Abstract
Statins reduce arterial stiffness but are also associated with mild muscle complaints. It is unclear whether individuals with muscle symptoms experience the same vascular benefit or whether statins affect striated and smooth muscle cells differently. We examined the effect of simvastatin treatment on arterial stiffness in patients who did versus those who did not exhibit muscle symptoms. Patients with a history of statin-related muscle complaints (n = 115) completed an 8 wk randomized, double-blind, cross-over trial of daily simvastatin 20 mg and placebo. Serum lipids and pulse wave velocity (PWV) were assessed before and after each treatment. Muscle symptoms with daily simvastatin treatment were reported by 38 patients (33%). Compared to baseline, central PWV decreased (P = 0.01) following simvastatin treatment but not placebo (drug ∗ time interaction: P = 0.047). Changes in central PWV with simvastatin treatment were not influenced by myalgia status or time on simvastatin (P ≥ 0.15). Change in central PWV after simvastatin treatment was inversely correlated with age (r = −0.207, P = 0.030), suggesting that advancing age is associated with enhanced statin-mediated arterial destiffening. In patients with a history of statin-related muscle complaints, the development of myalgia with short-term simvastatin treatment did not attenuate the improvement in arterial stiffness.
1. Introduction
Hydroxy-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) inhibit mevalonate production, effectively reducing low-density lipoprotein- (LDL-) cholesterol concentrations. Additionally, statins are associated with multiple vascular benefits [1, 2] that may contribute to reduced cardiovascular disease (CVD) morbidity and mortality [3–5]. Reductions in central arterial stiffness (assessed noninvasively by arterial pulse wave velocity (PWV)) with statin use [6–10] represent one such vascular benefit.
Statins are well-tolerated but can produce mild muscle complaints such as muscle pain (myalgia), cramps, weakness, and stiffness. It is not known whether patients who exhibit muscle symptoms with statin use demonstrate the same improvement in central arterial stiffness as nonmyalgic patients. Observation of unchanged central arterial stiffness with statin use in myalgic patients might support a generalized effect on muscle cells (both striated and smooth) by which statins influence skeletal muscle stiffness and fatigue. The present investigation examined the effect of simvastatin treatment on PWV in patients who did versus those who did not exhibit statin-associated muscle symptoms during the run-in phase of the Co-Enzyme Q10 in Statin Myopathy study, of which the methods have been described in detail [11, 12].
2. Methods
2.1. Study Design
Men and women ≥20 yrs of age with a history of muscle complaints during statin treatment were recruited and enrolled into a randomized, double-blind, crossover, run-in trial of simvastatin 20 mg/d or placebo to confirm statin myalgia [11, 12]. Following discontinuation of cholesterol medications for 4 wk, subjects were treated for 8 wks or until myalgia persisted for 1 wk or became intolerable. Subjects then underwent 4 wk washout period and received the alternative treatment for 8 wks or until myalgia persisted for 1 wk or became intolerable. Subjects were queried about muscle complaints using the Short-Form Brief Pain Inventory [13] at each study visit and were contacted weekly by study personnel to inquire about muscle complaints. Plasma samples were collected and arterial PWV measured at the beginning and end of each treatment phase. In order to maintain study blinding, plasma samples were analyzed for lipids upon study completion (Clinical Laboratory Partners, Hartford Hospital). The Institutional Review Board at Hartford Hospital approved the study and the study was monitored by a Data Safety Monitoring Board. This study was registered at ClinicalTrials.gov (NCT01140308).
2.2. Confirmation of Myalgia
Subjects were defined as myalgic if they developed muscle symptoms during simvastatin treatment only. If a participant developed muscle symptoms during both simvastatin and placebo treatments or reported no muscle symptoms during simvastatin treatment, they were considered nonmyalgic [11, 12].
2.3. Arterial Stiffness Assessment
Following a 10 min supine rest period, measurements of pulse wave analysis and pulse wave velocity (PWV) were performed with the SphygmoCor CPV Central Blood Pressure/Pulse Wave Velocity System (AtCor Medical, Sydney, Australia). Multiple pulse waveforms of the right carotid and right femoral artery were recorded sequentially by applanation tonometry to determine central PWV. The transit time was determined by measuring the distance between the points of measurement of the carotid and femoral pulses, recorded by taking measurements on the surface of the body from the suprasternal notch to the point where the right carotid pulse was found and from the suprasternal notch to the right femoral pulse via the umbilicus. Peripheral PWV was measured as the transit time between the right radial and the right femoral artery waveforms. Pulse waveforms obtained over a 10 sec period at the right radial artery were used to compute a corresponding central waveform using a validated mathematical transformation. Central systolic (CSBP) and diastolic blood pressure (CDBP), augmentation pressure (AP), and augmentation index (AIx) are reported as pulse wave analysis parameters.
2.4. Statistical Analyses
Data (means ± SD) were analyzed by SPSS Version 19.0 (SPSS Inc., Chicago, IL, USA). Prior to all analyses, normality of data was assessed using Shapiro-Wilk's W-test. Initial analyses were performed using 3-way repeated measures ANOVA to examine effects due to drug, time, and gender. All drug∗time∗gender interactions were not significant (all P > 0.05) and thus male and female data are combined. Independent samples t-test, Mann-Whitney U test, or chi-square test was performed to examine differences in participant characteristics between myalgics and nonmyalgics at baseline. Three-way repeated measures ANCOVA was used to examine main and interactive effects due to drug, time, and myalgia status, covarying for time on drug. A Student's paired t-test or Wilcoxon signed rank test was used to evaluate comparisons between study drugs at baseline and between the baseline and the end of the trial within each treatment group in the case of a statistically significant drug∗time interaction. Linear regression analyses were performed to evaluate if baseline variables or the magnitude of change in plasma lipids predicted changes in arterial stiffness. Further models were run controlling for sex and age. An α-level of P ≤ 0.05 was considered statistically significant for all analyses.
3. Results
3.1. Participant Characteristics
Characteristics of participants who did (n = 38, or 33%) versus those who did not (n = 77) meet the study definition for myalgia [11, 12] are summarized in Table 1. Compared to nonmyalgics, participants with myalgia were heavier and were treated with simvastatin for a shorter duration (P < 0.05). Five nonmyalgic participants were on simvastatin treatment for >8 wks (range = 8.3–11.7 wks) due to reported missed doses or antibiotic treatment.
Table 1.
Baseline characteristics of participants by myalgia statusa,b,c.
| Nonmyalgic sample (n = 77) | Myalgic sample (n = 38) | |
|---|---|---|
| Age, y | 60.9 ± 8.5 | 59.1 ± 10.0 |
| Men, n (%) | 42 (55%) | 25 (66%) |
| Height, m | 1.7 ± 0.1 | 1.7 ± 0.1 |
| Weight, kg | 80.8 ± 15.8 | 87.4 ± 17.9∗ |
| BMI, kg/m2 | 27.7 ± 4.2 | 29.8 ± 5.2∗ |
| SBP, mmHg | 123.4 ± 13.7 | 121.5 ± 13.1 |
| DBP, mmHg | 74.9 ± 6.4 | 75.1 ± 6.9 |
| Time on simvastatin, wks | 7.3 ± 1.6 | 3.8 ± 2.0∗ |
| TC, mmol/L | 6.56 ± 1.14 | 6.35 ± 1.04 |
| LDL-C, mmol/L | 4.23 ± 1.00 | 4.11 ± 0.80 |
| HDL-C, mmol/L | 1.41 ± 0.38 | 1.33 ± 0.39 |
| TG, mmol/L | 4.51 ± 2.85 | 4.57 ± 2.98 |
aData are means ± SD or proportions. bBMI, body mass index; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides. cPlasma lipid levels assessed prior to initiating simvastatin or placebo treatment did not differ (P ≥ 0.23) and therefore were averaged. ∗ P ≤ 0.05 between samples.
3.2. Changes in Plasma Lipids
Simvastatin treatment produced the expected reductions in plasma total cholesterol, LDL cholesterol, and triglycerides (Table 2). Neither myalgia status (P ≥ 0.31) nor time on simvastatin (P ≥ 0.17) influenced lipid changes.
Table 2.
Plasma lipid changes by drug assignment and myalgia statusa,b.
| Nonmyalgic sample (n = 77) | Myalgic sample (n = 38) | |||
|---|---|---|---|---|
| Simvastatin | Placebo | Simvastatin | Placebo | |
| ΔTC, mmol/L | −1.50 ± 0.84∗ | −0.04 ± 0.58 | −1.67 ± 0.61∗ | −0.03 ± 0.70 |
| ΔLDL-C, mmol/L | −1.44 ± 0.67∗ | −0.01 ± 0.50 | −1.51 ± 0.60∗ | −0.14 ± 0.60 |
| ΔHDL-C, mmol/L | 0.08 ± 0.18∗ | −0.01 ± 0.15 | 0.04 ± 0.18 | 0.02 ± 0.14 |
| ΔTG, mmol/L | −0.65 ± 2.47∗ | 0.04 ± 1.66 | −1.00 ± 2.50∗ | 0.48 ± 2.73 |
aData are means ± SD; Δ = absolute change from before to after the treatment intervention.
bLDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides. ∗ P < 0.01 from baseline. There were no differences (P ≥ 0.18) between nonmyalgic and myalgic participants within a treatment group.
3.3. Changes in Arterial Stiffness
No differences in arterial stiffness measures were observed between study drug groups at baseline (Table 3). Only central PWV showed a significant drug∗time interaction (P = 0.047). Compared to baseline (9.8 ± 2.9 m/s), central PWV decreased following simvastatin treatment (9.3 ± 2.4 m/s; P = 0.01) but not placebo (Figure 1(a)). Neither myalgia status (P = 0.62) (Figure 1(b)) nor time on simvastatin (P = 0.15) influenced central PWV suggesting that the development of myalgia did not attenuate the improvement in central arterial stiffness with simvastatin treatment.
Table 3.
Arterial stiffness changes by drug assignmenta,b.
| Simvastatin | Placebo | |||
|---|---|---|---|---|
| Baseline | Study end | Baseline | Study end | |
| Peripheral PWV, m/s | 10.3 ± 1.9 | 10.4 ± 1.8 | 10.7 ± 2.5 | 10.2 ± 1.7 |
| Central SBP, mmHg | 115.1 ± 14.6 | 112.7 ± 11.6 | 113.3 ± 12.2 | 112.1 ± 13.3 |
| Central DBP, mmHg | 75.7 ± 6.9 | 75.1 ± 7.6 | 74.9 ± 6.9 | 74.5 ± 8.9 |
| AP, mmHg | 11.9 ± 6.4 | 11.4 ± 5.0 | 12.1 ± 5.2 | 10.9 ± 5.2 |
| AIx, % | 23.4 ± 9.4 | 23.5 ± 8.9 | 24.9 ± 9.4 | 23.0 ± 8.9 |
aData are means ± SD (n = 96). bAIx, augmentation index; AP, augmentation pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure; PWV, pulse wave velocity. There were no differences at baseline (P ≥ 0.07) between nonmyalgic and myalgic participants within a treatment group.
Figure 1.
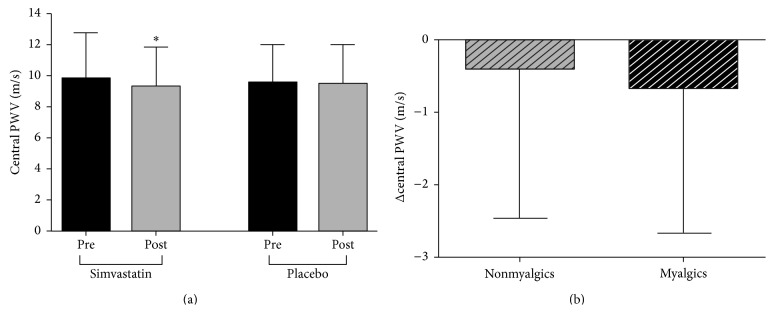
Data are means ± SD. (a) Central pulse wave velocity (PWV) before (Pre) and after (Post) 8 wks of simvastatin (n = 100) or placebo treatment (n = 100); ∗ P < 0.05 compared to Pre within a group. (b) Changes (Post-Pre) in central PWV of participants who completed the simvastatin intervention with no development of statin-related muscle symptoms (nonmyalgics; n = 76) compared with those who reported muscle symptoms with daily simvastatin treatment (myalgics; n = 34); P = 0.51 between groups.
Age was inversely correlated with the change in central PWV after simvastatin treatment (r = −0.207, R 2 = 0.043, P = 0.03) (Figure 2), suggesting that advancing age is associated with enhanced statin-mediated arterial destiffening. The magnitude of change in central PWV after simvastatin treatment was positively correlated with changes in plasma LDL cholesterol concentrations when sex and age were controlled (r = 0.336, R 2 = 0.113, P < 0.01).
Figure 2.
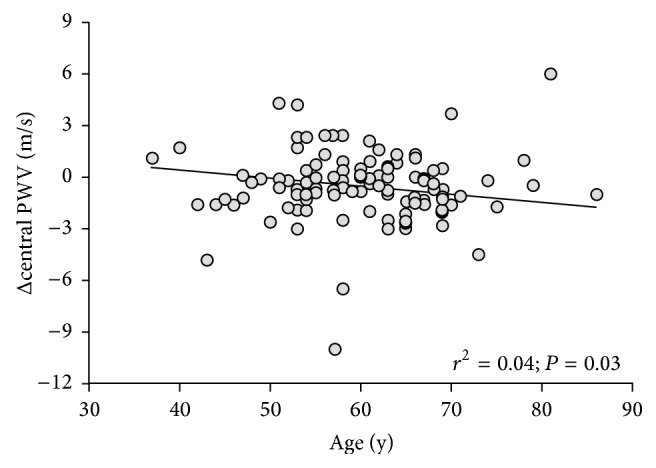
Relationship between changes (Post-Pre) in central pulse wave velocity (PWV) and age in simvastatin-treated participants (n = 110). Δ = absolute change from before to after the treatment intervention.
4. Discussion
The exact mechanism(s) by which statins induce muscle complaints is not known [14, 15]. Depletion of intramuscular metabolites produced by the mevalonate pathway with subsequent increase in cytosolic calcium and activation of mitochondrial-mediated skeletal myocyte apoptosis may contribute to statin myopathy [16]. Findings from the present study of improved central arterial stiffness with simvastatin treatment, irrespective of the development of myalgia, suggest that the mechanism(s) underlying statin muscle symptoms is specific to skeletal myocytes and does not impact vascular smooth muscle with statin therapy. Thus, these data do not support a generalized mechanism of statin myalgia (i.e., impacting both skeletal and smooth muscle cells) as one would expect a differential impact of simvastatin therapy on arterial stiffness in myalgics versus nonmyalgics to occur.
Increased central PWV independently predicts CVD and all-cause mortality [17], suggesting that interventions that reduce central arterial stiffness are clinically important. Central arterial destiffening with statin therapy has been observed in some [6–10] but not all [18–21] studies, an inconsistency potentially explained by differential effects of statin type on arterial stiffness [22] and/or heterogeneity between PWV protocols [23]. Findings from the present study are the first to show similar reductions in arterial stiffness in patients who did versus those who did not exhibit muscle symptoms with statin use as prior studies [6–10] did not assess muscle complaints.
The precise mechanism(s) underlying statin-mediated reductions in central arterial stiffness [6–10] remains unclear. Improvements in endothelial function [1] or suppression of sympathetic neural activity [24, 25] have been proposed as mechanisms by which statins reduce vascular smooth muscle tone and central arterial stiffness in humans. While defining the mechanism was beyond the scope of the present study, our observation of decreased central PWV with simvastatin treatment within a relatively short period of time (≤8 wks) suggests that statins mediate arterial destiffening through functional rather than structural mechanisms. Furthermore, our finding of a direct relationship between the degree of LDL cholesterol lowering and the magnitude of reduction in central PWV with simvastatin treatment suggests a contribution of lipid lowering to central arterial destiffening. In contrast, previous studies in healthy adults have reported reductions in central PWV to occur independently of lipid lowering [7]. Increased statin use [26] warrants future clinical studies aimed at determining the mechanism(s) responsible for statin-mediated central arterial destiffening and whether improvements in PWV with chronic statin therapy translate into lower CVD risk.
Our observation of an inverse relationship between age and reductions in central PWV after simvastatin treatment suggests that the beneficial effects of statins on arterial stiffness may be enhanced with aging. Statins may more beneficially impact older arteries through their antioxidant actions [1] based upon evidence that oxidative stress plays a pathophysiological role in the age-associated reductions in large artery compliance in humans [27]. Limited evidence exists regarding age-dependent pleiotropic effects of statins, an area that warrants future investigation due to the increasing proportion of older adults [28, 29].
Limitations of the present study, including statin type, dose, and treatment duration, have been described [12]. Nevertheless, our observation of reduced central arterial stiffness with simvastatin treatment for ≤8 wks, independent of the development of muscle symptoms, is strengthened by our large sample size and cross-over study design.
5. Conclusions
In summary, findings from this randomized, double-blind, cross-over study show that simvastatin reduces central arterial stiffness in patients with a history of statin-associated muscle complaints. Central PWV was reduced similarly in patients who did versus those who did not exhibit muscle symptoms despite differences in time on simvastatin. Reductions in central arterial stiffness occurred independently of the development of statin-related muscle symptoms, suggesting that statins do not differentially impact arterial stiffness in patients reporting skeletal muscle symptoms with statin treatment. Thus, our data do not support a generalized effect on muscle cells as a mechanism by which statins influence skeletal muscle stiffness and fatigue.
Acknowledgments
Co-Enzyme Q10 in Statin Myopathy study is funded by NCCAM Grant 1RC1AT005836 (P. D. Thompson). All authors made substantial contributions to conception or design of the study and/or acquisition, analysis, or interpretation of the data as well as drafting, revising, and reviewing the paper. The following individuals are acknowledged for their participation on the Data Safety Monitoring Board: JoAnne Foody, M.D., Pamela Hartigan, Ph.D., and Ira Ockene, M.D.
Conflict of Interests
Dr. Taylor served on the statin safety monitoring board for Amgen Pharmaceutical, Inc., Dr. Thompson has received research support from the National Institutes of Health, Genomas, Roche, Sanolfi, Regeneron, Esperion, Amarin, and Pfizer, has served as a consultant for Amgen, Regeneron, Merck, Esperion, and Sanolfi, has received speaker honoraria from Merck and AstraZeneca, owns stock in Abbvie, Abbott Labs, General Electric, Johnson & Johnson, and JA Wiley, and has provided expert legal testimony on exercise-related cardiac events and statin myopathy. The other authors report no conflict of interests regarding the publication of this paper.
References
- 1.Dilaveris P., Giannopoulos G., Riga M., Synetos A., Stefanadis C. Beneficial effects of statins on endothelial dysfunction and vascular stiffness. Current Vascular Pharmacology. 2007;5(3):227–237. doi: 10.2174/157016107781024091. [DOI] [PubMed] [Google Scholar]
- 2.Lahera V., Goicoechea M., Garcia de Vinuesa S., et al. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Current Medicinal Chemistry. 2007;14(2):243–248. doi: 10.2174/092986707779313381. [DOI] [PubMed] [Google Scholar]
- 3.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. The Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 4.Ridker P. M., Danielson E., Fonseca F. A., et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. The New England Journal of Medicine. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd J., Cobbe SM., Ford I., et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. The New England Journal of Medicine. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 6.Kanaki A. I., Sarafidis P. A., Georgianos P. I., et al. Effects of low-dose atorvastatin on arterial stiffness and central aortic pressure augmentation in patients with hypertension and hypercholesterolemia. American Journal of Hypertension. 2013;26(5):608–616. doi: 10.1093/ajh/hps098. [DOI] [PubMed] [Google Scholar]
- 7.Lunder M., Janić M., Habjan S., Šabovič M. Subtherapeutic, low-dose fluvastatin improves functional and morphological arterial wall properties in apparently healthy, middle-aged males—a pilot study. Atherosclerosis. 2011;215(2):446–451. doi: 10.1016/j.atherosclerosis.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 8.Mäki-Petäjä K. M., Booth A. D., Hall F. C., et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. Journal of the American College of Cardiology. 2007;50(9):852–858. doi: 10.1016/j.jacc.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 9.Orr J. S., Dengo A. L., Rivero J. M., Davy K. P. Arterial destiffening with atorvastatin in overweight and obese middle-aged and older adults. Hypertension. 2009;54(4):763–768. doi: 10.1161/hypertensionaha.109.138248. [DOI] [PubMed] [Google Scholar]
- 10.Pirro M., Schillaci G., Mannarino M. R., et al. Effects of rosuvastatin on 3-nitrotyrosine and aortic stiffness in hypercholesterolemia. Nutrition, Metabolism and Cardiovascular Diseases. 2007;17(6):436–441. doi: 10.1016/j.numecd.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Parker B. A., Gregory S. M., Lorson L., Polk D., White C. M., Thompson P. D. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. Journal of Clinical Lipidology. 2013;7:187–193. doi: 10.1016/j.jacl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor B. A., Lorson L., White C. M., Thompson P. D. A randomized trial of coenzyme Q10 in patients with confirmed Statin Myopathy. Atherosclerosis. 2015;238(2):329–335. doi: 10.1016/j.atherosclerosis.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan G., Jensen M. P., Thornby J. I., Shanti B. F. Validation of the brief pain inventory for chronic nonmalignant pain. Journal of Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Norata G. D., Tibolla G., Catapano A. L. Statins and skeletal muscles toxicity: from clinical trials to everyday practice. Pharmacological Research. 2014;88:107–113. doi: 10.1016/j.phrs.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Venero C. V., Thompson P. D. Managing statin myopathy. Endocrinology Metabolism Clinics of North America. 2009;38(1):121–136. doi: 10.1016/j.ecl.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Dirks A. J., Jones K. M. Statin-induced apoptosis and skeletal myopathy. The American Journal of Physiology—Cell Physiology. 2006;291:C1208–C1212. doi: 10.1152/ajpcell.00226.2006. [DOI] [PubMed] [Google Scholar]
- 17.Vlachopoulos C., Aznaouridis K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Ballard K. D., Taylor B. A., Capizzi J. A., Grimaldi A. S., White C. M., Thompson P. D. Atorvastatin treatment does not alter pulse wave velocity in healthy adults. International Scholarly Research Notices. 2014;2014:5. doi: 10.1155/2014/239575.239575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fassett R. G., Robertson I. K., Ball M. J., Geraghty D. P., Sharman J. E., Coombes J. S. Effects of atorvastatin on arterial stiffness in chronic kidney disease: a randomised controlled trial. Journal of Atherosclerosis and Thrombosis. 2010;17:235–241. doi: 10.5551/jat.2683. [DOI] [PubMed] [Google Scholar]
- 20.Raison J., Rudnichi A., Safar M. Effects of atorvastatin on aortic pulse wave velocity in patients with hypertension and hypercholesterolaemia: a preliminary study. Journal of Human Hypertension. 16(10):705–710. doi: 10.1038/sj.jhh.1001470. [DOI] [PubMed] [Google Scholar]
- 21.Shige H., Dart A., Nestel P. Simvastatin improves arterial compliance in the lower limb but not in the aorta. Atherosclerosis. 2001;155(1):245–250. doi: 10.1016/s0021-9150(00)00558-x. [DOI] [PubMed] [Google Scholar]
- 22.Ichihara A., Hayashi M., Koura Y., Tada Y., Kaneshiro Y., Saruta T. Long-term effects of statins on arterial pressure and stiffness of hypertensives. Journal of Human Hypertension. 2005;19(2):103–109. doi: 10.1038/sj.jhh.1001786. [DOI] [PubMed] [Google Scholar]
- 23.Rizos E. C., Agouridis A. P., Elisaf M. S. The effect of statin therapy on arterial stiffness by measuring pulse wave velocity: a systematic review. Current Vascular Pharmacology. 2010;8(5):638–644. doi: 10.2174/157016110792006950. [DOI] [PubMed] [Google Scholar]
- 24.Gao L., Wang W., Li Y. L., et al. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation. 2005;112:1763–1770. doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- 25.Gao L., Wang W., Zucker I. H. Simvastatin inhibits central sympathetic outflow in heart failure by a nitric-oxide synthase mechanism. The Journal of Pharmacology and Experimental Therapeutics. 2008;326(1):278–285. doi: 10.1124/jpet.107.136028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics and Centers for Disease Control and Prevention. Health, United States, 2012: With Special Feature on Emergency Care. Hyattsville, Md, USA: Substance Abuse and Mental Health Servic; 2013. (Health, United States). [PubMed] [Google Scholar]
- 27.Moreau K. L., Gavin K. M., Plum A. E., Seals D. R. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45(6):1107–1112. doi: 10.1161/01.hyp.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 28.Retooling for an Aging America: Building the Health Care Workforce. Committee on the Future Health Care Workforce for Older Americans, Institute of Medicine; 2008. [Google Scholar]
- 29.Forman D. E., Rich M. W., Alexander K. P., et al. Cardiac care for older adults. Time for a new paradigm. Journal of the American College of Cardiology. 2011;57:1801–1810. doi: 10.1016/j.jacc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


