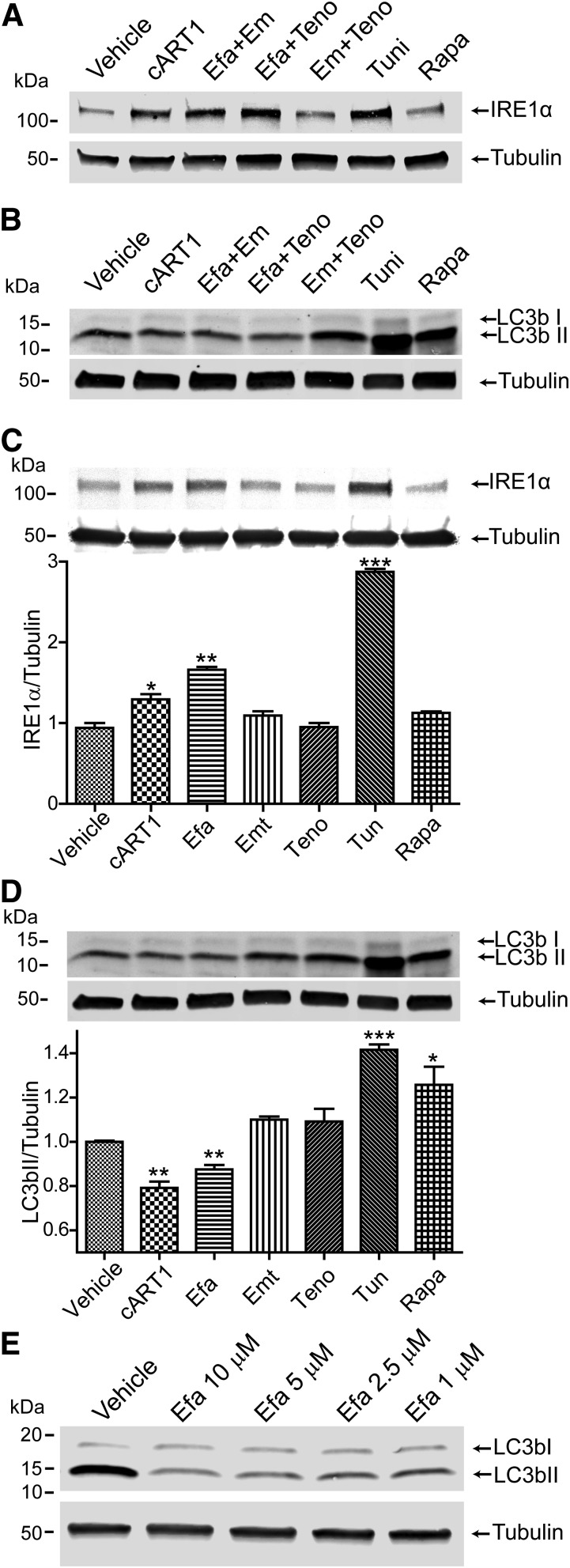
Cells were exposed to the ARVds composing cART1 individually or in pairs and tested for the induction of ER stress by evaluation of IRE1α expression levels and for autophagy by determination of the LC3bII/tubulin ratio. Only the pairs of drugs containing efavirenz (Fig. 3, A and B) or efavirenz alone (Fig. 3, C and D) mimicked the effects of cART1 by inducing ER stress and, at the same time, reducing autophagy. Since these observations are contrary to those reported in other publications, we analyzed LC3b expression in response to different concentration of efavirenz. We observed that with concentrations as low as 1 µM a reduction in LC3b expression and LC3bI/II conversion was still observed (Fig. 3E).
Fig. 3.
Efavirenz is responsible for ER stress induction and autophagy reduction. Brain endothelial cells were exposed to vehicle (DMSO), cART1, tandems of drugs composing cART1 (efavirenz, emtricitabine, and/or tenofovir), tunicamycin, or rapamycin for 48 hours. (A) Levels of IRE1α and (B) LC3b levels were analyzed by immunoblotting. (C) Immunoblotting for IRE1α in cells exposed to individual drugs composing cART1. Levels were normalized to tubulin expression. (D) Quantification of LC3b to tubulin ratio under the same conditions as in (C). (E) Western blot for LC3b in endothelial cells treated with different concentrations of efavirenz. The data are mean ± S.E.M., n = 3. *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle. Efa, efavirenz; EM/Emt, emtricitabine; Teno, tenofovir; Tuni, tunicamycin; Rapa, rapamycin.
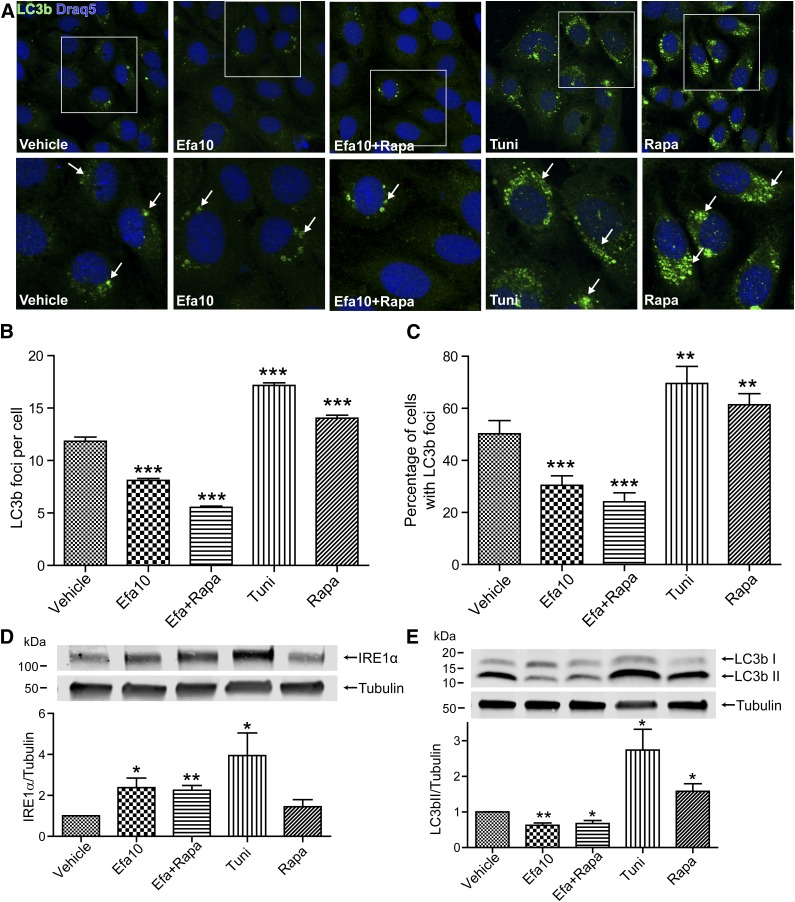
To confirm these results, we next analyzed and quantified LC3b foci formation in cells exposed to efavirenz (10 µM) or control treatments. The analyses were performed using foci picker 3D, a plug-in for the ImageJ software. Using this method, we demonstrated a significant reduction in both the numbers of foci (Fig. 4, A and B) per cell and the percentage of cells with foci when exposed to efavirenz compared with vehicle (Fig. 4, A and C). In addition, coexposure to rapamycin did not affect the efavirenz-induced stimulation of ER (measured by IRE1α immunoblotting) or reduction of autophagy (measured by LC3b foci or by immunoblotting) (Figs. 4, A–E).
Fig. 4.
Quantification of efavirenz-induced autophagy disruption. (A) Immunostaining for LC3b in brain endothelial cells exposed to the indicated drugs for 48 hours, lower images are enlarged fragments of the upper images indicated by the white squares. Arrows denote examples of LC3b immunoreactive foci. (B) Quantification of LC3b foci formation per cell and (C) the percentage of cells with LC3b-positive foci. (D) Coexposure to rapamycin does not protect against efavirenz-induced ER stress and (E) autophagic disruption. The data are mean ± S.E.M.; blots, n = 3 or 4; foci quantification, n = at least 200 cells/group. *P < 0.05 ; **P < 0.01 ; ***P < 0.001 versus DMSO. Efa, efavirenz; Rapa, rapamycin; Tuni, tunicamycin.
Efavirenz Dysregulates ER Stress Activity and Autophagy in Other BBB Cells and in Brain Microvessels of HIV Transgenic Mice.
To further support the observation that treatment with efavirenz-mediated dysfunction of autophagic processes at the BBB level, studies were performed using two additional BBB cell types, namely, primary human brain microvascular endothelial cells (PHBME) and an astrocytic cell line, SVG P12. Exposure to efavirenz (10 µM) markedly elevated levels of ER resident protein IRE1α in both PHBME (Fig. 5A) and SVG P12 cells (Fig. 5C). In addition, a slight but significant reduction in autophagic activity was observed in both cell types as measured with Cyto-ID detection dye (Fig. 5, B and D). In PHBME cells, the expression of LC3bI and LC3BII was not altered (Fig. 5A); however, a prominent decrease in the expression and conversion of LC3bI to LC3bII was observed in SVG P12 cells (Fig. 5C).
Fig. 5.
Confirmation of efavirenz-induced dysregulation of ER stress and autophagy responses in BBB cells. Primary human brain endothelial cells were exposed to efavirenz, vehicle (DMSO), and rapamycin (positive control), followed by immunoblotting for IRE1α levels and LC3b expression (A) and Cyto-ID detection for autophagosome formation (B). Similar analyses of IRE1α levels, LC3bI/LC3bII conversion and total expression (C) and Cyto-ID detection (D) in SVGP12 cells treated as in (A and B). Data are mean ± S.E.M.; n = 8. *P < 0.05 versus vehicle; **P < 0.01 versus vehicle; ***P < 0.001 versus vehicle. Efa, efavirenz; Rapa, rapamycin.
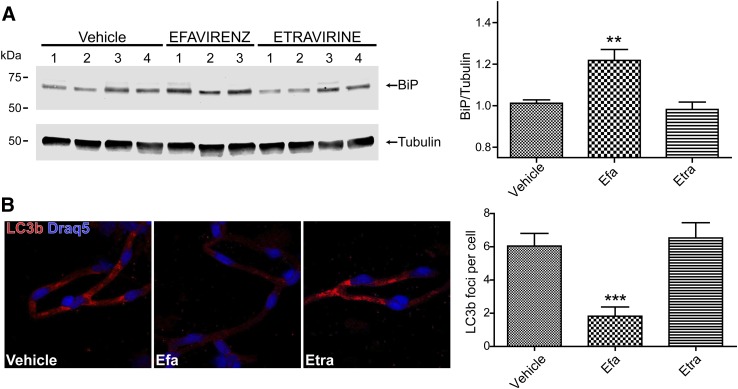
Efavirenz-induced induction of ER stress was also confirmed in brain microvessels of HIV transgenic mice exposed to this drug for 30 days. The treatment resulted in a significant upregulation of ER stress effector protein BiP, confirming the in vitro results. In contrast, similar exposure to etravirine, a second generation non-nucleoside reverse transcriptase inhibitor, did not affect BiP expression levels (Fig. 6A). Importantly, we also detected a significant reduction in LC3b-specific immunoreactivity and the number of foci in brain microvessels of mice treated with efavirenz (Fig. 6B) compared with vehicle or etravirine.
Fig. 6.
Efavirenz induces dysregulation of ER stress and autophagy responses in brain microvessels of HIV transgenic mice. Mice were administered with efavirenz (Efa), etravirine (Etra), or vehicle for 30 days as described in the Materials and Methods. (A) BiP was detected by immunoblotting (left panel) and quantified (right panel). (B) LC3b-positive immunostaining (left panel) was evaluated and quantified for foci formation. Data are mean ± S.E.M.; five mice per group, 18–20 microvessels per mouse. **P < 0.01; ***P < 0.001 versus vehicle.
Efavirenz Does Not Alter Expression of Proteins Bridging ER Stress and Autophagy Pathways.
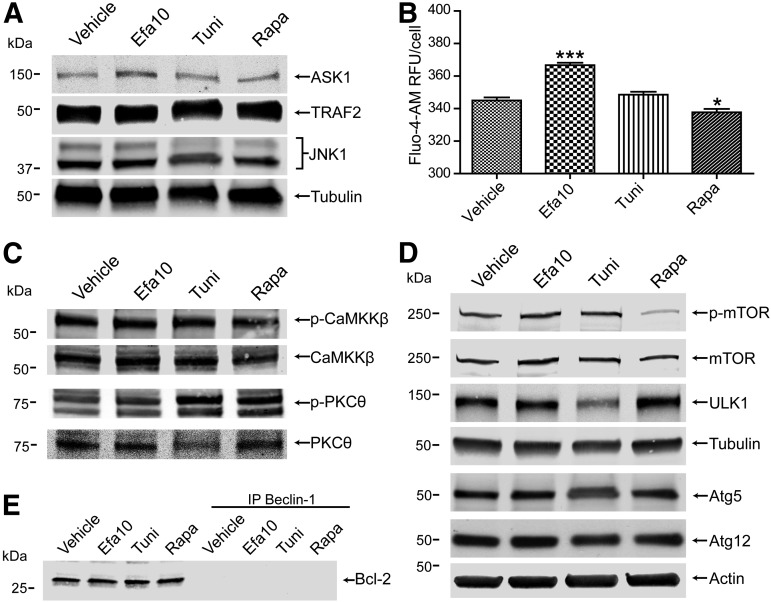
Several proteins and signaling pathways, which can bridge between ER stress and autophagy, were analyzed to identify a mechanism of efavirenz-induced disconnection between ER stress and autophagy. First, we determined expression of the ASK1/TRAF2 complex since this complex is activated by IREα and efavirenz induced a significant increase in IREα expression. Surprisingly, treatment with efavirenz did not alter expression of TRAF2 and slightly increased ASK1 expression compared with vehicle (Fig. 7A). Similarly, no change in JNK expression, a kinase responsible for the phosphorylation of Bcl-2, was observed (Fig. 7A). A common trigger of ER stress and autophagy induction is the alteration of intracellular Ca2+ levels. Consistent with observations by others (Apostolova et al., 2013), a significant increase in cytoplasmic calcium levels after treatment with efavirenz was detected (Fig. 7B); however, calcium-induced common mediators of autophagy, such as phosphorylation of PKCɵ or CaMKKβ, were not affected by efavirenz exposure (Fig. 7C).
Fig. 7.
Efavirenz does not affect expression of proteins of the autophagy pathway but increases intracellular calcium. Cells were exposed to efavirenz, tunicamycin, and rapamycin. (A) Immunoblot for the expression levels of proteins associated with IRE1α-dependent induction of autophagy, ASK1, TRAF2, and JNK. (B) Detection of intracellular calcium level using Fluo-4-AM. (C) Immunoblot depicting the expression of calcium-dependent CaMKKβ and PKCɵ and their phosphorylated forms (p-CaMKKβ and p-PKCɵ) levels. (D) Expression of proteins implicated in the autophagic pathway, mTOR, phosphorylated mTOR (p-mTOR), ULK1, Atg5, and Atg12. Actin and tubulin were detected as house-keeping proteins. (E) Immunoblot analysis of Bcl-2 expression in lysates and in beclin-1 immunoprecipitated (IP) samples. Data are mean ± S.E.M., n = 16. *P < 0.05; ***P < 0.001 versus vehicle. Efa, efavirenz; Rapa, rapamycin; Tuni, tunicamycin.
We next evaluated expression or activation of several proteins implicated in the autophagy pathway. Mammalian target of rapamycin (mTOR) is a central cell-growth regulator that integrates growth factor and nutrient signals. mTOR phosphorylation was reduced in cells exposed to rapamycin, providing a stimulus for autophagy induction; however, no apparent changes in mTOR levels or its phosphorylation were observed in cells exposed to efavirenz. In addition, treatment with efavirenz did not affect expression levels of several downstream autophagy effector proteins, such as ULK1, or Atg5 and Atg12, which are implicated in the formation of the autophagic vesicle (Fig. 7D). We also did not observe any changes in coimmunoprecipitation of Bcl-2 with Beclin-1, which could have an inhibitory effect on autophagy progression (Fig. 7E). Overall, these results indicate that reduction of autophagic activity by efavirenz is not due to decreased expression of proteins involved in the induction of autophagy or the signaling pathways which bridge autophagy with ER stress.
Efavirenz Inhibits Autophagy at the Vesicle Nucleation Stage.
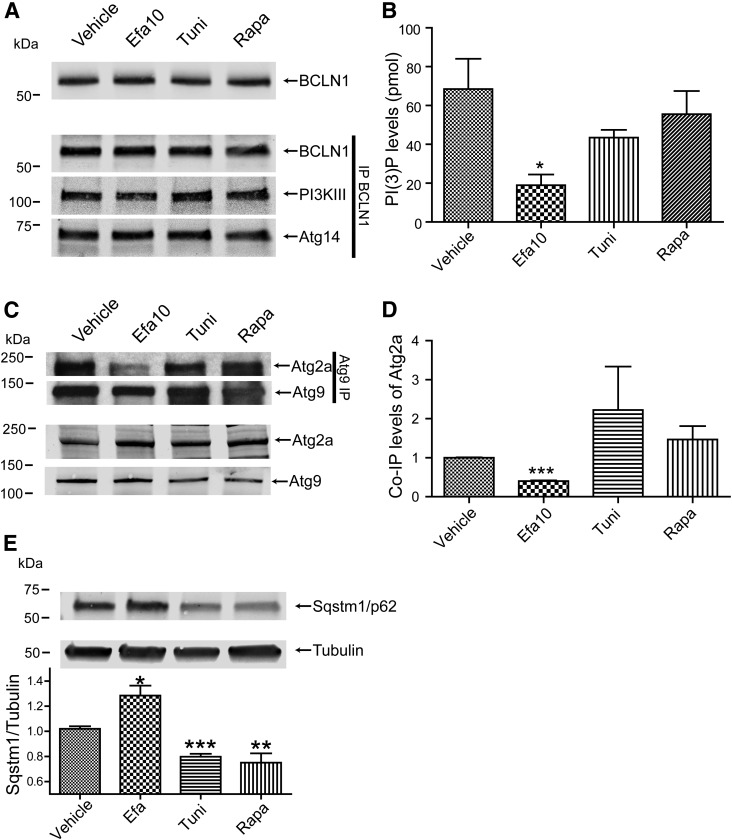
The lack of changes in the expression of the mediators of autophagic activity suggested that efavirenz-induced dysregulation of autophagy may be related to a blockage in the formation of autophagic vesicles. Therefore, we evaluated the formation of the complex composed of Beclin-1, Atg14, and PI3KIII, which is implicated in the membrane nucleation stage of autophagy. As indicated in Fig. 8A, the complex was formed properly under all treatment conditions as PI3KIII and Atg14 coimmunoprecipitated with Beclin-1.
Fig. 8.
Efavirenz inhibits autophagosome nucleation events and leads to the accumulation of SQSTM1/p62. Cells were exposed to the indicated drugs for 48 hours. (A) Analysis of the formation of the Beclin-1 complex implicated in the progression of autophagy. Top image represents input levels of Beclin-1 as determined by immunoblotting, whereas the bottom three images represent immunoprecipitation (IP) of Beclin-1 and coimmunoprecipitation of PI3 kinase class III and Atg14. (B) Cytosolic PI3P levels are decreased in efavirenz-treated cultures. (C) Association of Atg2a with Atg9 is decreased by efavirenz. Top images represent coimmunoprecipitation of Atg2a with Atg9. The lower images represent levels of Atg2a and Atg9 in cell lysates as determined by immunoblotting. (D) Quantification of Atg2a coimmunoprecipated with Atg9 shown in (C). (E) Accumulation of SQSTM1/p62 levels in efavirenz-treated cultures as determined by immunoblotting. Graph represents quantification of SQSTM1/p62 normalized to tubulin levels. Data are mean ± S.E.M., n = 3–5. *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle. BCLN1, Beclin-1; Efa, efavirenz; Rapa, rapamycin; Tuni: tunicamycin.
Because the Beclin-1/Atg14/PI3KIII complex controls synthesis of PI3P, which is implicated in the formation of the autophagosome, we next measured cellular levels of PI3P. PI3P levels were significantly reduced in cells treated with efavirenz compared with all other conditions (Fig. 8B), indicating a blockage which may be responsible for decreased autophagy upon treatment with this drug. To confirm these findings, the expression levels and interaction of Atg9 and Atg2a were analyzed, as these proteins are recruited in response to the PI3P synthesis. In line with previous results, the expression of both proteins remained unchanged in efavirenz-exposed cells; however, the association of Atg2a to Atg9 was reduced (Fig. 8, C and D). We then extended these analyses to evaluate the levels of sequestosome 1 (SQSTM1)/p62, a protein that is consumed during autophagy. Significant accumulation of SQSTM1/p62 in brain endothelial cells treated with efavirenz was significant, indicating an inhibition in the autophagy pathway (Fig. 8E). These results clearly indicate that exposure to efavirenz blocks autophagy progression by inhibiting the Beclin-1/Atg14/PI3KIII complex activity to synthesize PI3P, preventing the recruitment of the downstream proteins and the nucleation of autophagic membranes.
Discussion
The benefits resulting from the use of ARVds in the treatment of HIV are undeniable. They limit the progression of the disease and enable infected patients to survive while restricting the infection. With prolonged survival, however, these patients develop a variety of comorbidities, which are frequently linked to drug toxicity (Gakhar et al., 2013). Despite control of the infection, the virus is never fully suppressed, and circulating HIV proteins are present, albeit at low concentrations. This continuous exposure can lead to complications since some HIV proteins, such as Tat, can induce cellular stress, as well as inflammatory and oxidative responses (Toborek et al., 2003). Furthermore, the surviving patients are aging, making them more susceptible to ARVd toxicity and placing them at a higher risk of developing neurologic problems. These facts highlight the importance of evaluating and preventing the toxicity of ARVds, especially because patients are likely to be on these medications for the rest of their lives.
In the present study, we focused on two combinations of ARVds: efavirenz, tenofovir, and emtricitabine (referred herein as cART1) and atazanavir, ritonavir, emtricitabine, and tenofovir (referred to as cART2). Both cART1 and cART2 are drug combinations that are recommended as the initial regimens for antiretroviral-naïve patients (http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf ). In addition, cART1 components constitute the single-tablet regimen (Atripla). The drugs evaluated in this study were from different categories of ARVds, namely, NNRTIs (efavirenz and etravirine), nucleoside reverse transcriptase inhibitors (tenofovir and emtricitabine), and protease inhibitors (atazanavir and ritonavir).
Potential side effects of ARVds include hyperbilirubinemia, lipodystrophy, diarrhea, hypersensitivity, lactic acidosis, peripheral neuropathy, neuropsychiatric disorders, and pancreatitis (Blas-Garcia et al., 2011; Gutierrez et al., 2011; Domingo et al., 2012; Gakhar et al., 2013). Several reports also demonstrated hepatotoxicity and the blockage of proteasome activity by HIV protease inhibitors (Liang et al., 2001; Carr, 2003). In addition, secretion of proinflammatory cytokines (Diaz-Delfin et al., 2011) and neurotoxicity has been associated with the use of NNRTI, namely, efavirenz and nevirapine (Decloedt and Maartens, 2013). The neurotoxic impact of these drugs may be mediated, at least in part, by inhibition of creatine kinase and cytochrome C complex IV activity in the brain (Streck et al., 2008, 2011), mitochondria dysfunction (Purnell and Fox, 2014), or iNOS upregulation (Apostolova et al., 2014). Our studies focused on the toxicity of these drugs to brain endothelial cells as these cells are the main component of the BBB and play a critical role in the protection of the brain against the development of neurodegenerative disorders. Mechanistically, we focused on the induction of ER stress and autophagic responses.
ER stress is involved in maintaining cellular homeostasis and resolving dysregulation in protein folding, calcium and redox imbalance, and glucose deprivation. Furthermore, given its role in monitoring protein folding, the process is often activated after viral infection. Proper indication of ER stress responses induces expression of protein chaperones and enhances the degradation of ER-associated proteins (Xu et al., 2005). On the other hand, aberrant ER stress may lead to cell death and apoptosis. ER stress is induced by at least three signaling pathways, initiated by PERK, IRE1α, and ATF-6 (Chambers and Marciniak, 2014). Therefore, these pathways were evaluated in our model systems exposed to different cART1 and cART2 drug combination. IRE1α and PERK, but not ATF-6, were induced by cART1, with the subsequent studies indicating the role of efavirenz in this process. Induction of ER stress was associated with increased expression of ER resident chaperones BiP and CHOP. Efavirenz-induced ER stress was initiated as early as 6 hours after exposure and lasted as long as 96 hours (unpublished data). In addition, it was reversible upon removing efavirenz from the culture media (unpublished data).
Novel observations of the present study indicate that exposure to efavirenz (alone or in combination; concentration range, 1–20 µM) results in induction of ER stress and reduced autophagic activity in BBB cells, namely, in brain endothelial cells and astroglia. These findings were unexpected because induction of ER stress is usually associated with an increase in autophagic activity, as there are several mediators joining the two pathways. Therefore, it was surprising that treatment with efavirenz resulted in a decrease in the autophagic activity of BBB cells, as demonstrated by the reduced cleavage of LC3b, and a drastic reduction in the formation of LC3b foci, even with the addition of rapamycin. Our results also contrast with literature reports in which efavirenz was demonstrated to induce autophagy in hepatocytes, neurons, and human umbilical vein endothelial cells (Apostolova et al., 2011, 2013; Purnell and Fox, 2014; Weiβ et al., 2015). This discrepancy indicates a highly unique response of the BBB cells to this drug and may indicate specific toxicity of efavirenz toward the cerebrovascular system. Indeed, while some literature reports are based on treatment with efavirenz of up to 50 µM (Apostolova et al., 2011, 2013), we observed significant toxicity of this drug at 20 µM and 100% cell death at 30 µM after a 24-hour exposure of primary human brain microvessels endothelial cells (unpublished data). These individual responses and variable levels of sensitivity to efavirenz-induced toxicity may be related to the mitochondrial profile in different cell types since efavirenz affects mitochondrial functions. Whereas our in vitro experiments were conducted in basal media, which may affect drug toxicity, efavirenz-induced ER stress and reduced autophagy activity were also observed in brain microvessels of HIV transgenic mice chronically administered with a physiologically relevant dose of this drug, further demonstrating the relevance of our findings.
Autophagy is a protective mechanism of protein recycling and removal of defective proteins and organelles (Boya et al., 2005; Liang, 2010); therefore, a decrease in autophagy activity can potentiate efavirenz toxicity. As the mechanism of dysregulation of ER stress and autophagy, we determined that the complex composed of Beclin-1/Atg14/PI3KIII is deficient in the synthesis of PI3P in cells exposed to efavirenz. This deficiency results in a reduced efficiency in the nucleation step of the autophagy pathway, as demonstrated by the reduction in Atg9/Atg2a association and accumulation of protein SQSTM1/p62.
In line with several studies demonstrating that alterations of calcium metabolism can induce autophagy (Decuypere et al., 2011, 2013; Ghislat et al., 2012), we also considered this mechanism in the present study. Although an increase in cytoplasmic calcium levels was observed in brain endothelial cells exposed to efavirenz, this increase did not result in stimulation of autophagy. This apparent disconnection is consistent with the fact that efavirenz increases calcium levels by depletion of mitochondrial or ER calcium stores (Apostolova et al., 2013), which can inhibit autophagy (Gordon et al., 1993; Engedal et al., 2013). Indeed, several calcium-related mediators which may affect autophagy responses, such as CaMKKβ (Xi et al., 2013) or PKCɵ (Zhang et al., 2009), were not significantly affected by efavirenz exposure.
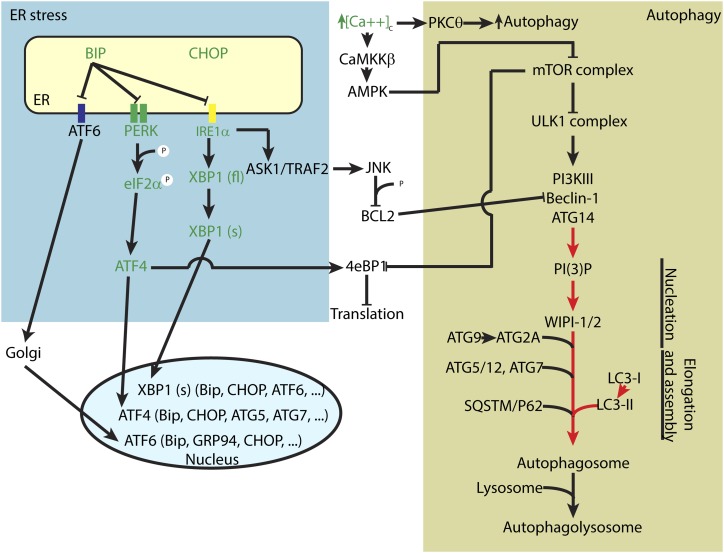
In summary, our findings demonstrate that exposure to efavirenz, but not to other common ARVds, induces ER stress responses but decreases autophagic activity in BBB cells and brain microvessels (Fig. 9). This dysregulation of ER stress and autophagic pathways is associated with a reduced PI3P synthesis activity. The observed changes may contribute to the dysfunction of brain endothelial cells, contributing to cerebrovascular toxicity frequently observed in HIV-infected patients.
Fig. 9.
Summary of the input of efavirenz on ER stress and autophagy signaling in brain endothelial cells. Green depicts an increase in expression or activation. Red represents inhibition of activity or processing.
Acknowledgments
The authors thank Gretchen Wolff and Jagoda Wrobel for their help in animal breeding and microvessel isolation.
Abbreviations
- ARVd
antiretroviral drugs
- BBB
blood-brain barrier
- cART
combined antiretroviral therapy
- CSC
Cell Systems Corporation
- DMSO
dimethylsulfoxide
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- HIV
human immunodeficiency virus
- NNRTI
non-nucleoside reverse transcriptase inhibitors
- PHBME
primary human brain microvessel endothelial cell
- PI3P
phosphatidylinositol 3-phosphate
Authorship Contributions
Participated in research design: Bertrand, Toborek.
Conducted experiments: Bertrand.
Performed data analysis: Bertrand, Toborek.
Wrote or contributed to the writing of the manuscript: Bertrand, Toborek.
Footnotes
This work was supported by the National Institutes of Health [Grants R01-MH098891, R01-MH072567, R01-MH063022, R01-HL126559, R01-DA039576, and R01-DA027569].
References
- András IE, Toborek M. (2013) Amyloid beta accumulation in HIV-1-infected brain: the role of the blood brain barrier. IUBMB Life 65:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova N, Funes HA, Blas-Garcia A, Alegre F, Polo M, Esplugues JV. (2014) Involvement of nitric oxide in the mitochondrial action of efavirenz: a differential effect on neurons and glial cells. J Infect Dis 211:1953–1958 DOI: 10.1093/infdis/jiu825. [DOI] [PubMed] [Google Scholar]
- Apostolova N, Gomez-Sucerquia LJ, Alegre F, Funes HA, Victor VM, Barrachina MD, Blas-Garcia A, Esplugues JV. (2013) ER stress in human hepatic cells treated with efavirenz: mitochondria again. J Hepatol 59:780–789. [DOI] [PubMed] [Google Scholar]
- Apostolova N, Gomez-Sucerquia LJ, Gortat A, Blas-Garcia A, Esplugues JV. (2011) Compromising mitochondrial function with the antiretroviral drug efavirenz induces cell survival-promoting autophagy. Hepatology 54:1009–1019. [DOI] [PubMed] [Google Scholar]
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ. (2004) Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 18 (Suppl 1):S11–S18. [PubMed] [Google Scholar]
- Blas-García A, Apostolova N, Ballesteros D, Monleón D, Morales JM, Rocha M, Victor VM, Esplugues JV. (2010) Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology 52:115–125. [DOI] [PubMed] [Google Scholar]
- Blas-Garcia A, Esplugues JV, Apostolova N. (2011) Twenty years of HIV-1 non-nucleoside reverse transcriptase inhibitors: time to reevaluate their toxicity. Curr Med Chem 18:2186–2195. [DOI] [PubMed] [Google Scholar]
- Boffito M, Jackson A, Amara A, Back D, Khoo S, Higgs C, Seymour N, Gazzard B, Moyle G. (2011) Pharmacokinetics of once-daily darunavir-ritonavir and atazanavir-ritonavir over 72 hours following drug cessation. Antimicrob Agents Chemother 55:4218–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann FM. (2013) Telomerase inhibition may contribute to accelerated mitochondrial aging induced by anti-retroviral HIV treatment. Med Hypotheses 81:285–287. [DOI] [PubMed] [Google Scholar]
- Boven LA, Middel J, Verhoef J, De Groot CJ, Nottet HS. (2000) Monocyte infiltration is highly associated with loss of the tight junction protein zonula occludens in HIV-1-associated dementia. Neuropathol Appl Neurobiol 26:356–360. [DOI] [PubMed] [Google Scholar]
- Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D, Souquere S, Yoshimori T, et al. (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25:1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman K, Smeitink JA, Romijn JA, Reiss P. (1999) Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet 354:1112–1115. [DOI] [PubMed] [Google Scholar]
- Carr A. (2003) Toxicity of antiretroviral therapy and implications for drug development. Nat Rev Drug Discov 2:624–634. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Marciniak SJ. (2014) Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 2. Protein misfolding and ER stress. Am J Physiol Cell Physiol 307:C657–C670. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Ances BM. (2013) HIV-associated neurocognitive disorder. Lancet Infect Dis 13:976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, et al. (2010) IKK connects autophagy to major stress pathways. Autophagy 6:189–191. [DOI] [PubMed] [Google Scholar]
- Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. (1999) Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol 155:1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decloedt EH, Maartens G. (2013) Neuronal toxicity of efavirenz: a systematic review. Expert Opin Drug Saf 12:841–846. [DOI] [PubMed] [Google Scholar]
- Decuypere JP, Kindt D, Luyten T, Welkenhuyzen K, Missiaen L, De Smedt H, Bultynck G, Parys JB. (2013) mTOR-controlled autophagy requires intracellular Ca(2+) signaling. PLoS ONE 8:e61020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere JP, Welkenhuyzen K, Luyten T, Ponsaerts R, Dewaele M, Molgó J, Agostinis P, Missiaen L, De Smedt H, Parys JB, et al. (2011) Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy 7:1472–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Havlir DV. (2013) The end of AIDS: HIV infection as a chronic disease. Lancet 382:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Delfín J, del Mar Gutiérrez M, Gallego-Escuredo JM, Domingo JC, Gracia Mateo M, Villarroya F, Domingo P, Giralt M. (2011) Effects of nevirapine and efavirenz on human adipocyte differentiation, gene expression, and release of adipokines and cytokines. Antiviral Res 91:112–119. [DOI] [PubMed] [Google Scholar]
- Domingo P, Estrada V, López-Aldeguer J, Villaroya F, Martínez E. (2012) Fat redistribution syndromes associated with HIV-1 infection and combination antiretroviral therapy. AIDS Rev 14:112–123. [PubMed] [Google Scholar]
- Droste JA, Verweij-van Wissen CP, Kearney BP, Buffels R, Vanhorssen PJ, Hekster YA, Burger DM. (2005) Pharmacokinetic study of tenofovir disoproxil fumarate combined with rifampin in healthy volunteers. Antimicrob Agents Chemother 49:680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engedal N, Torgersen ML, Guldvik IJ, Barfeld SJ, Bakula D, Sætre F, Hagen LK, Patterson JB, Proikas-Cezanne T, Seglen PO, et al. (2013) Modulation of intracellular calcium homeostasis blocks autophagosome formation. Autophagy 9:1475–1490. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Banks WA. (2013) Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab 33:1500–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 26:1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakhar H, Kamali A, Holodniy M. (2013) Health-related quality of life assessment after antiretroviral therapy: a review of the literature. Drugs 73:651–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galescu O, Bhangoo A, Ten S. (2013) Insulin resistance, lipodystrophy and cardiometabolic syndrome in HIV/AIDS. Rev Endocr Metab Disord 14:133–140. [DOI] [PubMed] [Google Scholar]
- Gallego-Escuredo JM, Del Mar Gutierrez M, Diaz-Delfin J, Domingo JC, Mateo MG, Domingo P, Giralt M, Villarroya F. (2010) Differential effects of efavirenz and lopinavir/ritonavir on human adipocyte differentiation, gene expression and release of adipokines and pro-inflammatory cytokines. Curr HIV Res 8:545–553. [DOI] [PubMed] [Google Scholar]
- Ghislat G, Patron M, Rizzuto R, Knecht E. (2012) Withdrawal of essential amino acids increases autophagy by a pathway involving Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β). J Biol Chem 287:38625–38636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta B, Ehrhart J, Obregon DF, Lam L, Le L, Jin J, Fernandez F, Tan J, Shytle RD. (2011) Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol Brain 4:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PB, Holen I, Fosse M, Røtnes JS, Seglen PO. (1993) Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J Biol Chem 268:26107–26112. [PubMed] [Google Scholar]
- Grammas P, Martinez J, Miller B. (2011) Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med 13:e19. [DOI] [PubMed] [Google Scholar]
- Gutierrez MdelM, Mateo MG, Vidal F, Domingo P. (2011) The toxicogenetics of antiretroviral therapy: the evil inside. Curr Med Chem 18:209–219. [DOI] [PubMed] [Google Scholar]
- Hecht M, Harrer T, Büttner M, Schwegler M, Erber S, Fietkau R, Distel LV. (2013) Cytotoxic effect of efavirenz is selective against cancer cells and associated with the cannabinoid system. AIDS 27:2031–2040. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Berman JW, Lyman WD. (1994) The role of the blood-brain barrier in HIV infection of the central nervous system. Adv Neuroimmunol 4:249–256. [DOI] [PubMed] [Google Scholar]
- Intelence. (2014) Package insert. Janssen Therapeutics, Titusville, NJ.
- Ivey NS, MacLean AG, Lackner AA. (2009) Acquired immunodeficiency syndrome and the blood-brain barrier. J Neurovirol 15:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MR, Kanazawa T, Daigaku Y, Fujimura S, Miotto G, Kadowaki M. (2007) Cytosolic LC3 ratio as a sensitive index of macroautophagy in isolated rat hepatocytes and H4-II-E cells. Autophagy 3:553–560. [DOI] [PubMed] [Google Scholar]
- Kebodeaux CD, Wilson AG, Smith DL, Vouri SM. (2013) A review of cardiovascular and renal function monitoring: a consideration of older adults with HIV. HIV AIDS (Auckl) 5:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, Soiza RL. (2013) Evidence of endothelial dysfunction in the development of Alzheimer’s disease: Is Alzheimer’s a vascular disorder? Am J Cardiovasc Dis 3:197–226. [PMC free article] [PubMed] [Google Scholar]
- Kitamura M, Hiramatsu N. (2011) Real-time monitoring of ER stress in living cells and animals using ESTRAP assay. Methods Enzymol 490:93–106. [DOI] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH. (2005) Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57:176–179. [DOI] [PubMed] [Google Scholar]
- Lake JE, Currier JS. (2013) Metabolic disease in HIV infection. Lancet Infect Dis 13:964–975. [DOI] [PubMed] [Google Scholar]
- Larochelle C, Alvarez JI, Prat A. (2011) How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett 585:3770–3780. [DOI] [PubMed] [Google Scholar]
- Liang C. (2010) Negative regulation of autophagy. Cell Death Differ 17:1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JS, Distler O, Cooper DA, Jamil H, Deckelbaum RJ, Ginsberg HN, Sturley SL. (2001) HIV protease inhibitors protect apolipoprotein B from degradation by the proteasome: a potential mechanism for protease inhibitor-induced hyperlipidemia. Nat Med 7:1327–1331. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS. (2010) HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol 69:801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda KR, Banerjee A, Banks WA, Ercal N. (2011) Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free Radic Biol Med 50:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. (2001) Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71–75. [DOI] [PubMed] [Google Scholar]
- Mishra R, Singh SK. (2014) HIV-1 Tat C phosphorylates VE-cadherin complex and increases human brain microvascular endothelial cell permeability. BMC Neurosci 15:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Castro V, Toborek M. (2012) Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J Cell Mol Med 16:2950–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardacci R, Amendola A, Ciccosanti F, Corazzari M, Esposito V, Vlassi C, Taibi C, Fimia GM, Del Nonno F, Ippolito G, et al. (2014) Autophagy plays an important role in the containment of HIV-1 in nonprogressor-infected patients. Autophagy 10:1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A, Calmy A, Delhumeau C, Mercier IK, Cavassini M, Fayet-Mello A, Elzi L, Genné D, Rauch A, Bernasconi E, et al. Swiss HIV Cohort Study (2011) A randomized crossover study to compare efavirenz and etravirine treatment. AIDS 25:57–63. [DOI] [PubMed] [Google Scholar]
- Overton ET, Arathoon E, Baraldi E, Tomaka F. (2012) Effect of darunavir on lipid profile in HIV-infected patients. HIV Clin Trials 13:256–270. [DOI] [PubMed] [Google Scholar]
- Park M, Kim HJ, Lim B, Wylegala A, Toborek M. (2013) Methamphetamine-induced occludin endocytosis is mediated by the Arp2/3 complex-regulated actin rearrangement. J Biol Chem 288:33324–33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinini M, Rinaudo MT, Anselmino A, Buccinnà B, Ramondetti C, Dematteis A, Ricotti E, Palmisano L, Mostert M, Tovo PA. (2005) The HIV protease inhibitors nelfinavir and saquinavir, but not a variety of HIV reverse transcriptase inhibitors, adversely affect human proteasome function. Antivir Ther 10:215–223. [PubMed] [Google Scholar]
- Proikas-Cezanne T, Pfisterer SG. (2009) Assessing mammalian autophagy by WIPI-1/Atg18 puncta formation. Methods Enzymol 452:247–260. [DOI] [PubMed] [Google Scholar]
- Purnell PR, Fox HS. (2014) Efavirenz induces neuronal autophagy and mitochondrial alterations. J Pharmacol Exp Ther 351:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wang Z, Tao L, Wang Y. (2010) ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 6:239–247. [DOI] [PubMed] [Google Scholar]
- Rodrigues MC, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, Sanberg PR, Voltarelli JC, Garbuzova-Davis S. (2012) Neurovascular aspects of amyotrophic lateral sclerosis. Int Rev Neurobiol 102:91–106. [DOI] [PubMed] [Google Scholar]
- Seelbach M, Chen L, Powell A, Choi YJ, Zhang B, Hennig B, Toborek M. (2010) Polychlorinated biphenyls disrupt blood-brain barrier integrity and promote brain metastasis formation. Environ Health Perspect 118:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhle L, Moberg L, Svensson JO, Sönnerborg A. (2004) Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit 26:267–270. [DOI] [PubMed] [Google Scholar]
- Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. (2011) Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res 1399:96–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streck EL, Ferreira GK, Scaini G, Rezin GT, Gonçalves CL, Jeremias IC, Zugno AI, Ferreira GC, Moreira J, Fochesato CM, et al. (2011) Non-nucleoside reverse transcriptase inhibitors efavirenz and nevirapine inhibit cytochrome C oxidase in mouse brain regions. Neurochem Res 36:962–966. [DOI] [PubMed] [Google Scholar]
- Streck EL, Scaini G, Rezin GT, Moreira J, Fochesato CM, Romão PR. (2008) Effects of the HIV treatment drugs nevirapine and efavirenz on brain creatine kinase activity. Metab Brain Dis 23:485–492. [DOI] [PubMed] [Google Scholar]
- Sustiva. (2015) Package insert. Bristol-Myers Squibb, Atlanta, GA.
- Toborek M, Lee YW, Pu H, Malecki A, Flora G, Garrido R, Hennig B, Bauer HC, Nath A. (2003) HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J Neurochem 84:169–179. [DOI] [PubMed] [Google Scholar]
- Valade E, Tréluyer JM, Bouazza N, Ghosn J, Foissac F, Benaboud S, Fauchet F, Viard JP, Urien S, Hirt D. (2014) Population pharmacokinetics of emtricitabine in HIV-1-infected adult patients. Antimicrob Agents Chemother 58:2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE. (2010) Aging with HIV: clinical considerations for an emerging population. Am J Nurs 110:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß M, Kost B, Renner-Müller I, Wolf E, Mylonas I, Brüning A. (2015) Efavirenz causes oxidative stress, endoplasmic reticulum stress, and autophagy in endothelial cells. Cardiovasc Toxicol DOI: 10.1007/s12012-015-9314-2. [DOI] [PubMed] [Google Scholar]
- Weksler B, Romero IA, Couraud PO. (2013) The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 10:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi H, Barredo JC, Merchan JR, Lampidis TJ. (2013) Endoplasmic reticulum stress induced by 2-deoxyglucose but not glucose starvation activates AMPK through CaMKKβ leading to autophagy. Biochem Pharmacol 85:1463–1477. [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wu Y, Tashiro S, Onodera S, Ikejima T. (2009) Involvement of PKC signal pathways in oridonin-induced autophagy in HeLa cells: a protective mechanism against apoptosis. Biochem Biophys Res Commun 378:273–278. [DOI] [PubMed] [Google Scholar]
- Zhou D, Masliah E, Spector SA. (2011) Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J Infect Dis 203:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201. [DOI] [PubMed] [Google Scholar]