Abstract
Accumulating evidence suggests that aquaporins (AQPs) may facilitate tumor development. The molecular pathways connecting the pathological functions of AQPs are unclear and need to be better defined. This study aimed to investigate whether AQP3, one of the AQPs expressed highly in breast cancer, had any clinical implication in estrogen-receptor (ER) positive breast cancer, and explore the regulatory mechanisms of AQP3 in estrogen-related breast cancer progression. Here we show that AQP3 is an important enforcer of migration and invasion in breast cancer. We, for the first time, reported that ER-positive breast cancer tissues obtained from premenopausal patients had higher AQP3 expression when compared to those obtained from postmenopausal patients. Estrogen directly upregulates AQP3 by activating ERE in the promoter of the AQP3 gene. The upregulation of AQP3 can influence the expression of molecules related to epithelial-mesenchymal transition and the reorganization of actin-cytoskeleton, resulting in enhancement of cell migration and invasion in ER-positive breast cancer cells.
Breast cancer is the most common cancer in women worldwide. The majority of breast cancers are estrogen-dependent for tumor progression1. Well understanding of the mechanisms of cell migration, invasion and proliferation in breast cancer is important for investigating possible anti-tumor therapies.
Aquaporins (AQPs) are a class of small integral membrane proteins distributed widely in organisms2,3. Thirteen members (AQP0-12) have been identified in mammals. AQP3, a member of the aquaglyceroporin subgroup, has broad tissue distribution in human body including renal collecting duct, epidermis, conjunctiva and mammary glands4,5. It has been reported that AQP3 could facilitate cell migration by transportation of water and glycerol for lamellipodia formation6, and lead to cellular proliferation by maintaining a high level of cellular glycerol used for the generation of ATP and lipid biosynthesis7. Mice lacking AQP3 showed defects in urinary-concentrating function8, skin wound healing6 and alimentary tract repairing9. Conversely, enhancement of AQP3 function, by upregulating AQP3 expression, may promote tumorigenesis and tumor development7,10,11.
Recent studies showed that several kinds of tumors including breast cancer overexpressed AQP312,13,14,15,16,17,18. However, whether high expression level of AQP3 in breast cancer has any clinical implication in patients is poorly understood. On the other hand, the mechanisms underlying AQP3 upregulation in breast cancer also remain unclear. Because estrogen has been shown to be an important determinant of the risk of breast cancer1,19, we firstly investigated the relationship between the expression level of AQP3 in estrogen receptor (ER)-positive breast cancer and the patient characteristics. We then examined whether estrogen could alter the expression level of AQP3 in breast cancer cell lines. Finally, we successfully identified an estrogen response element (ERE) in the promoter of AQP3 gene, which might mediate estrogen-induced AQP3 expression, cell migration and invasion in ER-positive breast cancer.
Results
Immunochistochemical analysis of AQP3 expression in the cancer tissues of patients with ER-positive breast cancer.
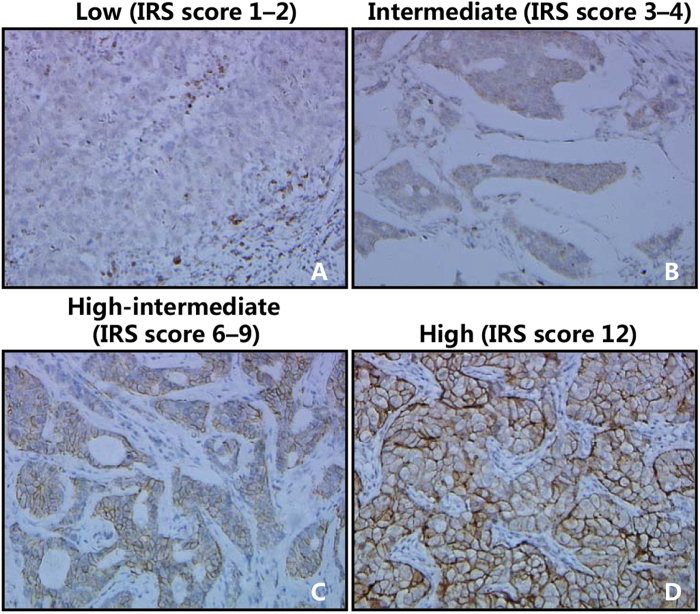
Using immunohistochemistry (IHC) and immunoreactivity scoring system (IRS), we examined the expression level of AQP3 protein in breast invasive ductal carcinoma samples obtained from 56 patients. Before the IHC experiments, the AQP3 antibody had been proofed appropriately validated for IHC (Supplementary Figure S1A). Fig. 1 shows different IRS scores in breast cancer samples. We found that AQP3 was mainly expressed in the cell membrane and cytoplasm (Fig. 1 and Supplementary Figure S1B). The IRS analysis showed that higher AQP3 expression level was associated with higher histopathological grade and more lymph node metastasis in the patients with ER-positive breast cancer (Table 1). On the other hand, AQP3 expression level in ER-positive breast cancer was higher in the premenopausal patients than which in the postmenopausal patients (Table 1).
Figure 1. Immunochistochemical analysis of AQP3 expression in cancer tissues of patients with breast cancer.
AQP3 IRS scores of representative examples of low (A), intermediate (B), high-intermediate (C), and high (D) are shown in ER-positive breast cancer, respectively (magnification: ×200). The tumor status (histopathological grade, nodal status, stage) of the representative examples is detailed as follows, (A) (1, N, 1), (B) (2, N, 1), (C) (3, P, 2) and (D) (3, P, 3). P: positive nodal metastasis; N: negative nodal metastasis.
Table 1. Patient characteristics and AQP3 expression in ER-positive.
| Characteristics | ER-positive (n = 56) | |
|---|---|---|
| Age (years, mean (range)) | 50 (31–69) | |
| BMI (kg/m2) | 23.06 ± 2.92 | |
| E2 (pmol/L, mean (range)) | 286.86 (18.35–2322) | |
| n (%) | AQP3 IRS score | |
| Menses status | ||
| Premenopausal | 31 (55.4%) | 6.25 ± 2.24 |
| Postmenopausal | 25 (44.6%) | 5.05 ± 1.84* |
| Histopathological grade | ||
| I-II | 32 (57.1%) | 4.72 ± 1.55 |
| II-III | 22 (39.3%) | 7.28 ± 2.04** |
| Nodal status | ||
| Negative | 29 (51.8%) | 5.10 ± 1.78 |
| Positive | 27 (48.2%) | 6.24 ± 2.39* |
| Stage | ||
| I-II | 49 (87.5%) | 5.38 ± 2.02 |
| III | 7 (12.5%) | 8.03 ± 1.53** |
Data are presented as mean ± SD, mean (range) or number (%). *P < 0.05, and **P < 0.01, compared with the corresponding controls, respectively.
Estrogen upregulated AQP3 expression in the ER-positive breast cancer cells
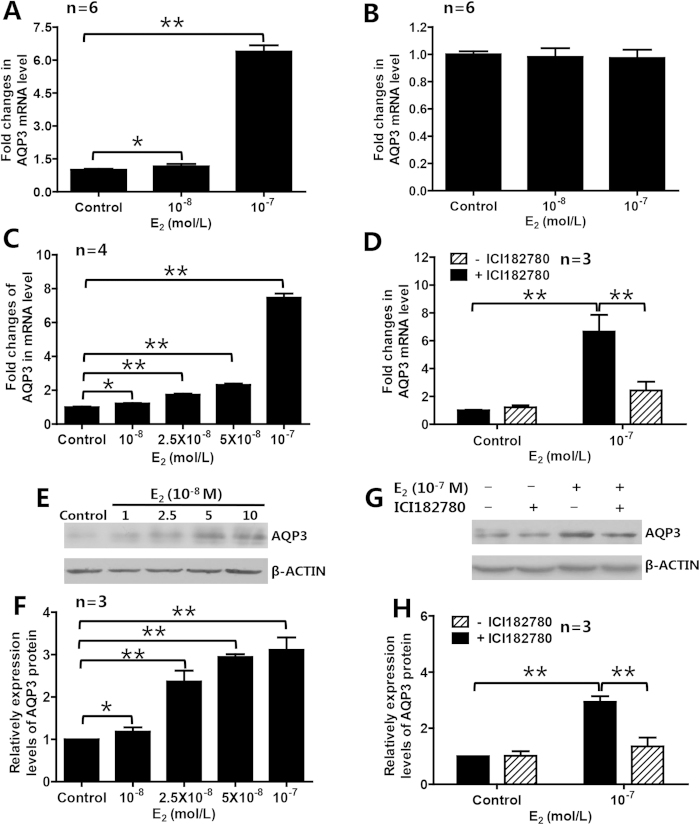
In order to determine whether and how estrogen regulates AQP3 expression in ER-positive breast cancer cells, we treated three breast cancer cell lines including ER-positive T47D and MCF7 cells and ER-negative MDA-MB-231 cells with estradiol (E2), and found that treatment with 10−8 M and 10−7 M E2 for 48 h significantly upregulated the expression level of AQP3 mRNA in ER-positive breast cancer cells (T47D, Fig. 2A; MCF7, Supplementary Figure S2A), but not in ER-negative breast cancer cells (MDA-MB-231, Fig. 2B). The E2-induced upregulation of AQP3 mRNA and protein expression in T47D cells was dose-dependent (Fig. 2C,E,F). The estrogenic effects on AQP3 mRNA and protein expression in T47D cells were blocked by 10−6 M ICI182780, an estrogen receptor antagonist20, suggesting that estrogen receptors may mediate the estrogen-induced upregulation of AQP3 in ER-positive breast cancer cells (Fig. 2D,G,H).
Figure 2. E2 upregulated AQP3 expression in ER-positive breast cancer cells.
E2 upregulated expression of AQP3 in ER-positive T47D cells (A), but not in ER-negative MDA-MB-231 cells (B). In T47D cells, E2 dose-dependently increased AQP3 mRNA (C) and protein (E,F) expression. ER antagonist ICI182780 could block the E2-induced expression of AQP3 mRNA (D) and protein (G,H). The data are presented as means ± SD, *P < 0.05 and **P < 0.01 (A–C,F One-way ANOVA and Turkey’s post hoc tests; D,H Student t-test).
Identification of a functional ERE in the promoter of AQP3 gene
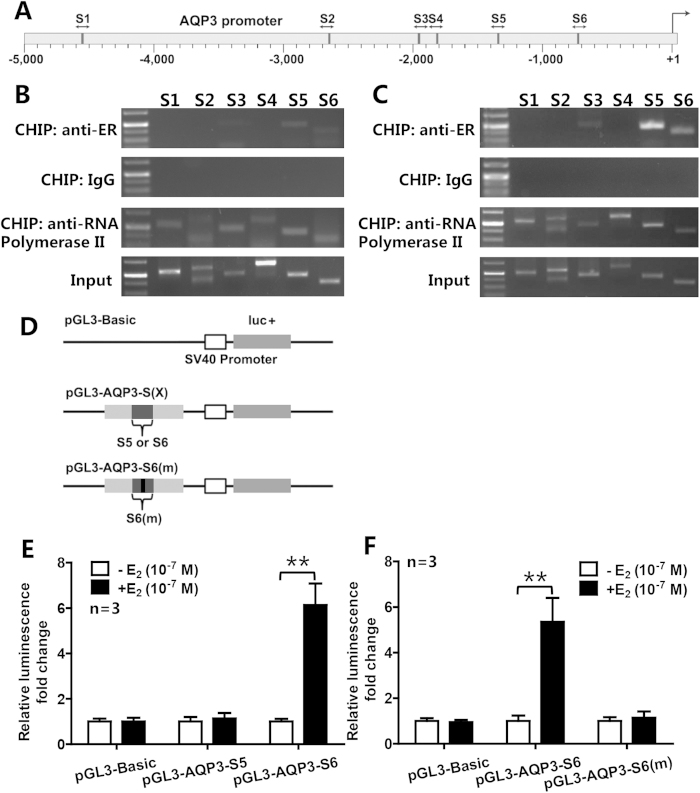
In order to determine whether the AQP3 gene in ER-positive breast cancer cells is regulated directly by estrogen via ER binding to ERE, we analyzed putative EREs in promoter of AQP3 gene using the Regulatory Sequence Analysis Tools (RSAT), and, obtained six high-score putative EREs (Fig. 3A). ChIP analysis showed that three fragments (S3, S5 and S6) in promoter of AQP3 could be pulled down by ERα antibody (Fig. 3B,C; Supplementary Figure S2B and S2C), and were brighter in the presence of E2 (Fig. 3C and Supplementary Figure S2C). After sequencing, S3 was excluded. To determine whether the pulled down fragments, including S5 and S6, had a functional role in estrogen-dependent transcriptional activation, we constructed two plasmids (pGL3-AQP3-S5 and pGL3-AQP3-S6, Fig. 3D) for luciferase reporter assay. The results showed that pGL3-AQP3-S6 that contained a putative ERE (ACATGGCTaggTGACCTAG) was activated by E2 (Fig. 3E), whereas E2 had no effect on pGL3-AQP3-S5 and the mutated pGL3-AQP3-S6(m) (ACATGGCTaggCCTAG) (Fig. 3E,F). This result indicates that the promoter of AQP3 gene contains a functional ERE motif, which may mediate estrogen-induced upregulation of AQP3 expression in ER-positive breast cancer cells.
Figure 3. Identification of the functional ERE in the promoter of AQP3 gene.
(A) Schematic depiction of the AQP3 promoter suggested six sequences (S1-S6, dark grey rectangles) which might contain putative EREs. (B,C) ChIP analysis included positive control (anti-RNA polymerase II), negative control (normal mouse IgG), ERα antibody and input groups. Three sequences (S3, S5 and S6) were pulled down by anti-ERα antibody, and were brighter in the presence of E2 (C) than in the absence of E2 (B). After sequencing, S3 was excluded. (D) Schematic of AQP3 promoter-driven luciferase reporter constructs was indicated. PGL3-Basic was used as a negative control. pGL3-AQP3-S5 and pGL3-AQP3-S6 contained S5 and S6, respectively. AQP3-luc-S6(m) contained a mutated S6, and the mutation site was in its putative ERE. (E,F) Luciferase activities of the report systems showed that only pGL3-AQP3-S6 could be activated by E2, and E2 had no effect on the mutated pGL3-AQP3-S6(m). The data are presented as mean ± SD, **P < 0.01 (Student t-test).
Knockdown or inhibition of AQP3 significantly reduced the E2-induced migration and invasion of ER-positive breast cancer cells
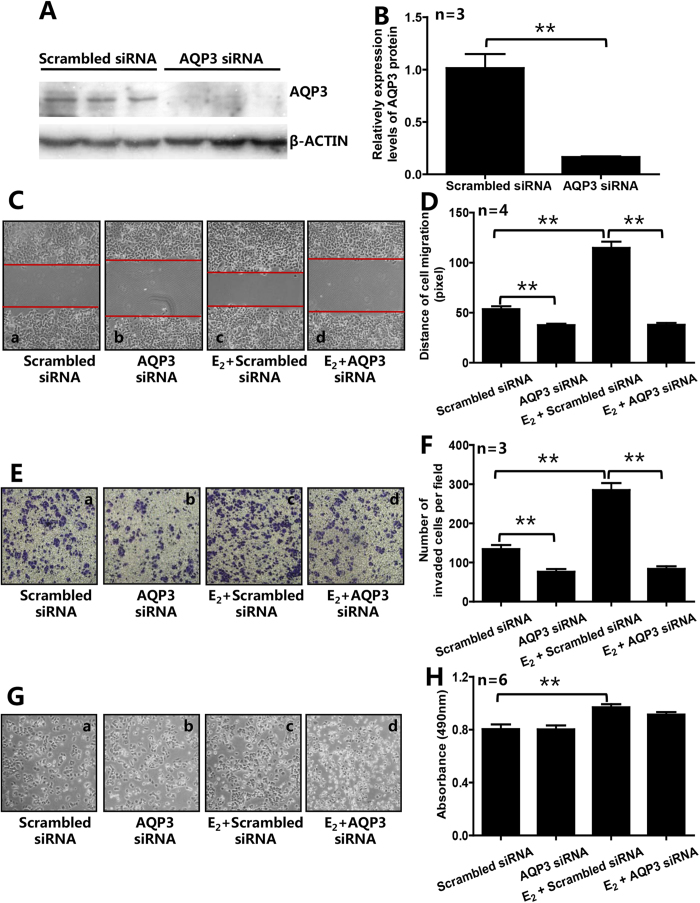
In order to determine the role of AQP3 in E2-induced migration, invasion and proliferation of ER-positive breast cancer cells, we transfected siRNA targeting AQP3 gene into T47D and MCF7 cells. Compared with the cells transfected with scrambled siRNA, treating T47D cells with AQP3 siRNA for 36 h significantly reduced the expression level of AQP3 protein by 84.9% (Fig. 4A,B). E2 (10−7 M) significantly promoted cell migration (2.14 ± 0.23-flod), invasion (2.12 ± 0.23-flod) and proliferation (1.21 ± 0.07-flod) in T47D cells transfected with scrambled siRNA (Fig. 4C–H). As shown in Fig. 4C,D, knockdown of AQP3 decreased 29.7% of the migration distance in AQP3 siRNA group (vs. scrambled siRNA group), and 66.9% in AQP3 siRNA + E2 group (vs. scrambled siRNA + E2 group). As shown in Fig. 4E,F, knockdown of AQP3 significantly reduced 43.2% of the invaded cells in AQP3 siRNA group (vs. scrambled siRNA group), and 70.6% in AQP3 siRNA + E2 group (vs. scrambled siRNA + E2 group). However, knockdown of AQP3 had no effect on cell proliferation (Fig. 4G,H). We also observed the similar results in another ER-positive breast cancer cell line MCF7 (Supplementary Figure S4). Treating MCF7 cells with E2 (10−7 M) significantly promoted cell migration (Supplementary Figure S4C and S4D) and invasion (Supplementary Figure S4E and S4F). As shown in Supplementary Fig. S4C and S4D, knockdown of AQP3 significantly decreased 39.2% of the migration distance in AQP3 siRNA group (vs. scrambled siRNA group), and 56.9% in AQP3 siRNA + E2 group (vs. scrambled siRNA + E2 group). As shown in Supplementary Fig. S4E and S4F, knockdown of AQP3 significantly reduced 55.1% of the invaded cells in AQP3 siRNA group (vs. scrambled siRNA group), and 66.5% in AQP3 siRNA + E2 group (vs. scrambled siRNA + E2 group).
Figure 4. Knockdown of AQP3 reduced E2-induced cell migration and invasion of ER-positive breast cancer cells.
(A,B) Treating T47D cells with AQP3-specific siRNA significantly reduced the expression level of AQP3. Knockdown of AQP3 attenuated migration (C,D) and invasion (E,F) of T47D cells in the absence of E2. E2 (10−7 M) significantly increased cell migration (C,D), invasion (E,F), and proliferation (G,H) in scrambled siRNA group, and, Knockdown of AQP3 in T47D cells inhibited the E2-promoted migration and invasion, but not proliferation (C–H). The data are presented as mean ± SD, **P < 0.01 (B Student t-test; D,F and H One-way ANOVA and Turkey’s post hoc tests).
Pretreatment of CuSO4, an inhibitor of AQP function, also significantly reduced the E2-induced migration and invasion in T47D cells (Supplementary Figure S3). As shown in Supplementary Fig. S3A and S3B, CuSO4 significantly decreased 41.2% of the migration distance in CuSO4 group (vs. control group), and 66.8% in CuSO4 + E2 group (vs. E2 group). As shown in Supplementary Fig. S3C and S3D, CuSO4 significantly reduced 40.6% of the invaded cells in CuSO4 group (vs. control group), and 65.1% in CuSO4 + E2 group (vs. E2 group).
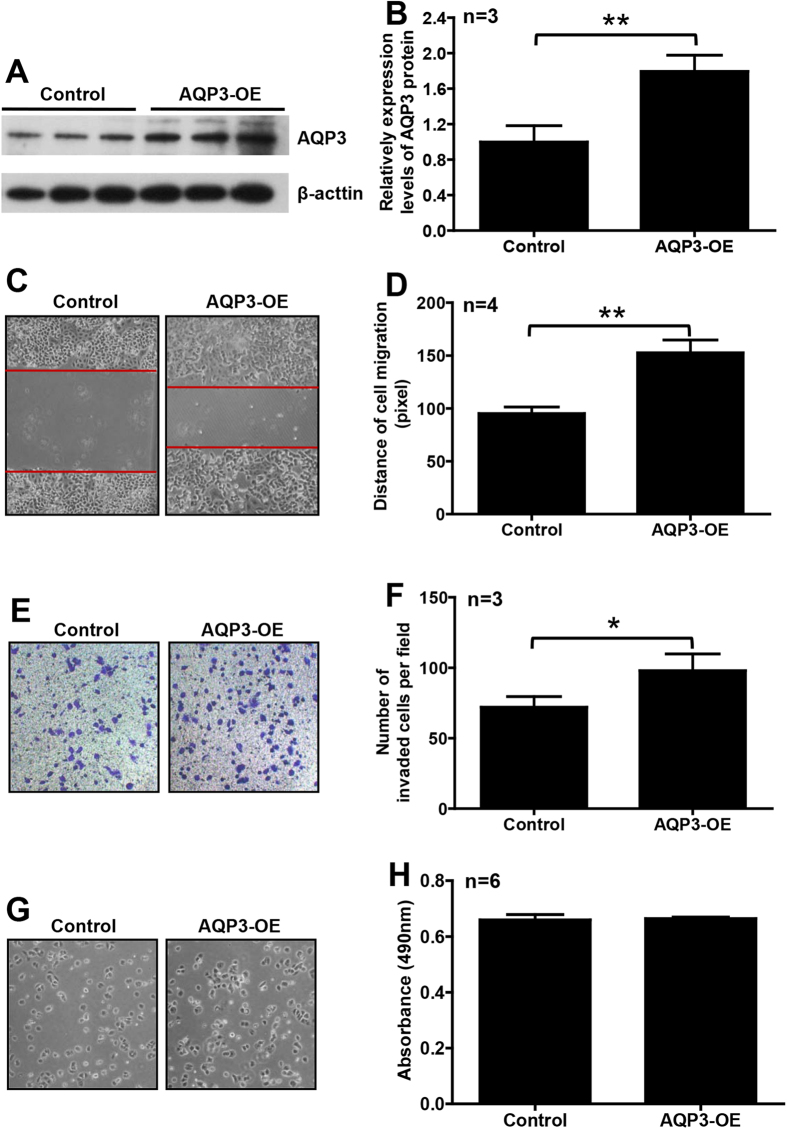
AQP3 overexpression enhanced the migration and invasion of T47D cells
To confirm that high AQP3 expression might play an important role in breast cancer progression, we overexpressed AQP3 in T47D cells. After AQP3 overexpression vector transfection for 48 h, AQP3 protein level was significantly increased (161.9%), compared with control (Fig. 5A,B). As shown in Fig. 5C–H, the cells overexpressing AQP3 showed significantly increased migration (1.60 ± 0.13-fold) and invasion (1.36 ± 0.16-fold), not proliferation (1.01 ± 0.02-fold).
Figure 5. Overexpression of AQP3 enhanced migration and invasion of ER-positive breast cancer cells.
The expression level of AQP3 was significantly increased in T47D cells with vector-mediated overexpression (AQP3-OE) (A,B). Overexpression of AQP3 significantly enhanced migration (C,D) and invasion (E,F), but not proliferation (G,H), of T47D cells. The data are presented as mean ± SD, *P < 0.05 and **P < 0.01 (Student t-test).
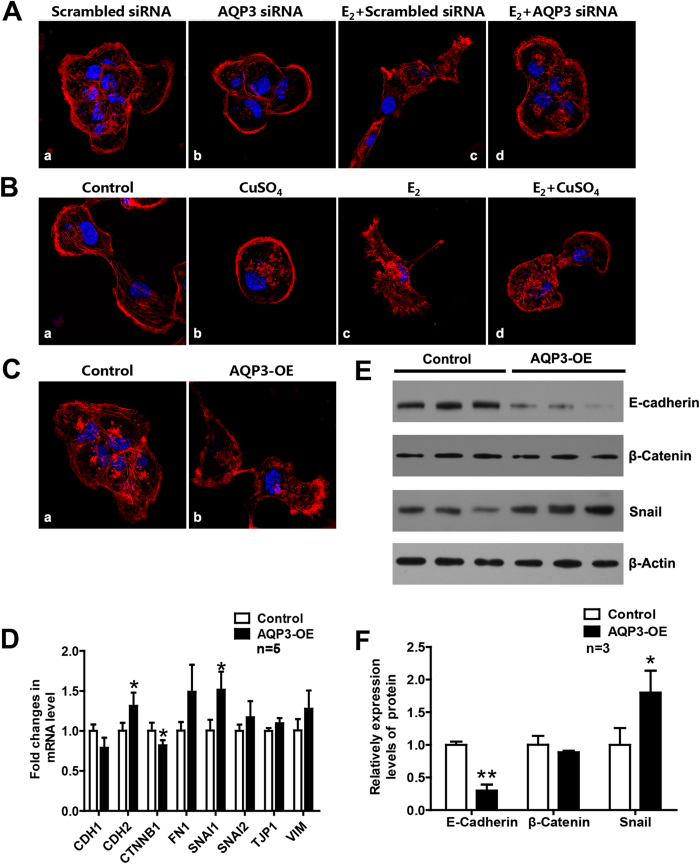
Knockdown or inhibition of AQP3 significantly reduced the E2-induced reorganization of actin cytoskeleton in T47D cells.
In T47D cells, E2 induced a marked reorganization of actin cytoskeleton characterized by formation of filopodia and rearrangement of stress fibers (Fig. 6Ac,Bc), which contributed to the E2-induced migration and invasion. Knockdown of AQP3 (Fig. 6Ab,Ad) or inhibition of AQP3 function with CuSO4 (Fig. 6Bb,Bd) significantly reduced E2-regulated reorganization of actin cytoskeleton.
Figure 6. AQP3 influenced reorganization of actin cytoskeleton and expression of EMT-related factors.
(A,B) Organization of the actin cytoskeleton in T47D cells treated with or without AQP3 siRNA or CuSO4, respectively. (Ac and Bc) E2 induced the reorganization of actin cytoskeleton (red) by promoting formation of filopodia and rearrangement of stress fibers. Treating T47D cells with AQP3 siRNA (Ab and Ad) or CuSO4 (Bb and Bd) significantly reduced E2-regulated reorganization of actin cytoskeleton. (C) AQP3 overexpression influence reorganization of actin cytoskeleton. (D–F) The expression of several MET-related factors changed after AQP3 overexpressed. The data are presented as mean ± SD, *P < 0.05 and **P < 0.01 (Student t-test).
AQP3 overexpression influenced reorganization of actin cytoskeleton and expression of EMT-related factors in T47D cells.
AQP3 overexpression induced reorganization of actin cytoskeleton in T47D cells, which characterized by formation of filopodia and rearrangement of stress fibers (Fig. 6Cb). To explore the mechanism underlying migration and invasion promoted by AQP3, we analyzed the expression of several molecules related to epithelial-mesenchymal transition (EMT), including the epithelial markers CDH1 (E-cadherin), CTNNB1 (β-catenin) and TJP1 (ZO-1), and, the mesenchymal markers CDH2 (N-cadherin), VIM (Vimentin), FN1 (Fibronectin 1), SNAI1 (Snail) and SNAI2 (Slug), between control and AQP3-overexpressed T47D cells by qPCR. As shown in Fig. 6D, the mRNA level of one factor (CTNNB1) was decreased and two (CDH2 and SNAI1) were increased significantly (P < 0.05) in AQP3-overexpression group. The results were further verified by Western blotting analysis (Fig. 6E,F), suggesting that AQP3 might act as a positive regulator of the EMT signaling pathways during the metastasis of breast cancer, although the mechanisms was yet unknown.
Discussion
The present study showed that higher AQP3 expression level in ER-positive breast cancer tissues was associated with higher histopathological grade and more lymph node metastasis. On the other hand, we found that AQP3 expression level was higher in samples obtained from premenopausal patients than those from postmenopausal patients. We identified an ERE in the promoter of AQP3 gene, and found that estrogen could promote cell migration and invasion in ER-positive breast cancer via activating the ERE in AQP3 gene. Knockdown of AQP3 significantly attenuated migration and invasion of breast cancer cells, and over-expression of AQP3 significantly enhanced these two processes. AQP3 mediated E2-enhanced migration and invasion through influencing the expression of EMT-related factors and the reorganization of actin cytoskeleton.
Several studies showed that some subtypes of aquaporins including AQP1, 3 and 5 were elevated in breast cancer tissues compared to normal tissues17,21,22. In clinical studies, it has been suggested that high AQP1 expression might be associated with poor prognosis22, and high AQP5 expression might be associated with more lymph node invasion17,21. In this study, we explored the relationship between AQP3 expression level in ER-positive breast cancer and the clinical characteristics of patients with breast invasive ductal carcinoma, by immunohistochemistry. Immunoreactivity analysis showed that ER-positive breast cancer tissues obtained from premenopausal patients had higher AQP3 level than those obtained from postmenopausal patients, and treating ER-positive breast cancer cells with E2 not only upregulated the expression of AQP3, but also promoted cell migration and invasion. The results suggest that AQP3 may mediate estrogen-promoted tumor development in ER-positive breast cancer.
The mechanisms underlying the regulation of AQP3 expression are complex and unclear. It was reported that natriuretic peptides could upregulate AQP3 in human colonic epithelial cells23, and, TNF-α could upregulate AQP3 in the epithelial lesion with chronic periodontitis24. Further studies demonstrated that growth factors like EGF and FGF-2 could upregulate AQP3 expression though MAPK/ERK pathway and/or PI3K/AKT pathway11,16,25. However, the regulatory mechanisms for AQP3 expression in breast cancer were still poorly understood. Previous researches showed that, in rat model, the expression of AQP3 in urothelium26 and vagina27 was significantly lower after ovariectomy and could be restored to the control levels after estrogen treatment. Since estrogen is critical for development and progression of breast cancer, we investigated whether E2 could enhance AQP3 expression in ER-positive breast cancer cell line (T47D) or ER-negative breast cancer cell line (MDA-MB-231). Our data showed that E2 could upregulate AQP3 expression in ER-positive cells only, and the ER antagonist inhibited its enhanced effects. Estrogen exerts its effects by directly binding to ER, which homodimerizes and interacts with ERE in genes to stimulate the transcription of target genes. We, for the first time, found a functional ERE in the promoter region of the AQP3 gene. Although this sequence is exactly same as the canonical ERE (GGTCAcagTGACC), the 13-nucleotide (TGGCTaggTGACC) can form an inverted repeat just like the classical ERE. We are now fully persuaded the expression of AQP3 is under the direct control of estrogen.
On the other hand, we found that higher AQP3 levels were associated with poorer cell differentiation and more lymph node metastasis in ER-positive breast cancer patients, suggesting that AQP3 may be an enhancer in breast cancer progression. It has been shown that AQP3 might mediate FGF-2-induced cell migration in two representative breast cancer cell lines (MDA-MB-231 and Bcap-37)25. This study demonstrated that AQP3 played an important role in E2-induced migration and invasion of ER-positive (T47D) breast cancer cells. Knockdown of AQP3 expression significantly attenuated migration and invasion of T47D cells, while overexpression of AQP3 promoted cell migration and invasion.
For metastasis to occur, tumor cells firstly detach from their tissue of origin, and then migrate and invade to another organ28. Previous studies indicated that AQP3 expression level might have relevance with molecules which were important in cell-cell and/or cell-matrix interactions24,29,30,31,32. Those Data showed a significant decrease in matrix metalloproteinases (MT1-MMP, MMP-2 and M MP-9)31 and cell adhesion molecules (ICAM-133, integrinα5β129 and E-cadherin30) after AQP3 knockdown, suggesting that these molecules may play roles in AQP3-mediated migration and invasion of breast cancer cells. Our data showed that, in breast cancer cells, increased expression of AQP3 would upregulate the expression of Snail, downregulate the expression of E-cadherin, and, influence the formation of filopodia and the rearrangement of stress fibers.
In conclusion, we used a combination of clinical patient samples and in vitro cellular systems to show that AQP3 plays an important role in cell migration and invasion of ER-positive breast cancer. Our clinical data indicated that higher AQP3 expression in ER-positive breast cancer was associated with poorer cell differentiation, more lymph node metastasis and premenopausal status. We, for the first time, identified an ERE in the promoter of AQP3 gene, and found that estrogen might promote breast cancer development through activating ERE in the promoter of AQP3 gene and upregulating AQP3 expression in ER-positive breast cancer. Upregulation of AQP3 by E2 increases the cell migration and invasion through regulating the expression of EMT-related factors and influencing the reorganization of actin cytoskeleton. Hence, in our view, AQP3 might be a potential target for anti-breast cancer treatment. However, the precise molecular mechanisms of AQP3-induced cell migration and invasion are still incompletely understood. Further studies are encouraged to investigate the mechanisms.
Methods
Patients and sample collection
This study included Fifty-six patients (median age, 50 yr; range: 31–69 yr) with breast invasive ductal carcinoma (IDC) confirmed by histopathological analysis after breast surgery at Women’s Hospital, School of Medicine, Zhejiang University, China, between January 2011 and June 2012. Histopathological grade was determined by modified Bloom-Richardson-Elston grading system. These patients did not receive hormone treatment in the previous three months. The study was approved by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University, and all patients provided the written informed consent for this study. All experiments were performed in accordance with relevant guidelines and regulations.
Tissue immunohistochemistry
Breast samples were sectioned at 4 μm intervals. Sections were heated in citrate buffer for 20 min for antigen retrieval, and, bathed in a 3% H2O2/PBS solution for 10 min at room temperature in the dark to quench endogenous peroxidase. Then, sections were incubated with AQP3 primary antibody (Abcam, San Francisco, CA, USA) at a 1:500 dilution overnight at 4 °C. After several washes, 50 μl of secondary antibodies (DakoCytomation, Carpinteria, CA, USA) were added to tissues and incubated for 30 min. After washing again, the sections were reacted with DAB, and, counterstained with hematoxylin. Immunostained sections were reviewed and scored semiquantitatively by expert pathologists on the basis of a well-established immunoreactivity scoring system (IRS)34. The IRS score was calculated by the product of the percentage of positive cells (0, 0%; 1, 1–10%; 2, 11–50%; 3, 51–80%; 4, >80%) and the intensity of the staining (0, no staining; 1, mild; 2, moderate and 3, strong), which gave an IRS score from 0 (no staining) to 12 (maximum staining).
Agar-paraffin double embedded technology
In order to validate the IHC results, the pellets of control, AQP3 knockdown and AQP3 overexpression T47D cells were embedded in the same paraffin block, and IHC was performed on the same panel. Cells were trypsinised and fixed for 3 h in 10% formalin, centrifuged at 2000 g for 10 minutes, then washed twice with PBS and stained with eosin, and finally re-suspended in 3% agarose. The cell pellets were processed through gradient concentrations of alcohols before being cleared in xylene and washed in molten paraffin. These cell pellets were embedded in paraffin and IHC was carried out on 4–5 μm sections.
Cells and cell culture
Breast cancer cell lines, T47D and MDA-231 cells were obtained from the Key Laboratory of Cancer Prevention and Intervention, Ministry of Education, China, and, were cultured in RPMI-1640 and L-15, respectively, supplemented with 10% fetal bovine serum (FBS) and antibiotics. Before and during the E2 treatment, cells were cultured in phenol red-free medium supplemented with 10% charcoal/dextran-treated FBS.
RT-qPCR
Total RNA was extracted from breast cancer cells using RNAiso Plus (Takara, Dalian, China) according to the manufacturer’s instructions. The cDNA was prepared by reverse transcription, using RT reagent Kit (Takara). qPCR was carried out with SYBR-Green premix Ex Taq (Takara) in an Applied Biosystems 7900 Fast (ABI, Carlsbad, CA, USA), using GAPDH as internal controls. Primer sequences used for qPCR are shown in Supplemental Table S2. qPCR was performed in a 10 μl reaction system containing 5 μl SYBR premix Ex Taq, 0.2 μl sense and 0.2 μl antisense primers, 0.2 μl Dye I, 3.4 μl ddH2O, 1.0 μl cDNA. The thermal cycling conditions were: 95 °C for 10 s, 95 °C for 5 s, 60 °C for 34 s, and for 40 cycles. Every sample was repeated at least three times. Data were analyzed by the comparative threshold cycle (CT) method.
Protein extraction and western blot analysis
Protein extracts from breast cancer cell lines were made in RIPA buffer containing protease inhibitors (1 μg/mL leupeptin and 1 μg/mL phenylmethylsulfonyl fluoride). The polyvinylidene fluoride transfer membranes containing separated samples were incubated with blocking buffer for 30 min. The membranes were then incubated with primary antibody (AQP3, 1:500 or β-actin, 1:2000) overnight at 4 °C, followed by horseradish peroxidase-linked secondary antibody (1:5000) for 1 h at room temperature, and visualized with ECL detection reagent. The grayscale of bands was measured with Quantity One software.
Bioinformation and chromatin immunoprecipitation (ChIP) analyses
The Regulatory Sequence Analysis Tools (RSAT) web server was used to analyze the promoter sequence of AQP3 gene to find high-score putative EREs. T47D cells were treated with 10−7 M E2 for 6 h and cross-linked with 1% formaldehyde and processed. The ChIP analyses were performed according to the ChIP Kit Instruction Manual (Millipore, Billerica, MA, USA). Condition for sonication was 15 sec pulse followed by 30 sec rest, and power setting at 30%. ERα antibody (Millipore) is functionally validated in the precipitation of ERα associated chromatin. Purified immunoprecipitated DNA were used for RT- PCR and the PCR products were confirmed by sequencing. Primer sequences used for ChIP PCR are shown in Supplemental Table S1.
Plasmid construction and luciferase reporter assay
AQP3 promoter fragments containing an ERE-like sequence were gained from ChIP analyses. They were digested with XhoI and KpnI, and ligated into promoter-less luciferase reporter plasmid pGL3-basic (Promega, Madison, WI, USA) to construct the AQP3 promoter-luciferase reporter systems. Luciferase reporter systems (0.8 μg AQP3 promoter-luciferase reporter plasmid or pGL3-basic, and 0.08 μg pRL-TK reporter plasmid) were transfected into T47D cells in 24-well plate. After transfection for 36 h, cells were treated with or without E2 (10−7 M). Cell lysates were prepared, and, luciferase activities were measured using the dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions. Luciferase values were normalized to the Renilla luciferase activity.
Interfering RNAs (siRNAs) knockdown and vector-mediated overexpression studies
T47D cells were seeded in 6-well plates. For knockdown experiments, siRNA targeting the AQP3 gene (100 pmol/well) and siRNA negative control were purchased from RiboBio (Guangzhou, China). For overexpression experiments, AQP3 overexpression vector (2 μg/well) was purchased from OriGene (Rockville, MD, USA). Cell transfection was conducted using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s guideline.
Wound healing assay
T47D cells (1 × 105/well) were seeded in 12-well plates pre-coated with 0.5% gelatin overnight at 4 °C. After pretreatment (knockdown or overexpression of AQP3), cells were cultured to confluence overnight. The monolayer cells were then scratched with a standard 200 μl pipette tip, and washed twice with PBS to remove floating cells. After scratching the lines, cells were cultured in medium supplemented with or without E2 (10−7 M) for 24 h. Mitocycin C (10 mg/ml) was included in the medium to prevent cell proliferation. Wound healing was quantified by measuring the migratory distance of cells.
Transwell invasion assay
A permeable filter of transwell system (Corning Incorporated, Midland, MI, USA) was used to study the invasion ability of cells. The inside compartment of the transwell inserts was coated with Matrigel (BD Biosciences, Bedford, MA, USA) at 4 °C overnight, and then blocked by 1% BSA/PBS solution for 30 min at room temperature. After pretreatment (knockdown or overexpression of AQP3), T47D cells (1 × 105/well) were loaded in the upper chamber in culture medium with 0.2% BSA, and with or without E2 (10−7 M). Cell migration to the other side of the membrane was induced by 30% FBS-containing medium in the lower chamber for 24 h. Cells were fixed in methanol for 30 min, and stained with 0.5% crystal violet for 15 min. After gently removing the cells on the up side of the top chamber, migrated cells were photographed and counted with Image-J software (National Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay
T47D cells (1 × 104/well) were plated in 96-well plates. After pretreatment (knockdown or overexpression of AQP3), cells were cultured for 24 h in culture medium supplemented with or without E2 (10−7 M). The MTT assay was applied to quantify cell proliferation, and, the absorbance of samples was measured at 490 nm.
Immunofluorescence analysis
T47D cells (1 × 104/well) were grown on coverslips and exposed to treatments. The fixed cells were blocked in 1% BSA and then incubated with a 1:300 dilution of primary AQP3 antibody at 4 °C overnight. They were then incubated with a 1:200 dilution of Alexa Fluor488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA) for 1 h. Actin-cytoskeleton was detected using rhodamine-conjugated phalloidin (Sigma, St Louis, MO, USA) diluted in phosphate buffer (50 μg/mL). Confocal images were taken by Olympus microscope (BX61W1-FV1000).
Statistical analysis
Statistical analysis was performed with two-tailed indirect Student’s t-test between two groups. One-way ANOVA and Turkey’s post hoc tests were used to evaluate the statistical significance of the difference between more than two groups. P-value < 0.05 was regarded as significant.
Additional Information
How to cite this article: Huang, Y.-T. et al. Identification of Estrogen Response Element in Aquaporin-3 Gene that Mediates Estrogen-induced Cell Migration and Invasion in Estrogen Receptor-positive Breast Cancer. Sci. Rep. 5, 12484; doi: 10.1038/srep12484 (2015).
Supplementary Material
Acknowledgments
The authors thank Ms. Cai-Yun Zhou for expert technical assistance, and Professor Jin-Biao Zhan, Zhejiang University School of Medicine, for reading this manuscript. This work was supported by the National Basic Research Program of China (No.2012CB944900 to H.F.H and No.2011CB944502 to J.Z.S.); the National Natural Science Foundation of China (No.81270708 to J.Z.S.); The National Science and Technology Support Program (No.2012BAI32B01 to H.F.H.).
Footnotes
Author Contributions Y.T.H., H.F.H. and J.Z.S. conceived and designed the experiments. Y.T.H., J.Z., H.Y.X., S.S., F.Q., Y.D.C., D.Z. and J.Y. performed the experiments. Y.T.H., H.Y.X., S.S., J.Z., F.Q., Z.D., J.Y., H.F.H. and J.Z.S. analyzed the data. Y.T.H., H.F.H. and J.Z.S. wrote the manuscript.
References
- Yager J. D. & Davidson N. E. Estrogen carcinogenesis in breast cancer. N Engl J Med. 354, 270–282 (2006). [DOI] [PubMed] [Google Scholar]
- Verkman A. S. & Mitra A. K. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 278, F13–F28 (2000). [DOI] [PubMed] [Google Scholar]
- King L. S., Kozono D. & Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 5, 687–698 (2004). [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Anderson M. O. & Papadopoulos M. C. Aquaporins: important but elusive drug targets. NAT REV DRUG DISCOV. 13, 259–277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobasheri A. & Barrett-Jolley R. Aquaporin water channels in the mammary gland: from physiology to pathophysiology and neoplasia. J Mammary Gland Biol Neoplasia. 19, 91–102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M. & Verkman A. S. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med (Berl). 86, 221–231 (2008). [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M. & Verkman A. S. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. MOL CELL BIOL. 28, 326–332 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Song Y., Yang B., Gillespie A. & Carlson E. J. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci USA. 97, 4386–4391 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajah J. R., Zhao D. & Verkman A. S. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. GUT. 56, 1529–1535 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Li Z., Zhang W., Wang H. & Zhi X. miR-874 Inhibits cell proliferation, migration and invasion through targeting aquaporin-3 in gastric cancer. J GASTROENTEROL. 49, 1011–1025 (2014). [DOI] [PubMed] [Google Scholar]
- Ji C., Cao C., Lu S., Kivlin R. & Amaral A. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother Pharmacol. 62, 857–865 (2008). [DOI] [PubMed] [Google Scholar]
- Liu Y. L., Matsuzaki T., Nakazawa T., Murata S. & Nakamura N. Expression of aquaporin 3 (AQP3) in normal and neoplastic lung tissues. HUM PATHOL. 38, 171–178 (2007). [DOI] [PubMed] [Google Scholar]
- Hwang I., Jung S. I., Hwang E. C., Song S. H. & Lee H. S. Expression and localization of aquaporins in benign prostate hyperplasia and prostate cancer. Chonnam Med J. 48, 174–178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. H., Chen R., Talafu T., Nijiati R. & Lalai S. Significance and expression of aquaporin 1, 3, 8 in cervical carcinoma in Xinjiang Uygur women of China. Asian Pac J Cancer Prev. 13, 1971–1975 (2012). [DOI] [PubMed] [Google Scholar]
- Rubenwolf P. C., Otto W., Denzinger S., Hofstadter F. & Wieland W. Expression of aquaporin water channels in human urothelial carcinoma: correlation of AQP3 expression with tumour grade and stage. WORLD J UROL. 32, 991–997 (2014). [DOI] [PubMed] [Google Scholar]
- Li A., Lu D., Zhang Y., Li J. & Fang Y. Critical role of aquaporin-3 in epidermal growth factor-induced migration of colorectal carcinoma cells and its clinical significance. ONCOL REP. 29, 535–540 (2013). [DOI] [PubMed] [Google Scholar]
- Shi Z., Zhang T., Luo L., Zhao H. & Cheng J. Aquaporins in human breast cancer: identification and involvement in carcinogenesis of breast cancer. J SURG ONCOL. 106, 267–272 (2012). [DOI] [PubMed] [Google Scholar]
- Chen J., Wang T., Zhou Y. C., Gao F. & Zhang Z. H. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J Exp Clin Cancer Res. 33, 38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang J. P., Santen R. J., Kim T. H. & Park H. Estrogen stimulation of cell migration involves multiple signaling pathway interactions. ENDOCRINOLOGY. 151, 5146–5156 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A., Osborne C. K., Morris C. & Wakeling A. E. ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. CANCER-AM CANCER SOC. 89, 817–825 (2000). [DOI] [PubMed] [Google Scholar]
- Jung H. J., Park J. Y., Jeon H. S. & Kwon T. H. Aquaporin-5: a marker protein for proliferation and migration of human breast cancer cells. PLOS ONE. 6, e28492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbach F., Callies R., Adamzik M., Kimmig R. & Siffert W. Aquaporin 1 (AQP1) expression is a novel characteristic feature of a particularly aggressive subgroup of basal-like breast carcinomas. Breast Cancer Res Treat. 120, 67–76 (2010). [DOI] [PubMed] [Google Scholar]
- Itoh A., Tsujikawa T., Yasuoka T., Nakahara T. & Sasaki M. Natriuretic peptides up-regulate aquaporin 3 in a human colonic epithelial cell line. INT J MOL MED. 14, 621–626 (2004). [PubMed] [Google Scholar]
- Tancharoen S., Matsuyama T., Abeyama K., Matsushita K. & Kawahara K. The role of water channel aquaporin 3 in the mechanism of TNF-alpha-mediated proinflammatory events: Implication in periodontal inflammation. J CELL PHYSIOL. 217, 338–349 (2008). [DOI] [PubMed] [Google Scholar]
- Cao X. C., Zhang W. R., Cao W. F., Liu B. W. & Zhang F. Aquaporin3 is required for FGF-2-induced migration of human breast cancers. PLOS ONE. 8, e56735 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. O., Song S. H., Hwang E. C., Oh K. J. & Ahn K. Changes in aquaporin (AQP)2 and AQP3 expression in ovariectomized rat urinary bladder: potential implication of water permeability in urinary bladder. WORLD J UROL. 30, 207–212 (2012). [DOI] [PubMed] [Google Scholar]
- Zhu J., Xia J., Jiang J., Jiang R., He Y. & Lin H. Effects of estrogen deprivation on expression of aquaporins in rat vagina. MENOPAUSE. 10.1097/gme.0000000000000403 (2015). [DOI] [PubMed] [Google Scholar]
- Patel L. R., Camacho D. F., Shiozawa Y., Pienta K. J. & Taichman R. S. Mechanisms of cancer cell metastasis to the bone: a multistep process. FUTURE ONCOL. 7, 1285–1297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusayama M., Wada K., Nagata M., Ishimoto S. & Takahashi H. Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. CANCER SCI. 102, 1128–1136 (2011). [DOI] [PubMed] [Google Scholar]
- Kim N. H. & Lee A. Y. Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J INVEST DERMATOL. 130, 2231–2239 (2010). [DOI] [PubMed] [Google Scholar]
- Xu H., Xu Y., Zhang W., Shen L. & Yang L. Aquaporin-3 positively regulates matrix metalloproteinases via PI3K/AKT signal pathway in human gastric carcinoma SGC7901 cells. J Exp Clin Cancer Res. 30, 86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejsum L. N. & Nelson W. J. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J CELL BIOL. 178, 323–335 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancharoen S., Matsuyama T., Abeyama K., Matsushita K. & Kawahara K. The role of water channel aquaporin 3 in the mechanism of TNF-alpha-mediated proinflammatory events: Implication in periodontal inflammation. J CELL PHYSIOL. 217, 338–349 (2008). [DOI] [PubMed] [Google Scholar]
- Gatto F., Feelders R. A., van der Pas R., Kros J. M. & Waaijers M. Immunoreactivity score using an anti-sst2A receptor monoclonal antibody strongly predicts the biochemical response to adjuvant treatment with somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 98, E66–E71 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.