Abstract
Purpose:
Penile lichen sclerosus (LS) is a nagging condition and its progression result in devastating urinary and sexual problems and reduction in the quality-of-life. This study has been carried out to present our experience about this disease with simultaneous review of the available literature.
Materials and Methods:
This retrospective study has been done at a tertiary care center of eastern India. The data of 306 patients affected with LS were analyzed for clinical presentation, physical examination, investigations, and treatment offered.
Results:
Presenting symptoms were non-specific. The prepuce was most commonly involved location followed by glans and meatus. Urethral involvement was not isolated as the primary site. Circumcision was done in 237 patients, while 63 patients underwent meatotomy. Thirty-six of 39 cases of LS induced stricture were treated with buccal mucosal graft (BMG) either in one stage or in two stages.
Conclusion:
LS varies from being a highly aggressive disease of the penis and anterior urethra to a burnt out condition affecting just the meatus and surrounding glans. Early diagnosis and treatment are required to prevent its complication and associated morbidity. Management depends on the anatomical location of lesion, extent of involvement, rapidity of progression and its severity. Use of BMG in LS induced urethral stricture has shown encouraging results.
Keywords: Balanitis xerotica obliterans, lichen sclerosus, meatal stenosis, penis, urethral stricture
INTRODUCTION
Penile lichen sclerosus (LS) is a progressive, sclerosing, inflammatory dermatosis of the glans penis and foreskin (prepuce).[1] The true incidence, epidemiology, and pathogenesis of this entity is not yet completely known.[1,2] If not recognized early, the progression of this disease may result to destructive scarring that can lead to devastating urinary and sexual problems and a dramatic reduction in the quality-of-life and of course a significant morbidity. Lichen sclerosis has not been given much emphasis in the urological research and there is a paucity of literature on this disease. The present study is an attempt to share our experience and to review the available literature about this disease entity.
MATERIALS AND METHODS
This retrospective study has been done in the Urology Department of IPGMER and SSKM Hospital, Kolkata, a tertiary care center of eastern India. Three hundred and sixty-five patients affected with LS were treated in our institute from January 2005 to December 2011. Patient data about their clinical presentation, physical examination, investigations, and treatment were analyzed. All patients who were investigated with urinalysis, uroflowmetry, retrograde urethrogram, micturating cystourethrogram, cysto-urethroscopic assessment and diagnosis confirmed with the biopsy from the prepuce, glans or meatus were included in the study. The offered treatment varied from topical drug application to circumcision, meatotomy, single or two stage urethroplasty or perineal urethrostomy. According to our institutional policy, post-operatively each patient was evaluated on every 4th month in the 1st year and annually thereafter; with the ultrasonography of kidney, ureter, bladder and prostate region with post-void residual urine measurement, uroflowmetry, and urine culture. Urethrography was also added and repeated whenever the patient had symptoms of decreased urinary flow and/or the maximum flow rate (Qmax) was less than 12 mL/s. Thirty-six patients who lost to follow-up and 23 patients who had incomplete investigational data were excluded and therefore effective study was done on 306 patients only.
RESULTS
The mean age of the presentation was 37 years (range 7-79 years). The presenting symptoms were non-specific; though the most common were pruritus, soreness, and dysuria. Sixty-six patients had chronic retention of urine, 52 patients had deranged renal function (serum creatinine > 1.5 mg/dL), and 91 patients had active UTI on initial evaluation in which Escherichia coli was the most common affecting organism [Table 1]. The prepuce was most commonly involved followed by glans and meatus. Though 39 patients had long segment penile or pan urethral stricture and another 56 patients had meatal stenosis and a small segment distal urethral stricture, urethral involvement was not isolated as the primary site in any of the case [Table 1]. Ninety-seven patients had their flow rate below 15 mL/min, but only 79 patients had proven stricture or stenosis. Urethrogram was able to identify all stricture cases, but in nine cases, it was falsely positive owing to associated urethral inflammation and incomplete opacification of the urethra due to distal stenosis.
Table 1.
The clinical summary of all the cases
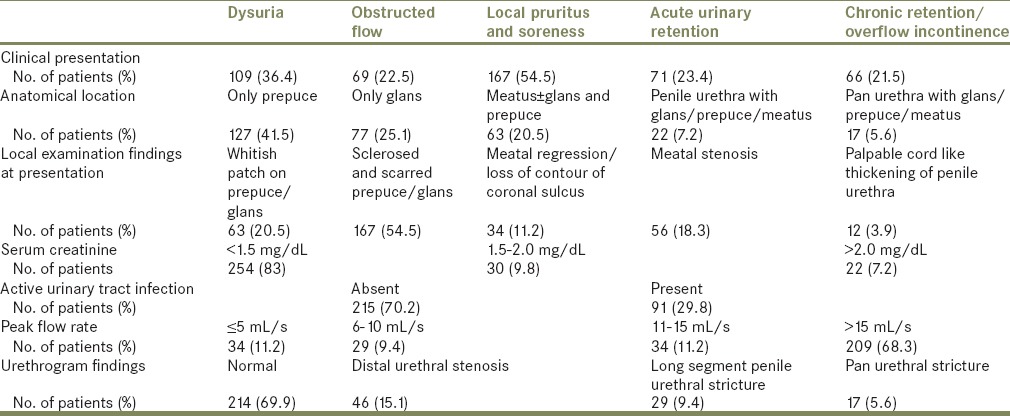
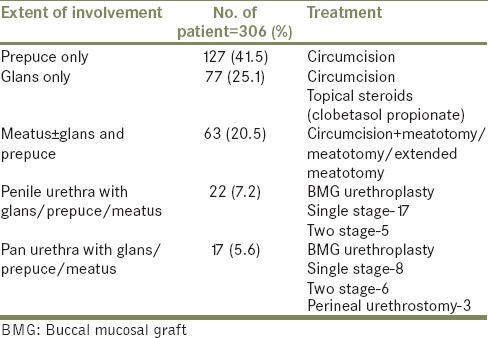
Circumcision was done in 237 patients while 33 patients underwent meatotomy along with circumcision. Thirty patients who were already circumcised, were undergone only meatotomy. Nine cases of meatotomy got restenosed over the follow-up; six underwent extended meatotomy, while rest three required buccal mucosal graft (BMG) uretroplasty. Three patients with pan urethral stricture underwent perineal urethrostomy due to associated cardiac morbidity and renal failure. Rest all cases of LS induced stricture were treated with BMG either in one stage or in two stages depending on local tissue condition and availability of urethral plate [Table 2]. On follow-up extended over 2-9 years, two patients developed meatal stenosis that required meatotomy and staged BMG urethroplasty in separate cases, while two other patients developed stricture at anastomosis site required repeated visual dilatation. One patient developed lateral urethral diverticulum.
Table 2.
The site of involvement and treatment for lichen sclerosus
DISCUSSION
LS, also known as balanitis xerotica obliterans (BXO), is a chronic lymphocyte mediated skin disease, which may affect any cutaneous surface, but shows a predilection for the ano-genital area in men and women. This disease process was first clinically described by Hallopeau in 1887 and given the name lichen plan atrophique. In 1892, Darier named it as lichen plan sclérux.[3] The male form of LS that is also called as BXO; was first defined in 1928 by Stuhmer[2] The name BXO has three components; balanitis, that is a chronic inflammation of the glans penis; xerotica, meaning abnormally dry appearance of the lesion; and obliterans, that indicates its association with occasional endarteritis. In 1976, the International Society for the study of Vulvovaginal Disease officially adopted the term LS to define this disease process in both males and females.[2,3,4]
Its exact incidence and prevalence is difficult to estimate because the disease is often unrecognized and such patients may go to various specialist doctors including pediatricians, surgeons, dermatologists, and urologists.[5] Reported incidence was 0.07% in an unselected cohort study of Kizer et al. while the prevalence was reported between 1:300 and 1:1000 of all patients that were referred to a community-based Dermatology Department.[6,7]
Penile LS most often occurs in patients of 30-49 years of age, though it has also been reported in adolescents and old age.[2,5] It is most common in white races.[2,5] The exact etiology remains unknown, multiple theories have been proposed including bacterial infection (acid fast bacilli and spirochetes),[2] viral infection (human papillomavirus, herpes zoster, hepatitis C virus),[1,8,9] local trauma (Koebner phenomenon),[10] chemical irritation (by intravasation of urine), auto-immune deregulation and hormonal dysfunction.[11]
In early stage of development of penile LS, there is lymphocytic infiltration in the basal epidermis and superficial dermis, associated with basal vacuolar change in the epidermis. The classic lesion of penile LS occurs relatively late and is characterized by hyperkeratosis of the epithelium, atrophy and loss of the rete pegs, degeneration of the basal cells, sclerosis of the sub epithelial collagen and lymphocytic infiltration of the dermis. Initially, the dermis is homogenized and edematous; later in the course of the disease it is hyalinized and sclerotic.[12]
LS in males may have an insidious or aggressive course. It typically starts as an itchy patch of the white discoloration on the inner aspect of the foreskin or glans [Figure 1]. Glans may be affected in diffused fashion or as mottled patches. Patches eventually coalesce and the affected skin becomes inelastic, brittle and sore rather than itchy.[11] Scarring of the glans and the prepuce can cause phimosis, difficulty in penile erection and sexual intercourse. The pathognomonic features of BXO also include a perimeatal whitish and erythematous area.[12] More severely affected patients may develop meatal regression and loss of contour of glans and coronal sulcus. Scarring around the meatus causes meatal stenosis that can spread proximally to involve the fossa navicularis, penile urethra and rarely the bulbar urethra with resultant urethral stricture [Figure 2]. The reason behind the involvement of the urethra is still controversial.[8,12] It has been suggested that panurethral strictures in patients with LS are caused by the trauma of repeated dilatation or instrumentation.[8] According to Depasquale et al.; LS involved the foreskin and glans in 57%, meatus in 4%, and 20% in urethra.[13] Ten cases of urethral involvement were first reported by Laymon in 1951.[14] According to Catteral and Oates, in their series of BXO with urethral involvement, urethral discharge was the main symptom while one-third of their patient presented with dysuria and obstructive voiding symptoms.[15] Bainbridge et al. in their study have reported the presence of obstruction in 47% patients.[16] In our series, most of time the presentation was non-specific; with prepucial involvement in 54.6%, glanular in 30.4%, and urethral in 18.7%.
Figure 1.
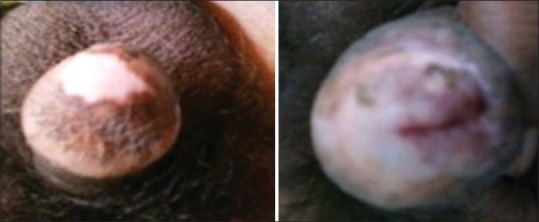
Hypopigmented lesion on glans
Figure 2.
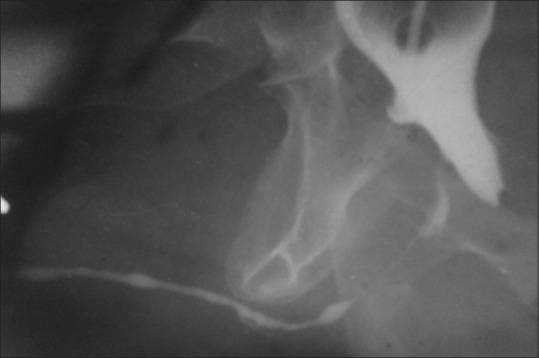
The involvement of urethra by balanitis xerotica obliterans
Unrecognized or ignored LS may lead to urinary retention, retrograde damage of posterior urethra, bladder, and kidney[12] [Figures 3 and 4]. LS has a rare risk of malignant transformation (4-8%).[17] In our series, 23% patients presented with acute urinary retention, while 17% patient had compromised renal function.
Figure 3.
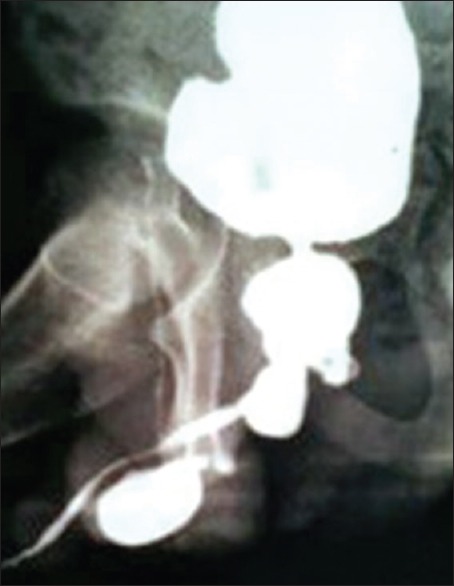
The diseased urethra with diverticulum in balanitis xerotica obliterans patients
Figure 4.
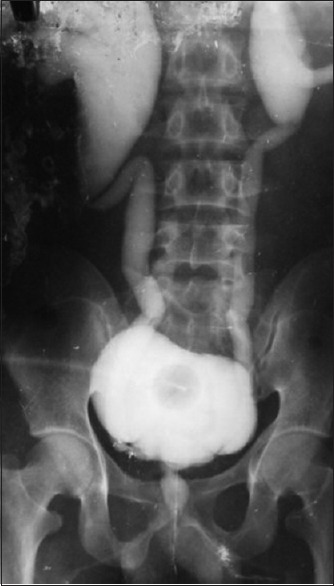
The back pressure changes in kidney, ureter, and bladder in patient of balanitis xerotica obliterans
The LS cannot be diagnosed clinically alone, since it resembles erythroplasia of querat, lichen planus, leukoplakia, and scleroderma. A skin biopsy should be considered to confirm the diagnosis in these circumstances and also to exclude associated subclinical in situ or invasive squamous cell carcinoma.[18] In all cases of LS, when there is clinical concern for urethral involvement, urethroscopy, and retrograde urethrography are mandatory.[13]
The goal for treatment of LS is to alleviate symptoms and discomfort, prevent anatomical changes such as stricture and prevent malignant transformation. Many advocate even asymptomatic patients should be treated to prevent progression of the disease and possible development of malignancy.[2,3]
Traditional treatment consists of periodic urethral dilation with the application of topical emollients or steroids.[15] In some series, 41% of those treated with steroids showed improvement in clinical symptoms.[19] A trial of a potent topical corticosteroid should always be undertaken in uncomplicated penile LS before surgery. There is no standard treatment protocol regarding the type and duration of topical steroid usage.[1] In adults, initially a potent steroid; clobetasol propionate (0.05%) twice daily application for 2-3 months with gradual dose lowering has been used with success, although this medication is not approved by the Food and Drug Administration for this indication.[1] If there is no improvement within 6 months, then the use of the potent topical steroid should be stopped. The topical calcineurin inhibitors pimecrolimus and tacrolimus have been used with success, but their long-term safety has not been established.[20] A combination of topical testosterone and progesterone preparation have also been tried. But topical steroids prove to be more effective in these cases.[2]
Systemic treatments have been used for LS, but they should be reserved for severe, unresponsive cases, or for those who are intolerant to topical high potency corticosteroids.[1,2] Penile dysaesthesia may respond to a low dose tricyclic antidepressant or gabapentin.[18] In our experience with topical steroids in 137 patients, none had complete cure. Cryotherapy, ultraviolet phototherapy, carbon dioxide laser, pulse dye laser and subcutaneous injection of absolute alcohol have also been successfully attempted for LS.[2,8,15] Further research into these and other alternative therapies is required.
In men, surgical treatments are generally required for the obstructive urethral stricture or painful erection and intercourse. Circumcision plays an important role in the management of early LS.[11] Depasquale et al. have reported success rate of 96% for patient with LS limited to the glans and foreskin.[13] In our experience, 11 out of 39 cases of urethral involvement which initially underwent circumcision, presented with urethral involvement later [Figure 5]. In previously circumcised patients with balano-preputial adhesion, the scleroatrophic tract intersecting the skin of glans base and residual prepuce are completely excised and resultant defect can be covered with full thickness non genital skin graft.[15] The reported outcome of this procedure has better results both symptomatically and cosmetically.
Figure 5.
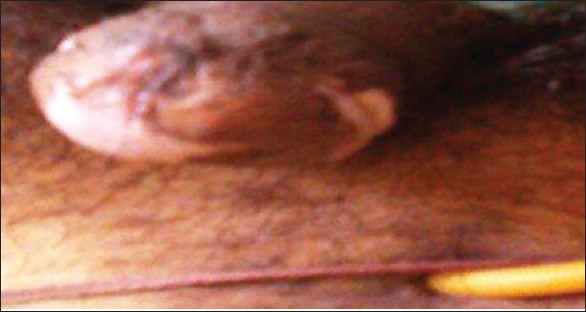
Development of lichen sclerosus despite early circumcision
Meatal stenosis can be treated with ventral meatotomy or dorsal V-meatoplasty. Due to its higher restenosis rate in LS, interposition techniques or extended meatotomy (EM) have been advised. In refractory cases of fossa navicularis strictures, reported EM success rate was 87%, as reported by Morey et al.[21] We have similar experience with extended meatotomy for recurrent fossa navicularis stricture, but four out of nine patients were uncomfortable with hypospadiac meatus.
The aim of the urologist in treating this entity should be to restore the integrity of the genitalia affected by LS and to permitting normal urinary micturition through an apical glandular meatus. Strictures related to lichen sclerosis are difficult to manage. The fibrotic process is usually tenacious and tends to increase inexorably in length and severity, creating a poor quality urethral plate. LS involving the anterior urethra can be treated with substitution urethroplasty or by two stage procedure. Surgical options should be selected according to patient's age, clinical presentation and histological features.[22] Single stage repair is preferred in patients who histologically show mild or moderate disease, without full involvement of the glans and penile skin and with a reasonably wide urethral plate. In patients histologically showing severity, full involvement of the glans and penile skin and with a narrow and scarred urethral plate, two-stage repair gives better outcome.[22] Genital skin should not be used because of the recurrence of disease and failure of urethroplasty.[4,13] Recurrence of stricture may occur between 6 months to 2 years, but it may even occur after 10 years of follow-up.[13] The use of a buccal mucosa graft has proven invaluable in the treatment of LS. Its use in penile urethroplasty as an one stage or two stage procedure results in less contracture and more reliable revascularization due to the thin and highly vascular lamina propria.[23] The use of other tissue as a urethral graft like bladder mucosa, rectal mucosa and tunica vaginalis, have been described with varied success rate.[11] Future developments in tissue engineering are likely to contribute to successful genital and urethral reconstruction in patients with LS by providing unlimited sources of graft tissue that may be resistant to LS. In our series, all the LS induced urethral stricture were treated with BMG in one or two stages with a 87.6% success rate over a minimum follow-up period of 2 years (range 2-9 years). Two cases of pan urethral stricture associated with periurethral infection were treated initially with marsupialization of the urethra as part of stage procedure.
The often extensive nature of LS, limited availability of non-penile skin sources and acceptance of voiding in a squatting position makes definitive perineal urethrostomy a viable treatment option. In older patients, with multiple unsuccessful prior repairs, serious co-morbidity, histologically severe disease, severely scarred urethral plate; the possibility of performing a definitive perineal urethrostomy should be discussed with the patient.[22] We follow the same rationale for perineal urethrostomy because heroic measures may not always be justified in extensive urethral stricture due to LS. The final choice, however, was made after consultation with a patient. In our series, nine patients underwent perineal urethrostomy by flap based technique using an inverted U incision given in the perineum. In six patients, it was done as a part of staged procedure and in three patients as permanent procedure.
The results of this study are subject to few limitations. The study is retrospective one and the quality-of-life assessment of LS patient was not done though the penile morphology is related to the patient's self-esteem, body image, confidence, and sexuality. Third, a mean follow-up of almost 4 years (range 1-9 years) may still be insufficient to conclude that many LS lesions are permanently cured.
CONCLUSION
LS is a scarcely known disease, though not rare. It varies from being a highly aggressive and inflammatory disease of the penis and anterior urethra to a burnt out condition affecting just the meatus and surrounding glans. The clinical presentation is nonspecific. Early diagnosis and treatment are required to prevent its complication and associated morbidity. Management depends on the anatomical location of lesion, extent of involvement, rapidity of progression and its severity. Use of BMG in LS induced urethral stricture has shown encouraging results.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393–416. doi: 10.1016/0190-9622(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 2.Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777–83. doi: 10.1016/s0140-6736(98)08228-2. [DOI] [PubMed] [Google Scholar]
- 3.Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808–17. doi: 10.1097/01.grf.0000179635.64663.3d. [DOI] [PubMed] [Google Scholar]
- 4.Venn SN, Mundy AR. Urethroplasty for balanitis xerotica obliterans. Br J Urol. 1998;81:735–7. doi: 10.1046/j.1464-410x.1998.00634.x. [DOI] [PubMed] [Google Scholar]
- 5.Yesudian PD, Sugunendran H, Bates CM, O’Mahony C. Lichen sclerosus. Int J STD AIDS. 2005;16:465–73. doi: 10.1258/0956462054308440. [DOI] [PubMed] [Google Scholar]
- 6.Kizer WS, Prarie T, Morey AF. Balanitis xerotica obliterans: Epidemiologic distribution in an equal access health care system. South Med J. 2003;96:9–11. doi: 10.1097/00007611-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9–30. [PubMed] [Google Scholar]
- 8.Beattie PE, Dawe RS, Ferguson J, Ibbotson SH. UVA1 phototherapy for genital lichen sclerosus. Clin Exp Dermatol. 2006;31:343–7. doi: 10.1111/j.1365-2230.2006.02082.x. [DOI] [PubMed] [Google Scholar]
- 9.Neill SM, Lessana-Leibowitch M, Pelisse M, Moyal-Barracco M. Lichen sclerosus, invasive squamous cell carcinoma, and human papillomavirus. Am J Obstet Gynecol. 1990;162:1633–4. doi: 10.1016/0002-9378(90)90942-z. [DOI] [PubMed] [Google Scholar]
- 10.Miller RA. The Koebner phenomenon. Int J Dermatol. 1982;21:192–7. doi: 10.1111/j.1365-4362.1982.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 11.Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: Review of the literature and current recommendations for management. J Urol. 2007;178:2268–76. doi: 10.1016/j.juro.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Mundy AR, Andrich DE. Urethral strictures. BJU Int. 2011;107:6–26. doi: 10.1111/j.1464-410X.2010.09800.x. [DOI] [PubMed] [Google Scholar]
- 13.Depasquale I, Park AJ, Bracka A. The treatment of balanitis xerotica obliterans. BJU Int. 2000;86:459–65. doi: 10.1046/j.1464-410x.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 14.Laymon CW. Lichen sclerosus et atrophicus and related disorders. AMA Arch Derm Syphilol. 1951;64:620–7. doi: 10.1001/archderm.1951.01570110090013. [DOI] [PubMed] [Google Scholar]
- 15.Das S, Tunuguntla HS. Balanitis xerotica obliterans – A review. World J Urol. 2000;18:382–7. doi: 10.1007/PL00007083. [DOI] [PubMed] [Google Scholar]
- 16.Bainbridge DR, Whitaker RH, Shepheard BG. Balanitis xerotica obliterans and urinary obstruction. Br J Urol. 1971;43:487–91. doi: 10.1111/j.1464-410x.1971.tb12073.x. [DOI] [PubMed] [Google Scholar]
- 17.Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911–4. doi: 10.1016/s0190-9622(99)70245-8. [DOI] [PubMed] [Google Scholar]
- 18.Clouston D, Hall A, Lawrentschuk N. Penile lichen sclerosus (balanitis xerotica obliterans) BJU Int. 2011;108(Suppl 2):14–9. doi: 10.1111/j.1464-410X.2011.10699.x. [DOI] [PubMed] [Google Scholar]
- 19.Kiss A, Csontai A, Pirót L, Nyirády P, Merksz M, Király L. The response of balanitis xerotica obliterans to local steroid application compared with placebo in children. J Urol. 2001;165:219–20. doi: 10.1097/00005392-200101000-00062. [DOI] [PubMed] [Google Scholar]
- 20.Neill SM, Lewis FM, Tatnall FM, Cox NH. British Association of Dermatologists. British Association of Dermatologists’ guidelines for the management of lichen sclerosus 2010. Br J Dermatol. 2010;163:672–82. doi: 10.1111/j.1365-2133.2010.09997.x. [DOI] [PubMed] [Google Scholar]
- 21.Morey AF, Lin HC, DeRosa CA, Griffith BC. Fossa navicularis reconstruction: Impact of stricture length on outcomes and assessment of extended meatotomy (first stage Johanson) maneuver. J Urol. 2007;177:184–7. doi: 10.1016/j.juro.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni S, Barbagli G, Kirpekar D, Mirri F, Lazzeri M. Lichen sclerosus of the male genitalia and urethra: Surgical options and results in a multicenter international experience with 215 patients. Eur Urol. 2009;55:945–54. doi: 10.1016/j.eururo.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 23.Zinman L. Muscular, myocutaneous, and fasciocutaneous flaps in complex urethral reconstruction. Urol Clin North Am. 2002;29:443–6623. doi: 10.1016/s0094-0143(02)00032-0. [DOI] [PubMed] [Google Scholar]


