Abstract
In the present study, we have elucidated the functional characteristics and mechanism of action of methaqualone (2-methyl-3-o-tolyl-4(3H)-quinazolinone, Quaalude), an infamous sedative-hypnotic and recreational drug from the 1960s–1970s. Methaqualone was demonstrated to be a positive allosteric modulator at human α1,2,3,5β2,3γ2S GABAA receptors (GABAARs) expressed in Xenopus oocytes, whereas it displayed highly diverse functionalities at the α4,6β1,2,3δ GABAAR subtypes, ranging from inactivity (α4β1δ), through negative (α6β1δ) or positive allosteric modulation (α4β2δ, α6β2,3δ), to superagonism (α4β3δ). Methaqualone did not interact with the benzodiazepine, barbiturate, or neurosteroid binding sites in the GABAAR. Instead, the compound is proposed to act through the transmembrane β(+)/α(–) subunit interface of the receptor, possibly targeting a site overlapping with that of the general anesthetic etomidate. The negligible activities displayed by methaqualone at numerous neurotransmitter receptors and transporters in an elaborate screening for additional putative central nervous system (CNS) targets suggest that it is a selective GABAAR modulator. The mode of action of methaqualone was further investigated in multichannel recordings from primary frontal cortex networks, where the overall activity changes induced by the compound at 1–100 μM concentrations were quite similar to those mediated by other CNS depressants. Finally, the free methaqualone concentrations in the mouse brain arising from doses producing significant in vivo effects in assays for locomotion and anticonvulsant activity correlated fairly well with its potencies as a modulator at the recombinant GABAARs. Hence, we propose that the multifaceted functional properties exhibited by methaqualone at GABAARs give rise to its effects as a therapeutic and recreational drug.
Introduction
Methaqualone (2-methyl-3-o-tolyl-4(3H)-quinazolinone) has a colorful history as a therapeutic and recreational drug. Methaqualone was marketed in the early 1960s as a nonbarbiturate hypnotic with a wide safety margin and low abuse potential under trade names like Quaalude, Parest, Somnafac, Revonal, and as the combination drug Mandrax (with the antihistamine diphenhydramine). In the subsequent years, methaqualone became one of the best-selling sedative-hypnotic drugs worldwide, with several structural analogs following in its trail (collectively referred to as “quaaludes”) (Carroll and Gallo, 1985; Gass, 2008). However, clinical use of the drug soon revealed that besides giving rise to serious adverse effects, it was highly addictive and induced tolerance and cross-tolerance with other hypnotics. Moreover, concomitantly with its therapeutic use, methaqualone became highly popular as a recreational drug, where it often was consumed in combination with alcohol (known as “luding out”) (Falco, 1976; McCarthy et al., 2005; Gass, 2008; Herzberg, 2011). These problems led to the implementation of tighter regulation of the drug, and by the mid-1980s, it had been withdrawn from most markets (Carroll and Gallo, 1985; Gass, 2008). Nevertheless, recreational use of illegally produced methaqualone still constitutes a substantial health problem in some parts of the world (Parry et al., 2004; McCarthy et al., 2005).
The overall clinical properties of methaqualone are very characteristic for a sedative-hypnotic drug; however, some of its in vivo effects differ from those induced by classic central nervous system (CNS) depressants. Methaqualone reportedly mediates a rapid induction of a more natural deep sleep that results in less severe dizziness/dullness and headaches in insomnia patients than benzodiazepines and barbiturates (Barcelo, 1961; Ionescu-Pioggia et al., 1988). Furthermore, unlike most sedatives, methaqualone is also quite efficacious as an antispasmodic (Gass, 2008). Finally, the euphoria and aphrodisiac properties constituting some of the major psychologic effects evoked by methaqualone in its recreational use are effects not typically associated with CNS depressants (Falco, 1976; Ionescu-Pioggia et al., 1988; Gass, 2008; Barceloux, 2012). Although the electroencephalographic effects induced by methaqualone in rodent and human brains largely resemble those produced by barbiturates and other CNS depressants, the fact that some qualitative differences have been observed between these drugs in these recordings seems to support the clinical observations (Pfeiffer et al., 1968; Saxena et al., 1977).
Whereas the therapeutic and psychotropic effects of methaqualone arguably have been comprehensively documented, the molecular basis for these effects has never been investigated. Based on the overall similarities between its behavioral effects and those induced by barbiturates and benzodiazepines, methaqualone has been assumed to act through the GABA type A receptors (GABAARs) (Carroll and Gallo, 1985; Gass, 2008). This family of ligand-gated anion channels comprises a plethora of receptors assembled from 19 subunits (α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3), and the complexity of GABAergic neurotransmission largely arises from differential regional and cellular expression of these subtypes (Whiting, 2003; Olsen and Sieghart, 2008; Brickley and Mody, 2012). The pentameric GABAAR complex is typically composed of two α subunits, two β subunits, and a γ or δ subunit; and the receptor comprises numerous allosteric sites through which GABA-evoked signaling can be modulated by various drugs, including barbiturates, benzodiazepines, neurosteroids, and anesthetics (Sieghart, 2015). In contrast to the well established role of GABAARs as the principal mediators of the effects of these drugs, however, the link between methaqualone and GABAergic neurotransmission is founded on strikingly sparse and largely inconclusive experimental data (Müller et al., 1978; Naik et al., 1978; Hicks et al., 1990).
In the present study, methaqualone has been subjected to an elaborate functional characterization at human GABAAR subtypes expressed in Xenopus oocytes, and its molecular mechanism of action at the receptors has been delineated. Furthermore, the functionality of methaqualone at native GABAARs has been elucidated by multiparametric analysis of its electrophysiologic effects at cortical neuron network activity. Finally, the correlation between the functional properties of methaqualone at GABAARs in vitro and its in vivo efficacy in mice models for locomotion and anticonvulsant activity has been investigated.
Materials and Methods
GABA, diazepam, ZnCl2, and chemicals for buffers were obtained from Sigma-Aldrich (St. Louis, MO). Methaqualone (Fig. 1A) was synthesized by the MedChem Department at H. Lundbeck A/S. Pentobarbital and allopregnanolone were purchased from May and Baker (Dagenham, UK) and Merck Chemicals (Nottingham, UK), respectively. Flumazenil and etomidate were purchased from Abcam Biochemicals (Cambridge, UK), and DS2 (4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridine-3-yl benzamide) was obtained from Tocris Cookson (Bristol, UK). Defolliculated stage V or VI oocytes harvested from female Xenopus laevis frogs (using MS222 as anesthetic) were obtained from Lohmann Research Equipment (Castrop-Rauxel, Germany).
Fig. 1.
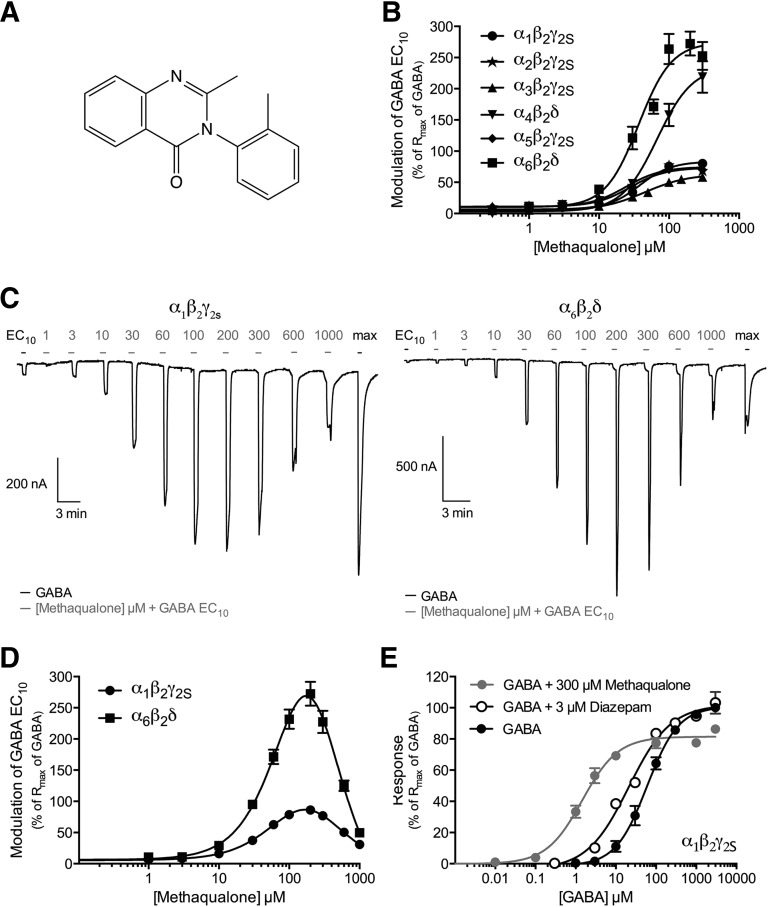
Functional properties of methaqualone at human GABAARs expressed in Xenopus oocytes. (A) Chemical structure of methaqualone. (B) Concentration-response curves for methaqualone at α1β2γ2S, α2β2γ2S, α3β2γ2S, α4β2δ, α5β2γ2S, and α6β2δ GABAARs in the presence of GABA EC10 (means ± S.E.M.; n = 5–9). (C) Representative traces for various concentrations of methaqualone coapplied with GABA EC10 at α1β2γ2S (left) and α6β2δ (right) GABAARs. The application bars in gray for the various methaqualone concentrations all represent a 30-second preincubation with methaqualone followed by coapplication of methaqualone and GABA EC10. The black application bars represent application of GABA EC10 and the GABA concentration eliciting the maximal response. (D) Concentration-response curves for methaqualone at α1β2γ2S and α6β2δ GABAARs in the presence of GABA EC10 (means ± S.E.M.; n = 5–6). (E) Concentration-response curves for GABA at the α1β2γ2S GABAAR in the absence or presence of 3 µM diazepam or 300 µM methaqualone (means ± S.E.M.; n = 4–6).
Molecular Biology
The subcloning of human α1-α6, β1, β2, δ, and γ2S cDNAs into pcDNA3.1 has been described previously (Jensen et al., 2010; Hoestgaard-Jensen et al., 2014), and the human β3 cDNA used in this study was in pGEMHE. Point mutations were introduced into cDNAs using the QuikChange mutagenesis kit (Stratagene, Santa Clara, CA) and oligonucleotides from TAG Copenhagen A/S (Copenhagen, Denmark). The integrity and the absence of unwanted mutations in all cDNAs created by polymerase chain reaction were verified by DNA sequencing (Eurofins MWG Operon, Ebersberg, Germany).
Xenopus laevis Oocytes and Two-Electrode Voltage Clamp Recordings
The functional characterization of methaqualone at wild-type (WT) and mutant GABAARs expressed in Xenopus oocytes was performed essentially as previously described (Hoestgaard-Jensen et al., 2013). The GABAAR cDNAs were linearized and applied as templates for in vitro cRNA synthesis using the T7 mMESSAGE mMACHINE High Yield Capped RNA transcription kit (Life Technologies Corp., Carlsbad, CA. Either 9 or 18 nl cRNA encoding for α1β2 (α1:β2 ratio: 0.06:0.06 µg/µl) and α1,2,3,5β2,3γ2S GABAARs (α:β:γ2S ratio: 0.01:0.01:0.01 µg/μl), or 46 nl cRNA encoding for α6β2 (α6:β2 ratio: 1:0.1 µg/µl) and α4,6β1,2.3δ GABAARs (α:β:δ ratio: 1:0.1:1 µg/μl) were injected into oocytes, which subsequently were incubated at 18°C in modified Barth’s solution [88 mM NaCl, 1 mM KCl, 15 mM HEPES (pH 7.5), 2.4 mM NaHCO3, 0.41 mM CaCl2, 0.82 mM MgSO4, 0.3 mM Ca(NO3)2, 100 U/ml penicillin and 100 μg/ml streptomycin]. Whole-cell currents in the α1β2/α1,2,3,5β2,3γ2S- and α6β2/α4,6β1,2,3δ-expressing oocytes were measured 1–4 and 3–6 days after cRNA injection, respectively. In the two-electrode voltage-clamp (TEVC) recordings, the oocytes were placed in a recording chamber continuously perfused with a saline solution [115 mM NaCl, 2.5 mM KCl, 10 mM HEPES (pH 7.5), 1.8 mM CaCl2, 0.1 mM MgCl2], and the test compounds were applied in the perfusate. Both voltage and current electrodes were agar-plugged with 3 M KCl and displayed resistances between 0.5–2.0 MΩ. Oocytes were voltage-clamped at −40 mV to −80 mV (depending on the current size) using an Oocyte Clamp OC-725C amplifier (Warner Instruments, Hamden, CT). The incorporation of the γ2S subunit into the GABAARs assembled at the cell surface of α1,2,3,5β2,3γ2S-expressing oocytes was confirmed on a routine basis with 100 µM ZnCl2 (Karim et al., 2013), and the presence of δ in cell surface-expressed receptors in α4,6β1,2,3δ-injected oocytes was verified using the δ-GABAAR selective positive allosteric modulator (PAM) DS2 (1 µM) and 1 µM ZnCl2 (Storustovu and Ebert, 2006; Wafford et al., 2009; Karim et al., 2012).
In the experiments where the functional properties of GABA or methaqualone as agonists at the various receptors were characterized, 10 µM GABA was applied to the perfusate until the peak of the response was observed, usually within 30 seconds. When two consecutive applications of GABA had elicited responses of comparable sizes (±5%), various concentrations of GABA or methaqualone were applied. In the experiments where the functional properties of various allosteric modulators were characterized, the GABA concentration to be used (GABA EC10 or GABA EC60–70) was determined on the day of the experiment by measurements on two oocytes expressing the specific receptor. Subsequently, when two consecutive applications of GABA EC10 or GABA EC60–70 were applied to the perfusate and observed to elicit currents of comparable sizes (±5%), the functional characteristics of the allosteric modulators at the GABAAR were determined by preapplication of the modulator to the perfusate 30 seconds before coapplication of the modulator and GABA. In all recordings, a 2.5-minute wash was executed between all applications to prevent receptor desensitization. At the end of each recording on an oocyte, a GABA concentration evoking the maximum response through the specific receptor was applied in the perfusate. Experiments were performed at room temperature, and each data point represents the mean ± S.E.M. value of recordings performed on at least three oocytes from at least two different batches of oocytes.
The recorded baseline-to-peak current amplitudes were analyzed using Clampfit 10.1 (Axon Instruments, Union City, CA), and data for the test compounds were normalized to the maximal response elicited by GABA on each oocyte. Data analysis and statistical analysis were performed using Prism GraphPad, version 6.0a (GraphPad Software, Inc. La Jolla, CA). Concentration-response and concentration-inhibition curves were fitted by nonlinear regression using the equation for sigmoidal dosage-response with variable slope. Comparison of best-fitting equation (monophasic versus biphasic) was carried out using the extra sum-of-squares F test, and the null hypothesis was rejected at P < 0.05. When a biphasic fit was the statistically better model, data were fitted to the equation for a biphasic dose-response curve using nonlinear regression. Unless otherwise stated in the figure legends, statistical analysis was performed using ordinary one-way analysis of variance. The null hypothesis was rejected at P < 0.05, and the differences between the means were analyzed by Dunnett´s multiple comparisons test with a single pooled variance.
In Silico Study
The modeling study was performed using the software package MOE 2013.08 (Molecular Operating Environment, Chemical Computing Group, Montreal, QC, Canada) using the built-in mmff94x force field and the GB/SA continuum solvation model. Etomidate, loreclezole, and methaqualone were submitted to a stochastic conformational search (standard setup) to enumerate low-energy conformations. Structurally collapsed conformations were discarded, and superimposition of selected low-energy conformations (up to ΔΔG = 3 kcal/mol) was done using the built-in function by fitting the carbonyl groups of etomidate and methaqualone and the vinylogous chlorine of loreclezole.
Screening of Methaqualone at Various CNS targets
In vitro binding profiling of methaqualone in competition radiobinding assays at a total of 53 CNS targets were performed by the National Institute of Mental Health’s Psychoactive Drug Screening Program (PDSP). Detailed information about the binding assay protocols is given at http://pdsp.med.unc.edu/pdspw/binding.php. In brief, most of the binding assays were performed to homogenates of mammalian cell lines transiently or stably expressing the different targets, with a few assays being performed using homogenized rat brain tissue. Methaqualone was tested in an assay concentration of 30 μM, and an assay concentration of the radioligand near or at the KD value for the specific target was used. The functional characterization of methaqualone at GABAB receptors and at the α4β2 nicotinic acetylcholine receptor in fluorescence-based Ca2+/Fluo-4 or membrane potential assays and at the human four GABA transporters in a conventional [3H]GABA uptake assay was performed essentially as described previously (Trattnig et al., 2012; Hoestgaard-Jensen et al., 2014).
Functional Phenotypic Characterization of Methaqualone at Neuronal Cell Cultures
The effects of methaqualone at cortical network activity in vitro were characterized essentially as previously described for the opioid ligand LP1 (Parenti et al., 2013).
Primary Cell Cultures.
Frontal cortex tissue was harvested from embryonic day 15/16 chr:NMRI mice (Charles River, Sulzfeld, Germany). The mice were sacrificed by cervical dislocation according to the German Animal Protection Act §4. Tissue was dissociated by enzymatic digestion (133.3 Kunitz U/ml DNase, 10 U/ml papain) and mechanical trituration, counted, vitality-controlled, and plated in Dulbecco’s modified Eagle’s medium containing 5% fetal bovine serum and 5% horse serum on poly-d-lysine– and laminin-coated microelectrode array (MEA) neurochips with 64 passive electrodes (Center for Network NeuroScience, University of North Texas, Denton, TX). Cultures on the MEA chips were incubated at 37°C in a 10% CO2 atmosphere until ready for use, usually 4 weeks after seeding. Culture media were replenished twice a week with Dulbecco’s modified Eagle’s medium containing 10% horse serum. The developing cocultures were treated with the mitosis inhibitor 5-fluoro-2′-deoxyuridine (25 µM) and uridine (63 µM) for 48 hours on day 5 after seeding to prevent further glial proliferation. After 4 weeks in culture, the activity pattern stabilizes and is composed of one coordinated main burst pattern with several coordinated subpatterns (Gramowski et al., 2004, 2006a,b). In this study, cultures between 28 and 35 days in vitro were used.
Multichannel and Data Analysis.
Extracellular recordings were performed using a computer-controlled 64-channel MEA workstation acquisition system (Plexon, Inc., Dallas, TX), where temperature control of 37°C and stable pH of 7.4 (10% CO2) enabled stable recording and cumulative concentration-response determinations for periods longer than 10 hours (Parenti et al., 2013). The neuronal networks were acutely treated with a series of accumulating increasing concentrations of the test compound (maximum assay concentration of dimethylsulfoxide: 0.1%). For each of the applied test compound concentrations, a stable activity phase of the last 30 minutes was analyzed, and real-time unit separation and spike identification were performed in real time as previously described (Parenti et al., 2013). Action potentials, termed spikes, were recorded as spike trains, which in cortical neurons are clustered in bursts (Gramowski et al., 2006a), and these were quantitatively described via direct spike train analysis using the programs NeuroEXplorer (Plexon Inc., Dallas, TX) and the proprietary tool NPWaveX. Bursts definition and high content analysis of the network activity patterns provided a multiparametric description characterizing the activity changes in four defined categories: overall activity, burst structure, oscillatory behavior and synchronicity (see Parenti et al., 2013) for more information). The parameters for each experiment and each experimental treatment were normalized to the corresponding values of the native reference activity. From each network, 21–158 separate neurons were simultaneously recorded.
Pattern Recognition and Classification.
Characteristics of the effects displayed by methaqualone on the activity of cortical networks were elucidated further by analysis of the electrophysiologic data using methods of pattern recognition and cross validation as previously described (Parenti et al., 2013). A total of 204 spike train features were calculated using NPWaveX (NeuroProof GmbH, Rostock, Germany). Activity changes within these 204 features over the tested concentration range generate a functional, phenotypic profile for a compound. Methaqualone data were subsequently classified using pattern recognition (software package PatternExpert, NeuroProof GmbH) by comparison with the phenotypic profiles of 69 reference compounds from the NeuroProof database. An artificial neuronal network was trained with the datasets from the reference compounds to establish a classifier (multilayer feed forward network and back propagation algorithm without hidden units). It uses a multilayer feed-forward perceptron and a resilient-propagation learning algorithm that uses as many input nodes as features and one output node for each class that has to be classified. Relatively high variation of our data justifies nonuse of hidden layers. The thereby obtained cross-validation delivers a ranking that reflects the functional similarity between methaqualone data sets and reference compound. This analysis was repeated 10 times. The values reflect “% of methaqualone datasets classified as a phenotypic reference profile,” named the similarity score. High values reflect high functional phenotypic similarity between reference compound profiles and methaqualone.
Animal Studies
Animals.
Male NMRI mice (20–24 g at the time of testing) from Charles River (Germany) were housed under controlled conditions (12 hours of light starting at 06:00 hours, 20 ± 2°C, 30–70% humidity) in Macrolon (type III) cages, with standard sawdust bedding and environmental enrichment (plastic house and wooden chew blocks) and food and water available ad libitum. The experiments were carried out in accordance with the Danish legislation regulating animal experiments, Law and Order on Animal experiments; Act no. 474 of 15/05/2014 and Order no. 88 of 30/01/2013, and with the specific license for this experiment issued by the National Authority.
Maximal Electroshock Seizures Threshold and Beam Walk Assays.
Methaqualone was tested 60 minutes and diazepam 30 minutes after a subcutaneous dose in the beam walk assay, which is a sensitive measure of sedation or ataxia side effects. Briefly, mice walk across a wooden beam, 8 mm in diameter and 60 cm long, to a goal box at the far end. The number of foot slips and number of falls from the beam are scored (Stanley et al., 2005). The same mouse is first tested in beam walking, and then the convulsion threshold is determined by the maximal electroshock seizures threshold (MEST) to tonic hind limb extension by electrical stimulation via corneal electrodes using the ‘up and down’ method of shock titration (Kimball et al., 1957; Löscher and Schmidt, 1988). Electrical stimulation was delivered by electrical stimulator (Ellegaard Systems, Faaborg, Denmark) as constant current for 0.4 seconds at 50 Hz starting at 14 mA, and the stimulation intensity was lowered or raised by 2 mA steps if the preceding mouse did or did not show hind limb extension, respectively. In the same mouse, plasma and brain samples were taken to directly link efficacy to exposure.
Plasma and Brain Exposure Analysis.
Mouse plasma and brain samples from the MEST study were analyzed for methaqualone using ultra-performance liquid chromatography followed by tandem mass spectrometry detection. Brain homogenate samples were prepared by homogenizing the brain 1:4 (v/v) with water: 2-propanol:dimethylsulfoxide (50:30:20 v/v/v), followed by centrifugation and collection of the supernatant. Sample preparation was performed by protein precipitation with acetonitrile, followed by centrifugation and the addition of 0.1% ammonium hydroxide. The mobile phase consisted of water/acetonitrile with ammonium hydroxide pumped through an analytical column (Acquity ultra performance liquid chromatography BEH phenyl column 1.8 µm, 2.1 × 30 mm, Waters, MA). Detection was performed using a Sciex-API 4000 MS (Applied Biosystems, The Netherlands) using electrospray with positive ionization mode with a parent > daughter molecular mass of 251.1 > 91.1 amu. The lower limit of quantification was 1 ng/ml in plasma and 5 ng/g in brain (peak S/N > 6). The free fraction of methaqualone was determined in vitro using standard equilibrium dialysis methods with freshly isolated mouse brain homogenate or plasma (Redrobe et al., 2012). Equilibrium dialysis was performed by incubating at 37°C for 5 hours in triplicate.
Results
Functional Characterization of Methaqualone at Human GABAARs Expressed in Xenopus Oocytes
The functional properties of methaqualone were characterized at 13 human GABAAR subtypes expressed in Xenopus oocytes by TEVC electrophysiology. Whereas GABA displayed monophasic concentration-response relationships at the most of these receptors, its concentration-response curves at the α4β3δ GABAAR were distinctly biphasic, and recordings from α4β1δ-oocytes resulted in both monophasic and biphasic concentration-response curves (Table 1). In agreement with previous reports (Karim et al., 2012; Jensen et al., 2013; Hoestgaard-Jensen et al., 2014), the receptors formed in α4β1δ- and α4β3δ-expressing oocytes also exhibited pronounced levels of constitutive activity (assessed by application of 10 μM picrotoxin; unpublished data). All in all, these basic functional properties of the receptors were in good agreement with those obtained in previous studies (Mortensen et al., 2011; Karim et al., 2012, 2013; Hoestgaard-Jensen et al., 2013, 2014).
TABLE 1.
Functional properties of GABA and methaqualone determined by two-electrode voltage-clamp electrophysiology at human GABAARs expressed in Xenopus oocytes
Unless otherwise indicated in the footnotes to this table, data given for methaqualone represents its properties as a positive allosteric modulator determined at the receptors in the presence of GABA EC10. EC50 values are given in μM with pEC50 ± S.E.M. values in brackets, and Rmax values are given in percentage of the Rmax value of GABA at the receptor (n indicates number of experiments performed).
| GABA |
Methaqualone |
||||
|---|---|---|---|---|---|
| EC50 (pEC50 ± S.E.M.) | n | EC50 (pEC50 ± S.E.M.) | Rmax ± S.E.M. | n | |
| α1β2 | 7.8 (5.11 ± 0.05) | 6 | 49 (4.31 ± 0.03) | 79 ± 3.7 | 6 |
| α1β2γ2S | 57 (4.25 ± 0.10) | 6 | 38 (4.41 ± 0.03) | 84 ± 2.1 | 7 |
| α1β3γ2S | 34 (4.46 ± 0.18) | 5 | 30 (4.52 ± 0.03) | 77 ± 2.2 | 6 |
| α2β2γ2S | 40 (4.40 ± 0.07) | 4 | 24 (4.61 ± 0.03) | 75 ± 2.1 | 9 |
| α3β2γ2S | 120 (3.94 ± 0.03) | 7 | 49 (4.31 ± 0.12) | 66 ± 2.4 | 4 |
| α4β1δa monophasic | 0.46 (6.33 ± 0.44) | 3 | n.d. | n.d. | |
| α4β1δa biphasic | 0.034 (7.47 ± 0.54) | ||||
| 1.2 (5.91 ± 0.51) | 4 | n.d. | n.d. | 12a | |
| α4β2δ | 2.2 (5.65 ± 0.10) | 7 | 68 (4.17 ± 0.06) | 240 ± 27 | 5 |
| α4β3δ | 0.012 (7.94 ± 0.16) | ||||
| 3.1 (5.51 ± 0.14) | 5 | 120 (3.91 ± 0.14)b | 230 ± 12b | 6b,c | |
| α5β2γ2S | 31 (4.50 ± 0.06) | 6 | 28 (4.56 ± 0.04) | 73 ± 1.6 | 6 |
| α6β1δ | 1.4 (5.85 ± 0.08) | 6 | ∼100 (∼4.0)d | 5d | |
| α6β2 | 0.28 (6.54 ± 0.07) | 7 | 74 (4.13 ± 0.02) | 220 ± 25 | 4 |
| α6β2δ | 0.30 (6.52 ± 0.04] | 6 | 36 (4.44 ± 0.04) | 280 ± 22 | 8 |
| α6β3δ | 0.60 (6.23 ± 0.03) | 8 | 31 (4.50 ± 0.04) | 110 ± 21 | 7 |
| α1M236Wβ2γ2S | 4.1 (5.39 ± 0.11) | 6 | 88 (4.06 ± 0.03)b | 60 ± 4.8b | 4b,c |
| α1β2M286Wγ2S | 5.4 (5.26 ± 0.07) | 6 | 25 (4.60 ± 0.22) | 41 ± 2.6 | 5 |
| α6β1S265Nδ | 0.90 (6.05 ± 0.02) | 5 | 96 (4.02 ± 0.03) | 230 ± 24 | 5c |
| α6β2N265Sδ | 0.30 (6.53 ± 0.02) | 4 | ∼300 (∼3.5)d | 7d | |
n.d., not determinable.
GABA displayed monophasic and biphasic concentration-response curves at three and four of seven α4β1δ-expressing oocytes, respectively. Methaqualone was tested as a positive and negative allosteric modulator at both oocyte populations.
Properties of methaqualone as an agonist at α4β3δ and α1M236Wβ2γ2S GABAARs. Agonist EC50 values are given in μM with pEC50 ± S.E.M. values in brackets, and Rmax values are given in percentage of the Rmax value of GABA at the receptor.
The concentration-response curve was not completely saturated at the highest methaqualone concentration tested. EC50 (pEC50) and Rmax values have been extracted from the fitted curve.
Properties of methaqualone as a negative allosteric modulator at α6β1δ and α6β2N265Sδ GABAARs determined in the presence of GABA EC60–70. Estimated IC50 values are given in μM with pIC50 in brackets.
In the initial round of characterization, the functional properties of methaqualone were determined at α1β2γ2S, α2β2γ2S, α3β2γ2S, α4β2δ, α5β2γ2S and α6β2δ GABAARs (Fig. 1). This selection of receptors not only represents the full spectrum of molecular diversity in terms of α-subunits but also comprises six major physiologic GABAAR subtypes (Olsen and Sieghart, 2008; Belelli et al., 2009; Brickley and Mody, 2012). Methaqualone displayed negligible agonism at the α1,2,3,5β2γ2S receptors (Rmax values of 1–4% of GABA Rmax), whereas it was more efficacious as an agonist at α4β2δ (Rmax ± S.E.M. = 5.5 ± 1.6%; n = 5) and α6β2δ (Rmax ± S.E.M. = 13 ± 1.6%; n = 8). In addition to its small intrinsic agonist activity, methaqualone was a PAM exhibiting midmicromolar EC50 values at all six receptors when coapplied with GABA EC10 (Fig. 1B; Table 1). The currents evoked by GABA EC10 in α1β2γ2S-, α2β2γ2S-, α3β2γ2S-, and α5β2γ2S oocytes were potentiated 6- to 8-fold by maximal potentiating concentrations of methaqualone. The compound was an even more efficacious PAM at the α4β2δ and α6β2δ GABAARs, potentiating GABA EC10-evoked currents through these receptors to amplitudes 2- to 3-fold greater than the maximal responses of GABA (Fig. 1B; Table 1). Interestingly, methaqualone displayed bell-shaped concentration-response curves as a PAM at all receptors when coapplied with GABA EC10 in concentrations ranging from 1 to 1000 μM (exemplified for α1β2γ2S and α6β2δ in Fig. 1, C and D). Furthermore, pronounced rebound currents were observed at methaqualone concentrations of 300 μM and greater (Fig. 1C).
To elucidate the nature of methaqualone-mediated modulation of αβγ and αβδ GABAARs, GABA concentration-relationships at the α1β2γ2S and α6β2δ receptors were determined in the absence or presence of the modulator. In agreement with previous studies (Campo-Soria et al., 2006; Gielen et al., 2012), the maximal current amplitude evoked by GABA through α1β2γ2S in the presence of a saturating diazepam concentration (3 µM) (Rmax ± S.E.M. = 110 ± 10%; n = 4) did not differ significantly from that elicited by GABA alone, whereas the benzodiazepine gave rise to a small but significant (2.4-fold) left shift of the concentration-response curve [EC50 (pEC50 ± S.E.M.) = 24 µM (4.62 ± 0.10) (n = 4) versus 57 µM (4.25 ± 0.10) (n = 7); P < 0.1] (Fig. 1E). In contrast, preincubation and coapplication of 300 µM methaqualone increased the potency of GABA at the receptor by 41-fold [(EC50 (pEC50 ± S.E.M.) = 1.4 µM (5.85 ± 0.08) (n = 6) versus 57 µM 4.25 ± 0.10] (n = 7); P < 0.001), whereas the maximal response evoked by GABA was significantly reduced [Rmax ± S.E.M. = 82 ± 2.3% (n = 6); P < 0.1] (Fig. 1E). Interestingly, it was impossible to determine the effect of 300 µM methaqualone at the GABA concentration-response relationship at the α6β2δ receptor, since the large currents elicited through this receptor by coapplications of the modulator and high GABA concentrations consistently resulted in failure to keep the holding potential of the oocytes. However, low GABA concentrations unable to evoke significant currents in α6β2δ-oocytes when applied alone were observed to induce substantial currents when coapplied with 300 μM methaqualone (unpublished data). Thus, although we were unable to quantify the degree of left shift of the GABA concentration-response curve brought on by the presence of methaqaulone, the drug clearly modulated both GABA potency and efficacy at this receptor.
With the first round of characterization having revealed distinctly different methaqualone functionalities at different α-containing GABAAR subtypes, the second round focused on the putative importance of β subunit identity and of the accessory γ2S/δ subunit for the modulation of α1βγ2S and α4,6βδ GABAARs. The methaqualone-mediated potentiation of the α1βγ2S GABAAR did not appear to be dependent on the presence of γ2S in the receptor, since the functionalities exhibited by the compound at α1β2 and α1β2γ2S receptors did not differ significantly (Fig. 2A; Table 1). Furthermore, substituting β2 for β3 in the α1βγ2S complex did not change the potency or efficacy of methaqualone as a PAM substantially (Fig. 2A; Table 1).
Fig. 2.
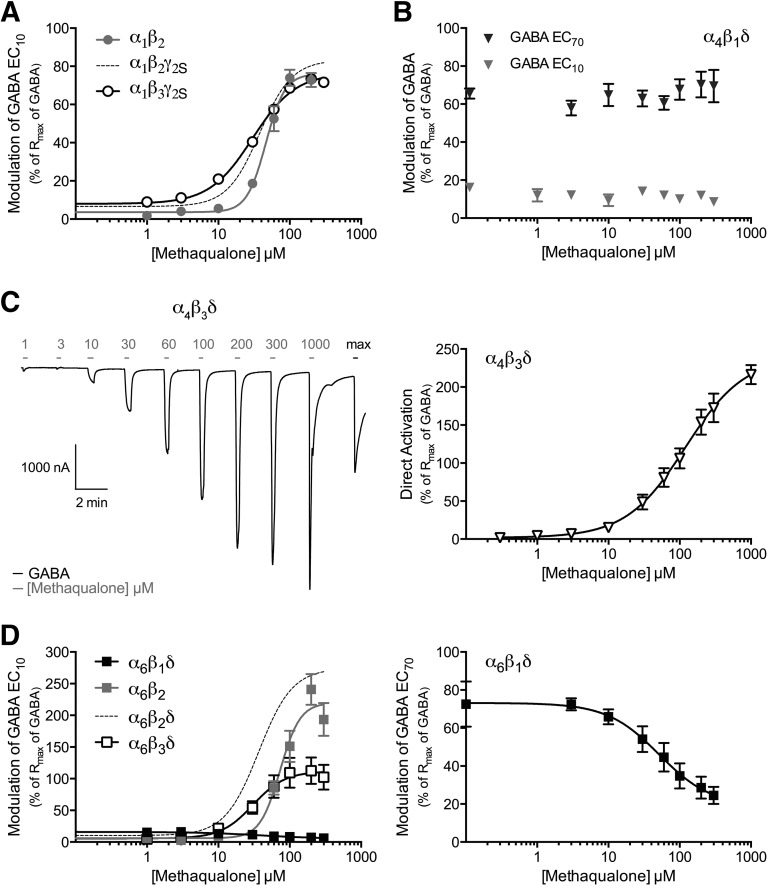
Functional properties of methaqualone at human GABAARs expressed in Xenopus oocytes. (A) Concentration-response curves for methaqualone at α1β2, α1β2γ2S, and α1β3γ2S GABAARs in the presence of GABA EC10 (means ± S.E.M.; n = 6–7). (B) Modulation of α4β1δ GABAAR signaling exerted by methaqualone in the presence of GABA EC10 or GABA EC70 (means ± S.E.M.; n = 4–8). (C) Representative trace and the concentration-response curve for methaqualone as an agonist at the α4β3δ GABAAR (means ± S.E.M.; n = 6). The gray application bars above the trace indicate application of the various methaqualone concentrations, and the black bar represents the application of a GABA concentration eliciting a maximal response. (D, left) Concentration-response curves for methaqualone at α6β2, α6β1δ, α6β2δ, and α6β3δ GABAARs in the presence of GABA EC10 (means ± S.E.M.; n = 4–8). (D, right) Concentration-inhibition curve for methaqualone at the α6β1δ GABAAR in the presence of GABA EC70 (means ± S.E.M.; n = 5). The hatched concentration-response curves for α1β2γ2S and α6β2δ in (A) and (D), respectively, are based on data displayed in Fig. 1B.
In contrast to its comparable modulation of α1β2γ2S and α1β3γ2S receptors, methaqualone displayed dramatically different functionalities at the different β subunit-containing α4βδ and α6βδ subtypes. Contrary to its PAM activity at the α4β2δ GABAAR (Fig. 1B), methaqualone did not modulate the responses evoked by GABA EC10 or GABA EC70 in α4β1δ-oocytes, and strikingly the presence of β3 in the α4βδ complex converted the compound into a superagonist with an efficacy comparable to that of the orthosteric agonist THIP (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol) (Fig. 2, B and C; Table 1) (Storustovu and Ebert, 2006; Hoestgaard-Jensen et al., 2014). The origin of the agonism mediated by methaqualone at this receptor will be addressed further in the Discussion section.
The functional properties exhibited by methaqualone at the three α6βδ GABAARs were just as diverse as those at the α4βδ receptors, but interestingly the pattern of functionalities determined for the modulator at these two receptor groups differed completely (Table 1). Methaqualone was a less efficacious PAM at α6β3δ than at the α6β2δ GABAAR, and the compound did not potentiate the GABA-evoked signaling through the α6β1δ GABAAR but instead acted as a weak negative allosteric modulator (NAM) at the receptor (Fig. 2D; Table 1). Finally, judging from the similar functional characteristics displayed by methaqualone at α6β2 and α6β2δ GABAARs, the presence of the δ subunit in the GABAAR assembly did not seem to be important for its modulation of αβδ receptors (Fig. 2D; Table 1).
Delineation of the Mechanism of Action of Methaqualone at the GABAAR
To elucidate the molecular basis for methaqualone modulation of the GABAAR, we investigated the putative interactions of the modulator with four known allosteric sites in the receptor complex.
The Benzodiazepine Site.
The high-affinity benzodiazepine site in the αβγ GABAAR is located at the extracellular α(+)/γ(–) subunit interface (Wieland et al., 1992; Sigel and Lüscher, 2011). Although the similar potencies displayed by methaqualone at the numerous GABAAR subtypes included in this study suggested that the modulator does not act through this site, the different structure of methaqualone compared with benzodiazepines hypothetically could enable it to bind to the α(+)/γ(–) interface in αβγ receptors, as well as to the corresponding interfaces in αβ and αβδ GABAARs. This possibility was investigated by two different approaches.
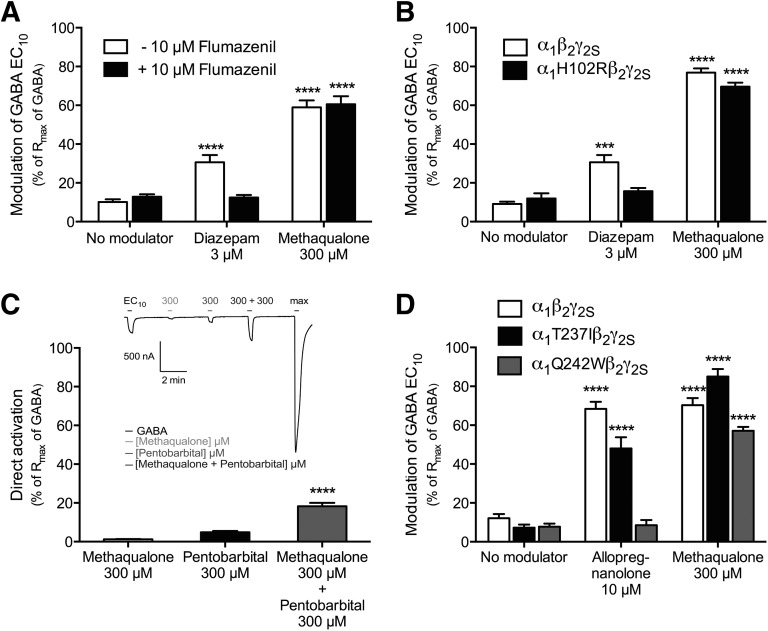
In the first experiment, the effect of the benzodiazepine-site antagonist flumazenil on methaqualone-mediated potentiation of α1β2γ2S receptor signaling was assessed. In concordance with the literature (Sigel and Lüscher, 2011), preincubation and coapplication of 10 μM flumazenil with GABA EC10 did not modulate the agonist-evoked response through the receptor significantly (10 ± 1.4% (n = 13) versus 13 ± 1.3% (n = 13)), whereas the potentiation of the GABA EC10-evoked response mediated by 3 μM diazepam (31 ± 3.7%, n = 11) was completely eliminated by the presence of the antagonist (12 ± 1.4%, n = 6) (Fig. 3A). In contrast, 10 μM flumazenil did not reduce the methaqualone-mediated potentiation of α1β2γ2S GABAAR signaling significantly (59 ± 3.5% (n = 7) versus 61 ± 4.2% (n = 7)) (Fig. 3A).
Fig. 3.
The potential interaction of methaqualone with three known allosteric sites in the GABAAR complex. The experiments were performed at human WT and mutant α1β2γ2S GABAARs expressed in Xenopus oocytes. (A) Effects of 10 μM flumazenil on the potentiation exerted by 3 μM diazepam or 300 μM methaqualone on the responses evoked by GABA EC10 through the α1β2γ2S GABAAR. Asterisks indicate significant differences between responses evoked by GABA EC10 in the presence of modulator (diazepam and methaqualone) and by GABA EC10 alone, either in the absence or presence of flumazenil: ****P < 0.0001 (means ± S.E.M.; n = 6–13). (B) The modulatory effects of 3 μM diazepam and 300 μM methaqualone on the GABA EC10-evoked responses through α1β2γ2S and α1H102Rβ2γ2S GABAARs. Asterisks indicate significant differences between responses evoked by GABA EC10 in the presence of modulator and by GABA EC10 alone at the same receptor (means ± S.E.M.; n = 5–12): ***P < 0.001; ****P < 0.0001. (C) Direct activation of the α1β2γ2S GABAAR evoked by 300 μM methaqualone, by 300 µM pentobarbital, and by coapplication of 300 μM methaqualone and 300 µM pentobarbital (means ± S.E.M.; n = 7). Asterisks indicate the significant difference between the responses evoked by 300 µM pentobarbital and by coapplication of 300 µM methaqualone and 300 µM pentobarbital: ****P < 0.0001. Insert: Representative trace for direct activation of the α1β2γ2S GABAAR by 300 μM methaqualone, 300 µM pentobarbital, and coapplication of 300 μM methaqualone and 300 µM pentobarbital. (D) The modulatory effects of 10 μM allopregnanolone and 300 μM methaqualone on the GABA EC10-evoked responses through α1β2γ2S, α1T237Iβ2γ2S, and α1Q241Wβ2γ2S GABAARs. Asterisks indicate significant differences between responses evoked by GABA EC10 in the presence of modulator and by GABA EC10 alone at the same receptor (means ± S.E.M.; n = 4–11): ****P < 0.0001.
In the second experiment, the impact of the α1-H102R mutation on the methaqualone-mediated modulation at α1β2γ2S receptor signaling was investigated. Substitution of this conserved histidine residue in the α1,2,3,5-subunit with an arginine (the corresponding residue in α4,6) has been shown to render α1,2,3,5βγ receptors insensitive to benzodiazepines (Wieland et al., 1992; Sigel and Lüscher, 2011). Whereas diazepam (3 μM) was completely inactive as a PAM at the α1H102Rβ2γ2S GABAAR, the potentiation of GABA EC10 evoked-signaling through WT α1β2γ2S and α1H102Rβ2γ2S receptors exerted by 300 μM methaqualone did not differ substantially (77 ± 2.1% (n = 6) versus 70 ± 2.1% (n = 5)) (Fig. 3B). In conclusion, these findings unequivocally rule out the high-affinity benzodiazepine site as the site of action for methaqualone.
The Barbiturate Site.
Although numerous residues and regions in GABAARs have been shown to be important for the barbiturate-mediated modulation of the receptors, the exact location of the binding site(s) for these ago-PAMs in the receptors has yet to be identified (Serafini et al., 2000; Greenfield et al., 2002; Feng and Macdonald, 2010; Chiara et al., 2013). To assess whether methaqualone targets the barbiturate site or an overlapping site in the GABAAR, we investigated whether the small but significant agonist response evoked by 300 µM pentobarbital through the α1β2γ2S receptor could be modulated by methaqualone. As can be seen in Fig. 3C, the current amplitudes elicited by 300 μM methaqualone (1.2 ± 0.17%, n = 6) and by 300 μM pentobarbital (4.9 ± 0.68%, n = 6) at α1β2γ2S-expressing oocytes were significantly smaller than that arising from coapplication of the two compounds at the receptor (18 ± 1.7%, n = 6). The ability of methaqualone to potentiate pentobarbital-evoked α1β2γ2S signaling demonstrates that it binds to a site that does not overlap with the site through which the barbiturate mediates its direct activation of the receptor. However, in view of the presently limited insight into the molecular basis for barbiturate modulation of GABAARs, we cannot exclude the possibility that barbiturate-mediated potentiation could arise from a distinct site in the GABAAR and that this site could overlap with the methaqualone binding site.
The Neurosteroid Sites.
Several endogenous and synthetic neurosteroids act as potent ago-PAMs of GABAARs (Herd et al., 2007). Smart and coworkers have proposed the existence of two discrete binding sites for neurosteroids in the transmembrane domains of the murine α1β2γ2 GABAAR: an intersubunit site at the β(+)/α(–) subunit interface (comprising the α1-TM1 residue Thr236) important for neurosteroid activation and an intrasubunit site in the α subunit (comprising the α1-TM1 residue Gln241), which is important for both neurosteroid-mediated potentiation and activation (Hosie et al., 2006, 2009). To investigate whether methaqualone mediates its effects on GABAAR signaling through one or both of these sites, the impact of mutations of these two α1 residues (Thr237 and Gln242, human α1 numbering) on the modulation exerted by the compound at the α1β2γ2S GABAAR was determined. Allopregnanolone was used as reference compound in these recordings, and in concordance with previous studies, the neurosteroid was an efficacious PAM of the GABAAR (Fig. 3D). In contrast to previous findings, however, allopregnanolone did not exhibit significant intrinsic agonist activity at the receptor at concentrations up to 10 μM (Hosie et al., 2006, 2009; Chen et al., 2014). The possible reasons for the absence of the direct activation component of allopregnanolone at the receptor are currently being investigated in our laboratory.
The absence of allopregnanolone-evoked agonism at the α1β2γ2S receptor obviously precluded us from verifying the previously reported effects of α1-Q242W and α1-T237I mutations on this activity component of the neurosteroid. However, in agreement with the previously reported importance of a highly conserved Gln residue in TM1 of the α subunit for neurosteroid-mediated potentiation of GABAARs (Hosie et al., 2006, 2009), allopregnanolone was completely inactive as a PAM at the α1Q242Wβ2γ2S receptor at concentrations up to 10 μM (Fig. 3D). In contrast, the presence of 10 μM allopregnanolone potentiated GABA EC10-evoked currents in WT α1β2γ2S- and α1T237Iβ2γ2S-expressing oocytes to similar degrees, which is also in agreement with the findings by Hosie et al. (2006) and with the notion of the proposed intersubunit site being responsible for neurosteroid-mediated activation exclusively (Fig. 3D). Interestingly, the degree of potentiation of the GABA EC10-evoked response through the α1β2γ2S receptor mediated by 300 μM methaqualone was not changed significantly by the introduction of neither the Q242W nor the T237I mutation in the α1 subunit (Fig. 3D).
Although the functional implications of the two α1 mutations on allopregnanolone-mediated potentiation of the GABAAR observed in this study are in concordance with those reported by the Smart group, the apparent discrepancy between our findings and the literature when it comes to the intrinsic agonist activity of the neurosteroid should obviously be kept in mind when interpreting the results obtained for methaqualone in these recordings. We propose that the dramatically different effects induced by the α1-Q242W mutation on the allopregnanolone- and methaqualone-mediated potentiation of α1β2γ2S receptor signaling unequivocally demonstrate that methaqualone does not act through the proposed intrasubunit neurosteroid site in the receptor. As for the proposed intersubunit neurosteroid site, the lack of intrinsic agonist activity of allopregnanolone at α1β2γ2S clearly devaluates it as a reference compound. Taken at face value, however, the WT-like properties exhibited by methaqualone at the α1T237Iβ2γ2S receptor does suggest that the modulator does not target a site comprising this residue.
The Transmembrane β(+)/α(–) Subunit Interface.
The transmembrane β(+)/α(–) interface in the GABAAR harbors binding sites for numerous allosteric modulators, but with the exception of the site targeted by the general anesthetic etomidate the compositions and locations of these sites are poorly elucidated (Wafford et al., 1994; Belelli et al., 1997; Hill-Venning et al., 1997; Halliwell et al., 1999; Walters et al., 2000; Krasowski et al., 2001; Bali and Akabas, 2004; Thompson et al., 2004; Khom et al., 2007). Interestingly, however, several of these modulators exhibit distinct selectivity between GABAAR subtypes on the basis of their respective β-subunits (Wafford et al., 1994; Belelli et al., 1997; Hill-Venning et al., 1997; Halliwell et al., 1999; Thompson et al., 2004; Khom et al., 2007). In this light, the differential functionalities displayed by methaqualone at the different β-subunit containing α4βδ and α6βδ receptors were intriguing and prompted us to probe the putative importance of three transmembrane residues in the GABAAR for the functional properties of methaqualone.
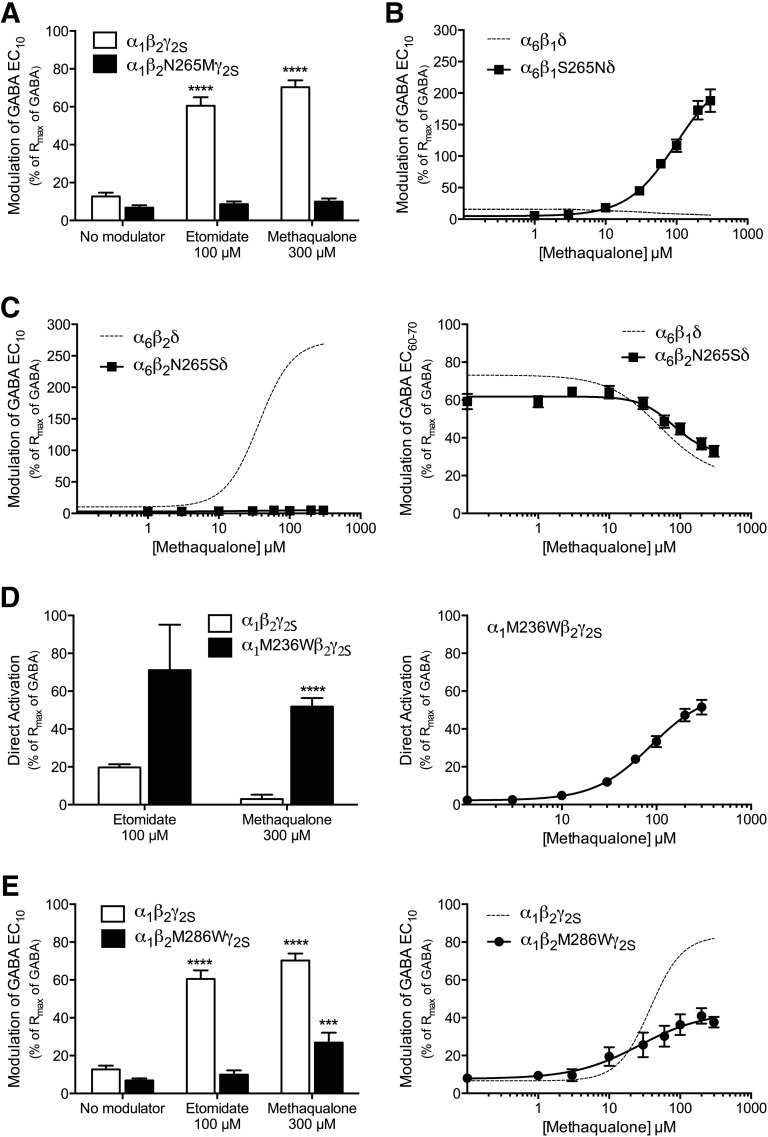
Residue 265 in TM2 of the β-subunit (β1-Ser265, β2/β3-Asn265) is a key molecular determinant of the β-selectivity (β2/β3-over-β1 or β1-over-β2/β3) displayed by several of the β(+)/α(−) interface modulators (Wingrove et al., 1994; Belelli et al., 1997; Hill-Venning et al., 1997; Halliwell et al., 1999; Khom et al., 2007). In the case of etomidate, the residue is believed not to participate in binding but rather to act as a transduction element between modulator binding and its effect on gating (Li et al., 2006; Desai et al., 2009; Chiara et al., 2012; Stewart et al., 2013; Stewart et al., 2014). In agreement with previous studies (Belelli et al., 1997; Siegwart et al., 2002; Desai et al., 2009; Stewart et al., 2014), the introduction of a N265M mutation in β2 eliminated etomidate-mediated potentiation of α1β2γ2S receptor signaling completely, and interestingly the mutation had a similar detrimental effect on the methaqualone-mediated potentiation (Fig. 4A). Furthermore, the PAM and NAM activities exhibited by methaqualone at the α6β2δ and α6β1δ receptors, respectively, were completely reversed by the introduction of the reciprocal residue in position 265 of the respective β subunits. In fact, methaqualone was roughly equipotent and equally efficacious as a PAM at the α6β2δ and α6β1S265Nδ receptors and as a NAM at the α6β1δ and α6β2N265Sδ receptors (Fig. 4, B and C; Table 1).
Fig. 4.
The potential interaction of methaqualone with the transmembrane β(+)/α(–) subunit interface in the GABAAR complex. The experiments were performed at human WT and mutant GABAARs expressed in Xenopus oocytes. (A) Modulatory effects of 100 μM etomidate and 300 μM methaqualone on the responses evoked by GABA EC10 through α1β2γ2S or α1β2N265Mγ2S GABAARs (means ± S.E.M.; n = 3–14). Asterisks indicate significant differences between the responses evoked by GABA EC10 in the presence of modulator and by GABA EC10 alone at the same receptor: ****P < 0.0001. (B) Concentration-response curves for methaqualone at α6β2δ and α6β2S265Nδ GABAARs in the presence of GABA EC10 (means ± S.E.M.; n = 5). (C, left) Concentration-response curves for methaqualone at α6β2δ and α6β2N265Sδ GABAARs in the presence of GABA EC10 (means ± S.E.M.; n = 3–8). (C, right) Concentration-inhibition curves for methaqualone at α6β1δ and α6β2N265Sδ GABAARs in the presence of GABA EC60-70 (means ± S.E.M.; n = 5–7). (D, left) Direct activation of α1β2γ2S and α1β2M236Wγ2S GABAAR signaling evoked by 100 μM etomidate and 300 μM methaqualone (means ± S.E.M.; n = 4–8). Asterisks indicate significant differences between the responses evoked by etomidate or methaqualone at the two receptors: ****P < 0.0001 (unpaired two-sided t test). (D, right) Concentration-response curve for methaqualone as an agonist at the α1β2M236Wγ2S GABAAR (mean ± S.E.M.; n = 4). (E, left) Modulatory effects of 100 μM etomidate and 300 μM methaqualone on the responses evoked by GABA EC10 at α1β2γ2S and α1β2M286Wγ2S GABAARs (means ± S.E.M.; n = 3–10). Asterisks indicate significant differences between the responses evoked by GABA EC10 in the presence of modulator and by GABA EC10 alone at the same receptor: ****P < 0.0001; ***P < 0.001. (E, right) Concentration-response curves for methaqualone at α1β2γ2S and α1β2M286Wγ2S GABAARs in the presence of GABA EC10 (means ± S.E.M.; n = 5–7). The hatched concentration-response curves for α6β1δ (B) α6β2δ (C) and α1β2γ2S (E) are based on data in Figs. 2D, 1B, and 1B, respectively.
Elaborate photolabeling, substituted cysteine accessibility method, and mutagenesis studies have demonstrated the key importance of the α1-TM1 Met236 and β2-TM3 Met286 residues for the GABAAR modulation exerted by etomidate, and the two residues are believed to form direct interactions with the modulator (Siegwart et al., 2002; Li et al., 2006; Stewart et al., 2008; Chiara et al., 2012; Stewart et al., 2013). In concordance with previous studies (Siegwart et al., 2002; Stewart et al., 2008), etomidate (100 μM) displayed higher intrinsic agonist activity at the α1M236Wβ2γ2S GABAAR than at the WT α1β2γ2S receptor, whereas it was completely inactive at the α1β2M286Wγ2S receptor (Fig. 4, D and E). Analogously to the increased intrinsic agonist activity of etomidate brought on by the α1-M236W mutation, the insignificant agonism of methaqualone at WT α1β2γ2S was converted into pronounced agonist activity at the α1M236Wβ2γ2S receptor (Fig. 4D). Conversely, the effect of the β2-M286W mutation on methaqualone functionality was considerably more subtle than that for etomidate, methaqualone being roughly equipotent albeit less efficacious as a PAM at the α1β2M286Wγ2S GABAAR compared with the WT receptor (Fig. 4E).
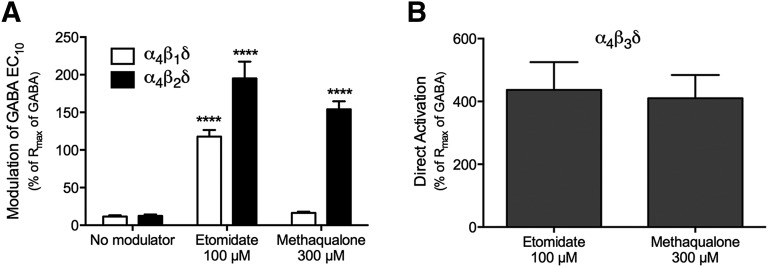
In a final experiment, we compared the modulation exerted by etomidate at the α4βδ GABAARs with the diverse functionalities exhibited by methaqualone at the three receptors. As mentioned previously, etomidate acts as a PAM at a plethora of α1,2,3,6βγ2 GABAARs, being ∼10-fold more potent and substantially more efficacious at β2-/β3-containing than at β1-containing subtypes of these receptors (Belelli et al., 1997; Hill-Venning et al., 1997). Etomidate has also been reported to potentiate GABA-evoked signaling through α4β3δ GABAARs expressed in Xenopus oocytes and in mammalian cell lines (Brown et al., 2002; Meera et al., 2009; Jensen et al., 2013), but to our knowledge, its modulatory effects at recombinant α4β1δ and α4β2δ receptors have not been reported. Etomidate (100 μM) displayed no significant agonist activity at the receptors formed in α4β1δ-expressing oocytes and only slightly higher intrinsic activity at the α4β2δ GABAAR (Rmax values of 1–5% of GABA Rmax). However, the compound robustly potentiated GABA EC10-evoked responses through the two receptors (Fig. 5A). Conversely, etomidate (100 μM) was a pronounced agonist at the α4β3δ GABAAR, evoking a response ∼4-fold higher than the GABA Rmax at the receptor (Fig. 5B). Although the fact that we only studied the effects of a single high etomidate concentration (100 μM) in these recordings combined with its reported bell-shaped concentration-response relationships at other GABAARs (Belelli et al., 1997; Hill-Venning et al., 1997) preclude us from drawing conclusions regarding whether etomidate exhibits differential modulatory potencies or efficacies at the three α4βδ receptors, the qualitative nature of its modulation of the receptors can be extracted from the data. Analogously to methaqualone, etomidate is a fairly pure PAM at α4β2δ and a superagonist at the α4β3δ receptor, whereas its PAM activity at the α4β1δ GABAAR contrasts the inactivity of methaqualone at this subtype (at concentrations up to 300 μM).
Fig. 5.
Functional properties of 100 μM etomidate at human α4βδ GABAARs expressed in Xenopus oocytes. The modulation exerted by etomidate and methaqualone was determined at the same α4β1δ-, α4β2δ-, or α4β3δ-expressing oocytes. (A) Modulatory effects of 100 μM etomidate and 300 μM methaqualone on the responses evoked by GABA EC10 through α4β1δ or α4β2δ GABAARs. Responses given as means ± S.E.M. in the percentage of Rmax of GABA; etomidate: 118 ± 9% (α4β1δ; n = 6) and 195 ± 23% (α4β2δ, n = 4); methaqualone: 16% ± 1.7 (α4β1δ; n = 6) and 154 ± 11% (α4β2δ, N = 4). ****P < 0.0001. (B) Direct activation of the α4β3δ GABAAR by 100 μM etomidate or 300 μM methaqualone. Responses given as means ± S.E.M. in percentage of Rmax of GABA; etomidate: 410 ± 88%; n = 6; methaqualone: 437 ± 74%; n = 6.
Putative Binding Modes of Etomidate, Loreclezole, and Methaqualone.
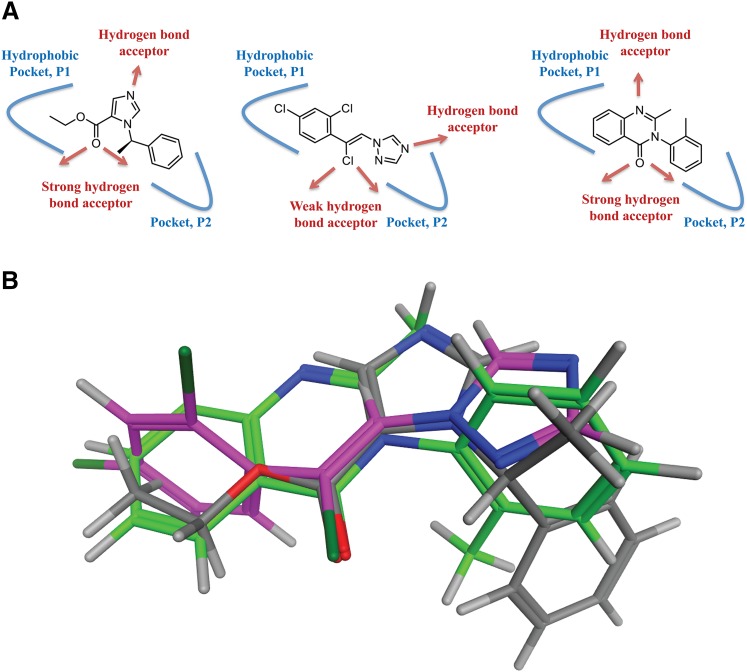
All in all, the results outlined above indicate that methaqualone acts through the same transmembrane β(+)/α(–) interface in the GABAAR complex targeted by etomidate, the anticonvulsant drug loreclezole, and several other modulators. An analysis of the physical-chemical properties of etomidate, loreclezole, and methaqualone shows that the three compounds share notable structural similarities. Both etomidate and methaqualone comprise two hydrophobic moieties, as well as a carbonyl group and an aromatic nitrogen capable of acting as hydrogen bond acceptors (Fig. 6A). Loreclezole comprises one hydrophobic moiety as well as a vinylogous chlorine and a triazole ring system as a potential hydrogen bond acceptors (Fig. 6A). This similarity prompted us to compare the putative binding modes of the three modulators with the aim of defining one common pharmacophore.
Fig. 6.
The putative shared binding mode of etomidate, loreclezole, and methaqualone. (A) Illustration of the structural similarities between etomidate (left), loreclezole (middle), and methaqualone (right). The putative pockets P1 and P2 are given in blue, and the hydrogen bond acceptors in the compounds are indicated with red arrows. (B) Superimposition of low-energy conformations of etomidate (type code), loreclezole (pink), and methaqualone (green) by fitting the carbonyl groups of etomidate and methaqualone and the vinylogous chlorine of loreclezole.
In view of the relatively high functional potencies of etomidate, loreclezole, and methaqualone as GABAAR modulators, all three modulators are expected to bind to the receptor in a low-energy conformation. Thus, the three molecules were initially submitted to a stochastic conformational search to enumerate their respective low-energy conformations. In the case of etomidate, the 2-phenethyl group adapted only one well defined conformation, and although three rotamers of the ester alkyl group was found the energy differences between these were not significant (<1 kcal/mol). Methaqualone is also a highly rigid molecule, and its low-energy conformation was readily determined. The same was true for loreclezole, which adapted only one low-energy conformation. The low-energy conformations of the three molecules were superimposed based on the assumption that carbonyl groups of etomidate and methaqualone and the vinylogous chlorine of loreclezole dictate the binding modes of the respective compounds in the putative shared site (Fig. 6B). From this overlay, it is clear that the ester alkyl group of etomidate, the 2,4-diclorophenyl ring of loreclezole, and the fused phenyl ring of methaqualone occupy the same area of space (designated hydrophobic pocket P1 in Fig. 6A). The 2-phenethyl group of etomidate and the N-phenyl group of methaqualone are oriented in the same area of space (designated pocket P2 in Fig. 6A). In this area, loreclezole comprises a triazole ring, which in comparison with the corresponding moieties in etomidate and methaqualone is less hydrophobic. Thus, the P2 pocket may be capable of accommodating the binding of ring systems with quite different physicochemical properties. Finally, the aromatic nitrogen atoms in the three molecules do not align perfectly in this superimposition; however, reorganization of the water molecules in the binding pocket (induced fit) could possibly adjust for the differences in hydrogen bond donating trajectories of the respective nitrogen atoms. Thus, the in silico study seems to support the hypothesis that etomidate, loreclezole, and methaqualone could bind to a common site in the transmembrane β(+)/α(–) interface of the GABAAR, although it should be noted that the superimposition of etomidate and methaqualone is more successful than the loreclezole/etomidate and loreclezole/methaqualone superimpositions.
Screening of Methaqualone at Other Putative CNS Targets
The possible existence of other CNS targets for methaqualone than GABAARs was investigated in an elaborate screening of the compound at a total of 50 recombinant neurotransmitter receptors and transporters and at native GABAARs and ionotropic glutamate receptors in rat brain homogenates, a selection that includes numerous key targets for known psychotropic drugs and psychostimulants. For most of these targets, the putative activity of methaqualone was assessed in competition binding assays (performed by PDSP), where radioligand concentrations near or at the KD value for the specific target in these assays was used to facilitate the detection of both inhibition and potentiation of radioligand binding by the test compound. Methaqualone (30 μM) did not display significant modulation (neither potentiation or inhibition) of radioligand binding to a wide range of serotonin, dopamine, norepinephrine, histamine, acetylcholine, glutamate, opioid, cannabinoid, and sigma receptors or at the three monoamine transporters in these assays (Table 2). Furthermore, the compound was inactive at concentrations up to 1 mM when tested in functional assays at the other plasma membrane-bound targets for GABA: the GABAB receptors and GABA transporters (Table 2).
TABLE 2.
Pharmacologic properties of methaqualone at various central nervous system targets
The binding affinities for methaqualone at numerous targets in a competition binding assays (using radioligand concentrations near or at the KD value for the specific target) were determined by National Institute of Mental Health’s PDSP. The IC50 values obtained for methaqualone in the binding assays are given in micromolar, and percent inhibition or percent potentiation of radioligand binding at 30 μM methaqualone is given in parentheses (positive and negative values represent percent inhibition and percent potentiation of control, respectively). An inhibition of >50% is considered significant by the PDSP. The data are based on four independent determinations. The functional properties of methaqualone at selected transporters and receptors were determined in in-house functional assays and are indicated by the # symbol. The IC50 and EC50 values obtained for methaqualone in these functional assays are given in micromolar.
| Target | Assay | IC50 [μM] (% Inhibition) |
|---|---|---|
| Serotonin | ||
| 5-HT1A (h) | [3H]8-OH-DPAT binding | >30 (6.3) |
| 5-HT1B (h) | [3H]GR125743 binding | >30 (−1.3) |
| 5-HT1D (h) | [3H]GR125743 binding | >30 (7.6) |
| 5-HT1e (h) | [3H]5-HT binding | >30 (−11) |
| 5-HT2A (h) | [3H]Ketanserin binding | >30 (-2.1) |
| 5-HT2B (h) | [3H]LSD binding | >30 (−8.3) |
| 5-HT2C (h) | [3H]Mesulergine binding | >30 (3.3) |
| 5-HT3A (h) | [3H]LY278584 binding | >30 (−7.7) |
| 5-HT5a (h) | [3H]LSD binding | >30 (−11) |
| 5-HT6 (h) | [3H]LSD binding | >30 (3.6) |
| 5-HT7 (h) | [3H]LSD binding | >30 (0.9) |
| SERT (h) | [3H]Citalopram binding | >30 (−1.5) |
| Dopamine | ||
| D1 (h) | [3H]SCH23390 binding | >30 (−2.4) |
| D2 (h) | [3H]N-Methylspiperone binding | >30 (30) |
| D3 (h) | [3H]N-Methylspiperone binding | >30 (9.9) |
| D4 (h) | [3H]N-Methylspiperone binding | >30 (10) |
| D5 (h) | [3H]SCH23390 binding | >30 (11) |
| DAT (h) | [3H]WIN35428 binding | >30 (28) |
| Norepinephrine | ||
| α1A (h) | [3H]Prazosin binding | >30 (−12) |
| α1B (h) | [3H]Prazosin binding | >30 (5.6) |
| α1D (h) | [3H]Prazosin binding | >30 (47) |
| α2A (h) | [3H]Rauwolscine binding | >30 (45) |
| α2B (h) | [3H]Rauwolscine binding | >30 (3.4) |
| α2C (h) | [3H]Rauwolscine binding | ∼30 (63) |
| β1 (h) | [125I]Pindolol binding | >30 (−5.8) |
| β2 (h) | [3H]CGP 12177 binding | >30 (−9.4) |
| β3 (h) | [3H]CGP 12177 binding | >30 (23) |
| NET (h) | [3H]Nisoxetine binding | >30 (2.3) |
| Histamine | ||
| H1 (h) | [3H]Pyrilamine binding | >30 (31) |
| H3 (h) | [3H]α-methylhistamine binding | >30 (−9.5) |
| Acetylcholine | ||
| m1 (h) | [3H]QNB binding | >30 (−9.5) |
| m2 (h) | [3H]QNB binding | >30 (−4.8) |
| m3 (h) | [3H]QNB binding | >30 (−13) |
| m4 (h) | [3H]QNB binding | >30 (−17) |
| m5 (h) | [3H]QNB binding | >30 (13) |
| α4β2 (m)# | FLIPR membrane potential sasay | EC50 > 1000, IC50 > 1000 |
| GABA | ||
| Rat forebrain | [3H]Muscimol binding | >30 (−8.9) |
| Rat brain | [3H]Flunitrazepam binding | >30 (−11) |
| Rat peripheral benzodiazepine receptor | [3H]PK11195 binding | >30 (21) |
| GAT1 (h)# | [3H]GABA uptake | >1000 |
| BGT1 (h)# | [3H]GABA uptake | >1000 |
| GAT2 (h)# | [3H]GABA uptake | >1000 |
| GAT3 (h)# | [3H]GABA uptake | >1000 |
| GABAB1a,2 (r)# | Ca2+/Fluo4 assay (with Gqi5) | EC50 > 1000, IC50 > 1000 |
| GABAB1b,2 (r)# | Ca2+/Fluo4 assay (with Gqi5) | EC50 > 1000, IC50 > 1000 |
| Glutamate | ||
| Rat brain NMDA-R | [3H]MK801 binding | >30 (8.1) |
| Rat brain AMPA-R | [3H]AMPA binding | >30 (23) |
| Rat brain KA-R | [3H]KA binding | >30 (18) |
| Opioid | ||
| δ (h) | [3H]DADLE binding | >30 (34) |
| κ (h) | [3H]U69593 binding | >30 (4.9) |
| μ (h) | [3H]DAMGO binding | ∼30 (50) |
| Cannabinoid | ||
| CB1 (h) | [3H]CP55940 binding | ∼30 (54) |
| CB2 (h) | [3H]CP55940 binding | >30 (6.6) |
| Sigma | ||
| Sigma1 (rat brain) | [3H]Pentazocine(+) binding | >30 (−16) |
| Sigma2 (rat, PC12) | [3H]DTG binding | >30 (49) |
CGP 12177, 4-[3-(tert-butylamino)-2-hydroxypropoxy]-1,3-dihydrobenzimidazol-2-one; CP55940, 2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-5-(2-methyloctan-2-yl)phenol; GR125743, N-[4-methoxy-3-(4-methylpiperazin-1-yl)phenyl]-3-methyl-4-(pyridin-4-yl)benzamide; h, human; LY278584, 1-methyl-N-[(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl]indazole-3-carboxamide; m, mouse; PK11195, N-butan-2-yl-1-(2-chlorophenyl)-N-methylisoquinoline-3-carboxamide; r, rat; SCH23390, 8-chloro-3-methyl-5-phenyl-1,2,4,5-tetrahydro-3-benzazepin-7-ol; U69593, N-methyl-2-phenyl-N-[(5R,7S,8S)-7-pyrrolidin-1-yl-1-oxaspiro[4.5]decan-8-yl]acetamide; WIN35428, methyl (1S,3S,4S,5R)-3-(4-fluorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate.
The inability of 30 μM methaqualone to compete with [3H]muscimol and [3H]flunitrazepam binding to rat brain tissue in the PDSP screening is in concordance with the binding site proposed for the modulator at GABAARs in this study. On the other hand, the insignificant modulation exerted by the compound on radioligand binding to native GABAARs in these assays could be argued to contrast with the augmentation of radioligand binding to the orthosteric and the benzodiazepine binding sites in native GABAARs previously reported for other allosteric modulators of these receptors. Most notably in connection with methaqualone, both etomidate and loreclezole have been reported to enhance [3H]muscimol and [3H]flunitrazepam binding to rat brain tissue (Quast and Brenner, 1983; Slany et al., 1995; Ghiani et al., 1996; Xue et al., 1996; Zhong and Simmonds, 1997; Sarantis et al., 2008). However, not all modulators targeting a common binding site may necessarily be capable of modulating ligand binding to other sites in the receptors. Furthermore, the reported degrees of radioligand binding enhancement induced by etomidate and loreclezole in these studies vary considerably, and the modulation has not been observed in all studies (Green et al., 1996). Thus, specific assay conditions seem to influence whether putative modulation of binding is detected in a radioligand binding assay.
An obvious caveat connected to the use of the competition binding assays in this screening is that not all ligands targeting an allosteric site in a certain target necessarily will compete with or modulate orthosteric radioligand binding to it. However, most targets assayed by radioligand binding in the screening were family A 7-transmembrane receptors (i.e., 37 of 48), and to our knowledge few (if any) allosteric modulators of these receptors have been reported not to affect orthosteric radioligand binding (Keov et al., 2011). Hence, although we cannot completely exclude the possibility that methaqualone could target an allosteric site in one (or several) of the receptors, the inactivity of the drug in the binding assays is likely to be a true reflection of its pharmacology at these receptors. In contrast, identification of ligands targeting multidomain receptor complexes such as the GABAARs or ionotropic glutamate receptors in a competition binding assay is likely to be more dependent on the specific radioligand and the experimental conditions used. Thus, the observed lack of effect of methaqualone on radioligand binding to these receptors should be seen only as a demonstration of the compound not binding to the specific site in the receptor complex targeted by the radioligand.
Multiparametric Description of the Effects of Methaqualone on Cortical Network Activity In Vitro
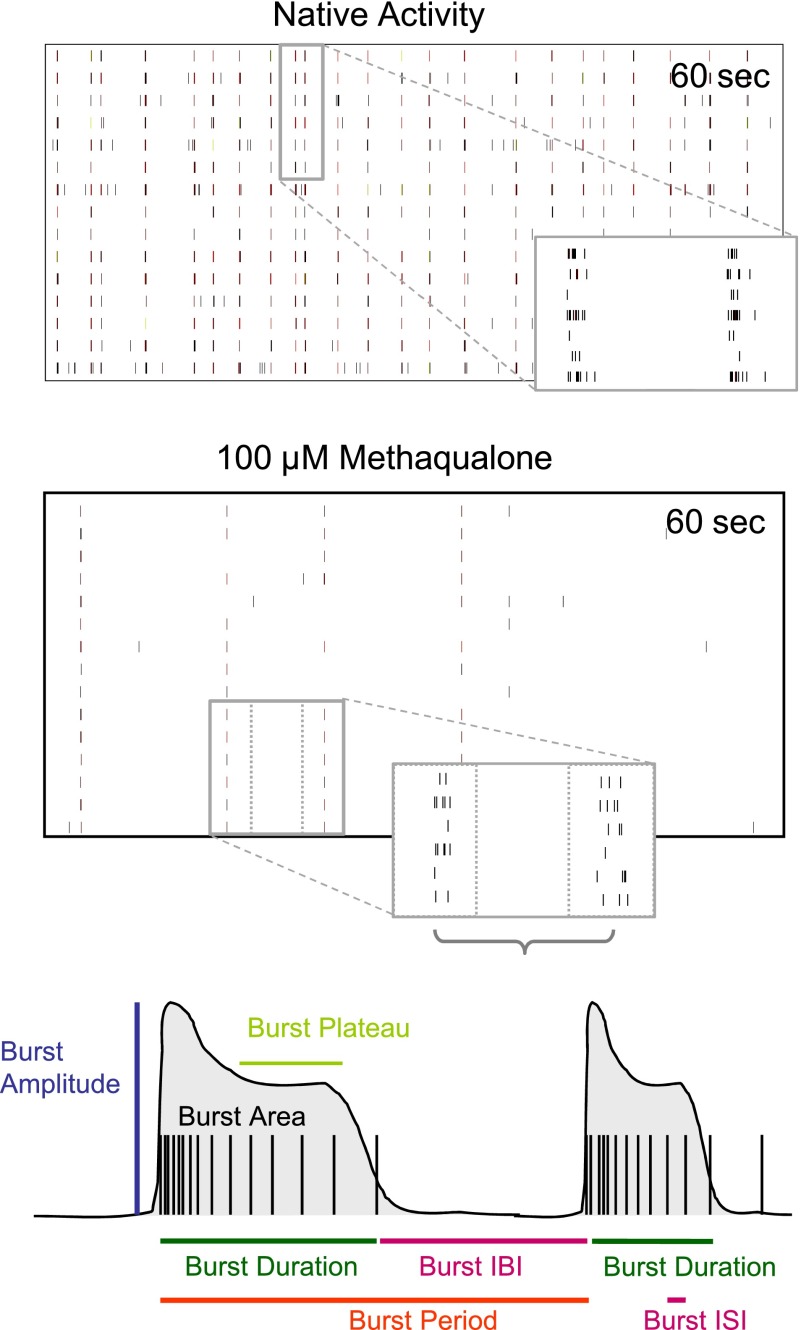
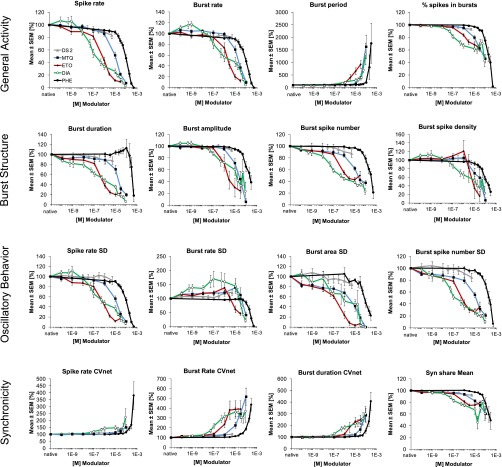
To investigate the effects of methaqualone at native GABAARs and on neuronal network activity, we analyzed the modulation exerted by the drug at the spontaneous activity pattern of primary neuronal networks from murine frontal cortex grown on MEA neurochips by multiparametric data analysis. This technology has previously been used extensively for neurotoxicity studies (Gramowski et al., 2006b; Johnstone et al., 2010; Novellino et al., 2011) but also for functional phenotypic screening of drugs (Gramowski et al., 2004, 2006a; Parenti et al., 2013). The 204 activity-describing parameters calculated based on the spike trains from these recordings can be divided into four categories. “General Activity” parameters represent global network activity descriptors such as spike rate, burst rate, percentage of spikes in bursts and burst period; “Burst Structure” parameters describe the internal structure of spikes within a high-frequency spiking phase (e.g., spike frequency in bursts, spike rate in bursts, spike density), as well as the overall burst structure (e.g., duration, area, plateau); “Oscillatory Behavior” parameters are the standard deviations associated with main general activity and burst structure parameters and illustrate the regularity of bursting events within experimental episodes, with higher values indicating less regular general activity or less regular burst structure. Finally, the “Synchronicity” parameters include those representing the coefficient of variation over the network, thus reflecting the level of synchronization among the neurons. Representative spike raster plots and some of the aforementioned extractable parameters from these recordings are given in Fig. 7.
Fig. 7.
Multiparametric analysis of cortical neuron network activity. Top panels: Representative spike raster plots of native cortical activity and cortical activity after acute treatment with 100 µM methaqualone. Reduction of overall spiking and bursting activity, as well as reduction of burst strength is observed (higher magnification). Bottom panel: Scheme of two simplified bursts outlining some of the parameters that can be extracted from the recordings. Parameters describing general activity [burst inter burst interval (IBI) and burst period] and burst structure [burst duration, burst plateau, burst amplitude, burst inter spike interval (ISI) and burst area] are indicated. Standard deviations of these parameters such as S.D. of burst rate and S.D. of burst duration are measures for regularity of general activity and burst structure, respectively.
Concentration-Effect Relationships for Methaqualone and Other GABAAR Modulators in the Network Recordings.
The overall profile of methaqualone in the recordings reflected in the 60 best-describing parameters was quite characteristic for a CNS depressant (Figs. 8 and 9). Application of the modulator in concentrations found to elicit significant effects at the recombinant GABAARs in the TEVC recordings (1–100 µM) resulted in significantly reduced spike and burst rates and increased the interval between bursts as well as the average burst period. Moreover, burst sizes were significantly reduced by the presence of these methaqualone concentrations (e.g., decreases in burst duration, burst area, and burst amplitude) (Figs. 8 and 9). The decreased variability observed for several of the burst structure parameters (e.g., burst area S.D. and burst spike number S.D.) indicated a more regular burst structure, whereas the overall bursting activity was observed to be more irregular (increase in burst rate S.D., interburst interval S.D., event period S.D.). Finally, increased network variability (e.g., burst rate CVnet and other CVnet parameters, decreased SynShare, the average number of units involved in population bursts) indicative of decreased synchronization within the network was observed at these modulator concentrations (Figs. 8 and 9). Interestingly, submicromolar concentrations of methaqualone also mediated significant effects on network activity although these were very subtle (Figs. 8 and 9).
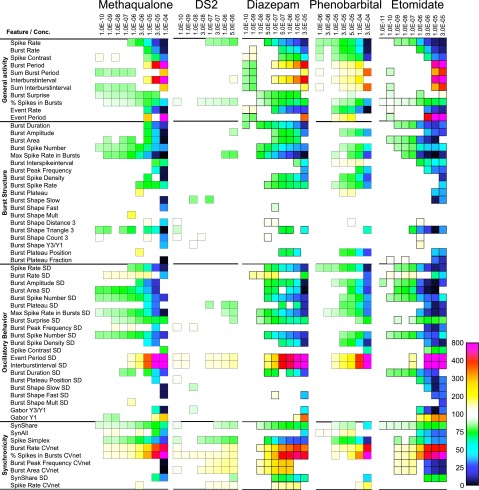
Fig. 8.
Summary of the changes induced by methaqualone, DS2, diazepam, phenobarbital, and etomidate on cortical network activity in vitro. The heat maps present the significant changes in 60 activity-describing parameters from four defined categories arising from eight or nine cumulatively increasing concentrations of the five modulators (concentrations are given in molar). The colors encode statistically significant modulator-induced changes (increases or decreases) in parameters relative to native activity (no drug, 100%).
Fig. 9.
Selected functional effects of methaqualone, DS2, etomidate, phenobarbital, and diazepam on cortical network activity in vitro. The effects of eight or nine cumulatively increasing concentrations of methaqualone (black square, blue line), DS2 (gray triangle, gray line), phenobarbital (black circle, black line), diazepam (open circle, green line), and etomidate (black diamond, red line) at 16 activity-describing parameters from four defined categories. Data are given as mean ± S.E.M. relative to native activity (no drug, 100%).
The functional characteristics of methaqualone in the MEA recordings were compared with those exhibited by four other GABAAR modulators: the benzodiazepine diazepam, the barbiturate phenobarbital, the general anesthetic etomidate, and DS2, a selective PAM of δ-containing GABAARs (Wafford et al., 2009). The multiparametric effects mediated by diazepam, etomidate, and phenobarbital at the cortical networks were quite similar to those induced by methaqualone, the different concentration-response relationships displayed by the four modulators being easily reconcilable with their different potencies as a GABAAR PAMs (Figs. 8 and 9). However, while the qualitatively trend in the changes induced by methaqualone, diazepam, etomidate, and phenobarbital was the same for most parameters, some interesting differences were observed. For example, high or saturating concentrations of etomidate or methaqualone induced more pronounced changes in some of the “General Activity”, “Burst Structure”, and “Oscillatory Behavior” parameters than high or saturating concentrations of phenobarbital or diazepam (Fig. 8). The general effects of DS2 on cortical network activity were much more subtle than those produced by the four other modulators, but most of the changes induced by this PAM were characterized by the same qualitative directions of parameter changes (Figs. 8 and 9).
Similarity Analysis and Classification.
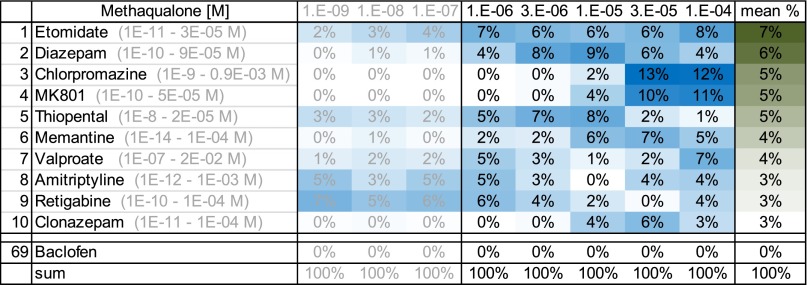
To assess the extent to which the effects of methaqualone at neuronal network activity resembled those induced by other types of CNS drugs and neuroactive compounds and whether its in vivo properties potentially could be ascribed to additional activity components than its GABAAR activity, the “phenotypic fingerprint” of the drug was compared with those exhibited by 69 reference compounds. This similarity analysis compares characteristics and patterns of the effects induced by the respective drugs on the activity-describing recording parameters, which means that compounds exhibiting different degrees of effects on a parameter can be classified as similar. The database compounds selected for the analysis in the present study comprised compounds targeting numerous different neurotransmitter systems through various mechanisms and included several classes of clinically administered therapeutics, including antidepressants, antipsychotics, anticonvulsants, antisedatives, analgesics, anesthetics, and procognitive drugs (Table 3). A training data set with the 204 spike train parameters was established using the data records for the 69 reference compounds, and subsequently the data records of methaqualone were classified as previously described (Parenti et al., 2013), resulting in a ranking list reflecting the functional similarity of each of the methaqualone concentrations with the database compounds. Thus, the effects mediated by specific methaqualone concentrations were compared with the profiles of each of the reference compounds (i.e., the averaged effects induced by multiple concentrations of the reference compound). With the exception of the classic antipsychotic drug chlorpromazine and the antidepressant amitriptyline, the database compounds giving rise to effects exhibiting the highest similarities to those mediated by methaqualone (1–100 µM) were GABAAR PAMs (etomidate, diazepam, thiopental, clonazepam), NMDA receptor antagonists (MK801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate], memantine), and other CNS depressants (valproate, retigabine) (Fig. 10).
TABLE 3.
The 69 NeuroProof database compounds compared with methaqualone in the similarity analysis
| Acetaminophen | Epibatidine | Muscimol |
| Agmatine | Eserine | Olanzapine |
| Amisulpride | Etomidate | Oxotremorine |
| Amitriptyline | Flumazenil | Pentylenetetrazolium |
| AMPA | Flunitrazepam | Phenytoin |
| Apomorphine | Fluoxetine | Picrotoxin |
| Aripiprazole | GABA | Propofol |
| Atropine | Galanthamine | Quetiapine |
| Baclofen | GS 39783 | Retigabine |
| Benzoquinone | Haloperidol | Risperidone |
| Carbamazepine | Ibuprofen | SB 205384 |
| CGP 7930 | Indatraline | SCH 50911 |
| Chlorpromazine | L-655708 | SKF-97541 |
| CL218872 | L-838417 | Sufentanil |
| Clobazam | Lamotrigine | Thiopental |
| Clonazepam | Levetiracetam | Thio-THIP |
| Clozapine | l-Polamidon | THIP |
| “Control” | LY341495 | Topiramate |
| d-Cycloserine | LY354740 | Tramadol |
| Diazepam | LY393558 | Valproate |
| Dimethylsulfoxide | Memantine | Wortmannin |
| Donepezil | MK801 | Xli093 |
| DS2 | Morphine | Zolpidem |
CGP 7930, 2,6-ditert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)phenol; CL218872, 3-methyl-6-[-3-(trifluoromethyl)phenyl]-1,2,4-triazolo[4,3-b]pyridazine; GS 39783, 4-N,6-N-dicyclopentyl-2-methylsulfanyl-5-nitropyrimidine-4,6-diamine; L-655708, ethyl (S)-11,12,13,13a-tetrahydro-7-methoxy-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylate; L-838417, 7-tert-butyl-3-(2,5-difluorophenyl)-6-[(2-methyl-1,2,4-triazol-3-yl)methoxy]-[1,2,4]triazolo[4,3-b]pyridazine; LY341495, (1S,2S)-2-[(1S)-1-amino-1-carboxy-2-(9H-xanthen-9-yl)ethyl]cyclopropane-1-carboxylic acid; LY354740, (1S,2S,5R,6S)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid; LY393558, 1-[2-[4-(6-fluoro-1H-indol-3-yl)-3,6-dihydro-1(2H)-pyridinyl]ethyl]-3,4-dihydro-3-(1-methylethyl)-6-(methylsulfonyl)-1H-2,1,3-benzothiadiazine-2,2-dioxide; SB 205384, but-2-ynyl 4-amino-7-hydroxy-2-methyl-5,6,7,8-tetrahydro-[1]benzothiolo[2,3-b]pyridine-3-carboxylate; SCH 50911, (2S)-(+)-5,5-dimethyl-2-morpholineacetic acid; SKF-97541, 3-aminopropyl(methyl)phosphinic acid; THIP, 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol; Xli093, bis[8-ethynyl-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid] 1,3-propanediyl ester hydrate.
Fig. 10.
Similarity analysis of the effects of methaqualone at cortical network activity. Top 10 ranks of the most phenotypically similar functional profiles of 69 reference compounds from the NeuroProof database (listed in Table 3) ranked based on the similarity score for methaqualone at concentrations ranging from 1 to 100 µM. Data for the methaqualone concentrations 1, 10, and 100 nM are given in shaded colors. The concentration-response profiles of the 69 reference compounds were used for training the classifier, and the methaqualone data sets were classified per concentration (10 per concentration). Table values correspond to similarity score per concentration (e.g., at 100 µM methaqualone, 8% of its data sets were classified as etomidate, 4% as diazepam, 12% as chlorpromazine, and so forth). High values reflect high functional phenotypic similarity between reference compound effects and methaqualone effects.
In Vivo Exposure and Efficacy of Methaqualone in Seizure Threshold and Motor Coordination Assays
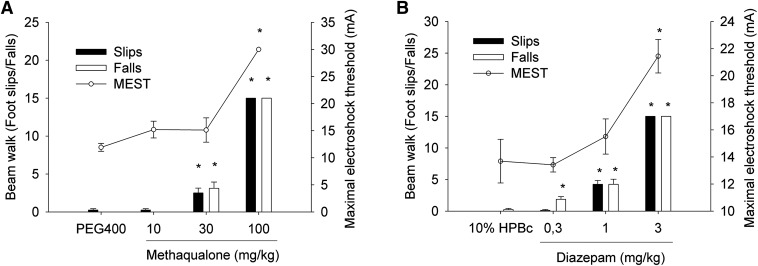
To investigate to what extent the in vitro properties displayed by methaqualone at GABAARs correlated with its in vivo efficacy, exposure studies of the drug were combined with testing it in MEST and beam-walk assays in mice using diazepam as a reference GABAAR modulator (Fig. 11).
Fig. 11.
Sedative or ataxic effects and anticonvulsant efficacy of methaqualone (A) and diazepam (B) in beam walk and MEST assays in mice. Data are given as average slips and falls (mean ± S.E.M.) and by the average current threshold (mean ± S.E.M.), respectively. *P < 0.05 analysis of variance and post hoc Dunnett’s test. HPBc; hydroxypropyl-β-cyclodextrin
In preliminary exposure studies, plasma and brain concentrations of methaqualone were determined 15, 30, 60, and 120 minutes after subcutaneous administration of 10 mg/kg of the drug, and since both concentrations peaked at 60 minutes, this study design was used for the subsequent experiments. After administration of 10-, 30-, and 100-mg/kg doses of methaqualone, plasma concentrations of the drug were determined to 2.79 ± 0.57, 12.3 ± 0.93, and 26.7 ± 0.63 μg/ml, respectively, and brain concentrations to 0.71 ± 0.10, 4.1 ± 0.23, and 16.1 ± 0.27 μg/g, respectively (means ± S.E.M.; n = 3). Since the levels of free (unbound) fraction of methaqualone in mouse brain homogenates were determined to be 13%, corresponding unbound concentrations of methaqualone in brain were estimated at 0.37 ± 0.05, 2.14 ± 0.12, and 8.38 ± 0.14 µM for the 10-, 30-, and 100-mg/kg doses, respectively (Fig. 11A). Plasma and brain concentrations of the reference drug diazepam were not determined in this study; however, a previous study has found subcutaneous administration of 3 mg/kg of diazepam in mice to produce a total brain concentration of 400 ng/g (30 minutes after administration) (Doran et al., 2005), and we have determined the fraction of free (unbound) diazepam in mouse brain to be 3%. Extrapolating from this finding, the 0.3-, 1-, and 3-mg/kg diazepam doses used in this study (Fig. 11B) would correspond to brain free diazepam concentrations of approximately 4, 15, and 40 nM, respectively.
Both methaqualone and diazepam displayed significant effects in the two animal models. Whereas subcutaneous administration of 100 mg/kg of methaqualone significantly increased seizure threshold in the animals in the MEST assay, administration of 30 or 100 mg/kg of methaqualone resulted in significantly increased numbers of slips and falls in the beam-walk assay (Fig. 11A). Analogously, diazepam and other benzodiazepines in these models induced significant sedative or ataxia effects at lower doses than those required to produce significant anticonvulsant effects (Fig. 11B and unpublished data). The doses of the two drugs needed to induce sedative or ataxia and anticonvulsant effects could seem somewhat high at first glance. However, it is well documented that behavioral readouts in rodents and humans on drug treatment do not always correlate, something that has been ascribed to the substantially faster hepatic clearance and drug metabolism in rodents (Lave et al., 1999; Sharma and McNeill, 2009).
Discussion
Investigations into the mechanisms of action of old CNS drugs hold interesting perspectives. Although a drug may have been shelved for good reasons, new insights into the molecular basis for its clinical efficacy can open new avenues of drug development, as exemplified by the current interest in ketamine as a lead for novel antidepressants (Sanacora and Schatzberg, 2015). Moreover, these explorations can shed light on previous observations for the drug and its target and could potentially reinvent the drug as a useful pharmacologic tool. In the present study, the notorious past and elusive mode of action of methaqualone (and other quaaludes) prompted us to explore the molecular basis underlying its therapeutic and recreational effects.
Methaqualone Is a Multifaceted GABAAR Modulator.
Methaqualone was found to be a pan-active GABAAR modulator exhibiting activity at 12 of 13 GABAAR subtypes (Table 1). Albeit a relatively pure PAM at most of these receptors, methaqualone exhibited everything from inactivity over negative or positive modulation to pronounced agonism within this selection of subtypes. Moreover, the nature of its potentiation of α1,2,3,5β2,3γ2S and α4β2δ/α6β2,3δ signaling differed, as it exclusively modulated GABA potency at the αβγ receptor, whereas it increased both GABA potency and efficacy at the αβδ receptor. The presence of γ2 or δ in the GABAAR was clearly not a prerequisite for methaqualone-mediated modulation, but these differential PAM characteristics nevertheless must be ascribed to the distinct functional properties of αβγ and αβδ receptors. Whereas the remarkable high-efficacious direct activation of the α4β3δ receptor mediated by methaqualone could be a reflection of true allosteric agonism, we cannot exclude that it could arise from potentiation of the pronounced spontaneous activity of this receptor, analogously to the mechanism proposed to underlie the apparent agonism displayed by DS2 at this receptor in a recent study (Jensen et al., 2013). The fact that etomidate also displays superagonism at α4β3δ seems to support this latter hypothesis (Fig. 5). Conversely, the inability of both modulators to potentiate the constitutive activity exhibited by the α4β1δ receptor suggests that they could possess true intrinsic agonist activity at α4β3δ or, alternatively, that the molecular basis for the spontaneous activity of α4β1δ may differ from that of α4β3δ (Fig. 5).
The comparable functional potencies displayed by methaqualone as PAM, NAM, or agonist at the various GABAARs suggest that the modulator targets a uniform binding site in the receptors, a hypothesis further supported by the complete reversal of its modulatory properties at the α6β1δ and α6β2δ receptors brought on by β1-S265N and β2-N265S mutations (Fig. 4, B and C). Thus, the diverse functionalities of methaqualone are more likely to arise from different energy barriers underlying allosteric transitions in the respective subtypes than from the modulator targeting different sites or having substantially different binding modes in the receptors. Although the inactivity of methaqualone at α4β1δ would constitute an outlier in this scenario if rooted in low binding affinity, the compound could also be envisioned to act as a neutral ligand (or silent allosteric modulator) at this subtype. We will refrain from further speculations about this, however, not having investigated the basis for the lack of modulation at this subtype. Analogously to the notion of methaqualone exerting its multifaceted pharmacology through a uniform site in the GABAARs, some benzodiazepine-site ligands display functional selectivity at α1,2,3,5βγ2 receptors (Dawson et al., 2006; de Lucas et al., 2015), and allosteric modulators of other receptor types have also been shown to mediate subtype-specific modulation (Mathiesen et al., 2003; Marlo et al., 2009; Costa et al., 2010).
The bell-shaped concentration-response curves and rebound currents obtained for methaqualone at the GABAARs are very similar to those observed for other PAMs/ago-PAMs acting through the transmembrane receptor domains (Fig. 1, C and D) (Hill-Venning et al., 1997; Wooltorton et al., 1997; Halliwell et al., 1999; Feng and Macdonald, 2004; Feng et al., 2004; Khom et al., 2007). In several of these cases, the submaximal potentiation observed at high modulator concentrations has been attributed to the existence of a low-affinity open-channel block site, with the rebound currents arising from the rapid unbinding of the modulator from this site (Wooltorton et al., 1997; Halliwell et al., 1999; Feng et al., 2004; Khom et al., 2007). This also seems a plausible explanation for methaqualone, although the submaximal potentiation at high modulator concentrations also could be caused by increased receptor desensitization.
The Methaqualone Binding Site.
Solid experimental evidence indicates that methaqualone does not act through the benzodiazepine, barbiturate, or neurosteroid binding sites in the GABAAR (Fig. 3). We propose that the key importance of β-subunit identity and of β-residue 265 for the functionality of methaqualone constitutes a strong case for the transmembrane β(+)/α(–) interface as the targeted receptor region. In contrast, there are compelling reasons to be cautious when speculating about the exact location of the binding site within this interface. Allosteric modulators bind quite differently to the transmembrane subunit interfaces in Cys-loop receptors (Hibbs and Gouaux, 2011; Trattnig et al., 2012; Sauguet et al., 2013; Stewart et al., 2013; Lansdell et al., 2015) and having only investigated two residues as putative binding partners, we can hardly claim to have obtained a detailed insight into the binding mode of methaqulone. Albeit the changed modulation displayed by methaqualone at α1M236Wβ2γ2S and α1β2M286Wγ2S receptors could indicate involvement of these residues in modulator binding, it could also arise from allosteric effects of the introduced mutations (Siegwart et al., 2002; Chiara et al., 2012). However, the structural similarities between methaqualone, etomidate, and loreclezole and the common pharmacophore identified for them support the notion of a shared binding site (Fig. 6), analogously to what has been proposed for etomidate, loreclezole, and mefenamic acid (Halliwell et al., 1999). Thus, these “β-residue 265 sensitive” modulators could be envisioned to act through such a common site, with ligands not sensitive to changes in this position possibly binding differently to this interface (Thompson et al., 2004).
Functional Characteristics of Methaqualone at Cortical Neurons.
All in all, the effects of methaqualone on neuronal firing patterns in cortical networks seem to be reconcilable with its GABAAR activity, and comparison of these effects to those induced by other GABAAR modulators serves to elucidate the contributions from the different activity components of the modulator (Figs. 8 and 9). The four modulators targeting αβγ receptors (methaqualone, diazepam, etomidate, and phenobarbital) all induced pronounced changes in the spontaneous activity patterns, and thus augmentation of synaptic GABAAR signaling appears to be key for overall effects on cortical network activity. In contrast, the much more subtle effects induced by DS2 indicate that the isolated contributions from the potentiation of δ-containing GABAARs to network activity are minor. Nevertheless, this activity component could be important for the observed effects of methaqualone since potentiation of these extrasynaptic GABAARs would be expected to affect the firing threshold of the neurons.
The key role of GABAARs for the methaqualone-mediated effects is further supported by the high-ranking of several GABAAR PAMs, NMDA receptor antagonists, and other known CNS depressants in the similarity analysis comparing the network activity effects of methaqualone to the phenotypic profiles of 69 reference compounds with diverse pharmacologic and therapeutic properties (Fig. 10; Table 3). The high-ranking of chlorpromazine constitutes a notable exception in this respect. Although micromolar chlorpromazine concentrations have been reported to modulate GABAergic currents in hippocampal neurons (Mozrzymas et al., 1999a,b), it is a highly promiscuous drug with potent activity at numerous receptors, transporters, and ion channels, and thus its effects on neuronal network activity are likely to arise from several other mediators.
Correlation between In Vitro GABAAR Activity and In Vivo Efficacy of Methaqualone.
As discussed above, the negligible activity displayed by methaqualone at a plethora of neurotransmitter targets in the screening and its effects on cortical network activity in the MEA recordings all in all seem to indicate that it is a fairly selective GABAAR modulator (Figs. 7–10; Table 2). This finding prompted us to investigate whether its GABAAR activity could account for its in vivo efficacy. The estimated free (unbound) methaqualone concentrations in mouse brain arising from in vivo effective doses (2–8 μM) were in the very low end of the effective concentration range for the modulator at GABAARs in the TEVC recordings. This apparent mismatch is unlikely to arise from substantially different pharmacologic properties of methaqualone at recombinant human and native rodent GABAARs, just as it seems improbable that its in vivo effects are mediated through another target. Instead, it could simply be a reflection of allosteric GABAAR modulators being efficacious in vivo at low levels of receptor occupancy. Interestingly, the estimated free concentrations of diazepam in mice brains produced by in vivo effective doses (4–40 nM) were also substantially lower than the functional potency of the benzodiazepine at recombinant αβγ GABAARs (EC50 ∼100 nM). In this light, our data could support the notion of GABAARs as the key mediators of the in vivo effects of methaqualone.
Conclusion.
The present delineation of the molecular basis for the behavioral effects of methaqualone does more than confirm what has been assumed for decades: that the drug mediates these effects through GABAARs. Methaqualone exhibits distinct functional properties at the GABAARs compared with other allosteric modulators, and it mediates these through a different mechanism than the barbiturates and benzodiazepines that it historically has been lumped together with. It is tempting to speculate that these differences could contribute to the reported differences in the in vivo effects induced by methaqualone and classic CNS depressants. In any case, the multifaceted functionality of methaqualone at GABAARs seems to be at the root of its clinical efficacy, as well as the addiction liability and recreational misuse associated with the drug.
Acknowledgments
The authors thank Dr. P. J. Whiting and Merck, Sharpe, and Dohme Research Laboratories for the generous gifts of the human GABAAR cDNAs, and Dr. S. Mennerick for kind advice.
Abbreviations
- CNS
central nervous system
- DS2
4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridine-3-yl benzamide
- GABAARs
GABA type A receptors
- MEA
microelectrode array
- MEST
maximal electroshock seizures threshold
- MK801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate, NAM, negative allosteric modulator
- PAM
positive allosteric modulator
- PDSP
Psychoactive Drug Screening Program
- TEVC
two-electrode voltage clamp
- WT
wild-type
Authorship Contributions
Participated in research design: Hammer, Bader, Schroeder, Bastlund, Gramowski-Voß, Jensen.
Conducted experiments: Hammer, Bader, Ehnert, Bundgaard, Bunch, Jensen.
Performed data analysis: Hammer, Bader, Ehnert, Hoestgaard-Jensen, Bastlund, Jensen.
Wrote or contributed to the writing of the manuscript: Hammer, Bader, Jensen (with contributions from Ehnert, Bundgaard, Bunch, Bastlund).
Footnotes
This study was supported financially by the Novo Nordisk Foundation. The in vitro binding profiling of methaqualone was generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP) [Grant HHSN-271-2008-025C]. The NIMH PDSP is directed by Bryan L. Roth at the University of North Carolina at Chapel Hill and project officer Jamie Driscol at NIMH, Bethesda, MD.
References
- Bali M, Akabas MH. (2004) Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol 65:68–76. [DOI] [PubMed] [Google Scholar]
- Barcelo R. (1961) A clinical study of methaqualone: a new nonbarbiturate hypnotic. Can Med Assoc J 85:1304–1305. [PMC free article] [PubMed] [Google Scholar]
- Barceloux DG (2012) Methaqualone and related compounds, in Medical Toxicology of Drug Abuse, pp 504–513, John Wiley & Sons, Inc., New York. [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. (2009) Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci 29:12757–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. (1997) The interaction of the general anesthetic etomidate with the γ-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci USA 94:11031–11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I. (2012) Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron 73:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. (2002) Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol 136:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo-Soria C, Chang Y, Weiss DS. (2006) Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol 148:984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M, Gallo G. (1985) Quaaludes: The Quest for Oblivion, Chelsea House, New York. [Google Scholar]
- Chen ZW, Wang C, Krishnan K, Manion BD, Hastings R, Bracamontes J, Taylor A, Eaton MM, Zorumski CF, Steinbach JH, et al. (2014) 11-trifluoromethyl-phenyldiazirinyl neurosteroid analogues: potent general anesthetics and photolabeling reagents for GABAA receptors. Psychopharmacology (Berl) 231:3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. (2012) Mapping general anesthetic binding site(s) in human α1β3 γ-aminobutyric acid type A receptors with [³H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry 51:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. (2013) Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 γ-aminobutyric acid type A (GABAA) receptor. J Biol Chem 288:19343–19357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Skifter DA, Jane DE, Monaghan DT. (2010) A novel family of negative and positive allosteric modulators of NMDA receptors. J Pharmacol Exp Ther 335:614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, et al. (2006) An inverse agonist selective for α5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther 316:1335–1345. [DOI] [PubMed] [Google Scholar]
- de Lucas AG, Ahring PK, Larsen JS, Rivera-Arconada I, Lopez-Garcia JA, Mirza NR, Munro G. (2015) GABAA α5 subunit-containing receptors do not contribute to reversal of inflammatory-induced spinal sensitization as indicated by the unique selectivity profile of the GABAA receptor allosteric modulator NS16085. Biochem Pharmacol 93:370–379. [DOI] [PubMed] [Google Scholar]
- Desai R, Ruesch D, Forman SA. (2009) γ-amino butyric acid type A receptor mutations at β2N265 alter etomidate efficacy while preserving basal and agonist-dependent activity. Anesthesiology 111:774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran A, Obach RS, Smith BJ, Hosea NA, Becker S, Callegari E, Chen C, Chen X, Choo E, Cianfrogna J, et al. (2005) The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: evaluation using the MDR1A/1B knockout mouse model. Drug Metab Dispos 33:165–174. [DOI] [PubMed] [Google Scholar]
- Falco M. (1976) Methaqualone misuse: foreign experience and United States drug control policy. Int J Addict 11:597–610. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Bianchi MT, Macdonald RL. (2004) Pentobarbital differentially modulates α1β3δ and α1β3γ2L GABAA receptor currents. Mol Pharmacol 66:988–1003. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Macdonald RL. (2004) Multiple actions of propofol on αβγ and αβδ GABAA receptors. Mol Pharmacol 66:1517–1524. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Macdonald RL. (2010) Barbiturates require the N terminus and first transmembrane domain of the δ subunit for enhancement of α1β3δ GABAA receptor currents. J Biol Chem 285:23614–23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT. (2008) Drugs The Straight Facts: Qualuudes, Chelsea House, New York. [Google Scholar]
- Ghiani CA, Tuligi G, Maciocco E, Serra M, Sanna E, Biggio G. (1996) Biochemical evaluations of the effects of loreclezole and propofol on the GABAA receptor in rat brain. Biochem Pharmacol 51:1527–1534. [DOI] [PubMed] [Google Scholar]
- Gielen MC, Lumb MJ, Smart TG. (2012) Benzodiazepines modulate GABAA receptors by regulating the preactivation step after GABA binding. J Neurosci 32:5707–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramowski A, Jügelt K, Stüwe S, Schulze R, McGregor GP, Wartenberg-Demand A, Loock J, Schröder O, Weiss DG. (2006a) Functional screening of traditional antidepressants with primary cortical neuronal networks grown on multielectrode neurochips. Eur J Neurosci 24:455–465. [DOI] [PubMed] [Google Scholar]
- Gramowski A, Jügelt K, Weiss DG, Gross GW. (2004) Substance identification by quantitative characterization of oscillatory activity in murine spinal cord networks on microelectrode arrays. Eur J Neurosci 19:2815–2825. [DOI] [PubMed] [Google Scholar]
- Gramowski A, Stüwe S, Jügelt K, Schiffmann D, Loock J, Schröder O, Gross GW, Weiss DG. (2006b) Detecting neurotoxicity through electrical activity changes of neuronal networks on multielectrode neurochips. ALTEX 23:410–415. [Google Scholar]
- Green AR, Misra A, Murray TK, Snape MF, Cross AJ. (1996) A behavioural and neurochemical study in rats of the pharmacology of loreclezole, a novel allosteric modulator of the GABAA receptor. Neuropharmacology 35:1243–1250. [DOI] [PubMed] [Google Scholar]
- Greenfield LJ, Jr, Zaman SH, Sutherland ML, Lummis SC, Niemeyer MI, Barnard EA, Macdonald RL. (2002) Mutation of the GABAA receptor M1 transmembrane proline increases GABA affinity and reduces barbiturate enhancement. Neuropharmacology 42:502–521. [DOI] [PubMed] [Google Scholar]
- Halliwell RF, Thomas P, Patten D, James CH, Martinez-Torres A, Miledi R, Smart TG. (1999) Subunit-selective modulation of GABAA receptors by the non-steroidal anti-inflammatory agent, mefenamic acid. Eur J Neurosci 11:2897–2905. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. (2007) Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol Ther 116:20–34. [DOI] [PubMed] [Google Scholar]
- Herzberg D. (2011) Blockbusters and controlled substances: Miltown, Quaalude, and consumer demand for drugs in postwar America. Stud Hist Philos Biol Biomed Sci 42:415–426. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks TP, Kaneko T, Oka JI. (1990) Receptive-field size of S1 cortical neurones is altered by methaqualone via a GABA mechanism. Can J Neurol Sci 17:30–34. [DOI] [PubMed] [Google Scholar]
- Hill-Venning C, Belelli D, Peters JA, Lambert JJ. (1997) Subunit-dependent interaction of the general anaesthetic etomidate with the γ-aminobutyric acid type A receptor. Br J Pharmacol 120:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoestgaard-Jensen K, Dalby NO, Krall J, Hammer H, Krogsgaard-Larsen P, Frølund B, Jensen AA. (2014) Probing α4βδ GABAA receptor heterogeneity: differential regional effects of a functionally selective α4β1δ/α4β3δ receptor agonist on tonic and phasic inhibition in rat brain. J Neurosci 34:16256–16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoestgaard-Jensen K, O’Connor RM, Dalby NO, Simonsen C, Finger BC, Golubeva A, Hammer H, Bergmann ML, Kristiansen U, Krogsgaard-Larsen P, et al. (2013) The orthosteric GABAA receptor ligand Thio-4-PIOL displays distinctly different functional properties at synaptic and extrasynaptic receptors. Br J Pharmacol 170:919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. (2009) Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology 56:149–154. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. (2006) Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444:486–489. [DOI] [PubMed] [Google Scholar]
- Ionescu-Pioggia M, Bird M, Orzack MH, Benes F, Beake B, Cole JO. (1988) Methaqualone. Int Clin Psychopharmacol 3:97–109. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Bergmann ML, Sander T, Balle T. (2010) Ginkgolide X is a potent antagonist of anionic Cys-loop receptors with a unique selectivity profile at glycine receptors. J Biol Chem 285:10141–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ML, Wafford KA, Brown AR, Belelli D, Lambert JJ, Mirza NR. (2013) A study of subunit selectivity, mechanism and site of action of the δ selective compound 2 (DS2) at human recombinant and rodent native GABAA receptors. Br J Pharmacol 168:1118–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone AF, Gross GW, Weiss DG, Schroeder OH, Gramowski A, Shafer TJ. (2010) Microelectrode arrays: a physiologically based neurotoxicity testing platform for the 21st century. Neurotoxicology 31:331–350. [DOI] [PubMed] [Google Scholar]
- Karim N, Wellendorph P, Absalom N, Bang LH, Jensen ML, Hansen MM, Lee HJ, Johnston GA, Hanrahan JR, Chebib M. (2012) Low nanomolar GABA effects at extrasynaptic α4β1/β3δ GABAA receptor subtypes indicate a different binding mode for GABA at these receptors. Biochem Pharmacol 84:549–557. [DOI] [PubMed] [Google Scholar]
- Karim N, Wellendorph P, Absalom N, Johnston GA, Hanrahan JR, Chebib M. (2013) Potency of GABA at human recombinant GABAA receptors expressed in Xenopus oocytes: a mini review. Amino Acids 44:1139–1149. [DOI] [PubMed] [Google Scholar]
- Keov P, Sexton PM, Christopoulos A. (2011) Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology 60:24–35. [DOI] [PubMed] [Google Scholar]
- Khom S, Baburin I, Timin E, Hohaus A, Trauner G, Kopp B, Hering S. (2007) Valerenic acid potentiates and inhibits GABAA receptors: molecular mechanism and subunit specificity. Neuropharmacology 53:178–187. [DOI] [PubMed] [Google Scholar]
- Kimball AW, Burnett WT, Jr, Doherty DG. (1957) Chemical protection against ionizing radiation. I. Sampling methods for screening compounds in radiation protection studies with mice. Radiat Res 7:1–12. [PubMed] [Google Scholar]
- Krasowski MD, Nishikawa K, Nikolaeva N, Lin A, Harrison NL. (2001) Methionine 286 in transmembrane domain 3 of the GABAA receptor β subunit controls a binding cavity for propofol and other alkylphenol general anesthetics. Neuropharmacology 41:952–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdell SJ, Sathyaprakash C, Doward A, Millar NS. (2015) Activation of human 5-hydroxytryptamine type 3 receptors via an allosteric transmembrane site. Mol Pharmacol 87:87–95. [DOI] [PubMed] [Google Scholar]
- Lavé T, Coassolo P, Reigner B. (1999) Prediction of hepatic metabolic clearance based on interspecies allometric scaling techniques and in vitro-in vivo correlations. Clin Pharmacokinet 36:211–231. [DOI] [PubMed] [Google Scholar]
- Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. (2006) Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci 26:11599–11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Schmidt D. (1988) Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res 2:145–181. [DOI] [PubMed] [Google Scholar]
- Marlo JE, Niswender CM, Days EL, Bridges TM, Xiang Y, Rodriguez AL, Shirey JK, Brady AE, Nalywajko T, Luo Q, et al. (2009) Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol 75:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen JM, Svendsen N, Bräuner-Osborne H, Thomsen C, Ramirez MT. (2003) Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol 138:1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Myers B, Siegfried N. (2005) Treatment for methaqualone dependence in adults. Cochrane Database Syst Rev (2):CD004146. [DOI] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. (2009) Etomidate, propofol and the neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAA receptors expressed in HEK cells. Neuropharmacology 56:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Patel B, Smart TG. (2011) GABA potency at GABAA receptors found in synaptic and extrasynaptic zones. Front Cell Neurosci 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozrzymas JW, Barberis A, Cherubini E. (1999a) Facilitation of miniature GABAergic currents by chlorpromazine in cultured rat hippocampal cells. Neuroreport 10:2251–2254. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Barberis A, Michalak K, Cherubini E. (1999b) Chlorpromazine inhibits miniature GABAergic currents by reducing the binding and by increasing the unbinding rate of GABAA receptors. J Neurosci 19:2474–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller WE, Schläfer U, Wollert U. (1978) Benzodiazepine receptor binding: the interactions of some non-benzodiazepine drugs with specific [3H] diazepam binding to rat brain synaptosomal membranes. Naunyn Schmiedebergs Arch Pharmacol 305:23–26. [DOI] [PubMed] [Google Scholar]
- Naik SR, Naid PR, Sheth UK. (1978) Involvement of γ-amino butyric acid (GABA) in the anticonvulsant action of methaqualone. Psychopharmacology (Berl) 57:103–107. [DOI] [PubMed] [Google Scholar]
- Novellino A, Scelfo B, Palosaari T, Price A, Sobanski T, Shafer TJ, Johnstone AF, Gross GW, Gramowski A, Schroeder O, et al. (2011) Development of micro-electrode array based tests for neurotoxicity: assessment of interlaboratory reproducibility with neuroactive chemicals. Front Neuroeng 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. (2008) International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev 60:243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti C, Turnaturi R, Aricò G, Gramowski-Voss A, Schroeder OH, Marrazzo A, Prezzavento O, Ronsisvalle S, Scoto GM, Ronsisvalle G, et al. (2013) The multitarget opioid ligand LP1’s effects in persistent pain and in primary cell neuronal cultures. Neuropharmacology 71:70–82. [DOI] [PubMed] [Google Scholar]
- Parry CD, Myers B, Morojele NK, Flisher AJ, Bhana A, Donson H, Plüddemann A. (2004) Trends in adolescent alcohol and other drug use: findings from three sentinel sites in South Africa (1997-2001). J Adolesc 27:429–440. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CC, Goldstein L, Murphree HB. (1968) Quantitative analysis of the effect of methqualone on the human EEG. J Clin Pharmacol J New Drugs 8:235–244. [DOI] [PubMed] [Google Scholar]
- Quast U, Brenner O. (1983) Modulation of [3H]muscimol binding in rat cerebellar and cerebral cortical membranes by picrotoxin, pentobarbitone, and etomidate. J Neurochem 41:418–425. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Elster L, Frederiksen K, Bundgaard C, de Jong IE, Smith GP, Bruun AT, Larsen PH, Didriksen M. (2012) Negative modulation of GABAA α5 receptors by RO4938581 attenuates discrete sub-chronic and early postnatal phencyclidine (PCP)-induced cognitive deficits in rats. Psychopharmacology (Berl) 221:451–468. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Schatzberg AF. (2015) Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology 40:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantis K, Sotiriou E, Papatheodoropoulos C, Matsokis N, Angelatou F. (2008) Differential pharmacological properties of GABAA/benzodiazepine receptor complex in dorsal compared to ventral rat hippocampus. Neurochem Int 52:1019–1029. [DOI] [PubMed] [Google Scholar]
- Sauguet L, Howard RJ, Malherbe L, Lee US, Corringer PJ, Harris RA, Delarue M. (2013) Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun 4:1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena RC, Thacore VR, Suri ML, Agarwal TN, Bhargava KP. (1977) Electroencephalographic studies with i.v. methaqualone in man. Electroencephalogr Clin Neurophysiol 43:876–879. [DOI] [PubMed] [Google Scholar]
- Serafini R, Bracamontes J, Steinbach JH. (2000) Structural domains of the human GABAA receptor β3 subunit involved in the actions of pentobarbital. J Physiol 524:649–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, McNeill JH. (2009) To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 157:907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. (2015) Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv Pharmacol 72:53–96. [DOI] [PubMed] [Google Scholar]
- Siegwart R, Jurd R, Rudolph U. (2002) Molecular determinants for the action of general anesthetics at recombinant α2β3γ2 γ-aminobutyric acidA receptors. J Neurochem 80:140–148. [DOI] [PubMed] [Google Scholar]
- Sigel E, Lüscher BP. (2011) A closer look at the high affinity benzodiazepine binding site on GABAA receptors. Curr Top Med Chem 11:241–246. [DOI] [PubMed] [Google Scholar]
- Slany A, Zezula J, Fuchs K, Sieghart W. (1995) Allosteric modulation of [3H]flunitrazepam binding to recombinant GABAA receptors. Eur J Pharmacol 291:99–105. [DOI] [PubMed] [Google Scholar]
- Stanley JL, Lincoln RJ, Brown TA, McDonald LM, Dawson GR, Reynolds DS. (2005) The mouse beam walking assay offers improved sensitivity over the mouse rotarod in determining motor coordination deficits induced by benzodiazepines. J Psychopharmacol 19:221–227. [DOI] [PubMed] [Google Scholar]
- Stewart D, Desai R, Cheng Q, Liu A, Forman SA. (2008) Tryptophan mutations at azi-etomidate photo-incorporation sites on α1 or β2 subunits enhance GABAA receptor gating and reduce etomidate modulation. Mol Pharmacol 74:1687–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DS, Hotta M, Li GD, Desai R, Chiara DC, Olsen RW, Forman SA. (2013) Cysteine substitutions define etomidate binding and gating linkages in the α-M1 domain of γ-aminobutyric acid type A (GABAA) receptors. J Biol Chem 288:30373–30386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DS, Pierce DW, Hotta M, Stern AT, Forman SA. (2014) Mutations at beta N265 in γ-aminobutyric acid type A receptors alter both binding affinity and efficacy of potent anesthetics. PLoS ONE 9:e111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stórustovu SI, Ebert B. (2006) Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther 316:1351–1359. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Wheat L, Brown NA, Wingrove PB, Pillai GV, Whiting PJ, Adkins C, Woodward CH, Smith AJ, Simpson PB, et al. (2004) Salicylidene salicylhydrazide, a selective inhibitor of β1-containing GABAA receptors. Br J Pharmacol 142:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trattnig SM, Harpsøe K, Thygesen SB, Rahr LM, Ahring PK, Balle T, Jensen AA. (2012) Discovery of a novel allosteric modulator of 5-HT3 receptors: inhibition and potentiation of Cys-loop receptor signaling through a conserved transmembrane intersubunit site. J Biol Chem 287:25241–25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Quirk K, McKernan RM, Wingrove PB, Whiting PJ, Kemp JA. (1994) A novel allosteric modulatory site on the GABAA receptor β subunit. Neuron 12:775–782. [DOI] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. (2009) Novel compounds selectively enhance δ subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology 56:182–189. [DOI] [PubMed] [Google Scholar]
- Walters RJ, Hadley SH, Morris KD, Amin J. (2000) Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosci 3:1274–1281. [DOI] [PubMed] [Google Scholar]
- Whiting PJ. (2003) GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov Today 8:445–450. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Lüddens H, Seeburg PH. (1992) A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem 267:1426–1429. [PubMed] [Google Scholar]
- Wingrove PB, Wafford KA, Bain C, Whiting PJ. (1994) The modulatory action of loreclezole at the γ-aminobutyric acid type A receptor is determined by a single amino acid in the β2 and β3 subunit. Proc Natl Acad Sci USA 91:4569–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Moss SJ, Smart TG. (1997) Pharmacological and physiological characterization of murine homomeric β3 GABAA receptors. Eur J Neurosci 9:2225–2235. [DOI] [PubMed] [Google Scholar]
- Xue BG, Friend JM, Gee KW. (1996) Loreclezole modulates [35S]t-butylbicyclophosphorothionate and [3H]flunitrazepam binding via a distinct site on the GABAA receptor complex. Eur J Pharmacol 300:125–130. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Simmonds MA. (1997) Interactions between loreclezole, chlormethiazole and pentobarbitone at GABAA receptors: functional and binding studies. Br J Pharmacol 121:1392–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]