Abstract
Auscultation of the lung is an important part of the respiratory examination and is helpful in diagnosing various respiratory disorders. Auscultation assesses airflow through the trachea-bronchial tree. It is important to distinguish normal respiratory sounds from abnormal ones for example crackles, wheezes, and pleural rub in order to make correct diagnosis. It is necessary to understand the underlying pathophysiology of various lung sounds generation for better understanding of disease processes. Bedside teaching should be strengthened in order to avoid erosion in this age old procedure in the era of technological explosion.
Keywords: Breath sound, bronchial breathing, crackles, rubs, wheeze
The auscultation of the respiratory system is an inexpensive, noninvasive, safe, easy-to-perform, and one of the oldest diagnostic techniques used by the physicians to diagnose various pulmonary diseases. History taking and a detailed physical examination, including the time-honored sequence of inspection, palpation, percussion, and auscultation should be considered an essential part of clinical examination, even in 21st century with explosive advancement in technology related to health sciences. Technologic advancement has led to erosion in the bedside teaching due to overreliance on laboratory testing; therefore, the clinical relevance of auscultation has receded significantly in recent years. It was Hippocrates who began the concept of auscultation by applying ear to the patient's chest to hear transmitted breath sounds and called this procedure as “immediate auscultation”. He described this as a method of direct auscultation. However, with the invention of stethoscope by Rene Theophile Hyac in the Laënnec in 1816; the art of auscultation not only became popular worldwide, but also comfortable for patients and physicians. Laënnec published his seminal work in 1819 in his masterpiece, “A Treatise on the Diseases of the Chest”.[1] Initially; he used rolled paper cone, and later on a wooden tube. Modern stethoscope had undergone several modifications before being molded into the current shape. Auscultation of the lungs includes breath sounds-its character and intensity, vocal resonance, and adventitious sounds. We will discuss the various types of breath sound, adventitious sounds, and vocal resonance; and their clinical importance and pathogenesis.
Physics of Breath Sounds
Breath sound has three characters; frequency, intensity, and timbre or quality; which helps us to differentiate two similar sounds.
Frequency and pitch
Frequency measures the number of the sound waves or vibrations per second and is measured objectively. It is measured in hertz (Hz). Frequency depends on the number of wavelengths per second. Wavelength is the distance from the peak of one pressure wave to the next pressure wave and is commonly designated by the Greek letter lambda (ë). Wavelength depends on the speed of the sound waves, the medium through which the sound waves are traversing, and the temperature of the medium. When wavelengths are shorter, there area greater number of sound waves per second, and the frequencies will be higher. On the other hand, with longer wavelengths, the frequencies are lower. Pitch is the subjective perception of sound's frequency. Pitch depends on the frequency and is within 5 Hz of the frequency usually.[2] The human ear can perceive sound waves over a wide range of frequencies, ranging from 20 to 20,000 Hz.
Amplitude or loudness
Amplitude is related to the energy of sound waves and is measured by the height of sound waves from the mean position. Loudness is the subjective perception of amplitude. The range of amplitude is extremely wide, so it is measured on a logarithmic scale and is depicted by decibels (dB). Sound measured at 10 dB has an increase in sound intensity of 10 times.
Quality or Timbre
Quality or timbre is an important property of sound that differentiates two sounds with the same pitch and loudness. Sound is made up of various frequencies. Fundamental frequency or primary frequency is the lowest frequency of a sound wave and it determines the pitch of the sound. Frequencies higher than the fundamental frequencies are called overtones. Harmonics are overtones whose frequencies are whole number multiples of the fundamental frequency.[3]
Methods of performing auscultation
Auscultation should be done in a quiet room, preferably in a sitting position. If the patient cannot assume sitting posture, roll the patient from one side to the other to examine the back.
Always warm up the cold stethoscope by rubbing the chest piece in your hands before placing it on naked body. Auscultation should never be done through the clothing.
Ask the patient to take deep breaths through the open mouth.
Using the diaphragm of the stethoscope, start auscultation anteriorly at the apices, and move downward till no breath sound is appreciated. Next, listen to the back, starting at the apices and moving downward. At least one complete respiratory cycle should be heard at each site.
Always compare symmetrical points on each side.
Listen for the quality of the breath sounds, the intensity of the breath sounds, and the presence of adventitious sounds.
Mechanism of Breath Sounds Production
The prerequisite for normal breath sound production is the air flow along the trachea-bronchial tree; however, not all types of airflow produce breath sound. Only turbulent and vorticose airflow are responsible for breath sound production.[4] Laminar flow occurs in low flow situations and is silent [Figure 1]. The streams of airflow are parallel to the walls. It is parabolic in shape as air in the central layers moves faster than air in the peripheral layers, with little or no transverse flow. Therefore, there is little mixing or collision between layers of gas. Laminar flow pattern follows the Poiseuille equation, as shown below [Figure 2].
Figure 1.
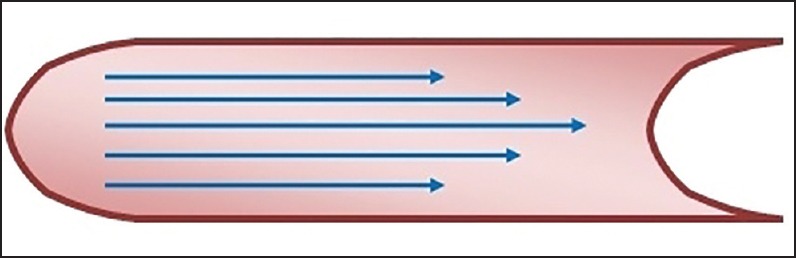
Showing laminar flow pattern
Figure 2.
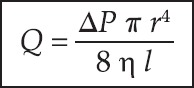
Showing Poiseuille equation
Where Q is the volume flow rate, P is the driving pressure, r the radius, n the viscosity, and l depicts length. Laminar flow is directly proportional to the driving pressure. Small airways (<2 mm) are not the site of breath sound production as flow here is laminar in nature, and therefore silent. Turbulent flow occurs when high velocity of flow passes through a large diameter airway, especially through an airway with irregular walls, for example, the trachea and bronchi or in the airway with sudden branching [Figure 3]. Unlike laminar flow, it does not have high axial flow velocity. Turbulent flow is disorganized and chaotic in nature. It depends on the density of air more than viscosity. Inhalation of a lighter gas mixture; for example, helium; reduces the turbulent flow and makes laminar flow more likely. Turbulency produces noise as the air molecules collide with each other and with the airway wall. Whether airflow becomes laminar or turbulent depends on Reynaud's number. Turbulency occurs when Reynaud's number exceeds 2,000.
Figure 3.
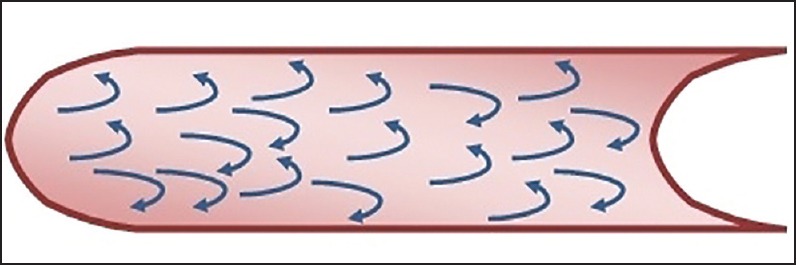
Showing turbulent flow
The development of vortices is another mechanism for breath-sound generation [Figure 4].[5] Vortices or whirlpools are formed when a stream of gas that emerges from a circular orifice to a wider channel. It occurs between the fifth and the 13th generations of the bronchial tree. Respiratory sounds heard in the chest wall undergo attenuation by the lungs and the chest wall. The lung parenchyma and chest wall act as a low-pass filter, not allowing high frequency sounds to pass through. Therefore, the sound heard over the chest wall consists mainly of low frequencies.[6] The low pass filtering function is responsible for a sharp drop in sound energy between 100 and 200 Hz.[7] Based on the frequency, respiratory sounds are classified into the following groups: Low (under 100 Hz), middle (200-600 Hz), and high frequency (600-1,200 Hz).[8]
Figure 4.
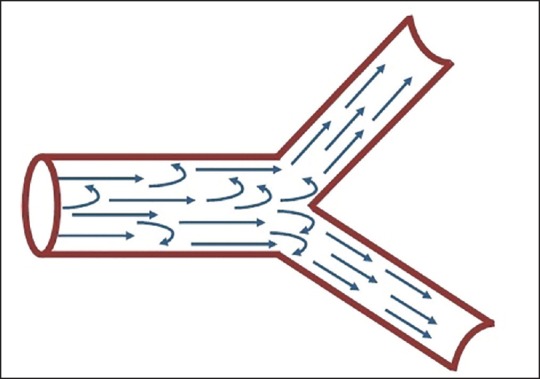
Showing transitional flow
Lung sounds are different from transmitted voice sounds. Lung sounds are generated within the lungs, unlike transmitted voice sounds, which are generated by the larynx. Lung sounds consist of breath sounds and adventitious, or abnormal, sounds heard or detected over the chest. Normal breath sounds are heard over the chest wall or trachea. Basically, breath sounds contains background noises, on which adventitious sounds are sometimes superimposed. Breath sounds are classified into normal tracheal sound, normal lung sound or vesicular breath sounds, and bronchial breath sound. Bronchial breath sounds are further subdivided into three types: Tubular, cavernous, and amphoric.
Normal lung or vesicular breath sounds
Vesicular breath sound is a misnomer as vesicles means alveoli, and this gives the impression that the breath sound is originating at the alveolar level. However, breath sounds cannot be generated at the alveolar level since airflow is laminar within the alveoli. The expiratory sound is audible only in the early phase. The short expiratory phase is due to the passive nature of expiration resulting in generation of less turbulent airflow. The origin of both phases of respiration is also indifferent sites. The inspiratory component originates in the lobar and segmental airways, whereas the expiratory component arises from more central airways.[9] Therefore, turbulence generated during expiration moves away from the chest wall and become fainter. Lung sounds normally peak at frequencies below 100 Hz,[10] with a sharp drop of sound energy occurring between 100 and 200 Hz,[11] but it can still be detected at or above 800 Hz with sensitive microphones.[12] There are regional variations in the intensity of breath sound. At the apex, intensity decreases with the progression of inspiration performed from residual volume whereas, at the base, initially the sound is less intense, and with the progression of inspiration, the intensity gradually increases.
Characteristics [Figure 5]
Figure 5.
Showing vesicular breath sound
Soft, low pitched, and rustling in quality
Inspiratory phase lasts longer than the expiratory phase with an inspiratory-expiratory ratio (I:E) of about 2:1 during tidal breathing
Intensity of inspiration is greater than that of expiration
Inspiration is higher pitch than expiration
No pause between inspiration and expiration
Various Types of Vesicular Breath Sound
Exaggerated or puerile vesicular breath sounds
It is normal vescicular breathing with relatively greater clarity. It is common in children and thin built individual. When a part of the lungs are damaged, other parts are functioning more; the latter area may produce exaggerated vesicular breath sounds.
Diminished or absent breath sounds
One important feature of auscultation is recording the intensity of the breath sound. Intensity can be reduced due to several factors: Weak sound generation and/or impaired transmission.[13] Various causes are shallow breathing, airway obstruction, bulla, hyperinflation, pneumothorax, pleural effusion or thickening, and obesity.
Normal vesicular breath sound with prolonged expiration
It can occur in obstructive airway diseases like asthma and chronic bronchitis. Sometimes inspiration becomes harsh in quality.
Breath sound intensity (BSI) score
It can be done by a quantitative system proposed by Pardee et al.,[14] in 1976. Patients are asked to perform rapid and deep breathing through mouth from residual volume to generate breath sound as loud as possible. Auscultation of chest is done to note the intensity of breath sound over six regions on the seated patient: Over upper anterior part of chest, mid axillary region, and posterior basal region bilaterally. Sound intensity is graded in each region as follows: 0-absent breath sound, 1-barely audible breath sound, 2-faint but definitely audible breath sound, 3-normal breath sound, and 4-louder than normal breath sound. Possible final BSI score may range from 0 (absent breath sound) to 24 (very loud breath sounds). Bohadana et al.,[13] evaluated BSI and found significant correlation with forced expiratory volume in 1 s (FEV1) and lung volumes. In areas where there is no pulmonary function laboratory, BSI can be of great use.
Inspiration is represented by upstroke and expiration by down stroke. Length of upstroke and down stroke indicates length of inspiration and expiration, respectively. Thickness indicates intensity of the sound. Pitch of inspiration is measured by the angle it makes with the perpendicular line.
Bronchial breath sound
It is normally heard anteriorly over the manubrium and posteriorly between the C7 and T3 vertebrae. Bronchial breath sounds contain much higher frequency components than normal breath sounds due to alteration of the low pass filtering function of the alveoli, as occurs in consolidation.
Characteristics [Figure 6]
Figure 6.
Showing bronchial breath sound
It is loud, hollow, and high pitch
Expiratory phase is longer than inspiratory phase with the I:E changing from normal 3:1 to 1:2
There is distinct pause between inspiration and expiration due to absent alveolar phase.
It is associated with whispering pectoriloquy.
It is normally heard over the manubrium and right upper chest and interscapular area.
Check for whispering pectoriloquy is absolutely essential in case of doubt about the presence of bronchial breathing as whispering pectoriloquy is always present along with bronchial breath sound.[15] Bronchial breath sounds are further subdivided into tubular, cavernous, and amphoric breath sound.
Tubular breath sound
It is a high pitch, bronchial breath sound. It can be seen in the following conditions:
Consolidation
Above the level of pleural effusion
Pulmonary fibrosis
In distal collapse, if collapse segment is in contact with chest wall and bronchus is patent, bronchial breathing may be present
Mediastinal tumor over a large patent bronchus.
Amphoric Breath Sound
It is a low pitch bronchial breath sound with high pitch overtones. It has a metallic character. Amphoric breathing can be produced by blowing over the mouth of an empty glass or clay jar. Greek word amphoreus means jar so it is called amphoric breathing. It occurs in the presence of a superficial large cavity (not less than 5-6 cm in diameter) with patent bronchi and open pneumothorax. Smooth wall is also a requirement as it is capable of reflecting sound. High pitch overtones occur because of strong resonance of sound waves within cavity wall or pleural cavity. Presence of fungal ball or fluid within cavity causes disappearance of amphoric breath sound. Amphoric breathing is also not heard if normal alveoli are present, so presence of amphoric breathing means alveolar destruction.
Cavernous breath sound
It is a low pitch bronchial breath sound heard over superficial large cavity with patent bronchus, abscess, and bronchiectatic cavity with patent bronchi.
Normal Tracheal Breath Sound
It is harsh, very loud, and high pitched sound heard over the trachea. Typical frequency of tracheal breath sound varies from 100 to 1,500 Hz, with a drop in power above a cutoff frequency of approximately 800 Hz sharply.[10] Tracheal breath sound has both phases of respiratory cycle equal with distinct gap between them. Frequency range of tracheal sound is much wider than normal lung sound with frequencies ranging from 100 to 5,000 Hz, with a sharp drop in energy at a frequency of approximately 800 Hz.[10] Auscultation over trachea is not done routinely, but it can be useful in certain specific conditions. First, it has hollow tubular quality so it is a good model for studying bronchial breath sound. Second, tracheal breath sound can be helpful in detecting upper airway obstruction (UAO). It becomes noisy in case of UAO. Extrathoracic UAO produces the characteristics stridor; whereas, intrathoracic UAO is associated with wheezing sound. Spectral analysis of tracheal sound is also helpful in this regard. Normally there is a small spectral peak observed at 1 kHz. Patients with significant tracheal stenosis; however, demonstrate an increase in the peak spectral power at 1 kHz and there is also an increase in the mean spectral power from 600 to 1,300 Hz.[16] Tracheal sound analysis is helpful also for the monitoring of patients with sleep apnea-hypopnea syndrome.[17]
Bronchovesicular breath sound
It is intermediate between bronchial and vescicular breathing. It has intermediate intensity and pitch with same duration of inspiratory and expiratory phase. It is normally heard anteriorly over 1st and 2nd intercostal spaces and between scapulae posteriorly. It is abnormal in other locations.
Interrupted or cogwheel breathing
Occasionally, vesicular breathing becomes interrupted during inspiration and is called cogwheel breathing, for example, bronchial obstruction by mediastinal lymph nodes or aortic aneurysm or nervousness and fatigue.
Breath Sounds At Mouth
Breath sound produced in the central airways can traverse both upwards and downwards. Though they originate from the same sites, they are different acoustically as frequencies above 200 Hz are filtered off in case of sound heard at chest wall by the alveolar air and chest wall. Breath sounds heard at mouth contain frequency distributed widely from 200 to 2,000 Hz like normal white noise.[18] In healthy person, breathing is silent at mouth, but it is easily audible even at a distance in patients with chronic bronchitis and asthma. However, this sign is less frequently used in modern day. One reason may be that stridor and wheeze are often confused with noisy breathing.[18] However, this simple method of observing noisy inspiration at mouth heard with the unaided ear can be an important clinical sign. Noisy inspiration is common in chronic bronchitis and asthma, but not in patients with emphysema. Noisy breath sound at mouth is due to increased turbulence caused by surface irregularities in the airways, abrupt changes in the direction of flow, or narrowing of the airways resulting in more rapid flow.[18] Forgacs reported a significant linear correlation between the intensity of the inspiratory sound at mouth and the degree of airflow obstruction except in patients with focal stenosis of one of the principal or lobar bronchi and emphysema.[19] The noise generated by turbulency at the stenotic segment is much louder than that predicted by FEV1. In emphysema, sound at mouth is either not audible or audible slightly above normal. Emphysema patients develop airflow obstruction due to loss of elastic recoil of the lung leading to small airway obstruction, and dynamic compression of the central airways. However, in chronic bronchitis and asthma patients, the caliber of the central airways remains normal during inspiration.
Vocal Resonance or Voice Sounds
Voice sounds are produced by the larynx. They are produced when puffs of air pass through the vocal folds, producing its vibration. Therefore; unlike breath sounds and adventitious sounds, they are not produced in the lungs. Voice sounds are subsequently modulated by filter function of the supralaryngeal airway. Voice sounds consist of a fundamental frequency and several overtones called together harmonics. Fundamental frequency is determined by the number of times the vocal folds vibrate in 1 s, and is measured in hertz. Fundamental frequency is the lowest resonant frequency of vibrating cords. Overtones are multiples of fundamental frequency. Vowel sound contains a mixture of high and low frequency overtones called formants. Normally, in healthy person, due to filtering effect of air-filled lungs, voice sounds are unintelligible as higher frequencies are lost. However, when air in the lungs is replaced by fluid or solid substances or the lungs undergo atelectasis, voice sounds are better transmitted and become well-distinct. There are three types of transmitted voice sounds: Whispered pectoriloquy, bronchophony, and egophony.
Bronchophony
Ask the patient to recite the word “ninety-nine” in a normal voice and listen to the chest via the stethoscope to each lung field. Bronchophony is present if sounds can be heard with an increase in intensity and clarity.
Whispered pectoriloquy
During whispering, vocal folds do not vibrate, but are held close together. This produces a turbulent flow of air resulting in a windy sound characteristic of whispering. Ask the patient to whisper a word such as “one-two-three” or “ninety-nine” and listen with a stethoscope. Normally, words are heard faintly. However, in cases of consolidation, the whispered sounds will be heard clearly and distinctly.
Egophony
The word egophony came from the Greek word “ego,” meaning goat. Laënnec in 1916 first described the sign “egophony”.[20] Egophony is elicitated by asking the patient to say the word ‘Ee’ and it will be transformed into ‘A’. It is present in cases of consolidation or pleural effusion. In pleural effusion; egophony is present just above the area of dullness. Sound E consists of high frequency of 2,000-3,500 Hz and low frequency of 100-400 Hz. In sound A, the low frequency is higher than E and reaches up to 600 Hz. Unlike normal lung, consolidated lungs transmit both higher and lower frequency well, but no significant transmission occurred above a frequency of 1,000 Hz. Therefore, consolidated lungs can not transmit the higher frequency of e, but can transmit the lower frequency of A well, so Ee becomes A. Patients of large pleural effusions have upward displacement and compression of the lung above the level of effusion. Fluid in the pleural space compresses the overlying lung parenchyma, making it more solid than normal. It results in modification of the acoustic properties of the lung, which becomes a better transmitter of high frequency sound and causes appearance of egophony. Animal study has shown that pleural effusion altered the transmission of sound from vocal cords to chest wall. Pleural fluid decreases the transmission of sound of wavelength between 100 and 300 Hz (fundamental frequency of speech) and increases transmissibility of higher frequencies.[21]
Adventitious lung sounds:
Wheezes
Crackles
Squeak
Pleural rub
Stridor
Adventitious sounds are additional respiratory sounds superimposed on normal breath sounds. As early as 1957, Robertson and Coope[22] proposed a simplified classification of adventitious lung sounds into two main categories; continuous and interrupted sounds. Continuous sounds were further classified into high- and low-pitched wheezes, and the interrupted sounds were divided into three categories: Coarse, medium, and fine crackles. International Lung Sound Association in 1976 further simplified the terminology: Discontinuous sound into fine and coarse crackles and continuous sound into wheeze and rhonchi.[23]
Continuous adventitious sound lasts more than 250 ms.[24] Wheezes and rhonchi are continuous musical lung sounds. The American Thoracic Society (ATS) Committee on pulmonary nomenclature defines wheezes as high-pitched continuous sounds with a dominant frequency of 400 Hz or more, and rhonchi as low-pitched continuous musical sounds with a dominant frequency of about 200 Hz or less.[24,25] Although the ATS definition of continuous sound includes a duration longer than 250 ms, wheeze does not necessarily need to extend beyond 250 ms and typically it is longer than 80-100 ms.3 Wheezes are usually louder than the underlying breath sounds, and are often audible at the patient's open mouth or by auscultation over the trachea and occasionally at some distance from the patient.[26] Rhonchi, being low pitch, are best heard over the chest wall. Rhonchi have a snoring quality as thepitch is typically near 150 Hz.[27] Both wheeze and rhonchi are characterized by sinusoidal waveforms.[27] Most often, wheeze is expiratory in nature, but it can be inspiratory or biphasic also. Severe obstruction of the intrathoracic lower airway or upper airways obstruction can be associated with inspiratory wheezes. Asthma and chronic obstructive pulmonary diseases (COPD) patients develop generalized airway obstruction; therefore, wheezes are heard all over the chest. Localized airway obstruction by a foreign body, mucous plug, or tumor produces focal wheezing. Wheezing is a nonspecific finding and may even be detected in a healthy person towards the end of expiration after forceful expirations. Pathological wheezing can be produced with a gentle expiratory maneuver.[27] Wheezes are not synonymous with asthma and can be found in variety of conditions. Wheezes may even be absent in asthma patients with severe airway obstruction. The production of wheezing sounds requires a certain degree of airflow. In acute severe asthma, respiratory flows become so low that they are unable to provide the energy necessary to generate wheezes (or any sounds). Wheezes may be absent in this condition, and this is called “silent chest.” Relief from the obstruction improves the airflow, resulting in the reappearance of wheeze and normal breath sounds. Therefore, there appearance of wheeze after a period of silent chest is a sign of improvement.
Mechanisms of Wheeze
The important prerequisite for the production of wheeze is airflow limitation,[28] but airflow limitation can occur in the absence of wheezes. Forgacs, in 1967, proposed that wheezes are generated by the oscillations of the bronchial walls initiated by airflow, and the pitch of the wheeze depends on the mechanical properties of the bronchial walls.[29] Wheeze is a musical sound and Forgacs compared it with a toy trumpet, whose sound is produced by the vibrating reed. The comparison with atoy trumpet reveals that vortices shedding near sharp edges are responsible for the bronchial wall vibration. The pitch of the trumpet is determined by the mass and elasticity of the reed. Similarly, the pitch of the wheeze depends on the mass and elasticity of the airway walls and the flow velocity, but not on the length or the size of the airway. Pitch does not depend eitheron the density of the flowing gas as there is no noticeable change in the character of wheezing when the patient is exposed to a helium-oxygen mixture.[29]
Flutter theory
Gavriely et al., subsequently used fluid dynamic flutter theory in an experimental mathematical model to explain the mechanisms of wheezing.[30] According to this hypothesis, wheezes are produced by the fluttering of the airways walls and fluid together. The fluttering begins when the airflow velocity reaches a critical value, called flutter velocity.[31] The magnitude of flutter velocity is dependent on theme chanical and physical characteristics of the tube andthe gas. Although the frequency of flutter increases with the narrow channel, a small airway is usually not the site of wheeze production as the speed of airflow is too low to reach the critical flutter velocity required for the production of wheeze.[30] The first five to seven generations of airway are the most probable site for wheeze production.[30] The mechanism of flutter can be explained by Bernoulli's principal. This principal states that when air flows through a narrow tube at high velocity, it causes a fall in pressure within the airway. Low intra-airway pressure causes a collapse of the airway. As the collapse worsens, it increases obstruction and intra-airway pressure. The increased intra-airway pressure decreases the obstruction by pushing the airway wall outside, and the fluttering cycle starts anew.
Wheezes are further classified into polyphonic or monophonic wheeze.
Monophonic wheezes
Monophonic wheezing consists of a single musical notes starting and ending at different times. A local pathology-like bronchial obstruction by tumor, bronchostenosis by inflammation, mucus accumulation, ora foreign body can produce it. In case of rigid obstruction, the wheeze is audible throughout the respiratory cycle, and when the obstruction is flexible, wheeze may be inspiratory or expiratory. The intensity may change with a change in posture, as occurs in patients with partial bronchial obstruction by tumor. Fixed monophonic wheeze has a constant frequency and a long duration, whereas random monophonic wheeze has a varying frequency and duration present in both phases of respiration. Random monophonic wheeze can be seen in asthma.
Polyphonic wheezing consists of multiple musical notes starting and ending at the same time and is typically produced by the dynamic compression of the large, more central airways. Polyphonic wheeze is confined to the expiratory phase only. The pitch of the polyphonic wheeze increases at the end of expiration as the equal pressure point moves towards the periphery.[19]
Squawks
Squawks are short inspiratory wheezes of less than 200 msduration and are also known as squeaks. Acoustic analysis shows the fundamental frequency varying between 200 and 300 Hz.[27] Squawks are found in pulmonary fibrosis of various causes, particularly in hypersensitivity pneumonitis.[32] Other causes are detected in pneumonia and bronchiolitis obliterans.[33,34] Squawks usually occur in late inspiration and areoften preceded by late inspiratory crackles. The exact mechanism is not known but, according to Forgacs, squawks are produced by the oscillations of peripheral airways in deflated lung zones when their walls remain in contact for a longer period of time and open in late inspiration.[35] Pneumonia should be suspected in patients with squawks if there is no evidence of restrictive lung disease.[34] Squawks in patients with extrinsic allergic alveolitis are of a shorter duration and higher frequency than thoseoccurring in other patients, as high frequency occurs in the vibration of the small airways.[32]
Crackles
Crackles are discontinuous, explosive, and nonmusical adventitious lung sounds normally heard in inspiration and sometimes during expiration. Crackles are usually classified as fine and coarse crackles based on their duration, loudness, pitch, timing in the respiratory cycle, and relationship to coughing and changing body position. Medium crackles have also been mentioned.[36,37] According to Crofton, type of the crackle is related to the size of the airways.[37]
Fine crackles are produced within the small airways, medium crackles are caused by air bubbling through mucus in small bronchi, and coarse crackles arise from the large bronchi or the bronchiectatic segments.[38] By definition, ‘continuous’ lung sounds last for 250 ms or more, whereas ‘discontinuous’ sounds last for 25 ms or less.[38] Crackles being discontinuous sounds are typically less than 20 ms in duration.[39] Differentiation of the crackles can be done objectively by the time expanded waveform analysis as proposed by Murphy et al. [Figure 7].[40]
Figure 7.
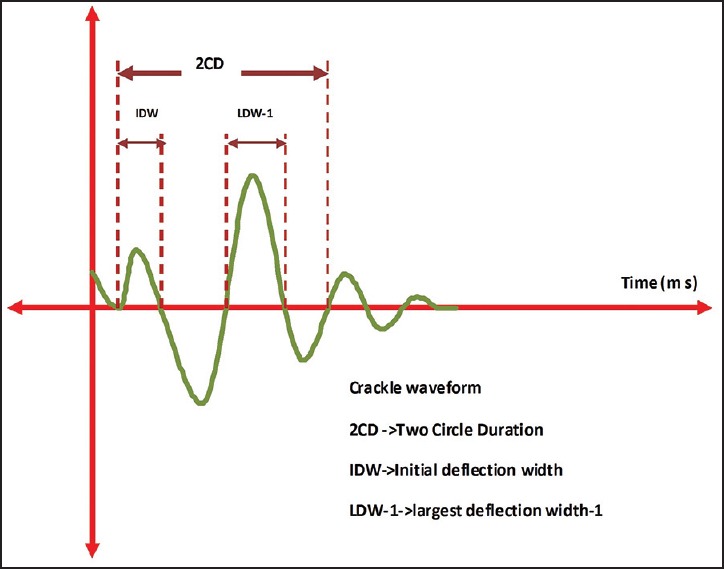
Showing crackles waveform
Initial deflection width (IDW) is the time duration (ms) between the beginning and the first deflection of the crackle above or below the baseline. Two-cycle duration (2CD) is the duration between the beginning of the crackles and the first two cycles of the crackle [Figure 3].[41] TDW means the total duration of the crackle. According to the ATS criteria, coarse crackles have the mean durations of IDW and 2CD of 1.5 and 10 ms, and those of fine crackles are 0.7 and 5 ms, respectively.[42] Computerized Respiratory Sound Analysis (CORSA) guideline defined coarse crackle as 2CD >10 ms, and fine crackle as 2CD <10 ms.[43] The frequency range of the crackles sound is 60-2,000 Hz, with the major contribution being in the range of 60-1,200 Hz.[44] Hoevers and Loudon proposed a different set of parameters to characterize crackles, namely, largest deflection width (LDW1); which is the duration of the largest deflection of the crackle.[45]
Mechanisms of the production of crackles
Initially, production of crackles was attributed to the passage of air through the accumulated secretions within the large and medium-size airways, creating the bubbling sounds. However, persistence of crackles after coughing in many patients and predominant localization in inspiration argues against this theory.[46] Moreover, air passing through secretions would produce both inspiratory and expiratory sounds. Later on, Forgacs proposed the second theory of crackles. According to Forgacs, small airways that were collapsed during expiration snap open during inspiration as a gradient of gas pressure is developed across the collapsed airways. Sudden explosive opening of the collapsed airways induces a rapid equalization of gas pressures resulting in oscillations of the gas column and development of crackles.[29]
Stress relaxation quadrupole hypothesis
Fredberg and Holford proposed the stress relaxation quadrupole hypothesis of crackles generation in 1983.[47] According to this hypothesis, crackles are produced by the vibration in the walls of small airways not by the air column within airways. Crackles arise due to the sudden opening and closing of airway, resulting in stress waves' propagation in the lung parenchyma. Vyshedskiy et al.,[48] demonstrated that expiratory crackles are produced by sudden airway closure that is far less energetic than the inspiratory crackles that are generated by the explosive opening of the airways. Almeida et al.,[49] proposed the liquid bridge hypothesis to explain crackles. Liquid bridges are formed due to abnormal mechanical instabilities in the small airways. Liquid bridge ruptures are responsible for the inspiratory crackle sound, and formation of the liquid bridge explains the expiratory crackle.
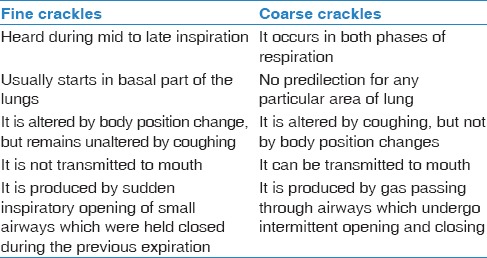
Spectral and clinical differences between fine and coarse crackles are given in Tables 1 and 2. Coarse crackles are loud, low-pitched, and fewer in number per breath, whereas, fine crackles are soft, higher-pitched, and greater in number per breath. Crackles are noted in pulmonary disorders, for example, pneumonia, COPD, pulmonary edema, interstitial lung disease, and heart failure. Fine crackles are seen in interstitial lung disease and early congestive heart failure and coarse crackles are observed in patients with chronic bronchitis and severe pulmonary edema.[50]
Table 1.
Spectral differentiation between fine and coarse crackles

Table 2.
Showing clinical differences between fine and coarse crackles
Crackles in Relation to Respiratory Phase
Early inspiratory crackles
They appear at the beginning of inspiration and end before mid-inspiration, for example, COPD. Crackles in COPD patients are scanty, gravity-independent, usually audible at the mouth, and strongly associated with severe airway obstruction. Crackles start early in inspiration and usually end before the midpoint of inspiration. Crackles are coarse with 2CD more than 9ms.[51] Crackles are usually due to airway secretions within large airway, and disappear on coughing. Sometimes, crackles may be heard in both phases of respiration. It is probably related to sequential closing and opening of proximal bronchi, narrowed due to inflammation mediated loss of their cartilaginous support.[46]
Bronchiectasis
Crackles in bronchiectasis are loud and present in both phases of respiration. Crackles start early in inspiration, continue to mid inspiration, and fade by the end of inspiration. They do not continue to the end of inspiration unlike crackles in idiopathic pulmonary fibrosis (IPF).[52] There are two reasons why crackles in bronchiectasis extend into the mid-phase of inspiration. First, longer time period is required to open bronchiectatic segments. Second; in bronchiectasis, especially in the varicose and cystic variety, the emptying of dilated sacs is prevented by collapse of the bronchi downstream (towards the mouth) so that the secretions are retained in them. In this situation, early inspiratory crackles may coincide with the opening of bronchiectatic airways and their continuation in the middle phase of inspiration results from bubbling in retained secretions as inspiration progresses.
Unlike chronic bronchitis patients with early inspiratory crackles, which occur when the airways obstruction is severe, crackles in bronchiectasis usually occur with mild airways obstruction.[49] Crackles are more profuse in early and mid phase than in late phase of inspiration. Similar to COPD, crackles are audible at mouth indicating proximal airway involvement. Coughing reduces the number of the crackles. Bronchiectasis crackles are coarse with 2CD 9 ms.
Late inspiratory crackles
They appear any time after the beginning of inspiration and last till the end of inspiration, for example, diffuse parenchymal lung disease (DPLD). Crackles are commonly seen in patients with IPF/usual interstitial pneumonia (UIP), asbestosis, and desquamative interstitial pneumonia (DIP). Epler at al.,[53] in a study involving 272 cases with DPLD documented by lung biopsy, reported bilateral fine crackles in 60% of IPF with a pathological diagnosis and asbestosis in 53% cases of DIP. However, fine crackles are less common in intra-alveolar filling disease such as pulmonary alveolar proteinosis and granulomatous diseases, such as sarcoidosis, eosinophilic granuloma, and miliary tuberculosis. It was heard only in 20% of those with sarcoidosis and other granulomatoses. There are various reasons why crackles are less common in sarcoidosis unlike patients with IPF. First of all; distribution of fibrosis is different. IPF usually predominates at the base of the lungs unlike sarcoidosis which is more common in the upper lobe with characteristics peribronchial location. Crackles are more frequent with honeycombing and subpleural fibrosis both are seen more commonly with IPF. Moreover; pulmonary fibrosis is less extensive in sarcoidosis than in patients with IPF.[53,54] The fine crackles in IPF patients are also called “velcro crackles” as the sound is similar to the sound produced while trying to gently separate the joined strip of velcro adhesive present on the blood pressure cuff (or jogging shoes).[55] Crackles in IPF have a unique characteristic. They may start in the middle and even early phase of inspiration, but continue till the end of inspiration.[52] Therefore; they may be heard throughout the inspiration. Crackles also become profuse at the end of inspiration. Unlike COPD and bronchiectasis, crackles in IPF are not transmitted to the mouth. In IPF patients, crackles are detected early in the course of the disease. If crackles are present throughout the inspiratory time and persist after several deep breaths, and if remain present on several occasions several weeks apart in a subject aged ³60 years, it should raise the suspicion of IPF and should lead to further investigations.[56] Crackles are fine with 2CD <8 ms, frequency around 200Hz.[46] Crackles in IPF first appear at the bases and with disease progression, it extends upwards.
Pneumonia
The characters of the crackles depend on the stages of pneumonia. In acute pneumonia, crackles tend to be mid-inspiratory and fairly coarse (2CD 9-11 ms). However, during resolution phase, they are more end-inspiratory and shorter in duration, resembling those in IPF. In acute pneumonia, reopening of airways closed by edema and infiltration of inflammatory cells produces coarse crackles. During resolution of pneumonia, the lung parenchyma gradually becomes drier and stiffer due to reduction in edema and healing process. It needs greater pressure and volume to open the airways so; crackles gradually shift towards the end of inspiration and become fine.[57]
Crackles in heart failure
In heart failure, crackles occur due to opening of the airways narrowed by peribronchial edema.[35] Forgacs described the crackles in heart failure as late, high-pitched inspiratory and expiratory crackles. Piirilä et al., in a computerized lung sound analysis noted that the crackles in heart failure have been the coarsest compared to that of IPF, bronchiectasis, or COPD with 2CD of 11.8 ms. The total crackling period is also long with rather late timing of crackles in the respiratory cycle.[51] Crackles disappear quickly with resolution in heart failure. In heart failure, crackles are typically posterior basal but in a supine patients, if anterior crackles are detected, look for alternate causes of crackles.[58] Pulmonary edema due to congestive heart failure can generate pan-inspiratory crackles which appear at the beginning of inspiration and last till the end of inspiration.[36]
Expiratory crackles
Crackles are predominantly inspiratory in nature, but can also occur during expiration. It has been described in COPD, bronchiectasis, and IPF.[35,59] Apart from the Fredberg and Holford stress-relaxation quadrupole theory,[47] another mechanism to explain the presence of expiratory crackles is the “trapped gas hypothesis”.[48] According to this hypothesis, some airways collapse during early expiration and air trapping develops. As expiration progresses, the pressure in the trapped region gradually increase, leading to a widened pressure difference across the airways. Once this pressure difference reaches the critical opening pressure, the airway pops open during expiration, producing crackles.
Posture induced crackles (PIC)
These are fine crackles induced by the change in postures, for example, from sitting to supine and/or from supine to passive leg elevation.[60] The procedure to measure these crackles are written below: Patient is asked to sit in the bed for 3 min and auscultation is done along the posterior axillary line at the 8th, 9th, and 10th intercostal spaces. Presence or absence of fine crackles is noted for at least five consecutive breaths. Patient is then asked to assume a supine position and after 3min, auscultation is done again in the same location. After that patient's both legs are elevated passively at 30° angle and auscultation is done again after 3 min. PIC is present when fine crackles are detected in the supine and passive leg raising position, but not detected in the sitting posture. PIC is frequently detected in patients with ischemic heart disease[61] and carries a poor prognosis in this group. Yasuda et al., reported an increased incidence of airway closure in patients with PIC at the base of the lungs and proposed that PIC may be related to the airway closure at the base of the lungs in the supine position.[62]
Post-tussive crackles
These crackles are not present normally on auscultation but can appear after a bout of cough. Crackles appear as the cough dislodges the thick secretions. It is present in early pneumonia, early tuberculosis, and lung abscess.
Stridor
Stridor is a loud, high-pitched, musical sound produced by upper respiratory tract obstruction.[27] It is different from wheezing by the following reasons. It is louder over the neck than chest wall. Secondly; stridor is mainly inspiratory. If occurs in expiration, it is usually biphasic. On the other hand; wheeze is mainly expiratory and occurs during both phases. It indicates extrathoracic upper-airway obstruction (supraglottic lesions like laryngomalacia, vocal cord lesion) when heard on inspiration. It occurs in expiration if associated with intrathoracic tracheobronchial lesions (tracheomalacia, bronchomalacia, and extrinsic compression). It occurs in both phases if lesion is fixed, for example, stenosis.[27,63] Stridor is caused by the turbulent flow passing through a narrowed segment of the upper respiratory tract.
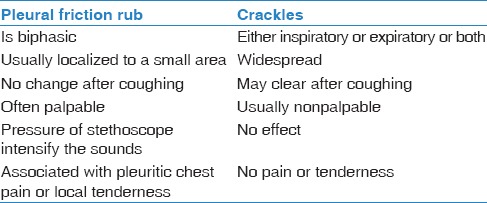
Pleural rub
it is a nonmusical, short explosive sounds, grating, rubbing, creaky, or leathery in character and present in both phases of respiration. Typically the expiratory component mirrors the inspiratory component.[35] It occurs due to inflamed pleural surface rubbing each other during breathing. Clinically, it is important to differentiate it from crackles [Table 3].
Table 3.
Showing differences between pleural friction rub and crackles
Other Auscultatory Signs
Coin test
It may be useful occasionally in cases of pneumothorax, large bulla and hydropneumothorax. Patients should be in sitting or standing position. Place a metallic coin flat against the chest just below the mid-clavicle and strikes the coin with edge of another coin with the help of an assistant. Place the diaphragm of your stethoscope at the same point on back of the chest. Coin test is positive if high-pitch, metallic, bell-like sounds are heard.
Scratch sign test
This is another infrequently used sign in pneumothorax. The testing should be done in sitting or supine position. Place the diaphragm of your stethoscope at the midpoint of sternum and surface of the chest wall at point equidistant to the left and right of the instrument is scratched with the fingers. When the site containing pneumothorax is scratched, the sound heard is louder.
Hippocratic succusion
It is also known as succusion splash. This sign is detected in cases of hydro- or pyo-pneumothorax. It can also be detected in cases of herniation of stomach or intestine through the diaphragm and a large cavity containing air and fluid in the lungs. Select the site containing air and fluid by percussion and place the stethoscope onto that side. Physician then shakes the patient side to side. A splashing sound will be heard on auscultation or even with unaided air.
Hamman's sign
This sign was first described by Louis Hamman in the year 1939.[64] This sign is seen in patients with pneumomediastinum and pneumothorax, particularly left-sided pneumothorax.[65,66] Hamman's sign or mediastinal crunch is a crunching, crackling sound best heard over the precordium from 3rd to 5th intercostal spaces and is synchronous with the heartbeat. Hamman's sign is pathognomic of pneumomediastinum and is usually more sensitive than chest radiograph in the detection of pneumomediastinum, it is present in only about 20% patients.[67] According to Hamman[68] presence of mediastinal air and its continuity with the beating heart was the genesis of this sign. Contraction of the heart within the mediastinum leads to tissue displacement of air bubbles and produces this classic raspy sound. Baumann et al., explained the development of Hamman's sign in patients with pneumothorax without mediastinal air. They proposed that cardiac filling-emptying cycle, anterior-posterior cardiac motion, or a combination of both may pulse pleural air cyclically into pleural fissure and chest wall, generating this sign in pneumothorax.[69]
Forced expiratory time (FET)
FET is a simple, inexpensive, and sensitive test to detect airflow obstruction at bedside. The FET is defined as the time taken for an individual tocomplete a forceful exhalation after maximal inspiration. It is a simple, sensitive, inexpensive, reproducible bedside procedure to measure airflow obstruction.
Method
Instruct the patient to inhale up to total lung capacity and blow it as fast and complete as possible. Place the bell of the stethoscope in the suprasternal notch and measure the duration of audible expiration to the nearest half second. A FET of less than 5 s indicated FEV1: VC of more than 60%; whereas, a FET more than 6 s indicates a FEV1:VC ratio of <50%.[70]
d'Espine's Sign
Jean Henri Adolphe D'Espine, a French physician, first noted that in some patients a whispered sound may be heard over the spinous processes of the upper thoracic vertebrae and considered this to be the earliest physical sign of the trachea-bronchial lymph nodes enlargement.[71]
Methods
Listen on either side of the vertebral column and compare the quality and intensity of these sounds with those over the spinous process at the same level. Normally, vesicular breath sounds on both sides of the spine are louder than sounds heard over the spine at the same level. The d'Espine's sign is positive, if the breath sounds over the vertebra are louder and greater intensity than the corresponding lateral lung sounds. A positive d'Espine's sign means presence of mass lesions in the posterior mediastinum, for example, lymph node enlargement (malignancy, lymphoma, metastatic cancer, tuberculosis, sarcoidosis, and other causes of mediastinal lymphadenopathy). Solid tissues improve the conductance of the breath sound including the high frequency sound. This sign is positive below the level of tracheal bifurcation, level of which depends on the age of the patients. In infant, tracheal bifurcation occurs below the level of seventh cervical spine and at 10 years, it reached the level of third thoracic vertebra, while in adults, bifurcation occurs below fourth thoracic vertebra.[72] In severe kyphosis, one may get false positive d'Espine's sign. Patients with positive d'Espine's sign may develop red spots over the spinous processes of T1-T5 after percussion. This is called Cattaneo's sign and suggests presence of bronchial lymphadenopathy.[73]
Auscultatory percussion
Auscultatory percussion is a technique of physical assessment of the respiratory system where a combination of auscultation and percussion are used. Guarino developed this technique for the detection of nodules, infiltrates, and effusions.[74] The technique is performed with patient in sitting or standing posture. Patient manubrium is tapped lightly with the distal phalanx of the index or middle finger, while the posterior thorax is auscultated with a stethoscope. The sound of the tapping heard over normal lungs is equally resonant bilaterally. The lungs are examined from the apex to the base. Guarino modified this technique in case of pleural effusion. Patients are asked to sit or stand in upright posture for 5 min to allow fluid to settle to the base. Stethoscope is placed 3 cm below the 12th rib in midclavicular line. The posterior thorax is directly percussed from apex to base with the free hand. Normally, the percussion note heard through the stethoscope is dull, but changing sharply to a loud note upon striking the last rib. In case of a pleural effusion, a similar loud note is heard with the level being higher than the last rib. A sensitivity of 95.8% and a specificity of 100% were obtained in diagnosing pleural effusions.[75]
I am grateful to Mrs Nagaveni Irappa Madabhavi, Lecturer, Government Polytechnic Zalaki, Bijapur, Karnataka, India for drawing the figures.
Footnotes
Source of Support: Nil
Conflicts of interest: None declared.
References
- 1.Laennec RT. De l'auscultation mediate ou traité du diagnostic des maladies des poumons et du coeur. Paris: Brosson & Chaudé; 1819. [Google Scholar]
- 2.Chasin M. Musicians and the prevention of hearing loss. San Diego: Singular Publishing Group; 1996. [Google Scholar]
- 3.Pelech AN. The physiology of cardiac auscultation. Pediatr Clin North Am. 2004;51:1515–35. doi: 10.1016/j.pcl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Forgacs P. Lung sounds. Br J Dis Chest. 1969;63:1–12. doi: 10.1016/s0007-0971(69)80039-2. [DOI] [PubMed] [Google Scholar]
- 5.Hardin JC, Patterson JL., Jr Monitoring the state of the human airways by analysis of respiratory sound. Acta Astronaut. 1979;6:1137–51. doi: 10.1016/0094-5765(79)90061-4. [DOI] [PubMed] [Google Scholar]
- 6.Forgacs P. Breath sounds. Thorax. 1978;33:681–3. doi: 10.1136/thx.33.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsby PD, Earis JE. Some high pitched thoughts on chest examination. Postgrad Med J. 2001;77:617–20. doi: 10.1136/pmj.77.912.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez I, Vizcaya C. Tracheal and lung sounds repeatability in normal adults. Respir Med. 2003;97:1257–60. doi: 10.1016/s0954-6111(03)00251-8. [DOI] [PubMed] [Google Scholar]
- 9.Kraman SS. Determination of the site of production of respiratory sounds by subtraction phonopneumography. Am Rev Respir Dis. 1980;122:303–9. doi: 10.1164/arrd.1980.122.2.303. [DOI] [PubMed] [Google Scholar]
- 10.Gavriely N, Palti Y, Alroy G. Spectral characteristics of normal breath sounds. J Appl Physiol Respir Environ Exerc Physiol. 1981;50:307–14. doi: 10.1152/jappl.1981.50.2.307. [DOI] [PubMed] [Google Scholar]
- 11.Gavriely N, Nissan M, Rubin AH, Cugell DW. Spectral characteristics of chest wall breath sounds in normal subjects. Thorax. 1995;50:1292–300. doi: 10.1136/thx.50.12.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasterkamp H, Powell RE, Sanchez I. Lung sound spectra at standardized air flow in normal infants, children, and adults. Am J Respir Crit Care Med. 1996;154:424–30. doi: 10.1164/ajrccm.154.2.8756817. [DOI] [PubMed] [Google Scholar]
- 13.Bohadana AB, Peslin R, Uffholtz H. Breath sounds in the clinical assessment of airflow obstruction. Thorax. 1978;33:345–51. doi: 10.1136/thx.33.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardee NE, Martin CJ, Morgan EH. A test of the practical value of estimating breath sound intensity. Breath sounds related to measured ventilatory function. Chest. 1976;70:341–4. doi: 10.1378/chest.70.3.341. [DOI] [PubMed] [Google Scholar]
- 15.Crofton J. Physical signs in the chest. Res Medica. 1963;4:9–10. 12-6. [Google Scholar]
- 16.Yonemaru M, Kikuchi K, Mori M, Kawai A, Abe T, Kawashiro T, et al. Detection of tracheal stenosis by frequency analysis of tracheal sounds. J Appl Physiol. 1993;75:605–12. doi: 10.1152/jappl.1993.75.2.605. [DOI] [PubMed] [Google Scholar]
- 17.Nakano H, Hayashi M, Ohshima E, Nishikata N, Shinohara T. Validation of a new system of tracheal sound analysis for the diagnosis of sleep apnea-hypopnea syndrome. Sleep. 2004;27:951–7. doi: 10.1093/sleep/27.5.951. [DOI] [PubMed] [Google Scholar]
- 18.Forgacs P, Nathoo AR, Richardson HD. Breath sounds. Thorax. 1971;26:288–95. doi: 10.1136/thx.26.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forgacs P. The functional basis of pulmonary sounds. Chest. 1978;73:399–405. doi: 10.1378/chest.73.3.399. [DOI] [PubMed] [Google Scholar]
- 20.Sapira JD. About egophony. Chest. 1995;108:865–7. doi: 10.1378/chest.108.3.865. [DOI] [PubMed] [Google Scholar]
- 21.Yonemaru M, Abe T, Kobayashi H, Kawashiro T, Yokoyama T. Changes in sound transmissibility through the canine thorax to the two experimental pleural effusion. Nihon Kyobu Shikkan Gakkai Zasshi. 1991;29:829–35. [PubMed] [Google Scholar]
- 22.Robertson AJ, Coope R. Rales, rhonchi, and Laennec. Lancet. 1957;2:417–23. doi: 10.1016/s0140-6736(57)92359-0. [DOI] [PubMed] [Google Scholar]
- 23.Loudon R, Murphy RL., Jr Lung Sounds. Am Rev Respir Dis. 1984;130:663–73. doi: 10.1164/arrd.1984.130.4.663. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society Ad Hoc Committee on Pulmonary Nomenclature. Updated nomenclature for membership reaction. ATS News. 1977;3:5–6. [Google Scholar]
- 25.Mikami R, Murao M, Cugell DW, Chretien J, Cole P, Meier-Sydow J, et al. International symposium on lung sounds. Synopsis of proceedings. Chest. 1987;92:342–5. doi: 10.1378/chest.92.2.342. [DOI] [PubMed] [Google Scholar]
- 26.Pasterkamp H, Kraman SS, Wodicka GR. Respiratory sounds. Advances beyond the stethoscope. Am J Respir Crit Care Med. 1997;156:974–87. doi: 10.1164/ajrccm.156.3.9701115. [DOI] [PubMed] [Google Scholar]
- 27.Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med. 2014;370:744–51. doi: 10.1056/NEJMra1302901. [DOI] [PubMed] [Google Scholar]
- 28.Nagasaka Y. Lung sounds in bronchial asthma. Allergol Int. 2012;61:353–63. doi: 10.2332/allergolint.12-RAI-0449. [DOI] [PubMed] [Google Scholar]
- 29.Forgacs P. Crackles and wheezes. Lancet. 1967;22:203–5. doi: 10.1016/s0140-6736(67)90024-4. [DOI] [PubMed] [Google Scholar]
- 30.Gavriely N, Palti Y, Alroy G, Grotberg JB. Measurement and theory of wheezing breath sound. J Appl Physiol. 1984;57:481–92. doi: 10.1152/jappl.1984.57.2.481. [DOI] [PubMed] [Google Scholar]
- 31.Gavriely N, Kelly KB, Grotberg JB, Loring SH. Critical pressures required for generation of forced expiratory wheezes. J Appl Physiol. 1989;66:1136–42. doi: 10.1152/jappl.1989.66.3.1136. [DOI] [PubMed] [Google Scholar]
- 32.Earis JE, Marsh K, Pearson MG, Ogilvie CM. The inspiratory “squawk” in extrinsic allergic alveolitis and other pulmonary fibroses. Thorax. 1982;37:923–6. doi: 10.1136/thx.37.12.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paciej R, Vyshedskiy A, Bana D, Murphy R. Squawks in pneumonia. Thorax. 2004;59:177–9. doi: 10.1136/thorax.2003.014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geddes DM, Corrin B, Brewerton DA, Davies RJ, Turner-Warwick M. Progressive airway obliteration in adults and its association with rheumatoid disease. Q J Med. 1977;46:427–43. [PubMed] [Google Scholar]
- 35.Forgacs P. Lung Sounds. 1st ed. London: Bailliere Tindall; 1978. pp. 1–72. [Google Scholar]
- 36.AlJarad N, Davies SW, Logan-Sinclair R, Rudd RM. Lung crackle characteristics in patients with asbestosis, asbestos-related pleural disease and left ventricular failure using a time-expanded waveform analysis: A comparative study. Respir Med. 1994;88:37–46. doi: 10.1016/0954-6111(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 37.Crofton J. Respiratory Diseases. 3rd edition. Blackwell Scientific Publications; 1981. Douglas A. Common clinical manifestations in respiratory disease; pp. 102–8. [Google Scholar]
- 38.Jones A. A brief overview of the analysis of lung sounds. Physiotherapy. 1995;81:37–42. [Google Scholar]
- 39.Bahoura M, Lu X. Separation of crackles from vesicular sounds using wavelet packet transform. Acoustics, Speech and Signal Processing ICASSP. 2006;2:1076–9. [Google Scholar]
- 40.Murphy RL, Jr, Holford SK, Knowler WC. Visual lung-sound characterization by time-expanded wave-form analysis. N Engl J Med. 1977;296:968–71. doi: 10.1056/NEJM197704282961704. [DOI] [PubMed] [Google Scholar]
- 41.Reichert S, Gass R, Brandt C, Andrès E. Analysis of respiratory sounds: State of the art. Clin Med Circ Respirat Pulm Med. 2008;2:45–58. doi: 10.4137/ccrpm.s530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Thoracic Society. Updated nomenclature for membership relation. ATS News. 1977;3:5–6. [Google Scholar]
- 43.Sovijärvi AR, Dalmasso F, Vanderschoot J, Malmberg LP, Righini G, Stoneman SA. Definition of terms for applications of respiratory sounds. Eur Respir Rev. 2000;10:77 597–610. [Google Scholar]
- 44.Abbas A, Fahim A. An automated computerized auscultation and diagnostic system for pulmonary diseases. J Med Syst. 2010;34:1149–55. doi: 10.1007/s10916-009-9334-1. [DOI] [PubMed] [Google Scholar]
- 45.Hoevers J, Loudon RG. Measuring crackles. Chest. 1990;98:1240–3. doi: 10.1378/chest.98.5.1240. [DOI] [PubMed] [Google Scholar]
- 46.Piirilä P, Sovijärvi AR. Crackles: Recording, analysis and clinical significance. Eur Respir J. 1995;8:2139–48. doi: 10.1183/09031936.95.08122139. [DOI] [PubMed] [Google Scholar]
- 47.Fredberg JJ, Holford SK. Discrete lung sounds: Crackle (rales) as stress-relaxation quadrupoles. J Acoust Soc Am. 1983;73:1036–46. doi: 10.1121/1.389151. [DOI] [PubMed] [Google Scholar]
- 48.Vyshedskiy A, Alhashem RM, Paciej M, Ebril I, Rudman JJ, Fredberg R, et al. Mechanism of inspiratory and expiratory crackles. Chest. 2009;135:156–64. doi: 10.1378/chest.07-1562. [DOI] [PubMed] [Google Scholar]
- 49.Alexandre B, Almeidaa, Sergey V, Buldyrev B, Alencar AM. Crackling sound generation during the formation of liquid bridges: A lattice gas model. Physica A. 2013;392:3409–16. [Google Scholar]
- 50.Munakata M, Ukita H, Doi I, Ohtsuka Y, Masaki Y, Homma Y, et al. Spectral and waveform characteristics of fine and coarse crackles. Thorax. 1991;46:651–7. doi: 10.1136/thx.46.9.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piirilä P, Sovijärvi AR, Kaisla T, Rajala HM, Katila T. Crackles in patients with fibrosingalveolitis, bronchiectasis, COPD, and heart failure. Chest. 1991;99:1076–83. doi: 10.1378/chest.99.5.1076. [DOI] [PubMed] [Google Scholar]
- 52.Nath AR, Capel IH. Lung crackles in bronchiectasis. Thorax. 1980;35:694–9. doi: 10.1136/thx.35.9.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epler GR, Carrington CB, Gaensler EA. Crackles (rales) in the interstitial pulmonary diseases. Chest. 1978;73:333–9. doi: 10.1378/chest.73.3.333. [DOI] [PubMed] [Google Scholar]
- 54.Baughman RP, Shipley RT, Loudon RG, Lower EE. Crackles in interstitial lung disease. Comparison of sarcoidosis and fibrosing alveolitis. Chest. 1991;100:96–101. doi: 10.1378/chest.100.1.96. [DOI] [PubMed] [Google Scholar]
- 55.Dines DE, DeRemee RA. Meaningful clues and physical signs in chest disease. Mod Treat. 1970;7:821–39. [PubMed] [Google Scholar]
- 56.Cottin V, Cordier JF. Velcro crackles: The key for early diagnosis of idiopathic pulmonary fibrosis? Eur Respir J. 2012;40:519–21. doi: 10.1183/09031936.00001612. [DOI] [PubMed] [Google Scholar]
- 57.Piirilä P. Changes in crackle characteristics during the clinical course of pneumonia. Chest. 1992;102:176–83. doi: 10.1378/chest.102.1.176. [DOI] [PubMed] [Google Scholar]
- 58.Sud M, Barolet A, McDonald M, Floras JS. Anterior crackles: A neglected sign? Can J Cardiol. 2013;29:1138.e1–2. doi: 10.1016/j.cjca.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 59.Walshaw MJ, Nisar M, Pearson MG, Calverley PM, Earis JE. Expiratory lung crackles in patients with fibrosingalveolitis. Chest. 1990;97:407–9. doi: 10.1378/chest.97.2.407. [DOI] [PubMed] [Google Scholar]
- 60.Deguchi F, Hirakawa S, Gotoh K, Yagi Y, Ohshima S. Prognostic significance of posturally induced crackles. Long-term follow-up of patients after recovery from acute myocardial infarction. Chest. 1993;103:1457–62. doi: 10.1378/chest.103.5.1457. [DOI] [PubMed] [Google Scholar]
- 61.Iida M, Gotoh K, Yagi Y, Ohshima S, Yamamoto N, Deguchi F, et al. Study on the genesis of posturally induced crackles from hemodynamic data--in patients with ischemic heart disease having normal respiratory function. Kokyu To Junkan. 1989;37:1009–14. [PubMed] [Google Scholar]
- 62.Yasuda N, Gotoh K, Yagi Y, Nagashima K, Sawa T, Nomura M, et al. Mechanism of posturally induced crackles as predictor of latent congestive heart failure. Respiration. 1997;64:336–41. doi: 10.1159/000196701. [DOI] [PubMed] [Google Scholar]
- 63.Ida JB, Thompson DM. Pediatric stridor. Otolaryngol Clin North Am. 2014;47:795–819. doi: 10.1016/j.otc.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Hamman L. Spontaneous interstitial emphysema of the lungs. Trans Assoc Am Physicians. 1937;52:311–9. [Google Scholar]
- 65.Scadding JG, Wood P. Systolic clicks due to left-sided pneumothorax. Lancet. 1939;2:1208–1. [Google Scholar]
- 66.Semple T, Lancaster WM. Noisy pneumothorax. Observations based on 24 cases. Br Med J. 1961;1:1342–6. doi: 10.1136/bmj.1.5236.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel PA, Chiu A, Nagurka R, Lamba S. A case of Hamman's Sign: Value of auscultation. Cardio Vasc Syst. 2014;2:4. [Google Scholar]
- 68.Hamman L. Mediastinal emphysema. JAMA. 1945;128:1–6. [Google Scholar]
- 69.Baumann MH, Sahn SA. Hamman's sign revisited. Pneumothorax or pneumomediastinum? Chest. 1992;102:1281–2. doi: 10.1378/chest.102.4.1281. [DOI] [PubMed] [Google Scholar]
- 70.Kern DG, Patel SR. Auscultated forced expiratory time as a clinical and epidemiologic test of airway obstruction. Chest. 1991;100:636–9. doi: 10.1378/chest.100.3.636. [DOI] [PubMed] [Google Scholar]
- 71.Espine D. Bulletin de 1 'Acad de Med. vol. 57. Paris: 1907. p. 167. [Google Scholar]
- 72.Sienkiewicz TM. The clinical view of D'Espine's sign. Can Med Assoc J. 1923;13:890–2. [PMC free article] [PubMed] [Google Scholar]
- 73.Williams ME. The Basic Geriatric Respiratory Examination. [Last accessed on 2009 Nov 25]. Available from: http://www.medscape.com/Medscape Family Medicine/Assessing the Geriatric Patient .
- 74.Guarino JR. Auscultation percussion: A new aid in the examination of the chest. J Kans Med Soc. 1974;75:193–4. [PubMed] [Google Scholar]
- 75.Guarino JR, Guarino JC. Auscultatory percussion: A simple method to detect pleural effusion. J Gen Intern Med. 1994;9:71–4. doi: 10.1007/BF02600204. [DOI] [PubMed] [Google Scholar]


