Abstract
Aim:
In this study, we compared and valued efficacy and safety of percutaneous echoguided sclerotherapy (PES) using 3% polidocanol with that using 99% ethanol in the treatment of patients with simple renal cysts.
Materials and Methods:
PES was performed for 65 simple renal cysts. Under ultrasonographic guidance the cyst was punctured using an 18 gauge needle. Sclerotherapy was performed with ethanol in 55% (36/65) of cases and with polidocanol in the remaining 45% (29/65). Patients were followed up with an ultrasound examination at 4 months, 8 months, and then at yearly intervals. A reduction of 50% or greater in cyst diameter was considered successful.
Results:
The median followup period for the ethanol and polidocanol groups was 24.6 and 22.8 months, respectively. The successful outcome ratio of the polidocanol group was significantly higher (90% vs. 61%, respectively) than the one of the ethanol group (P = 0.003). The partial regression of the ethanol and polidocanol groups were 6% versus 7%, respectively. The failure ratio of the polidocanol group was significantly lower (3% vs. 33%, respectively) than that of the ethanol group (P = 0.004). Neither infectious complications nor hyperthermia occurred in all treated cases. However, these methods are not completely free from symptoms. All these symptoms disappeared few hours after the procedure.
Conclusions:
Polidocanol is a safe and effective sclerosing agent for renal cysts, with superior clinical results than ethanol. Therefore, polidocanol can be an alternative to ethanol in sclerotherapy of renal cysts.
Keywords: Ethanol, polidocanol, renal cyst, sclerotherapy
INTRODUCTION
Simple renal cyst is a nonneoplastic disease within the kidney (typically cortical) extending outside the parenchyma and distorting the renal contour. A distinct characteristic of simple cysts is their increased occurrence with aging.[1] They are quite common in adults, with an incidence estimated around the 20% of the population by the age of 40 years and 33% by the age of 60 years.[1,2]
Currently is not clear the etiopathogenic origin of this disease, that can be caused by congenital or acquired disorders. It is thought that renal cysts originate by the weakening of tubular basement membrane of the distal convoluted or collecting duct cells. As a results, a diverticula is formed that can subsequently develop in to a simple renal cyst. Simple renal cysts usually remain untreated requiring intervention only when they cause symptoms or undergo a complication.[3] Radiographic surveillance with ultrasounds is an effective method to manage patients with simple renal cysts.[4] Ultrasonography (US) is commonly used to exclude the possibility of benign or malignant pathology because the kidney is readily accessible for US examination. However, the characteristics appearance of simple renal cysts on computer tomography (CT) and magnetic resonance imaging allows the radiologist to make an accurate diagnosis.
The main indications to start treating the patient are: Size ≥9 cm, symptoms of mass effect (lumbar pain, sense of heaviness, renal colic), compression of the urinary and/or intestinal tract, hypertension.
Symptomatic simple renal cysts can be treated in various ways ranging from simple aspiration with or without the use of sclerotic agents, to surgical excision via open, endoscopic, laparoscopic or robotic surgery.[2,3] Several agents have been used to injure the epithelial cells of the cyst wall, such as pantopaque, povidone iodine, bismuth-phosphate, minocycline hydrochloride, acetic acid, tetracycline and ethanol, which is a effective sclerosant for simple renal cysts.[3] However, alcohol injection is associated with complications, including pain, fever and systematic reactions, e.g., intoxication and shock.[5]
Polidocanol has been used in the treatment of venous malformations.[6] It has also been reported to be an effective sclerosing agent for renal cysts owing to its faster and more complete sclerosing effect compared with ethanol.[7] In this study, we compared and valued efficacy and safety of percutaneous echoguided sclerotherapy (PES) using 3% polidocanol with that using 99% ethanol to treat patients with simple renal cysts.
MATERIALS AND METHODS
Between September 2006 and October 2011, PES was performed for 65 simple renal cysts. The patients subsumed 37 men and 28 women, with ages ranging from 52 years old to 81 years old (mean, 63 years old). The cysts were graded according to the Bosniak renal cyst classification system.[8]
All patients were studied with uro-CT before the procedure with sclerosing agent in order to exclude the presence of a connection between the urinary tract and the cysts.
Percutaneous echoguided sclerotherapy is performed under local anesthesia with 2% lidocaine hydrochloride. Surgical access is posterior and patient is supine and the puncture is performed below the 12th rib, about 10 cm away from vertebral spinal process.
Under ultrasonographic guidance the cyst was punctured using an 18 gauge needle and 10 ml aspired from the cyst cavity were sent to the laboratory for cytological and microbiological examination [Figure 1]. After fluid's aspiration inside the cyst, we inject the sclerosant agent, fixing a nephrostomy catheter (8F) closed to the skin. We ask the patient to change often his positions.
Figure 1.
Nephrostomy catheter position under ultrasound guidance
The patient's position is changed from supine, to prone and both lateral decubitus positions, for 10 min each, to enhance contact of the ethanol with the entire wall of the cyst. After 40 min we open the nephrostomy catheter and we removed it after about 5 h. For the patients treated with ethanol (n = 36) we inject 99% ethanol in an amount equal to 30% volume aspirate, never exceeding 60 ml. Instead, for the group of patients treated with polidocanol (n = 29), 2 ml of 3% polidocanol (Atossisclerol®) was mixed using 5 ml of physiological saline solution and injected after as much fluid as possible was aspired. Polidocanol suspension was injected into the cystic cavity according to cyst diameter (range 4-6 cm: 2 ml, 6-10 cm: 3 ml and >10 cm: 4 ml). All patients were hospitalized for about 7 h after the procedure. First voided urine was tested for haematuria.
Patients were followed up with an ultrasound examination at 4 months, 8 months, and then at yearly intervals. The reduction rate was estimated through the comparison of the cyst volume. A reduction of 50% or greater in cyst diameter was considered successful, a reduction between <50% and 30% was considered partial regression and a reduction <30% was considered failure during the followup of 24 months approximately.
Chi-square test was used to assess differences in the response between the two groups and Fisher's test if necessary. P < 0.05 was considered statistically significant.
RESULTS
The mean size of the renal cysts in the pretreatment imaging study was 82 mm (range 68-145 mm) for the ethanol group and 77 mm (range 57-138 mm) for polidocanol group. In 79% of cases we found individual cysts with sizes from 73 to 145 mm.
27 (41%) cysts were localized in the right kidney and 38 (59%) cysts in the left.
We found lower polar cysts in 41 (63%) cases, upper polar cysts in 17 (26%) cases, cysts in the middle of kidney in 6 (9%) cases and cysts near renal pelvis just in 1 case (2%).
Sclerotherapy was performed with ethanol in 55% (36/65) of cases and with polidocanol in the remaining 45% (29/65).
The correlation between cyst size and patient age was significant (P < 0.05).
The patients’ symptoms related to the presence of cysts were: A continuous lumbar pain and a sense of heaviness 63% (41/65), renal colic 6% (4/65), hypertension 7% (5/65) and constipation 2% (1/65). Otherwise in 14 patients (22%) the diagnosis was made during routine investigations for other reasons.
The median followup period for the ethanol and polidocanol groups was 24.6 and 22.8 months, respectively. In all patients, no abnormalities were detected in the cystic fluid after the bacteriological studies, the cytological examination was negative for neoplastic cells and biochemical analysis showed findings similar to those of the corresponding plasma.
The number of patients in the ethanol and polidocanol groups with successful outcome (reduction ≥50% in cyst diameter after followup of 2 years) were 22 and 26, respectively. The successful outcome ratio of the polidocanol group was significantly higher (90% vs. 61%, respectively) than the one of the ethanol group (P = 0.003). There were two patients each with partial regression (reduction between <50% and 30%) both in the ethanol and polidocanol groups. The partial regression of the ethanol and polidocanol groups were 6% versus 7%, respectively. The number of failures of the ethanol and polidocanol groups was 12 and 1, respectively. The failure ratio of the polidocanol group was significantly lower (3% vs. 33%, respectively) than that of the ethanol group (P = 0.004) [Figure 2].
Figure 2.
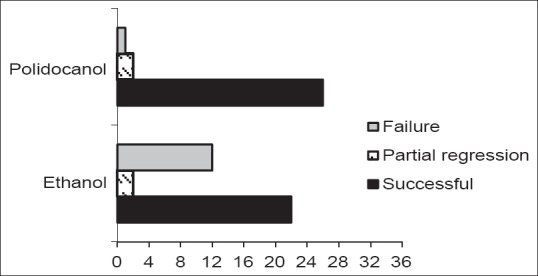
The number of patients in the ethanol and polidocanol groups with successful outcome, partial regression and failure
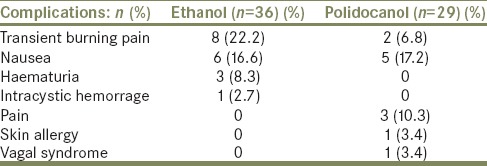
Neither infectious complications nor hyperthermia occurred in all treated cases. However, these methods are not completely free from complications, such as transient burning pain (n = 8), nausea (n = 6), haematuria (n = 3) and intracystic hemorrhage (n = 1) for ethanol group; pain with paracetamol or nonsteroidal anti-inflammatory analgesics request (n = 3), transient burning pain (n = 2), nausea (n = 5), allergic cutaneous reaction (n = 1) and vagal syndrome (n = 1) for polidocanol group [Table 1]. All these symptoms disappeared few hours after the procedure, while the followup urine analysis and ultrasonographic at 3 months was normal.
Table 1.
Percentage of complications in all treated cases with two agents
During the sclerotherapy, three patients of the polidocanol group developed mild flank pain that required medical management with analgesic drug during the filling of the cystic cavity with the sclerosant. However, the pain was not strong enough to interrupt the procedure.
The symptoms related to the presence of cysts after sclerotherapy in 2 years of followup were regressed completely in all patients except in four cases (treated with ethanol group) for persistent hypertension and in seven cases (six treated with ethanol and one treated with polidocanol) for persistent lumbar pain.
DISCUSSION
Simple renal cyst is a benign, common, and often asymptomatic disease. Treatment of simple renal cyst is indicated when the cyst is sufficiently large and causes complaints or when associated with complications.[2] In 2-4% of the cases, simple renal cysts become symptomatic due to enlargement or the development of a complication such as hemorrhage, infection or rupture. In addition, they may cause calyceal or renal pelvic obstruction.[9,10]
The association between simple renal cysts and the incidence of hypertension is controversial. Since the initial report about the development of hypertension by simple renal cyst by Ferrell and Young,[11] several authors reported cure or improvement of hypertension after decompression of a large cyst.[12] Other authors highlighted that the number and size of the cysts are independent risk factors from the prevalence of hypertension.[13] In our study, 7% of patients had hypertension, but only in one patient resolved after sclerotherapy treatment in the followup at 4 months.
Imaging guided percutaneous renal cyst aspiration with or without sclerosing therapy has been performed to treat simple renal cysts since 1970. The method has been considered minimally invasive, safe and low cost.[14] However, the procedure has not been standardized yet.
Simple aspiration without sclerotherapy has a low success rate and frequently recurs because of the fact that cysts are lined by secretory epithelium.[15] Sclerosing agents are believed to produce local inflammation on cysts’ luminal surface resulting in the adhesion of the cyst to the walls. Hanna and Dahniya[16] reported a recurrence rate of 80% in cysts treated with aspiration only and 32% in cysts after a single ethanol injection. Ethanol is the most widely used among the available sclerosant agents.[16,17] However, ethanol injection is associated with complications, including pain, fever, haematuria and systematic reactions, e.g. intoxication and shock.[5,16] In the present study, some patients reported a transient burning pain in 22.2% of cases and 8.3% haematuria. Nevertheless, all the side effects (tachycardia, nausea and cold sweats) were mild and transient, and they resolved without treatment. Alcohol intoxication is an extremely rare complication. Ethanol sclerotherapy of renal cysts may lead to measurable alcohol levels in patients’ blood. Increased levels were detected in cases with some hemorrhage into the cyst caused by the puncture.[18] Some researchers reported that multiple sessions of sclerotherapy are better than a single injection of sclerosant to reduce the recurrence of simple renal cysts. They also reported that 6 months after single-session sclerotherapy, the procedure might be safetly repeated to treat any symptomatic cyst that has reoccured.[5,19] In our department we handle failures as a partial regression or a reduction <30% or a persistence of symptoms for renal cysts after sclerotherapy with laparoscopic transperitoneal approach.[2,20] For the accuracy two patients previously subjected to sclerotherapy with ethanol have been treated with this surgical technique, with no evidence of difficulty for a tissue reaction around the cyst.
Recently Agarwal et al.[20] realized a prospective randomized study with 40 patients to evaluate aspiration and sclerotherapy versus laparoscopic deroofing in the management of symptomatic simple renal cysts. Sclerotherapy with polidocanol was an effective, safe, and minimally invasive therapeutic option for symptomatic simple renal cysts, with equal efficacy and lower morbidity and hospital stay in comparison with laparoscopic deroofing.
Polidocanol has been used in the treatment of venous malformations, aneurysmal bone cysts and hepatic cysts.[6,21] In literature, few studies have examined polidocanol as a sclerosing agent for renal cysts and all of them have a short followup except for the study at 26 months reported by Brunken et al.,[22]132 patients with 151 kidney cysts were treated by percutaneous sclerotherapy with polidocanol as sclerosing agent. In 56% of the cysts treated the cystic cavity disappeared completely, and in 30% the remaining volume was <10% of the initial one. The existing symptoms before intervention remained unchanged only in 4 (3.4%) patients.
In our study the failure ratio of the polidocanol group was significantly lower 3% than that of the ethanol group 33% (P = 0.004) and surgical reintervention was not necessary.
Most studies defined efficacy based on the change in cyst diameter and reduction in cyst volume. Some studies defined successful treatment as complete regression of the renal cyst or more than 70% reduction of cyst volume with no symptoms.[23] Our study defined a successful outcome as the complete disappearance of the cyst, or a ≥50% reduction in cyst diameter.
In this study, the overall success rate of complete and partial remission was significantly better in the polidocanol group than in the ethanol group (P = 0.003). This means that polidocanol is more effective as a sclerosing agent than ethanol, even if it is a more expensive procedure, but avoids another treatment (surgery or sclerosing). In addition, polidocanol was a safe sclerosant because there were no major procedure-related complications. One minor complication was the high rate of treatment-related mild flank pain in the polidocanol group. However, this problem was resolved with analgesics in all patients after few hours form the procedure.
Polidocanol is a safe and effective sclerosing agent for renal cysts, with clinical results superior to ethanol. However, despite having higher costs, three times more than ethanol, but with a pack of 5 vials (polidocanol 25 mg) I can treat more patients.
CONCLUSION
We can say that polidocanol may be a good alternative to ethanol for sclerotherapy of renal cysts, as it avoids possible systemic complications and reoperations on patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Skolarikos A, Laguna MP, de la Rosette JJ. Conservative and radiological management of simple renal cysts: A comprehensive review. BJU Int. 2012;110:170–8. doi: 10.1111/j.1464-410X.2011.10847.x. [DOI] [PubMed] [Google Scholar]
- 2.Kilciler M, Istanbulluoğlu MO, Basal S, Bedir S, Avci A, Ozgök Y. Finger assisted laparoscopic renal cyst excision: A simple technique. Urol J. 2010;7:90–4. [PubMed] [Google Scholar]
- 3.Terada N, Arai Y, Kinukawa N, Terai A. The 10-year natural history of simple renal cysts. Urology. 2008;71:7–11. doi: 10.1016/j.urology.2007.07.075. [DOI] [PubMed] [Google Scholar]
- 4.Gabr AH, Gdor Y, Roberts WW, Wolf JS., Jr Radiographic surveillance of minimally and moderately complex renal cysts. BJU Int. 2009;103:1116–9. doi: 10.1111/j.1464-410X.2008.08171.x. [DOI] [PubMed] [Google Scholar]
- 5.Falci-Júnior R, Lucon AM, Cerri LM, Danilovic A, Da Rocha PC, Arap S. Treatment of simple renal cysts with single-session percutaneous ethanol sclerotherapy without drainage of the sclerosing agent. J Endourol. 2005;19:834–8. doi: 10.1089/end.2005.19.834. [DOI] [PubMed] [Google Scholar]
- 6.Qiu Y, Chen H, Lin X, Hu X, Jin Y, Ma G. Outcomes and complications of sclerotherapy for venous malformations. Vasc Endovascular Surg. 2013;47:454–61. doi: 10.1177/1538574413492390. [DOI] [PubMed] [Google Scholar]
- 7.Ohta S, Fujishiro Y, Fuse H. Polidocanol sclerotherapy for simple renal cysts. Urol Int. 1997;58:145–7. doi: 10.1159/000282971. [DOI] [PubMed] [Google Scholar]
- 8.Israel GM, Bosniak MA. An update of the Bosniak renal cyst classification system. Urology. 2005;66:484–8. doi: 10.1016/j.urology.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Caglioti A, Esposito C, Fuiano G, Buzio C, Postorino M, Rampino T, et al. Prevalence of symptoms in patients with simple renal cysts. BMJ. 1993;306:430–1. doi: 10.1136/bmj.306.6875.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eknoyan G. A clinical view of simple and complex renal cysts. J Am Soc Nephrol. 2009;20:1874–6. doi: 10.1681/ASN.2008040441. [DOI] [PubMed] [Google Scholar]
- 11.Ferrell JI, Young RH. Hypertension caused by unilateral renal compression. J Am Med Assoc. 1942;118:711–2. [Google Scholar]
- 12.Lüscher TF, Wanner C, Siegenthaler W, Vetter W. Simple renal cyst and hypertension: Cause or coincidence? Clin Nephrol. 1986;26:91–5. [PubMed] [Google Scholar]
- 13.Chin HJ, Ro H, Lee HJ, Na KY, Chae DW. The clinical significances of simple renal cyst: Is it related to hypertension or renal dysfunction? Kidney Int. 2006;70:1468–73. doi: 10.1038/sj.ki.5001784. [DOI] [PubMed] [Google Scholar]
- 14.Choi YD, Ham WS, Kim WT, Cho KS, Lee JH, Cho SY, et al. Clinical experience of single-session percutaneous aspiration and OK-432 sclerotherapy for treatment of simple renal cysts: 1-year follow-up. J Endourol. 2009;23:1001–6. doi: 10.1089/end.2008.0137. [DOI] [PubMed] [Google Scholar]
- 15.Delakas D, Karyotis I, Loumbakis P, Daskalopoulos G, Charoulakis N, Cranidis A. Long-term results after percutaneous minimally invasive procedure treatment of symptomatic simple renal cysts. Int Urol Nephrol. 2001;32:321–6. doi: 10.1023/a:1017566723756. [DOI] [PubMed] [Google Scholar]
- 16.Hanna RM, Dahniya MH. Aspiration and sclerotherapy of symptomatic simple renal cysts: Value of two injections of a sclerosing agent. AJR Am J Roentgenol. 1996;167:781–3. doi: 10.2214/ajr.167.3.8751700. [DOI] [PubMed] [Google Scholar]
- 17.Xu XX, Du Y, Yang HF, Zhang Q, Li Y, Zee CS. CT-guided sclerotherapy with ethanol concentration monitoring for treatment of renal cysts. AJR Am J Roentgenol. 2011;196:W78–82. doi: 10.2214/AJR.10.4671. [DOI] [PubMed] [Google Scholar]
- 18.Cho DS, Ahn HS, Kim SI, Kim YS, Kim SJ, Jeon GS, et al. Sclerotherapy of renal cysts using acetic acid: A comparison with ethanol sclerotherapy. Br J Radiol. 2008;81:946–9. doi: 10.1259/bjr/41664864. [DOI] [PubMed] [Google Scholar]
- 19.Chung BH, Kim JH, Hong CH, Yang SC, Lee MS. Comparison of single and multiple sessions of percutaneous sclerotherapy for simple renal cyst. BJU Int. 2000;85:626–7. doi: 10.1046/j.1464-410x.2000.00508.x. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal M, Agrawal MS, Mittal R, Sachan V. A randomized study of aspiration and sclerotherapy versus laparoscopic deroofing in management of symptomatic simple renal cysts. J Endourol. 2012;26:561–5. doi: 10.1089/end.2011.0559. [DOI] [PubMed] [Google Scholar]
- 21.Brosjö O, Pechon P, Hesla A, Tsagozis P, Bauer H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop. 2013;84:502–5. doi: 10.3109/17453674.2013.850013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunken C, Pfeiffer D, Tauber R. Long term outcome after percutaneous sclerotherapy of renal cysts with polidocanol. Urologe A. 2002;41:263–6. doi: 10.1007/s00120-001-0144-4. [DOI] [PubMed] [Google Scholar]
- 23.Ham WS, Lee JH, Kim WT, Yu HS, Choi YD. Comparison of multiple session 99% ethanol and single session OK-432 sclerotherapy for the treatment of simple renal cysts. J Urol. 2008;180:2552–6. doi: 10.1016/j.juro.2008.08.026. [DOI] [PubMed] [Google Scholar]


