Abstract
Group II activator of G-protein signaling (AGS) proteins contain one or more G-protein regulatory motifs (GPR), which serve as docking sites for GαiGDP independent of Gβγ and stabilize the GDP-bound conformation of Gαi, acting as guanine nucleotide dissociation inhibitors. The GαGPR interaction is regulated by seven-transmembrane-spanning (7TM) receptors in the intact cell as determined by bioluminescence resonance energy transfer (BRET). It is hypothesized that a 7TM receptor directly couples to the GαGPR complex in a manner analogous to receptor coupling to the Gαβγ heterotrimer. As an initial approach to test this hypothesis, we used BRET to examine 7TM receptor–mediated regulation of GαGPR in the intact cell when Gαi2 yellow fluorescent protein (YFP) was tethered to the carboxyl terminus of the α2A adrenergic receptor (α2AAR-Gαi2YFP). AGS3– and AGS4–Renilla luciferase (Rluc) exhibited robust BRET with the tethered GαiYFP, and this interaction was regulated by receptor activation localizing the regulation to the receptor microenvironment. Agonist regulation of the receptor-Gαi-GPR complex was also confirmed by coimmunoprecipitation and cell fractionation. The tethered Gαi2 was rendered pertussis toxin–insensitive by a C352I mutation, and receptor coupling to endogenous Gαi/oβγ was subsequently eliminated by cell treatment with pertussis toxin (PT). Basal and agonist-induced regulation of α2AAR-Gαi2YFPC352I:AGS3Rluc and α2AAR-Gαi2YFPC352I:AGS4Rluc BRET was not altered by PT treatment or Gβγ antagonists. Thus, the localized regulation of GαGPR by receptor activation appears independent of endogenous Gαi/oβγ, suggesting that GαiAGS3 and GαiAGS4 directly sense agonist-induced conformational changes in the receptor, as is the case for 7TM receptor coupling to the Gαβγ heterotrimer. The direct coupling of a receptor to the GαiGPR complex provides an unexpected platform for signal propagation with broad implications.
Introduction
The discovery of the group of proteins defined as activator of G-protein signaling (AGS) in a yeast-based functional screen for mammalian cDNAs that activated G-protein signaling in the absence of a receptor, revealed both unexpected regulatory mechanisms for G-protein signaling systems and expanded functional roles for the G-protein subunits (Cismowski et al., 1999; Takesono et al., 1999; Cao et al., 2004; Sato et al., 2006, 2011). Group I AGS proteins encompass nonreceptor guanine nucleotide exchange factors, whereas group II AGS proteins, all of which contain a G-protein regulatory (GPR) motif, engage Gαi/o/t as guanine nucleotide dissociation inhibitors. Group III AGS proteins appear to engage Gβγ, whereas group IV AGS proteins, which were just recently identified, interact with Gα16 (Sato et al., 2011).
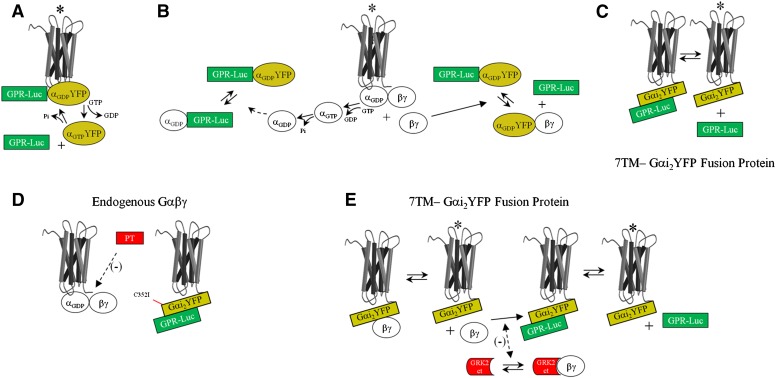
We recently reported that the GαiGPR interaction is regulated by agonist-bound cell surface seven-transmembrane-span (7TM) receptors in the intact cell as determined by bioluminescence resonance energy transfer (BRET) (Oner et al., 2010a,b). As the GPR motif stabilizes the GDP-bound conformation of Gα free of Gβγ, it was hypothesized that a 7TM receptor may directly couple to the GαGPR complex in a manner that is analogous to direct receptor coupling to the Gαβγ heterotrimer (Fig. 1A) (Oner et al., 2010a,b; Blumer and Lanier, 2014). Indeed, the GαGPR complex appears to be positioned in close proximity to the 7TM receptor, and this positioning, which is regulated by agonist, is dependent upon interaction of the GPR protein with Gαi (Oner et al., 2010a,b; Vellano et al., 2011). Alternatively, the regulation of GαGPR observed with receptor activation may be secondary to canonical 7TM receptor coupling to Gαβγ subsequent to G-protein subunit flux within the microenvironment of a signaling complex (Fig. 1B). It was also recently postulated that groups I–III AGS proteins may actually represent a signaling triad that parallels that of the well characterized 7TM receptor—Gαβγ—effector system (Blumer and Lanier, 2014).
Fig. 1.
Schematic representation of hypotheses regarding regulation of GαiGPR by a 7TM receptor. Agonist-induced reductions in GαiYFP–GPR-Rluc BRET may reflect the following different scenarios. (A) Direct coupling of the receptor to the GαiGPR module, which is regulated by agonist-induced nucleotide exchange on GαiYFP. (B) Competitive inhibition of Gαi binding to GPR proteins by endogenous Gα or Gβγ liberated subsequent to receptor coupling to endogenous Gαβγ. (C) Agonist-induced regulation of the interaction of GPR proteins with an α2AAR-Gαi2YFP fusion protein. (D) To determine the influence of endogenous Gαβγ on basal and agonist-induced regulation of α2AAR-Gαi2YFP:GPR-Rluc BRET, Cys352 in Gαi2 was mutated to Ile (C352I), rendering the tethered Gαi2YFP insensitive to pertussis toxin. Receptor coupling to endogenous Gαβγ could then be blocked by cell treatment with PT (Burt et al., 1998). (E) Endogenous Gβγ subunits may also engage the α2AAR-Gαi2YFP fusion protein (Burt et al., 1998). GRK2-CT was expressed as a scavenger for free Gβγ released subsequent to receptor activation of an α2AAR-Gαi2YFP fusion protein complexed with endogenous Gβγ. *Receptor activated by agonist.
As part of a broader approach to explore these concepts, we examined the 7TM receptor–mediated regulation of the Gαi-GPR complex when Gαi was actually tethered to the 7TM receptor itself (Fig. 1C). Thus, the GαiGPR interaction would be highly localized and could also be monitored independent of endogenous Gαβγ, as the tethered G-protein could be rendered pertussis toxin–insensitive by a single point mutation (Fig. 1D). The results of these studies suggest direct coupling of a 7TM receptor to the GαGPR complex, which has broad implications for G-protein signal processing.
Materials and Methods
Polyethylenimine (25 kDa molecular mass, linear form) was obtained from Polysciences, Inc. (Warrington, PA). Benzyl-coelenterazine was obtained from NanoLight Technology (Pinetop, AZ). UK14304 [5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline], pertussis toxin, and β-actin antiserum were purchased from Sigma-Aldrich (St. Louis, MO). Gray 96-well Optiplates were obtained from PerkinElmer (Waltham, MA). Green fluorescent protein (GFP) antiserum was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Gαi1/2 antiserum was provided by Dr. Thomas Gettys (Pennington Biomedical Research Center, Baton Rouge, LA). G-protein–coupled receptor kinase 2 (GRK2) antibody and anti–GFP-Sepharose were obtained from Abcam (Cambridge, MA). n-Dodecyl-β-d-maltoside was obtained from Cayman Chemical (Ann Arbor, MI). Protease inhibitor mixture tablets (Complete Mini) were obtained from Roche Applied Science (Indianapolis, IN). AGS3 and AGS4 fused at the carboxyl terminus to Renilla luciferase (Rluc) and α2A adrenergic receptor (α2AAR) constructs were generated as previously described (Oner et al., 2010a,b, 2013a). Rat Gαi2–yellow fluorescent protein (YFP) was generated by Dr. Scott Gibson (Gibson and Gilman, 2006) and provided by Dr. Nathan Dascal (Tel Aviv University, Tel Aviv, Israel). YFP was inserted within the αB–αC loops in the helical domain of Gαi as previously described (Gibson and Gilman, 2006; Oner et al., 2010a,b). pcDNA3::GRK2–carboxyl terminus (CT) (GRK2-CT), which encodes amino acids Tyr466–Leu689 in the carboxyl terminus of GRK2, was provided by Dr. Jeffrey Benovic (Thomas Jefferson University, Philadelphia, PA). All other reagents and materials were obtained as described elsewhere (Oner et al., 2010a,b, 2013a).
Site-Directed Mutagenesis and Plasmid Construction.
The α2AAR-Gαi2YFP fusion protein was generated by polymerase chain reaction using the human α2AAR as template and primer sets containing specific sites for restriction enzyme digest as follows: XhoI, α2AAR forward primer: 5′-AAA CTC GAG GCC GCC ACC ATG GGC TCC CTG CAG CCG GAC-3′; EcoRI, α2AAR reverse primer: 5′-CAT GGA ATT CTG CAA GCT TCC TCC TCC TCC GGA CAC GAT CCG CTT-3′. The reverse primer also encodes an SGGGS linker between α2AAR and Gαi2YFP. Digestion of pcDNA3::Gαi2YFP or pcDNA3::Gαi2YFPC352I constructs at upstream XhoI/EcoRI sites followed by ligation with the digested receptor linker resulted in in-frame construction of the α2AAR-Gαi2YFP fusion proteins. C352 in Gαi2, which is the site of ADP-ribosylation by pertussis toxin (PT), was converted to isoleucine to render the protein PT insensitive by site-directed mutagenesis using the pcDNA3::Gαi2YFP construct with the following primer set: Gαi2YFPC352I forward primer: 5′-AAC AAC CTG AAG GAC ATT GGC CTC TTC TGA-3′; Gαi2YFPC352I reverse primer: 5′-TCA GAA GAG GCC AAT GTC CTT CAG GTT GTT-3′.
Cell Culture, Transfection, Immunoblotting, and BRET.
BRET measurements and immunoblotting were performed as previously described (Oner et al., 2010a,b, 2013a). In experiments measuring BRET between AGS3Rluc or AGS4Rluc and α2AAR-Gαi2YFP or α2AAR-Gαi2YFPC352I, HEK293 cells were transfected with 10 ng of phRLucN3::AGS3 or 2 ng of phRLucN3::AGS4, respectively, and 750 ng of pcDNA3::α2AAR-Gαi2YFP or pcDNA3::α2AAR-Gαi2YFPC352I per well in a six-well plate. Based upon a series of preliminary experiments, we optimized the system to generate levels of α2AAR-Gαi2YFP and α2AAR-Gαi2YFPC352I that bracketed the levels of endogenous Gαi2 as determined by immunoblotting. For BRET saturation experiments, AGS3Rluc and AGS4Rluc were expressed as described earlier with increasing amounts (0–1000 ng) of pcDNA3::α2AAR-Gαi2YFP or pcDNA3::α2AAR-Gαi2YFPC352I. Forty-eight hours after cell transfection, cells were dispensed in triplicate at 1 × 105 cells/well in gray 96-well Optiplates (Perkin Elmer). Fluorescence and luminescence signals were measured using a TriStar LB 941 plate reader (Berthold Technologies, Oak Ridge, TN) with MikroWin 2000 software (Mikrotek Laborsysteme GmbH, Overath, Germany). Cells were incubated with the α2AR agonist (UK14304, 10 μM) or vehicle in Tyrode’s solution [140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 0.37 mM NaH2PO4, 24 mM NaHCO3, 10 mM HEPES (pH 7.4), and 0.1% glucose (w/v)] for 5 minutes prior to the addition of coelenterazine h. Coelenterazine h (5 μM final concentration; Nanolight Technology) was added to each well and luminescence measured after 2 minutes (donor: 480 ± 20 nm; acceptor: 530 ± 20 nm) with the TriStar LB 941 plate reader. Gαi2YFP or α2AAR-Gαi2YFP fusion protein expression was monitored by measuring YFP fluorescence (excitation 485 nm, emission 535 nm). AGS3 and AGS4Rluc expression was monitored by measuring the intensity of the luminescence signal. BRET signals were determined by calculating the ratio of the light intensity emitted by the YFP divided by the light intensity emitted by Rluc. Net BRET values were determined by first calculating the 530 ± 20:480 ± 20 nm ratio and then subtracting the background BRET signal determined from cells transfected with the donor plasmids phRLucN3::AGS3 or phRLucN3::AGS4 alone. Cell lysates and immunoblotting were performed as previously described (Oner et al., 2010a,b). Where indicated, cells were incubated with pertussis toxin (100 ng/ml) for 18 hours prior to BRET measurements. Cellular fractionation of UK14304- or vehicle-treated cells by hypotonic lysis and centrifugation were performed as previously described (Oner et al., 2013b), using HEK293 cells transfected with AGS3 and AGS4 donor plasmids (10 and 2 ng, respectively) and α2AAR-Gαi2YFP acceptor plasmid (750 ng) as described earlier.
Immunoprecipitation.
HEK293 cells expressing α2AAR-Gαi2YFP and AGS3Rluc (1.4 and 0.1 μg of plasmid per well in a six-well plate, respectively) for 24 hours were treated with the α2AR agonist UK14304 at a final concentration of 10 μM or with vehicle (Tyrode’s solution) for 5 minutes at room temperature and harvested in 4.5 ml of Tyrode’s solution. Cells were centrifuged at 500g for 5 minutes, resuspended in 0.5 ml of immunoprecipitation buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, protease inhibitor cocktail], and sonicated at 50% amplitude for three intervals at 5 seconds each. n-Dodecyl-β-d-maltoside was added to a final concentration of 2%, and membrane proteins were extracted by rotating for 3 hours at 4°C followed by centrifugation at 21,000g for 30 minutes at 4°C. The supernatant was collected and an input sample (1/20 volume; 82.5 μg) taken; to the remaining supernatant (1.65 mg), 25 μl of 50% anti–GFP-Sepharose (Abcam) was added and rotated overnight at 4°C followed by 6 × 500 μl resin washes with immunoprecipitation buffer containing 0.2% n-dodecyl-β-d-maltoside. Twenty-five microliters of 5× Laemmli sample buffer was added to the washed resin, incubated at room temperature for 5 minutes, processed for SDS-PAGE (7% polyacrylamide), transferred to polyvinylidene difluoride membranes, and immunoblotted with AGS3 antisera followed by stripping and reprobing with GFP antisera.
Data Analysis.
Statistical significance for differences involving a single intervention was determined by one-way analysis of variance using GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA).
Results and Discussion
As a first step to address the hypothesis regarding direct receptor coupling to Gαi2GPR, we generated a fusion protein in which Gαi2YFP was tethered to the carboxyl terminus of the α2AAR via a flexible glycine linker (Bertin et al., 1994; Wise et al., 1997; Bahia et al., 1998; Burt et al., 1998; Seifert et al., 1999). We also generated a variant of the α2AAR-Gαi2YFP fusion protein that was PT-insensitive (α2AAR-Gαi2YFPC352I). We then examined the ability of GPR proteins to interact with the tethered Gαi2. AGS3 and AGS4 were selected as representative members of two distinct subgroups of AGS proteins. AGS3 has four GPR motifs downstream of a series of tetratricopeptide repeat domains involved in protein interactions and intramolecular regulatory events, whereas AGS4 is a smaller protein with three full GPR motifs without any clearly defined protein interaction motifs upstream of the GPR motifs.
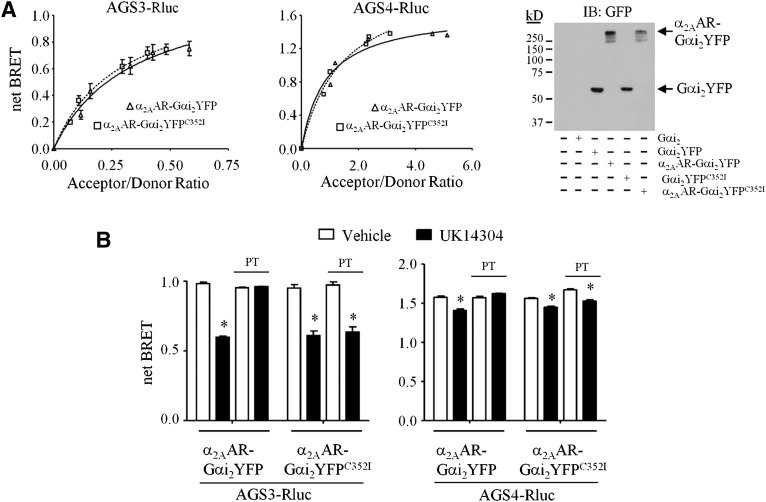
Both AGS3 and AGS4 interacted with the tethered wild-type and PT-insensitive Gαi2 as indicated by the robust basal levels of BRET (Fig. 2A). Expression and functionality of α2AAR-Gαi2YFP and α2AAR-Gαi2YFPC352I were confirmed by immunoblotting (Fig. 2A) and agonist-induced phosphorylation of extracellular signal-regulated kinase 1/2 (W. G. Robichaux, III and J. B. Blumer, unpublished data). α2AAR-Gαi2YFP:AGS3Rluc BRET and α2AAR-Gαi2YFP:AGS4Rluc BRET were not observed with the GPR-insensitive GαiN149I mutant or with AGS3 or AGS4 that was rendered incapable of binding Gαi by mutation of a conserved glutamate residue in each of the GPR motifs (AGS3-Q/A and AGS4-Q/A), thus demonstrating the specificity of the interaction (Peterson et al., 2002; Sato et al., 2004; Willard et al., 2008; Oner et al., 2010a,b; W. G. Robichaux, III and J. B. Blumer, unpublished data).
Fig. 2.
Agonist-induced regulation of an α2AAR-Gαi2 fusion protein complexed with the GPR proteins AGS3 and AGS4. (A, left panel) HEK293 cells expressing a fixed amount of AGS3Rluc (left) or AGS4Rluc (right) and increasing amounts of α2AAR-Gαi2YFP (squares) or α2AAR-Gαi2YFPC352I (triangles) were processed for BRET measurements as described in Materials and Methods. (Right panel) Lysates (50 μg) from control HEK293 cells or HEK293 cells expressing Gαi2, Gαi2YFP, α2AAR-Gαi2YFP, or α2AAR-Gαi2YFPC352I (750 ng of each plasmid) were subjected to SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted (IB) with GFP antiserum. (B) HEK293 cells expressing AGS3Rluc (left panel) or AGS4Rluc (right panel) and α2AAR-Gαi2YFP or α2AAR-Gαi2YFPC352I were incubated in the absence or presence of PT (100 ng/ml) for 18 hours, as described in Materials and Methods. Cells were then washed and incubated with vehicle (Tyrode’s solution) or α2AAR agonist UK14304 (10 μM) for 5 minutes followed by fluorescence and luminescence readings to obtain net BRET signals, as described in Materials and Methods. (B, left panel) AGS3Rluc relative luminescence units: AGS3Rluc + α2AAR-Gαi2YFP: 335,234 ± 9929; AGS3Rluc + α2AAR-Gαi2YFP + PT: 327,626 ± 15,110; AGS3Rluc + α2AAR-Gαi2YFPC352I : 385,996 ± 22,073; AGS3Rluc + α2AAR-Gαi2YFPC352I + PT: 373,388 ± 17,790. Relative fluorescence units: α2AAR-Gαi2YFP: 111,523 ± 3246; α2AAR-Gαi2YFP + PT: 112,991 ± 2545; α2AAR-Gαi2YFPC352I: 110,420 ± 2416; α2AAR-Gαi2YFPC352I + PT: 112,565 ± 3072. (B, right panel) AGS4Rluc relative luminescence units: AGS4Rluc + α2AAR-Gαi2YFP: 87,143 ± 6516; AGS4Rluc + α2AAR-Gαi2YFP + PT: 71,193 ± 5723; AGS4Rluc + α2AAR-Gαi2YFPC352I: 148,939 ± 7362; AGS4Rluc + α2AAR-Gαi2YFPC352I + PT: 133,482 ± 11,038. Relative fluorescence units: α2AAR-Gαi2YFP: 106,882 ± 5325; α2AAR-Gαi2YFP + PT: 109,976 ± 5497; α2AAR-Gαi2YFPC352I: 142,380 ± 2980; α2AAR-Gαi2YFPC352I + PT: 166,057 ± 8005. All BRET data are expressed as mean ± S.E. from at least three independent experiments with triplicate determinations. Immunoblots are representative of three independent experiments. *P < 0.05 compared with vehicle-treated control group.
Incubation of cells with the α2AAR agonist UK14304 reduced the α2AAR-Gαi2YFP:AGS3Rluc BRET by ∼40% (Fig. 2B, left panel). Significant agonist-induced reductions in α2AAR-Gαi2YFP:AGS4Rluc BRET were also observed, although not to the same magnitude as that observed for AGS3Rluc (Fig. 2B, right panel). Both the basal α2AAR-Gαi2YFP:AGS3Rluc BRET and the magnitude of the agonist-induced decrease in BRET observed for AGS3Rluc or AGS4Rluc with tethered Gαi2YFP were similar to that observed with untethered Gαi2YFP (similar results were obtained with the α2AAR-Gαi1-YFP fusion protein; W. G. Robichaux, III and J. B. Blumer, unpublished data). Thus, these data indicate that a 7TM agonist is regulating a GαGPR complex that is directly anchored to the receptor.
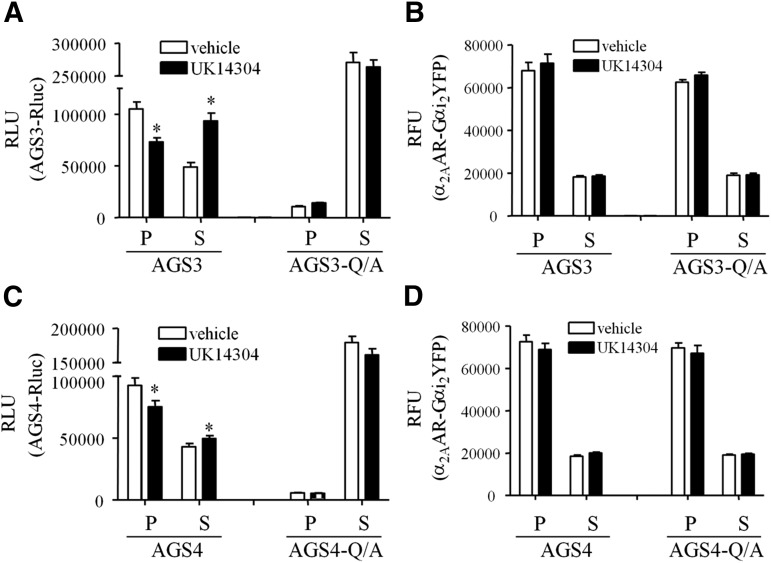
A similar distinction between AGS3 and AGS4 with respect to the magnitude of agonist-induced changes in BRET was also observed with untethered Gαi1YFP (Oner et al., 2010a,b). It is not clear if the differences in the magnitude of the agonist-induced changes in GαiYFP:AGS3Rluc versus GαiYFP:AGS4Rluc BRET reflect different coupling efficiencies, stoichiometric considerations, and/or the relative spatial positioning of the acceptor and donor for AGS3 versus AGS4. As an initial approach to address this issue and to verify that the agonist-induced changes in BRET were the result of translocation of GPR proteins away from the receptor-Gαi complex, we monitored the subcellular distribution of AGS3Rluc or AGS4Rluc and α2AAR-Gαi2YFP by cellular fractionation into crude membranes and cytosol (Fig. 3). These data indicate that activation of α2AAR-Gαi2YFP resulted in translocation of GPR proteins away from the membrane fraction and into the cytosol while α2AAR-Gαi2YFP remained in the membrane fraction, suggesting that the observed agonist-induced changes in BRET result from a physical dissociation of GPR proteins from the receptor-Gα complex. The relative extent of AGS3 and AGS4 translocation was almost directly related to the degree of agonist-induced reductions in BRET between AGS3 or AGS4 and α2AAR-Gαi2YFP, as shown in Fig. 2B.
Fig. 3.
Agonist-induced changes in GPR protein distribution. AGS3Rluc (A and B) or AGS4Rluc (C and D) and α2AAR-Gαi2YFP were expressed in HEK293 cells as described in Materials and Methods. Cells were incubated with vehicle (Tyrode’s solution) or UK14304 (10 μM) for 5 minutes followed by hypotonic lysis, and AGS3Rluc (A) or AGS4Rluc (C) relative luminescence units (RLU) and α2AAR-Gαi2YFP relative fluorescence units (RFU) (B and D) were measured in supernatant (S) and pellet (P) fractions representing crude cytosol and membrane fractions, respectively. AGS3-Q/A and AGS4-Q/A refer to the mutation of a conserved glutamine residue in each of the GPR motifs to alanine, which renders them incapable of binding Gαi. *P < 0.05 compared with vehicle.
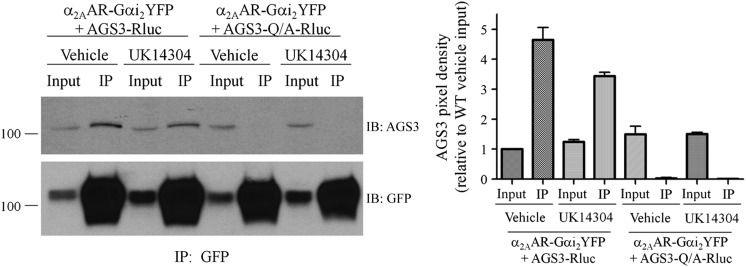
As an additional approach to observe agonist-regulated interaction of GPR proteins with Gαi-coupled 7TM receptors, we asked if AGS3 coimmunoprecipitates with α2AAR-Gαi2YFP and if this complex was also regulated by agonist. Indeed, AGS3Rluc coimmunoprecipitated with α2AAR-Gαi2YFP (Fig. 4). AGS3-Q/A-Rluc, which cannot bind Gαi (Oner et al., 2010a), did not coimmunoprecipitate with α2AAR-Gαi2YFP, thus serving as an important internal negative control. Treatment with the α2AAR agonist UK14304 resulted in an ∼30% decrease in coimmunoprecipitation of AGS3Rluc with α2AAR-Gαi2YFP compared with vehicle treatment. These data further support our hypothesis of an agonist-sensitive 7TM receptor–Gαi–GPR complex and the magnitude of the agonist-mediated effects on the complex in the context of coimmunopreciation is roughly similar to the relative magnitude of agonist-mediated effects observed with the BRET studies (Fig. 2B).
Fig. 4.
Coimmunoprecipitation of AGS3 with the 7TM receptor–Gαi2 fusion protein is regulated by agonist. (Left panel) HEK293 cells expressing α2AAR-Gαi2YFP and AGS3Rluc for 24 hours were treated with α2AR agonist UK14304 at a final concentration of 10 μM or with vehicle (Tyrode’s solution) for 5 minutes at room temperature, as described in Materials and Methods. Cell pellets were sonicated in immunoprecipitation (IP) buffer and cell membranes extracted with 2% n-dodecyl-β-d-maltoside followed by immunoprecipitation with anti–GFP-Sepharose overnight at 4°C. Immunoprecipitates were washed and resolved by SDS-PAGE and immunoblotted (IB) with AGS3 antisera (upper panel) followed by stripping of the blots and reprobing with GFP antisera (lower panel) as described in Materials and Methods. “Input” represents 1/20 of the total volume of cellular lysate taken prior to immunoprecipitation. AGS3-Q/A refers to the mutation of a conserved glutamine residue in each of the GPR motifs to alanine, which renders them incapable of binding Gαi. (Right panel) Densitometric analysis from the means of two independent immunoprecipitation experiments as shown in the left panel, with pixel density set relative to the AGS3–wild type (WT) vehicle-treated input.
Regulation of the α2AAR-Gαi2YFP:GPR-Rluc complex by agonist may reflect the ability of the Gαi2GPR cassette to directly sense agonist-induced conformational changes in the receptor (Fig. 1C), as is the case for 7TM receptor coupling to the Gαβγ heterotrimer. Alternatively, the agonist-induced reduction of α2AAR-Gαi2YFP:GPR-Rluc BRET may reflect displacement of AGS3Rluc or AGS4Rluc from the 7TM receptor–Gαi2YFP fusion protein by Gβγ or Gα subsequent to receptor coupling to either endogenous Gαβγ heterotrimer (Burt et al., 1998) or the α2AAR-Gαi2YFP fusion protein where endogenous Gβγ is bound to the tethered Gαi2YFP (Fig. 1E).
To address these questions, we conducted two sets of experiments. First, we studied the effect of agonist on α2AAR-Gαi2YFP:GPR-Rluc BRET after rendering the tethered Gα subunit PT-insensitive by mutation of the cysteine that is actually ADP ribosylated by pertussis toxin (Fig. 1D). Such an approach would allow us to eliminate receptor coupling to endogenous Gαβγ, but retain the coupling integrity of the α2AAR-Gαi2YFPC352I fusion protein (Bahia et al., 1998). Thus, we have an experimental platform that provides a highly localized readout of receptor-mediated regulation of Gαi2GPR.
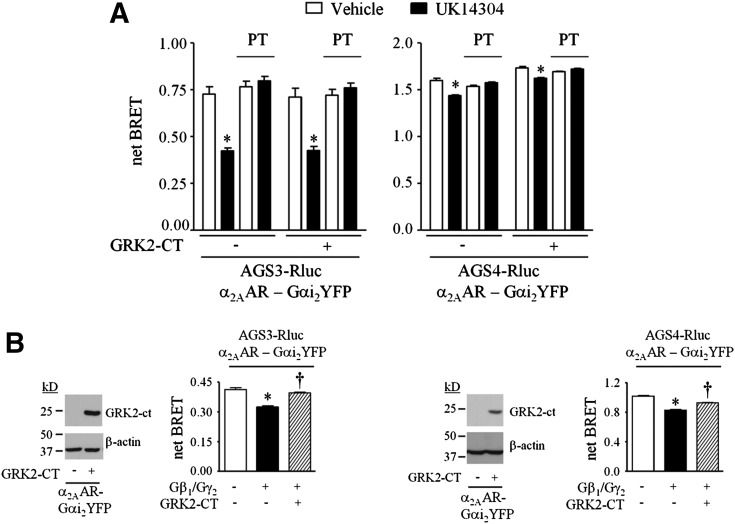
The agonist-induced regulation of α2AAR-Gαi2YFP:AGS3Rluc or α2AAR-Gαi2YFP:AGS4Rluc BRET observed with untethered (W. G. Robichaux, III and J. B. Blumer, unpublished data) or tethered Gα was completely blocked by incubation of cells with PT (Fig. 2B). However, the agonist-induced regulation of untethered (W. G. Robichaux, III and J. B. Blumer, unpublished data) or tethered Gαi2C352I was not altered by PT pretreatment, which blocked receptor coupling to endogenous Gαi/oβγ (Fig. 2B). These data indicate that the agonist-induced regulation of α2AAR-GαiYFP:AGS3Rluc or α2AAR-GαiYFP:AGS4Rluc BRET is spatially localized and not likely due to exchange of endogenous Gαi/o for GαYFP bound to the GPR protein or to the displacement of GαYFP bound to the GPR protein by Gβγ subsequent to receptor-mediated coupling to the Gαβγ heterotrimer.
In addition to interacting with the GPR proteins AGS3 and AGS4, the α2AAR-Gαi2YFP fusion protein may also interact with endogenous Gβγ. Agonist-induced activation of the α2AAR-Gαi2YFP:Gβγ complex may “release” Gβγ, which could potentially displace AGS3 or AGS4 from the α2AAR-Gαi2YFP fusion protein, reducing α2AAR-Gαi2YFP:GPR-Rluc BRET (Fig. 1E). To address this issue, we used GRK2-CT to scavenge any Gβγ that may be “released” by agonist-induced activation of α2AAR-Gαi2YFP:Gβγ (Fig. 5). GRK-CT expression was confirmed by immunoblotting (Fig. 5B). Expression of GRK2-CT did not alter the agonist-induced regulation of the BRET observed with AGS3Rluc or AGS4Rluc and the untethered (W. G. Robichaux, III and J. B. Blumer, unpublished data) or tethered Gαi2YFP (Fig. 5A). Under similar experimental conditions with untethered Gαi2YFP, expression of Gβγ reduces basal Gαi2YFP:GPR-Rluc BRET (Oner et al., 2010a,b), and this effect of Gβγ was reversed by GRK2-CT, providing an internal control that indicates effective Gβγ scavenging (Fig. 5B). The lack of effect of GRK2-CT on agonist-induced regulation of the interaction of GPR proteins with the tethered GαiYFP is consistent with previous observations using untethered GαiYFP (Oner et al., 2010a). Furthermore, the Gβγ inhibitor gallein also did not alter the basal or agonist-regulated BRET between AGS3Rluc or AGS4Rluc and either untethered Gαi2YFP or Gαi2YFP tethered to the α2AAR (W. G. Robichaux, III and J. B. Blumer, unpublished data). These data suggest that the agonist-induced regulation of the interaction of Gαi with GPR proteins does not involve subunit flux subsequent to receptor coupling to Gαβγ.
Fig. 5.
Influence of a Gβγ scavenger on the agonist-induced regulation of GαiGPR, where Gαi is tethered to the receptor. (A) Net BRET values obtained from HEK293 cells expressing AGS3Rluc (left panel) or AGS4Rluc (right panel) and α2AAR-Gαi2YFP, as described in Fig. 2 and Materials and Methods. Where indicated, cells also expressed GRK2-CT. Cells were incubated with vehicle (Tyrode’s solution) or UK14304 (10 μM) for 5 minutes. For experiments involving PT, cells were incubated with PT (100 ng/ml) for 18 hours prior to agonist exposure. (Left panel) relative luminescence units: AGS3Rluc: 195,791 ± 15,175; AGS3Rluc + PT: 178,887 ± 24,596; AGS3Rluc + GRK2-CT: 218,392 ± 12,663; AGS3Rluc + GRK2-CT + PT: 220,238 ± 19,824. Relative fluorescence units: α2AAR-Gαi2YFP: 110,414 ± 2294; α2AAR-Gαi2YFP + PT: 104,532 ± 2263; α2AAR-Gαi2YFP + GRK2-CT: 106,967 ± 2562; α2AAR-Gαi2YFP + GRK2-CT + PT: 116,045 ± 3266. (Right panel) Relative luminescence units: AGS4Rluc: 147,140 ± 7740; AGS4Rluc + PT: 150,290 ± 8165; AGS4Rluc + GRK2-CT: 155,576 ± 8972; AGS4Rluc + GRK2-CT + PT: 147,944 ± 10,565. Relative fluorescence units: α2AAR-Gαi2YFP: 109,090 ± 2942; α2AAR-Gαi2YFP + PT: 112,983 ± 3019; α2AAR-Gαi2YFP + GRK2-CT: 124,288 ± 2273; α2AAR-Gαi2YFP + GRK2-CT + PT: 112,371 ± 2189. *P < 0.05 compared with vehicle-treated control group. (B, left panel) Lysates (50 μg) from a representative experiment as described in (A) were subjected to SDS-PAGE and immunoblotting with GRK2 and β-actin antisera as indicated. (Left panel bar graph) HEK293 cells expressing AGS3Rluc (10 ng of plasmid) and α2AAR-Gαi2YFP (250 ng of plasmid) in the absence and presence of Gβ1, Gγ2, and/or GRK2-CT (500 ng of each plasmid) as indicated were subjected to BRET measurements, as described in Materials and Methods. (Right panel) Lysates (50 μg) from a representative experiment as described in (A) were subjected to SDS-PAGE and immunoblotting with GRK2 and β-actin antisera as indicated. (Right panel bar graph) HEK293 cells expressing AGS4Rluc (2 ng of plasmid) and α2AAR-Gαi2YFP (250 ng of plasmid) in the absence and presence of Gβ1, Gγ2, and/or GRK2-CT (500 ng of each plasmid) as indicated for 48 hours were subjected to BRET measurements, as described in Materials and Methods. All BRET data are expressed as means ± S.E. from at least three independent experiments with triplicate determinations. Immunoblots are a representative image of three independent experiments. *P < 0.001 compared with control group; †P < 0.001 compared with Gβ1γ2-expressing group.
Our data suggest that a 7TM receptor couples directly to a GαiGPR complex, ostensibly promoting exchange of GDP for GTP in a manner that may be similar to 7TM receptor engagement of the Gαβγ heterotrimer. Agonist-mediated activation of a 7TM receptor coupled to GαiGPR apparently results in reversible dissociation of the GPR protein from Gαi (Oner et al., 2010a,b). Upon termination of agonist-induced activation, the GPR protein then reassociates with GαiGDP, representing a cycle that is conceptually analogous to the Gαβγ activation-deactivation cycle (Oner et al., 2010a,b, 2013a,c). There are several interesting conceptual thoughts that emanate from this work. As regulation of both the GαiGPR complex and the Gαiβγ heterotrimer is PT-sensitive (Figs. 2 and 3) (Oner et al., 2010a,b), this raises the intriguing possibility that functional effects associated with PT may be mediated in part by 7TM regulation of GαiGPR complexes. Second, as group II AGS proteins may complex with multiple Gα subunits simultaneously (Bernard et al., 2001; Adhikari and Sprang, 2003; Kimple et al., 2004; Jia et al., 2012), AGS3 and AGS4 may scaffold receptors and Gα subunits within a larger signaling complex (Jahangeer and Rodbell, 1993; Blumer and Lanier, 2014). It is interesting to speculate on the relative ratio of receptors coupling to Gαβγ versus GαGPR. Regulation of GPR protein expression levels may play a role in determining this stoichiometry, as AGS3 and AGS4 levels are responsive to changes in environmental and pathophysiological conditions, including withdrawal from drugs of abuse, ischemia/reperfusion injury, and leukocyte activation (Bowers et al., 2004, 2008; Yao et al., 2005; Nadella et al., 2010; Regner et al., 2011; Kwon et al., 2012; Giguere et al., 2013; Branham-O'Connor et al., 2014; W. G. Robichaux, III and J. B. Blumer, unpublished data). Additional signals regulating the interaction of Gα with GPR proteins and the subcellular distribution of GPR proteins may also be involved and may provide more rapid and dynamic control of cellular responses (Blumer et al., 2003; An et al., 2008; Nadella et al., 2010; Oner et al., 2010a,b, 2013c; Vural et al., 2010; Giguere et al., 2012). Finally, of particular interest, the coupling of a receptor to the GαGPR complex or the Gαβγ heterotrimer may be differentially regulated by hormones, neurotransmitters, and small molecules.
Acknowledgments
The authors thank Dr. Thomas Gettys (Pennington Biomedical Research Center, Baton Rouge, LA) for Gαi1/2 antiserum and Dr. Jeffrey Benovic (Thomas Jefferson University) for the pcDNA3::GRK2-CT plasmid. The authors acknowledge the contribution of Dr. Scott Gibson in generating the Gαi2-YFP plasmid used in this study (Gibson and Gilman, 2006), which was provided by Dr. Nathan Dascal (Tel Aviv University).
Abbreviations
- AGS
activator of G-protein signaling
- BRET
bioluminescence resonance energy transfer
- CT
carboxyl terminus
- GFP
green fluorescent protein
- GPR
G-protein regulatory
- PT
pertussis toxin
- Rluc
Renilla luciferase
- 7TM
seven transmembrane span receptor
- UK14304
5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline
- YFP
yellow fluorescent protein
Authorship Contributions
Participated in research design: Robichaux, Oner, Lanier, Blumer.
Conducted experiments: Robichaux, Oner, Blumer.
Contributed new reagents or analytic tools: Robichaux, Oner, Lanier, Blumer.
Performed data analysis: Robichaux, Oner, Lanier, Blumer.
Wrote or contributed to the writing of the manuscript: Robichaux, Oner, Lanier, Blumer.
Footnotes
This work was supported by the National Institutes of Health National Institute for General Medical Sciences [Grant R01-GM086510 to J.B.B.], the National Institutes of Health National Institute for Neurologic Diseases and Stroke [Grant R01-NS24821 to S.M.L.], and the National Institutes of Health National Institute for Drug Abuse [Grant R01-DA025896 to S.M.L.].
References
- Adhikari A, Sprang SR. (2003) Thermodynamic characterization of the binding of activator of G protein signaling 3 (AGS3) and peptides derived from AGS3 with G alpha i1. J Biol Chem 278:51825–51832. [DOI] [PubMed] [Google Scholar]
- An N, Blumer JB, Bernard ML, Lanier SM. (2008) The PDZ and band 4.1 containing protein Frmpd1 regulates the subcellular location of activator of G-protein signaling 3 and its interaction with G-proteins. J Biol Chem 283:24718–24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia DS, Wise A, Fanelli F, Lee M, Rees S, Milligan G. (1998) Hydrophobicity of residue351 of the G protein Gi1 alpha determines the extent of activation by the alpha 2A-adrenoceptor. Biochemistry 37:11555–11562. [DOI] [PubMed] [Google Scholar]
- Bernard ML, Peterson YK, Chung P, Jourdan J, Lanier SM. (2001) Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J Biol Chem 276:1585–1593. [DOI] [PubMed] [Google Scholar]
- Bertin B, Freissmuth M, Jockers R, Strosberg AD, Marullo S. (1994) Cellular signaling by an agonist-activated receptor/Gs alpha fusion protein. Proc Natl Acad Sci USA 91:8827–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer JB, Bernard ML, Peterson YK, Nezu J, Chung P, Dunican DJ, Knoblich JA, Lanier SM. (2003) Interaction of activator of G-protein signaling 3 (AGS3) with LKB1, a serine/threonine kinase involved in cell polarity and cell cycle progression: phosphorylation of the G-protein regulatory (GPR) motif as a regulatory mechanism for the interaction of GPR motifs with Gi alpha. J Biol Chem 278:23217–23220. [DOI] [PubMed] [Google Scholar]
- Blumer JB, Lanier SM. (2014) Activators of G protein signaling exhibit broad functionality and define a distinct core signaling triad. Mol Pharmacol 85:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, Chou JK, Guillory AM, Chang SJ, Janak PH, Bonci A, Diamond I. (2008) Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proc Natl Acad Sci USA 105:12533–12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. (2004) Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron 42:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branham-O’Connor M, Robichaux WG, 3rd, Zhang XK, Cho H, Kehrl JH, Lanier SM, Blumer JB. (2014) Defective chemokine signal integration in leukocytes lacking activator of G protein signaling 3 (AGS3). J Biol Chem 289:10738–10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt AR, Sautel M, Wilson MA, Rees S, Wise A, Milligan G. (1998) Agonist occupation of an alpha2A-adrenoreceptor-Gi1alpha fusion protein results in activation of both receptor-linked and endogenous Gi proteins. Comparisons of their contributions to GTPase activity and signal transduction and analysis of receptor-G protein activation stoichiometry. J Biol Chem 273:10367–10375. [DOI] [PubMed] [Google Scholar]
- Cao X, Cismowski MJ, Sato M, Blumer JB, Lanier SM. (2004) Identification and characterization of AGS4: a protein containing three G-protein regulatory motifs that regulate the activation state of Gialpha. J Biol Chem 279:27567–27574. [DOI] [PubMed] [Google Scholar]
- Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, Fuernkranz H, Lanier SM, Duzic E. (1999) Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol 17:878–883. [DOI] [PubMed] [Google Scholar]
- Gibson SK, Gilman AG. (2006) Gialpha and Gbeta subunits both define selectivity of G protein activation by alpha2-adrenergic receptors. Proc Natl Acad Sci USA 103:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère PM, Billard MJ, Laroche G, Buckley BK, Timoshchenko RG, McGinnis MW, Esserman D, Foreman O, Liu P, Siderovski DP, et al. (2013) G-protein signaling modulator-3, a gene linked to autoimmune diseases, regulates monocyte function and its deficiency protects from inflammatory arthritis. Mol Immunol 54:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère PM, Laroche G, Oestreich EA, Duncan JA, Siderovski DP. (2012) Regulation of the subcellular localization of the G-protein subunit regulator GPSM3 through direct association with 14-3-3 protein. J Biol Chem 287:31270–31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangeer S, Rodbell M. (1993) The disaggregation theory of signal transduction revisited: further evidence that G proteins are multimeric and disaggregate to monomers when activated. Proc Natl Acad Sci USA 90:8782–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M, Li J, Zhu J, Wen W, Zhang M, Wang W. (2012) Crystal structures of the scaffolding protein LGN reveal the general mechanism by which GoLoco binding motifs inhibit the release of GDP from Gαi. J Biol Chem 287:36766–36776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimple RJ, Willard FS, Hains MD, Jones MB, Nweke GK, Siderovski DP. (2004) Guanine nucleotide dissociation inhibitor activity of the triple GoLoco motif protein G18: alanine-to-aspartate mutation restores function to an inactive second GoLoco motif. Biochem J 378:801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M, Pavlov TS, Nozu K, Rasmussen SA, Ilatovskaya DV, Lerch-Gaggl A, North LM, Kim H, Qian F, Sweeney WE, Jr, et al. (2012) G-protein signaling modulator 1 deficiency accelerates cystic disease in an orthologous mouse model of autosomal dominant polycystic kidney disease. Proc Natl Acad Sci USA 109:21462–21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadella R, Blumer JB, Jia G, Kwon M, Akbulut T, Qian F, Sedlic F, Wakatsuki T, Sweeney WE, Jr, Wilson PD, et al. (2010) Activator of G protein signaling 3 promotes epithelial cell proliferation in PKD. J Am Soc Nephrol 21:1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oner SS, An N, Vural A, Breton B, Bouvier M, Blumer JB, Lanier SM. (2010a) Regulation of the AGS3·Galphai signaling complex by a seven-transmembrane span receptor. J Biol Chem 285:33949–33958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oner SS, Blumer JB, Lanier SM. (2013a) Group II activators of G-protein signaling: monitoring the interaction of Gα with the G-protein regulatory motif in the intact cell. Methods Enzymol 522:153–167. [DOI] [PubMed] [Google Scholar]
- Oner SS, Maher EM, Breton B, Bouvier M, Blumer JB. (2010b) Receptor-regulated interaction of activator of G-protein signaling-4 and Galphai. J Biol Chem 285:20588–20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oner SS, Maher EM, Gabay M, Tall GG, Blumer JB, Lanier SM. (2013b) Regulation of the G-protein regulatory-Gαi signaling complex by nonreceptor guanine nucleotide exchange factors. J Biol Chem 288:3003–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oner SS, Vural A, Lanier SM. (2013c) Translocation of activator of G-protein signaling 3 to the Golgi apparatus in response to receptor activation and its effect on the trans-Golgi network. J Biol Chem 288:24091–24103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson YK, Hazard S, 3rd, Graber SG, Lanier SM. (2002) Identification of structural features in the G-protein regulatory motif required for regulation of heterotrimeric G-proteins. J Biol Chem 277:6767–6770. [DOI] [PubMed] [Google Scholar]
- Regner KR, Nozu K, Lanier SM, Blumer JB, Avner ED, Sweeney WE, Jr, Park F. (2011) Loss of activator of G-protein signaling 3 impairs renal tubular regeneration following acute kidney injury in rodents. FASEB J 25:1844–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Cismowski MJ, Toyota E, Smrcka AV, Lucchesi PA, Chilian WM, Lanier SM. (2006) Identification of a receptor-independent activator of G protein signaling (AGS8) in ischemic heart and its interaction with Gbetagamma. Proc Natl Acad Sci USA 103:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Gettys TW, Lanier SM. (2004) AGS3 and signal integration by Galpha(s)- and Galpha(i)-coupled receptors: AGS3 blocks the sensitization of adenylyl cyclase following prolonged stimulation of a Galpha(i)-coupled receptor by influencing processing of Galpha(i). J Biol Chem 279:13375–13382. [DOI] [PubMed] [Google Scholar]
- Sato M, Hiraoka M, Suzuki H, Bai Y, Kurotani R, Yokoyama U, Okumura S, Cismowski MJ, Lanier SM, Ishikawa Y. (2011) Identification of transcription factor E3 (TFE3) as a receptor-independent activator of Gα16: gene regulation by nuclear Gα subunit and its activator. J Biol Chem 286:17766–17776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K, Kobilka BK. (1999) GPCR-Galpha fusion proteins: molecular analysis of receptor-G-protein coupling. Trends Pharmacol Sci 20:383–389. [DOI] [PubMed] [Google Scholar]
- Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S, 3rd, Duzic E, Lanier SM. (1999) Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem 274:33202–33205. [DOI] [PubMed] [Google Scholar]
- Vellano CP, Maher EM, Hepler JR, Blumer JB. (2011) G protein-coupled receptors and resistance to inhibitors of cholinesterase-8A (Ric-8A) both regulate the regulator of g protein signaling 14 RGS14·Gαi1 complex in live cells. J Biol Chem 286:38659–38669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vural A, Oner S, An N, Simon V, Ma D, Blumer JB, Lanier SM. (2010) Distribution of activator of G-protein signaling 3 within the aggresomal pathway: role of specific residues in the tetratricopeptide repeat domain and differential regulation by the AGS3 binding partners Gi(alpha) and mammalian inscuteable. Mol Cell Biol 30:1528–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, Zheng Z, Guo J, Digby GJ, Kimple AJ, Conley JM, Johnston CA, Bosch D, Willard MD, Watts VJ, et al. (2008) A point mutation to Galphai selectively blocks GoLoco motif binding: direct evidence for Galpha.GoLoco complexes in mitotic spindle dynamics. J Biol Chem 283:36698–36710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise A, Carr IC, Milligan G. (1997) Measurement of agonist-induced guanine nucleotide turnover by the G-protein Gi1alpha when constrained within an alpha2A-adrenoceptor-Gi1alpha fusion protein. Biochem J 325:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Inoue Y, Diamond I. (2005) Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc Natl Acad Sci USA 102:8746–8751. [DOI] [PMC free article] [PubMed] [Google Scholar]