Abstract
BACKGROUND AND AIM:
Obstructive sleep apnea syndrome (OSAS) is an independent risk factor for cardiovascular disease. Recent studies showed endothelial dysfunction and pentraxin-3 both of an early marker for development of cardiovascular disease. The aim of the study was to evaluate the relationship between severity of OSAS and endothelial dysfunction and inflammatory markers including pentraxin-3 and high-sensitivity C-reactive protein (hs-CRP).
METHODS:
This was a cross-sectional study in which patients who had undergone a polysomnographic study for diagnosis of OSAS were recruited. Included patients were grouped according to apnea-hypopnea index (AHI) as mild (AHI between 5 and 14.9) and moderate-severe OSAS (AHI ⩾ 15). Patients with AHI < 5 served as control group. Endothelial function was evaluated by flow-mediated dilatation (FMD). Serum pentraxin-3 and hs-CRP levels were measured.
RESULTS:
Eighty-three patients enrolled for the study. We found a significant increment in pentraxin-3 and hs-CRP levels and a significant decrement in FMD as the severity of OSAS increased. There was a negative correlation between FMD and AHI, pentraxin, and hs-CRP.
CONCLUSION:
OSAS patients have significantly elevated pentraxin-3 levels and endothelial dysfunction. Furthermore, both pentraxin-3 and endothelial dysfunction were independently associated with severity of OSAS defined by AHI.
Keywords: Endothelial dysfunction, flow-mediated dilatation, hs-CRP, obstructive sleep apnea, pentraxin-3
Obstructive sleep apnea Syndrome (OSAS) is characterized by recurrent upper respiratory tract obstruction episodes lasting 10 s (total) or less (partial) during sleep.[1] Hypoxemia due to apneas causes an increase in sympathetic system activation, oxygen radicals, and inflammation; thus causing vascular injury and eventually endothelial dysfunction.[2] As a result of this activation, prevalence of hypertension, diabetes mellitus, cardiovascular, and metabolic disease are significantly increased in OSAS patients.[3] All these indicate that OSAS is a systemic disorder, rather than a local pathology.[3]
Endothelial dysfunction, of which is independently associated with adverse outcome, is the preliminary pathophysiological finding of vascular system abnormality before the clinical atherosclerosis appears. It is characterized by decreased and/or total loss in endothelial dependent vasodilation. Flow-mediated dilatation (FMD) assessed from brachial artery with ultrasound is a reliable and one of the most commonly used method to evaluate endothelial function.[4]
It is well-documented that inflammation increases the risk of development of cardiovascular disease. High sensitivity C-reactive protein (hs-CRP) is an inflammatory marker, which belongs to pentraxin family and released from liver and macrophages as a response to systemic mediators in the course of inflammation.[5] Pentraxin-3 is a newly discovered member of pentraxin family. This new acute phase protein is released from liver, endothelial cells, atherosclerotic lesions, macrophages, and neutrophils as a result of inflammation. In comparison with hs-CRP, pentraxin-3 is a more specific and sensitive marker for prediction of development cardiovascular diseases.[6,7] In addition, it has been postulated that hs-CRP primarily reflects systemic inflammation; whereas, pentraxin-3 specifically synthesized locally and reflect vascular system abnormality due to predominantly released from vascular endothelial cells.[8] Therefore, pentraxin-3 might be associated with endothelial dysfunction better than hs-CRP. Kasai et al., have recently showed that pentraxin-3 levels are significantly high in patients with moderate to high OSAS and they also showed that pentraxin-3 level is associated the cardio-ankle index.[9] However, this association has yet to be clarified in OSAS patients.
The aim of the study was to evaluate the relationship between severity of OSAS and endothelial dysfunction and inflammatory markers including pentraxin-3 and hs-CRP.
Methods
This cross-sectional study was conducted at Erciyes University School of Medicine, Department of Pulmonary Medicine Sleep Disorders Center. The study protocol was approved by Erciyes University Clinical Research Ethics Committee. All participants gave written informed consent.
Study population
Subjects younger than 18 years, patients with central sleep apnea syndrome (CSAS) diagnosed with PSG, diagnosis of upper airway resistance syndrome (UARS), those with history of smoking, having active infections, malignancy, diagnosis of chronic obstructive pulmonary disease, interstitial lung disease, and asthma were excluded from the study. We also excluded subjects with diabetes mellitus, hypertension, coronary artery disease, heart failure, and subjects using drugs (statin, renin-angiotensin system blockers, and multivitamins) affecting endothelium were excluded. Overall 83 participants (50 males and 33 females) were recruited. Patients with an apnea-hypopnea index AHI < 5 were recruited as control group. The AHI cutoffs for mild and moderate-severe OSAS were 5-14.9 and ≥15 events per hour of sleep, respectively.
Biochemical analysis
Venous blood samples were collected from all subjects after PSG at 7 am using gel tubes. The blood samples were centrifuged and then stored at -80°C till measurement. Serum pentraxin-3 levels were measured by enzyme-linked immunosorbent assay (ELISA) using Quantikine® ELISA (Human Pentraxin-3 Immunoassay, R&D Systems, Inc., Minneapolis, USA). Values were expressed as ng/mL. Serum hs-CRP levels were measured with an immunonephelometric method (hsCRP, Siemens, Erlangen, Germany) and expressed as mg/dl.
Assessment of endothelial function
Patients were instructed to fast and abstain from caffeinated beverages, tobacco products, and vitamin supplements for 12 h prior to the investigations. They were also instructed to abstain from exercise after waking up until the end of the examination. FMD measurements were obtained in the morning after PSG by a trained cardiologist of those who were blinded about the severity of OSAS. Vivid 7 Ultrasound System (General Electric) and 7.5 Mhz linear Doppler probe were used for the assessment. The technique described by Celermajer et al., was used.[10] The segment before brachial artery branches in antecubital fossa was used for measurement. The arterial segments where anterior and posterior surfaces are clearly delineated were chosen. Basal diameter of brachial artery was noted, then cuff of sphygmomanometer was inflated to 200 mmHg and it remained inflated for 5 min occluding the artery. Cuff was deflated quickly allowing reactive hyperemia. Measurements were obtained during 1 min after deflation and repeated three times during maximal diameter. FMD was defined as the ratio (%) change in arterial diameter at 1 min after cuff deflation compared with baseline resting diameter.
Polysomnography
Overnight PSG was performed in all patients with a 44-channel polysomnograph (Compumedics E series, Melbourne, Australia) at the Erciyes University Sleep Disorders Center, and included the following variables: Electrooculogram (two channels), electroencephalogram (two channels), electromyogram of the submental muscles (one channel), electromyogram of the anterior tibialis muscle of both legs (two channels), electrocardiogram, and measurement of airflow with an oronasal thermistor. Chest and abdominal efforts (two channels) were recorded using inductive plethysmography and arterial oxyhemoglobin saturation (SaO2: One channel) by pulse oximetry with a finger probe. Sleep staging and respiratory events were scored by experienced personnel blind to the patient's history using published criteria. Apneas were classified as a complete cessation of airflow for at least 10 s and hypopneas were defined as either >50% airflow reduction for a minimum of 10 s or a <50% airflow reduction with associated >3% oxygen desaturation or arousal.[11] AHI was calculated as the total number of apneas and hypopneas per hour of sleep. Oxygen desaturation index (ODI) was defined as the number of incidents of oxygen desaturation occupying more than 3% per hour of sleep, as determined by pulse oximetry. AHI was calculated as the number of apneas and hypopneas per hour of sleep.[12] Patients with AHI who experienced at least five events per hour were diagnosed as having OSAS.
Statistical analysis
Continuous variables are given as the mean ± standard deviation (SD). Parametric values were compared between the two groups using Mann-Whitney U test, categorical variables were also compared by the chi-square (χ2) test. Pearson correlation test was performed to identify the independent predictors. A value of P < 0.05 was considered statistically significant. Statistical Package for Social Sciences (SPSS) 15.0 software was used for all the statistical analysis (version 15, SPSS Inc, Chicago, IL, USA).
Result
Characteristics of the study population
Eighty-three participants took part in the study. Demographic and clinical characteristics of the study population were summarized in Table 1. Fifty subjects (60.2%) were male and 33 subjects (39.8%) were female. Using AHI sores for classification, there were 19 controls (22.9%), 14 subjects with mild OSAS (16.8%), and 50 subjects with moderate-severe OSAS group (60.3%). Age was significantly different between three groups (P = 0.01), whereas body mass index (BMI) and gender were not (P = 0.05 and 0.93, respectively).
Table 1.
Characteristics of patients with obstructive sleep apnea (OSAS) and healthy control patients
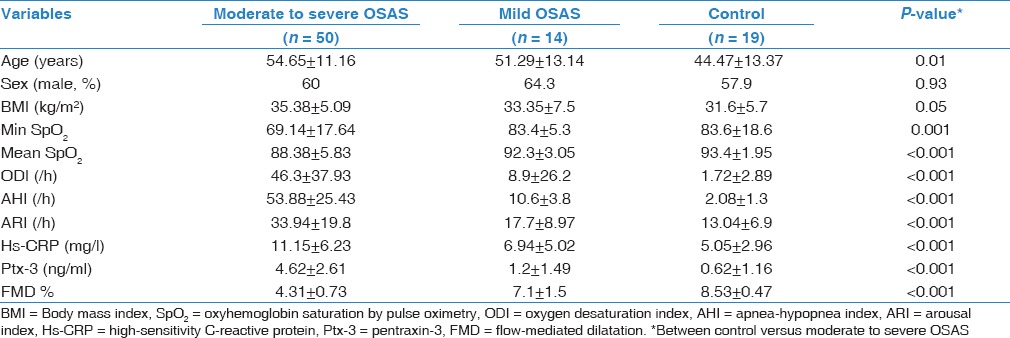
The polysomnographic findings are shown in Table 1. AHI and arousal index (ARI) were significantly different between groups (P < 0.001). As expected, AHI and ARI were worsened as the severity of OSAS increased.
Association of pentraxin-3, hs-CRP, and endothelial dysfunction
Hs-CRP and pentraxin-3 levels were significantly different between control group and mild and moderate-severe OSAS (P < 0.001) [Table 1]. We found a positive correlation between hs-CRP, pentraxin-3, and AHI [Table 2] (P < 0.001 for both]. Figure 1a and Table 1 shows that when we compared serum pentraxin-3 levels between control, mild OSAS, and moderate-severe OSAS groups; we found a significant increment in pentraxin-3 levels as the severity of OSAS increased.
Table 2.
Correlation coefficients between Hs-CRP, pentraxin-3, FMD% levels, and various variables in study population
Figure 1.
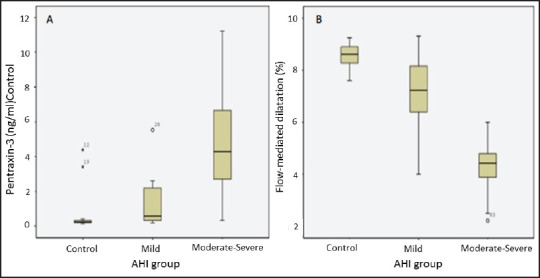
(a) Pentraxin levels in patients with obstructive sleep apnea and control group. (b) Flow-mediated dilatation percentage in patients with obstructive sleep apnea and control group. AHI = Apnea-hypopnea index
As Figure 1b and Table 1 shows, when we compared FMD measurement between control, mild OSAS, and moderate-severe OSAS groups, we found a significant decrement in FMD as the severity of OSAS increased. Univariate analysis showed that FMD values negatively correlated with AHI, pentraxin-3, and hs-CRP [Table 2 and Figure 2].
Figure 2.
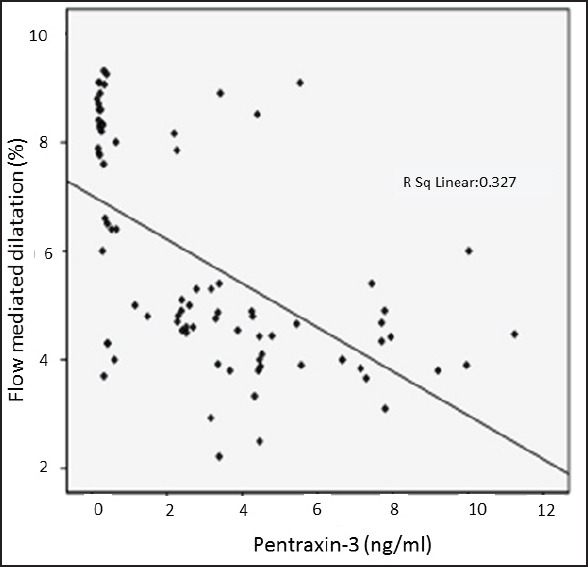
Distribution of flow-mediated dilatation percentage regarding pentraxin-3 values
Discussion
In the present study, we showed that serum pentraxin-3 and hs-CRP levels are significantly higher in OSAS patients compared to healthy controls. Secondly, we demonstrated that endothelial dysfunction is significantly increased in OSAS patients compared to healthy controls. Thirdly, we revealed a significant negative correlation between endothelial dysfunction and inflammatory markers, especially with serum pentraxin-3 level in OSAS patients.
Endothelial dysfunction is accepted as an initial step in the atherosclerotic process.[13] Previous studies showed that endothelial dysfunction predicts risk of both future fatal and nonfatal cardiovascular events.[14] The study conducted by Ip et al., included 28 OSAS patients and 12 control subjects, showed that moderate-severe OSAS patients have endothelial dysfunction and treatment with continuous positive airway pressure reversed the endothelial dysfunction.[15] Another study conducted by Oflaz et al., compared FMD measurements of OSAS patients and healthy control group. Both groups were composed of subjects without hypertension and the groups were similar with respect to gender, BMI, smoking, and level of blood lipids. FMD measurements were found significantly lower in subjects with OSAS. Additionally, diurnal variation of FMD, which was resulted in lower measurements in the morning due to hypoxia caused by recurrent nocturnal apneas was documented.[16] In our study, we also excluded all risk factors that may affect endothelial functions; which made our finding more significant and found a strong association between severity of OSAS and endothelial dysfunction. Endothelial dysfunction is associated with increased endothelial cell permeability, a procoagulant state, enhanced leukocyte adhesion due to increased endothelial expression of adhesion molecules, increased vascular tone due to a reduction in nitric oxide production, and the proliferation of smooth muscle cells.[17] It was widely accepted that OSAS can directly worsen the endothelial function via several mechanisms.[18,19,20] Impairment in endothelial function in OSAS patients is due to ongoing stimulated inflammation, oxygen radicals, sympathetic system mainly triggered by hypoxia.[21] In the light of aforementioned studies, mechanisms, and our findings; OSAS might be defined as a ‘cardiovascular toxin’.
Inflammation is a risk factor for endothelial dysfunction through the inhibition of endothelial nitric oxide synthase activity and downregulating the number of endothelial progenitor cells.[22,23,24] Pentraxin-3 is produced in the site of inflammation and it is associated with endothelial dysfunction.[25] In an experimental study, Rolph et al., showed a strong pentraxin-3 staining in macrophages and endothelial cells in advanced atherosclerotic lesions. In contrast, sections from nonatherosclerotic internal mammary arteries did not express pentraxin-3.[26] The major factors that regulate pentraxin-3 expression from atherosclerotic lesions are interleukin-1, tumor necrosis factor, and oxidized low density lipoprotein (LDL) cholesterol whose expression also increased during atherosclerotic lesions.[26] The increased secretion of pentraxin-3 in atherosclerotic endothelium might explain its role in the pathogenesis of cardiovascular disease. In contrast to these experimental studies, some experimental studies have showed that pentraxin-3 may behave as an anti-inflammatory mediator to regulate proinflammatory cytokine levels. Specifically, pentraxin-3 deficiency, as indicated by pentraxin-3 knockout mice, resulted in the upregulation of proinflammatory cytokine levels.[27] Furthermore, it has been shown that transgenic mice overexpressing pentraxin-3 had significantly increased anti-inflammatory cytokine production such as interleukin-10.[28]
In the present study, we showed that pentraxin-3 and hs-CRP are significantly correlated with endothelial dysfunction assessed by FMD in OSAS patients. To our knowledge, only three studies investigated the predictive value of pentraxin-3 in OSAS patients.[9,29,30] Kasai et al., conducted a study with 50 OSAS patients and 25 healthy controls and showed that serum pentraxin-3 levels and arterial stiffness is significantly high in OSAS patients compared to control subjects. They also showed a significant improvement in pentraxin-3 and arterial stiffness after 1 month continuous positive airway pressure treatment.[9] In addition some other studies also showed a higher serum pentraxin-3 levels in OSAS patients.[29,30] In this regard, our findings in OSAS patients and healthy subjects are consistent with previous studies. Moreover, we also showed a significant independent association between pentraxin-3 and endothelial dysfunction, which was evaluated by FMD in OSAS patients for the first time in the literature.
There are contradictory reports regarding plasma pentraxin-3 levels and its role in development of cardiovascular disease in obese patients.[31,32] Some previous studies showed that obese subjects have low serum pentraxin-3 levels compared to nonobese subjects.[32] In contrast, our study and previous studies that were done in OSAS patients[9,29,30] showed that serum pentraxin-3 levels are high in obese subjects with OSAS. However, we have to keep in mind that OSAS may causes several metabolic disarrangements that could affect serum pentraxin-3 levels and this might explain the why pentraxin-3 levels high in obese OSAS patients. Therefore, future studies with big sample size and long-term follow-up are warranted to elucidate whether high pentraxin-3 level is protective or not in OSAS patients.
However, some limitations of our study need to be mentioned. First of all, our study does not allow elucidating the role of pentraxin-3 in endothelial dysfunction; nevertheless, a significant association with endothelial dysfunction suggests that pentraxin-3 can be considered as an early marker of vascular damage in OSAS patients. The second important limitation is the sample size of the study groups. We had a large sample of OSAS patients, but we wanted to be sure about independent association between OSAS and endothelial dysfunction and vascular inflammation marker pentraxin-3; therefore, we excluded all subjects with risk factors that may affect endothelial function.
In conclusion, OSAS patients have significantly elevated pentraxin-3 levels and endothelial dysfunction. Furthermore, both pentraxin-3 and endothelial dysfunction were independently associated with severity of OSAS defined by AHI. Pentraxin-3, hs-CRP, and FMD could be used as markers of early cardiovascular damage in OSAS patients without known cardiovascular disease. Further studies with large sample size and long-term follow-up are warranted to elucidate the predictive value of pentraxin-3 and endothelial dysfunction on adverse outcome in OSAS patients.
Footnotes
Source of Support: Nil
Conflicts of interest: None declared.
References
- 1.Stradling JR. Sleep-related breathing disorders. 1. Obstructive sleep apnoea: Definitions, epidemiology, and natural history. Thorax. 1995;50:683–9. doi: 10.1136/thx.50.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;27:1113–20. doi: 10.1093/sleep/27.6.1113. [DOI] [PubMed] [Google Scholar]
- 3.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. American J Respir Crit Care Med. 2001;164:2147–65. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 4.Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Lee T, et al. Endothelial dysfunction and C-reactive protein in relation with the severity of obstructive sleep apnea syndrome. Sleep. 2007;30:997–1001. doi: 10.1093/sleep/30.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 6.Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–72. [PubMed] [Google Scholar]
- 7.Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–41. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 8.Yasunaga T, Ikeda S, Koga S, Nakata T, Yoshida T, Masuda N, et al. Plasma pentraxin 3 is a more potent predictor of endothelial dysfunction than high-sensitive C-reactive protein. Int Heart J. 2014;55:160–4. doi: 10.1536/ihj.13-253. [DOI] [PubMed] [Google Scholar]
- 9.Kasai T, Inoue K, Kumagai T, Kato M, Kawana F, Sagara M, et al. Plasma pentraxin3 and arterial stiffness in men with obstructive sleep apnea. Am J Hypertens. 2011;24:401–7. doi: 10.1038/ajh.2010.248. [DOI] [PubMed] [Google Scholar]
- 10.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 11.Rechtschaffen AK. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda: National Institute for Neurological Disease and Blindness; 1968. Report No: NIH 204. [Google Scholar]
- 12.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 13.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kullo IJ, Malik AR. Arterial ultrasonography and tonometry as adjuncts to cardiovascular risk stratification. J Am Coll Cardiol. 2007;49:1413–26. doi: 10.1016/j.jacc.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 16.Oflaz H, Cuhadaroglu C, Pamukcu B, Meric M, Ece T, Kasikcioglu E, et al. Endothelial function in patients with obstructive sleep apnea syndrome but without hypertension. Respir Int Rev Thorac Dis. 2006;73:751–6. doi: 10.1159/000094183. [DOI] [PubMed] [Google Scholar]
- 17.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61. [PubMed] [Google Scholar]
- 18.Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling: Association with the severity of apnea-induced hypoxemia during sleep. Chest. 2001;119:1085–91. doi: 10.1378/chest.119.4.1085. [DOI] [PubMed] [Google Scholar]
- 19.Damiani MF, Lacedonia D, Resta O. Influence of obstructive sleep apnea on cognitive impairment in patients with COPD. Chest. 2013;143:1512. doi: 10.1378/chest.12-2997. [DOI] [PubMed] [Google Scholar]
- 20.Ciccone MM, Favale S, Scicchitano P, Mangini F, Mitacchione G, Gadaleta F, et al. Reversibility of the endothelial dysfunction after CPAP therapy in OSAS patients. Int J Cardiol. 2012;158:383–6. doi: 10.1016/j.ijcard.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 21.Atkeson A, Jelic S. Mechanisms of endothelial dysfunction in obstructive sleep apnea. Vasc Health Risk Manag. 2008;4:1327–35. doi: 10.2147/vhrm.s4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hein TW, Singh U, Vasquez-Vivar J, Devaraj S, Kuo L, Jialal I. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis. 2009;206:61–8. doi: 10.1016/j.atherosclerosis.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh U, Devaraj S, Vasquez-Vivar J, Jialal I. C-reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J Mol Cell Cardiol. 2007;43:780–91. doi: 10.1016/j.yjmcc.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: Further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–67. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 25.Savchenko A, Imamura M, Ohashi R, Jiang S, Kawasaki T, Hasegawa G, et al. Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol. 2008;215:48–55. doi: 10.1002/path.2314. [DOI] [PubMed] [Google Scholar]
- 26.Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–4. doi: 10.1161/01.atv.0000015595.95497.2f. [DOI] [PubMed] [Google Scholar]
- 27.Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F, et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 28.Breviario F, d'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–7. [PubMed] [Google Scholar]
- 29.Ciccone MM, Scicchitano P, Zito A, Cortese F, Boninfante B, Falcone VA, et al. Correlation between inflammatory markers of atherosclerosis and carotid intima-media thickness in Obstructive Sleep Apnea. Molecules. 2014;19:1651–62. doi: 10.3390/molecules19021651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Gozal D, Bhattacharjee R, Kheirandish-Gozal L. TREM-1 and pentraxin-3 plasma levels and their association with obstructive sleep apnea, obesity, and endothelial function in children. Sleep. 2013;36:923–31. doi: 10.5665/sleep.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyaki A, Maeda S, Yoshizawa M, Misono M, Sasai H, Shimojo N, et al. Is pentraxin 3 involved in obesity-induced decrease in arterial distensibility? J Atheroscler Thromb. 2010;17:278–84. doi: 10.5551/jat.2741. [DOI] [PubMed] [Google Scholar]
- 32.Yamasaki K, Kurimura M, Kasai T, Sagara M, Kodama T, Inoue K. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab Med. 2009;47:471–7. doi: 10.1515/CCLM.2009.110. [DOI] [PubMed] [Google Scholar]


