Abstract
Overweight–mortality paradox and impact of six-minute walk distance (SMWD) in lung transplantation
BACKGROUND:
The objective of this study was to examine combined prognostic influence of body mass index (BMI) and SMWD on mortality in lung transplant recipients.
METHODS:
Consecutive isolated lung transplant recipients were identified. Preoperative BMI and SMWD data were collected. The cohort was followed for all-cause mortality.
RESULTS:
The study included 324 lung transplant recipients with mean age of 57 ± 13 years and 58% were male (27% obstructive, 3% vascular, 6% cystic fibrosis, and 64% with restrictive lung diseases). In the total cohort; 37% had normal BMI, 10% were underweight, 33% were overweight, and 20% were obese. The median SMWD was 700 feet. The lower SMWDgroup was defined as the patients who had SMWD <237 feet as determined by receiver operating characteristic (ROC). Based on this definition, 66 patients (20%) had lower SMWD. There were 71 deaths during a median follow-up of 2.3 years. In multivariate analysis, both BMI and SMWD were independently associated with death. Being overweight was associated with reduced mortality risk (hazard ratio (HR) 0.50, P = 0.042) compared to the normal BMI group, and this was primarily driven by early mortality posttransplant. This paradoxical overweight–mortality relationship remained significant in the lower SMWD group (HR 0.075, P = 0.018), but not in the higher SMWD group (P = 0.552).
CONCLUSION:
In lung transplant recipients under lung allocation score (LAS) era, pretransplant BMI and SMWD were independent predictors for mortality after the transplant. The lowest mortality risk was noted in a group of transplant recipients identified as overweight; whereas, being underweight or obese was associated with increased mortality.
Keywords: Body mass index, body composition, lung transplant, six-minute walk, obesity paradox
Introduction
Obesity and being overweight are increasingly being recognized as major public health problems. More than two-thirds of adults in the United States are overweight or obese and the overall rates have doubled since 1970's.[1] Traditionally, body mass index (BMI) has been shown to be positively associated with poor outcomes; however, paradoxical J-shaped relationship of BMI with outcomes is a well-known phenomenon in various populations.[2] In pursuit of an explanation for the BMI paradox, cardiorespiratory fitness has been studied[3] as it is theoretically possible that the BMI paradox could be explained by high muscle mass leading to high BMI which could be reflected by fitness. In a recent meta-analysis[4] exploring the role of fitness, determined by maximal VO2 exercise testing and obesity in mortality; it has been shown that fitness ameliorates the deleterious effects of overweight and obesity. While maximal VO2 exercise testing gives the most comprehensive cardiorespiratory fitness information, it is a relatively sophisticated assay. Six-minute walk distance (SMWD) testing, on the other hand, is a very simple test which has been shown to provide prognostic performance comparable to maximal VO2 exercise testing.[5] In patients with systolic heart failure, fitness determined by SMWD has also been demonstrated to modify paradoxical relationship between BMI and mortality.[6]
In lung transplant recipients, BMI has been reported to have an increased association with mortality[4,7,8,9,10,11,12] and grade 3 primary graft dysfunction.[13] The paradoxical relationship between BMI and mortality, as well as, impact of SMWD on the BMI–mortality relationship has never been described in a lung transplant recipient cohort. Accordingly, the objectives of this study were to i) investigate impact of BMI as categorized by the National Heart, Lung and Blood Institute (NHLBI) classification on all-cause mortality and ii) inspect the interaction of SMWD with BMI and all-cause mortality in lung transplant recipients.
Methods
Study design and population
This is a single-center, retrospective, observational study of consecutive patients who underwent isolated lung transplantation between June 2007 and February 2013. Patients with multiorgan transplantation were excluded. A final cohort of 324 isolated lung transplant recipients was included in the study. Regulatory approval was obtained from theInstitutional Review Board of our institution.
Patient characteristics
Data was retrieved from the Houston Methodist Hospital's lung transplant clinical database. Baseline patient characteristics and perioperative data were collected. We categorized primary lung pathology according to United Network for Organ Sharing (UNOS) classification of lung diseases;[14] Group A (obstructive lung diseases), Group B (pulmonary vascular diseases), Group C (cystic fibrosis or other immunodeficiency disorders), and Group D (restrictive lung diseases). In double lung transplant recipients, the ischemic time was an averageof the ischemic time of each lung. History of coronary artery disease (CAD) was obtained by review of the medical record documentation (n = 44) and data from available preoperative coronary angiography results (n = 280). Presence of coronary plaque of any severity satisfied the definition of CAD. Smoking history was defined as any amount of cigarette smoking regularly prior to the lung transplantation. Preoperative oxygen support was determined at rest at the time of index hospitalization.
Classification of BMI
The BMI data used in the analysis was collected at time of index hospital admission for lung transplant surgery. BMI classification was followed according to the NHLBI categories.[15] Normal BMI was defined as BMI 18.5-24.9kg/m2. Underweight was defined as BMI <18.5kg/m2. Overweight was defined as BMI 25.0-29.9kg/m2 and obese was defined as BMI 30kg/m2. Normal BMI was used as a reference group for the analyses.
Six-minute walk distance (SMWD)
At least one SMWD within 6 months of transplantation was available for most patients, except for the transferred patients on mechanical ventilation or extracorporeal membrane oxygenation (ECMO) machine at the time of listing and did not have prior SMWD evaluation performed (n = 7). In that case, SMWD was determined as 0 to reflect the poorest-performing pretransplant functional status group. SMWD was not routinely repeated if there was no significant change in functional status per patients' reports during pretransplant clinic visits. If SMWD was repeated and there were multiple SMWD data in one patient, the closest SMWD to lung transplantation was chosen for the analyses. The SMWD test employed a standard protocol by measuring the distance that patients were able to walk within a six-minute time limit. Patients are asked to cover as much distance as possible at a self-determined pace and were provided with enough oxygen to maintain a minimum oxygen saturation of 90% as measured by a portable pulse oximeter. The SMWD test was performed at the time of transplant evaluation by an experienced respiratory therapist using American Thoracic Society standards at a dedicated pulmonary function laboratory at Houston Methodist Hospital. In our lung transplant program; while regular exercise as tolerated was encouraged during pretransplant visits, there was not a routine pre-transplant rehabilitation program.
Study outcomes
The primary study outcome was all-cause mortality post-lung transplant surgery at any time point. Death was examined by systematically searching through medical record database, lung transplantation registry records, and social security death index. Time to death was enumerated from the date of the transplantation to the date of death.
Statistical analysis
Descriptive statistics for studied variables are presented as mean with standard deviation (SD) for normally distributed continuous variables, median with interquartile range (IQR) for non-normally distributed continuous variables, and frequency with percentage for categorical variables. Independent Student's t-test and analysis of variance (ANOVA) were used to compare normally distributed continuous variables. Wilcoxon–Mann–Whitney U test and Kruskal–Wallis H test were utilized to compare non-normally distributed continuous variables. For comparison of categorical variables, chi-square and Fisher's exact tests were performed. The optimal cut-off SMWD value selected to determined cardiorespiratory fitness status was determined from a receiver operating characteristic (ROC), using maximal Youden index.
To assess for independent associations of BMI classifications and SMWD with mortality, univariate and multivariate Cox proportional regression analyses were performed. For SMWD, both continuous SMWD value after logarithmic transformation and dichotomous SMWD value using the optimal cut-off were examined in multivariate modeling. All covariates with P-value < 0.10 in univariate modeling were included into a multivariate analysis. The regression analysis results are presented as HR with 95% confidence interval (CI). The adjusted Kaplan–Meier statistic survival curves categorized by BMI and SMWD were plotted.
All statistical analyses were performed with IBM Statistical Package for Social Sciences (SPSS)/PASW Statistics 20 (SPSS Inc, Chicago, IL). A two-tailed P-value of <0.05 was considered statistically significant.
Results
The final cohort was comprised of 324 recipients with a mean age of 57 ± 13 years and 58% were male. Primary lung pathologies were; obstructive lung diseases (Group A, 26.9%); pulmonary vascular diseases (Group B, 2.5%); cystic fibrosis or immunodeficiency disorders (Group C, 6.5%); and restrictive lung diseases (Group D, 64.2%). Median lung allocation score (LAS) prior to the lung transplantation was 38 (IQR 34-45). A minority of the patients (2.8%) were on mechanical ventilators or ECMO prior to the transplant. Double lung transplantation was performed in 62.3% of the patients with the mean ischemic time of 205 ± 66 minutes.
BMI classifications
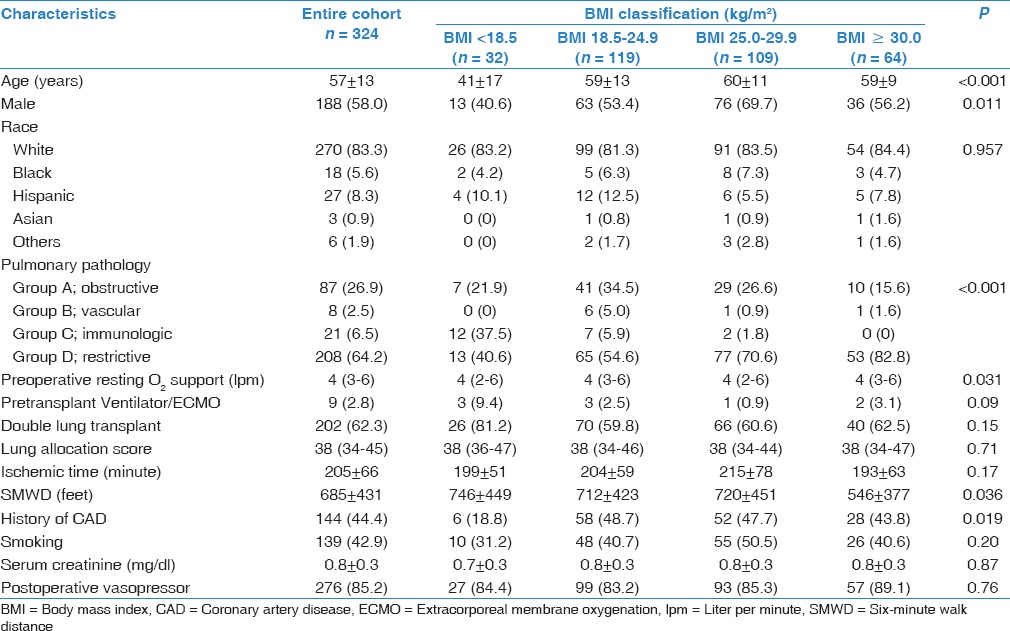
Mean BMI was 25.5 ± 5.4 kg/m2. Using the NHLBI BMI classification,[2] 119 patients (36.7%) were categorized as having normal BMI. There were 32 underweight (9.9%), 109 overweight (33.6%), and 64 obese patients (19.8%). Differences in patient characteristics in each BMI classification are shown in Table 1. Statistically significant differences of the characteristics between the BMI classifications were observed in age (P < 0.001), male gender (P = 0.011), primary pulmonary pathology (P < 0.001), preoperative oxygen support (P = 0.031), and history of CAD (P = 0.019).
Table 1.
Clinical characteristics categorized by BMI classification
Six-minute walk distance (SMWD)
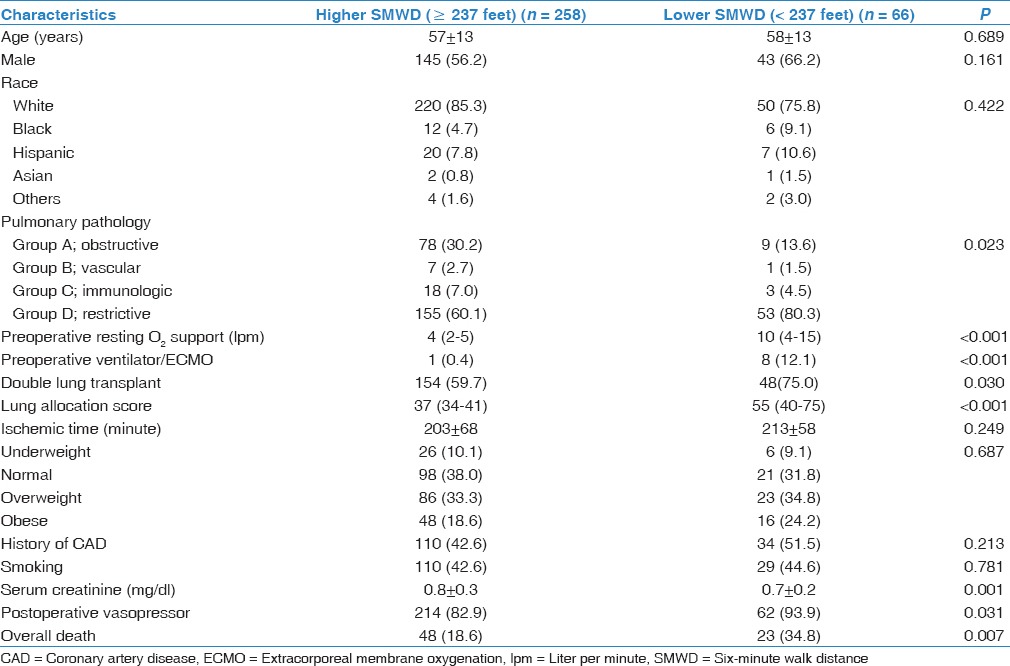
The median SMWD of the whole cohort was 700 feet (IQR 305–1,018 feet). There were a total of nine ventilator/ECMO patients in our cohort. Of these patients, seven did not have prior SMWD data and their SMWD were determined as zero. The remaining two patients had SMWD of 600 and 100 feet. The median time interval between SMWD and lung transplantation was 30 days (IQR 9-99 days). The optimal cut-off SMWD value determined by ROC was 237 feet (sensitivity = 68%, specificity = 17%, Area under curve (AUC) = 0.58). Based on this cut-off, 66 patients (20.4%) were classified as lower SMWD (SMWD <237 feet). Median SMWDs in the lower and higher SMWD groups were 81 feet (IQR 0-200 feet) and 850 feet (IQR 600-1,082 feet), respectively (P < 0.001). Baseline demographics and operative data of the cohort, categorized by SMWD are summarized in Table 2. Between the lower and higher SMWD group, statistically significant differences were observed in primary lung pathology groups (P = 0.023), Preoperative O2 support at rest (P < 0.001), preoperative mechanical ventilation or ECMO use (P < 0.001), double lung transplantation (P = 0.030), LAS (P < 0.001), serum creatinine (P < 0.001) and postoperative vasopressor use (P = 0.031). There was no significant difference in proportion of patients in each BMI classifications among the two groups (P = 0.687).
Table 2.
Clinical characteristics classified by cardiorespiratory fitness
Mortality rate and causes of death
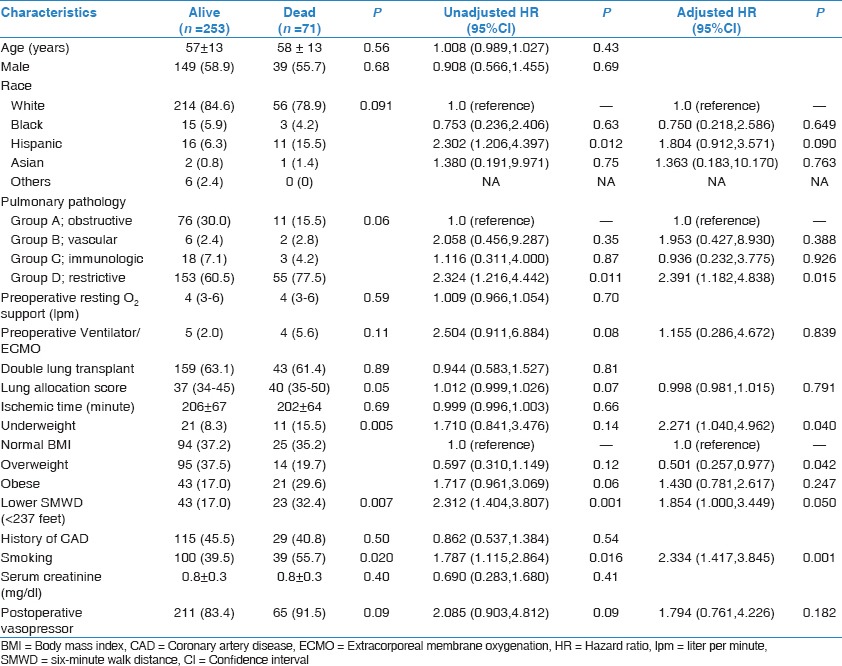
There were 71 patients (21.9%) who died during the median follow-up period of 2.3 years (IQR 1.0–3.6 years). The annualized mortality rate was significantly different among the BMI classifications (P = 0.005). The overweight group had the lowest mortality rate (5.6%/year) followed by normal BMI group (9.1%/year), obese group (14.3%/year), and underweight group (15.0%/year). Additionally, those who died had a significantly higher prevalence of unfitness (P = 0.007), cigarette smoking (P = 0.020), and higher LAS (P = 0.050). Other characteristics according to death and their mortality risks are shown in Table 3.
Table 3.
Clinical characteristics according to death and their mortality risk
Causes of death were available in 90% (64/71) of the patients. Deaths were due to 29 infections, 13 cardio-cerebrovascular causes, 8 graft failures, 8 respiratory failure, 4 operative bleedings, and 2 malignancies. The causes of death were not significantly different among different BMI groups (P = 0.73) as following: Normal BMI (11/23 infections, 3/23 cardio-cerebrovascular causes, 2/23 graft failures, 3/23 respiratory failure, 2/23 operative bleedings, and 2/23 malignancy), underweight (5/9 infections, 1/9 cardio-cerebrovascular causes, 1/9 graft failures and 2/9 respiratory failure), overweight (7/14 infections, 3/14 cardio-cerebrovascular causes, 2/14 graft failures and 2/14 respiratory failure), obese (6/18 infections, 6/18 cardio-cerebrovascular causes, 3/18 graft failures, 1/18 respiratory failure, 2/18 operative bleedings).
Predictors of mortality
Multivariate analysis of potential predictors for death is shown in Table 3. While the underweight patients were at higher risk of death compared to the normal BMI patients (HR 2.271, P = 0.040), the overweight patients had an approximate 50% reduced risk of death (HR 0.501, P = 0.042). The divergence in the survival curves began to show very early on after the transplantation and became relatively more stable after the first few months as shown in Figure 1a. Subgroup analyses classified by SMWD showed that the inverse relationship between overweight and mortality was attenuated in the higher SMWD group (SMWD ≥237 feet; P = 0.552), but not the lower SMWD group (SMWD <237 feet; HR 0.075, 95% CI 0.009-0.642, P = 0.018).
Figure 1.
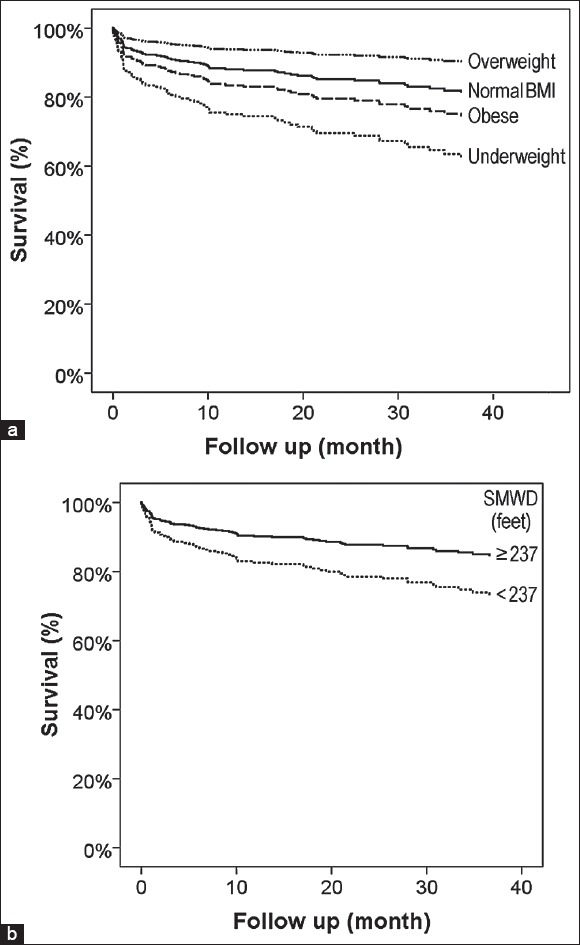
Survival curves of lung transplant patients categorized by body mass index (a) and six-minute-walk distance (SMWD) (b)
Separate analyses evaluating the impact of BMI on early mortality (30-day mortality) showed that overweight was independently associated with increased mortality after lung transplantation (HR 0.20, 95% CI 0.04-0.93, P = 0.040) with adjustment for covariates with univariate P < 0.10 (primary pulmonary pathology (P = 0.58), unfitness (HR 2.64, 95% CI 1.06-6.61, P = 0.038), and smoking history (HR 4.03, 95% CI 1.49-10.96, P = 0.006)). The risk of mortality associated with underweight, but not overweight, remained statistically significant after censoring 30-day death posttransplant (underweight HR 2.57, 95% CI 1.02-6.50, P = 0.046; overweight HR 0.67, 95%CI 0.31-1.43, P = 0.30; and obesity HR 1.37,95%CI 0.73-3.17, P = 0.27).
Other independent predictors for death were Group D, restrictive diseases (HR 2.391, P = 0.015) compared to Group A, obstructive diseases; lower SMWD (<237 feet) HR 1.854, P = 0.050); and smoking history (HR 2.334, P = 0.001). SMWD value as continuous variable after logarithmic transformation was also independently associated with mortality in multivariate analysis (HR 0.692, 95% CI 0.524-0.913, P = 0.009) as shown in Figure 1b.
Discussion
The primary finding of this study is the overweight–mortality paradox in the lung transplant recipients. In our cohort, overweight patients experienced lower mortality than patients with normal BMI, underweight, or obese. This overweight paradox was attenuated when the analysis was performed in the patients with higher SMWD, but not in the patients with lower SMWD.
Previous studies investigating the impact of pretransplant BMI on mortality after lung transplantation have shown that BMI was positively associated with mortality.[4,7,8,9,10,11,12] This includes the two studies[7,10] which examined the UNOS database between 1998-2008 and 1995-2003,respectively. Recent evidence,[16] which investigated impact of BMI on mortality after implementation of LAS-based organ allocation system, also showed that class II–II obesity (BMI ≥35.0 kg/m2) was associated with increased 1-year mortality posttransplant. Other data from single-center studies with short-term mortality (90 days) and intermediate-long-term mortality also reported consistent findings.[8,11]
However, the controversial issue in regard to the association between BMI and mortality is in the overweight and underweight BMI ranges as current evidence is conflicting. A few studies have shown increased mortality risk associated with overweight and underweight patients;[7,10] while others have shown no significant relationship,[4,8,9,12] which included the study investigating lung transplantation under LAS-organ allocation system that showed increased mortality risk associated with undernutrition, but not overweight and class I obesity (BMI 30.0-34.9 kg/m2).[16] In our study, we detected increased mortality associated with the underweight recipients compared to the normal BMI group. Our overweight group was paradoxically associated with a decreased mortality risk compared to the normal BMI group and this was primarily driven by early mortality (30 days). To the best of our knowledge, this paradoxical relationship has never been described in the lung transplant recipient population. We further examined the influence of SMWD on the overweight–mortality relationship. Currently after the establishment of LAS, the prognostic data of SMWD for mortality in lung transplant population is limited. The studies showing the prognostic value of SMWD in lung transplant patients were conducted prior to the LAS era.[17,18] Our study determined that SMWD, both as continuous variable and dichotomous variable (< or ≥237 feet) based on ROC, was independently associated with mortality in multivariate analyses. Furthermore, by using the SMWD cut-off value of 237 feet, we demonstrated that the overweight–mortality paradoxical association was attenuated in the higher SMWD group. This interaction of SMWD and overweight–mortality paradox is consistent with multiple previous publications in other healthy and nontransplant disease cohorts.[3]
Traditionally, increases in BMI have been shown to be positively associated with poor outcomes; however, paradoxical J-shaped relationship of BMI with outcomes is a well-known phenomenon in various populations;[2,19,20,21] including patients with chronic obstructive pulmonary disease and patients undergoing lung resection.[22,23] BMI is an overall index of body composition and it takes into account both fat and fat-free body mass. Recently in the literature, cardiorespiratory fitness has been used as a marker for fat-free body mass,[3] since fit individuals should theoretically have higher BMI than those who are unfit due to increased muscle mass. This paradigm has been shown in previous literature to be a potential explanation for the obesity paradox in the fit patients.[21] Nevertheless, the paradox still exists in the unfit patients for which the mechanisms are yet to be explained. In our study, we observed a survival advantage in overweight recipients compared to normal BMI recipients, but the trend is towards lower survival in obese recipients. This could potentially be explained by the time variance of dynamic changes between competing baseline overnutrition of the hosts versus undernutrition/wasting process secondary to chronic lung diseases leading to the transplant.[24] Additionally, we also observed that the patients with higher SMWD had significantly higher normal-range serum creatinine levels compared to the patients with lower SMWD. This may reflect the amount of muscle mass in overweight subjects; however, further research is needed to prove this hypothesis.
The strength of our study is that the study is the first demonstration of the overweight–mortality paradox in a lung transplant recipient cohort and the demonstration of influence on the paradox by SMWD. SMWD-determined fitness has been shown to have important prognostic value in lung transplant candidates before LAS.[17,18] The low cost intervention to improve fitness is regular exercise. Exercise in lung transplant recipients has been shown to have a positive effect on maximal and functional exercise capacity, skeletal muscle function, bone mass density, and health-related quality of life.[25] Our study additionally provides evidence that higher SMWD prior to lung transplantation is associated with survival benefit post-lung transplantation under LAS-based organ allocation system. This current study can be an incentive for prescribing a structured exercise regimen to all patients listed for lung transplantation; however, our findings need confirmation from larger randomized prospective studies and future research should also focus on effect of pretransplant structured rehabilitation on SMWD and clinical outcomes following the transplant.
We acknowledge some limitations in our study. While our data was from one of the largest single-center cohorts in the field, the number of patients was still modest and the risk of type II error should be considered. Second, we used SMWD as the surrogate for cardiorespiratory fitness instead of a cardiopulmonary test, the most scientifically accepted method to evaluate for fitness. While SMWD is a well-known prognosticator in COPD patients, the prognostic data of SMWD in lung transplant population under LAS is limited; however, we demonstrated the prognostic value of SMWD under LAS in this present study. Third, although the preoperative SMWD value used in the study was consistent with current UNOS guidelines, it is a one-time measurement; therefore the chronicity of the disease and potential effect of exercise leading to changes in SMWD could not be ascertained. This may also be a potential confounding factor as patients with rapid course of illness may still retain beneficial effects of fitness from respective healthy state and vice versa. Last, BMI is an overall index of body composition. A more specific marker of body composition such as dual-energy X-ray absorptiometry, which was not available in our study, should be considered in future studies.
Conclusion
In lung transplant recipients under LAS era, pretransplant BMI and SMWD were independent predictors for mortality after the transplant. The lowest mortality risk was noted in a group of transplant recipients identified as overweight, whereas being underweight or obese was associated with increased mortality. This paradoxical overweight–mortality relationship was diminished in the recipients with higher SMWD, but not in the lower SMWD group.
Footnotes
Source of Support: Nil
Conflicts of interest: None declared.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon CG, Manson JE. Obesity and mortality: A review of the epidemiologic data. Am J Clin Nutr. 1997;66:1044S–50. doi: 10.1093/ajcn/66.4.1044S. [DOI] [PubMed] [Google Scholar]
- 3.Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: A meta-analysis. Prog Cardiovasc Dis. 2014;56:382–90. doi: 10.1016/j.pcad.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Castro A, Llorca J, Suberviola B, Diaz-Reganon G, Ordonez J, Minambres E. Influence of nutritional status in lung transplant recipients. Transplant Proc. 2006;38:2539–40. doi: 10.1016/j.transproceed.2006.08.084. [DOI] [PubMed] [Google Scholar]
- 5.Forman DE, Fleg JL, Kitzman DW, Brawner CA, Swank AM, McKelvie RS, et al. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;60:2653–61. doi: 10.1016/j.jacc.2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafrir B, Salman N, Amir O. Joint impact of body mass index and physical capacity on mortality in patients with systolic heart failure. Am J Cardiol. 2014;113:1217–21. doi: 10.1016/j.amjcard.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Allen JG, Arnaoutakis GJ, Weiss ES, Merlo CA, Conte JV, Shah AS. The impact of recipient body mass index on survival after lung transplantation. J Heart Lung Transplant. 2010;29:1026–33. doi: 10.1016/j.healun.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Culver DA, Mazzone PJ, Khandwala F, Blazey HC, Decamp MM, Chapman JT. CCF LungTransplant Group. Discordant utility of ideal body weight and body mass index as predictors of mortality in lung transplant recipients. J Heart Lung Transplant. 2005;24:137–44. doi: 10.1016/j.healun.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 9.Kanasky WF, Jr, Anton SD, Rodrigue JR, Perri MG, Szwed T, Baz MA. Impact of body weight on long-term survival after lung transplantation. Chest. 2002;121:401–6. doi: 10.1378/chest.121.2.401. [DOI] [PubMed] [Google Scholar]
- 10.Lederer DJ, Wilt JS, D'Ovidio F, Bacchetta MD, Shah L, Ravichandran S, et al. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med. 2009;180:887–95. doi: 10.1164/rccm.200903-0425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madill J, Gutierrez C, Grossman J, Allard J, Chan C, Hutcheon M, et al. Nutritional assessment of the lung transplant patient: Body mass index as a predictor of 90-day mortality following transplantation. J Heart Lung Transplant. 2001;20:288–96. doi: 10.1016/s1053-2498(00)00315-6. [DOI] [PubMed] [Google Scholar]
- 12.Ruttens D, Verleden SE, Vandermeulen E, Vos R, van Raemdonck DE, Vanaudenaerde BM, et al. Body mass index in lung transplant candidates: A contra-indication to transplant or not? Transplant Proc. 2014;46:1506–10. doi: 10.1016/j.transproceed.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, et al. Lung Transplant Outcomes Group. Obesity and primary graft dysfunction after lung transplantation: The Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med. 2011;184:1055–61. doi: 10.1164/rccm.201104-0728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(UNOS) UNfOS. A Guide to Calculating the Lung Allocation Score.
- 15.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855–67. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 16.Singer JP, Peterson ER, Snyder ME, Katz PP, Golden JA, D'Ovidio F, et al. Body composition and mortality after adult lung transplantation in the United States. Am J RespirCrit Care Med. 2014;190:1012–21. doi: 10.1164/rccm.201405-0973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinu T, Babyak MA, O'Connell CF, Carney RM, Trulock EP, Davis RD, et al. INSPIRE Investigators. Baseline 6-min walk distance predicts survival in lung transplant candidates. Am J Transplant. 2008;8:1498–505. doi: 10.1111/j.1600-6143.2008.02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadikar A, Maurer J, Kesten S. The six-minute walk test: A guide to assessment for lung transplantation. J Heart Lung Transplant. 1997;16:313–9. [PubMed] [Google Scholar]
- 19.Tobias DK, Pan A, Jackson CL, O'Reilly EJ, Ding EL, Willett WC, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–44. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah R, Gayat E, Januzzi JL, Jr, Sato N, Cohen-Solal A, diSomma S, et al. GREAT (Global Research on Acute Conditions Team) Network. Body mass index and mortality in acutely decompensated heart failure across the world: A global obesity paradox. J Am Coll Cardiol. 2014;63:778–85. doi: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 21.Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: Implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345–54. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Attaran S, McShane J, Whittle I, Poullis M, Shackcloth M. A propensity-matched comparison of survival after lung resection in patients with a high versus low body mass index. Eur J Cardiothorac Surg. 2012;42:653–8. doi: 10.1093/ejcts/ezs135. [DOI] [PubMed] [Google Scholar]
- 23.Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: A meta-analysis. PLoS One. 2012;7:e43892. doi: 10.1371/journal.pone.0043892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433–42. doi: 10.1097/MCO.0b013e3281a30594. [DOI] [PubMed] [Google Scholar]
- 25.Wickerson L, Mathur S, Brooks D. Exercise training after lung transplantation: Asystematic review. J Heart Lung Transplant. 2010;29:497–503. doi: 10.1016/j.healun.2009.12.008. [DOI] [PubMed] [Google Scholar]


