Abstract
Aim:
We aimed to evaluate the prevalence of Candida and Streptococci species in the oral cavity of Down syndrome patients.
Materials and Methods:
50 children/adolescents with Down syndrome with a karyotype of 47 XX, 21+ (female) and 47 XY, 21+ (male), and 50 normal children/adolescents were included in our study. Oral swab/saliva was used to culture and identify Candida and Streptococci species based on gram and periodic acid schiff staining.
Results:
Of the 50 study group samples, which were cultured, 37 (74%) showed growth of Candida colonies, whereas in the 50 control samples only 18 (36%) were positive for Candida growth. In 4 Sabouraud's dextrose agar culture slopes of the study group, more than one morphological type of colonies were observed. 23 out of 50 samples in our study group had Streptococcus viridans colonies. In the 23 samples positive for Streptococci 16 had many streptococcal colonies, and 7 had few streptococcal colonies in the primary culture. 32 out of 50 samples from the control group had S. viridans colonies. In these 32 samples positive for Streptococci, 29 had predominantly streptococcal colonies while 3 had few streptococcal colonies in the primary culture.
Conclusion:
The oral cavity is an environment heavily colonized by microorganisms, however, the Down syndrome patients run a greater risk of having opportunistic infections especially from Candida species. Hence to improve the quality of life of an individual with Down syndrome, it is necessary to diagnose and treat these infections by more frequent oral microbial assessment.
Keywords: Candida, culture, Down syndrome, microbes, Streptococcus
INTRODUCTION
Down syndrome (trisomy 21) is the most common and best known of all malformation syndromes. It is an easily recognized congenital, autosomal anomaly characterized by generalized growth deficiency and mental deficiency affecting 1 in 600-1 in 1000 live births. It accounts for about one-third of all mentally handicapped children.[1] Many systemic diseases are responsible for mortality of infants and children with Down syndrome. A compromised immune system is characteristic of these individuals, and it is this immunodeficiency that contributes to an increased susceptibility to infections. Various microorganisms are generally seen as normal commensals in the oral cavity, and these may become pathogenic under favorable conditions. Candida is one such endogenous pathogen, the prevalence of which has been reported to be 45-65% in healthy children[2] and 30-45% in healthy adults.[3] Opportunistic infections occur mainly when the host defenses are inadequate, candidiasis is the most common among them. Streptococci form a large portion of the resident oral micro flora. In the general population, half the isolates from the tongue and saliva are Streptococci, but in Down syndrome individual's oral streptococcal levels are relatively lower.[4] This probably contributes to a disturbance in the normal microbial homeostasis and promotes the growth of other commensal organisms like Candida. Here, we evaluated the candidial/Streptococci carriage and the various species of Candida/Streptococci isolated from the Down syndrome patients and correlated it with normal oral micro flora from health subjects.
MATERIALS AND METHODS
Fifty Down syndrome individuals were selected for this study based on a karyotype of 47 XX, 21+ (female) and 47 XY, 21+ (male). A total of 50 normal children/adolescents were selected as a control group. To isolate Candida and Streptococci, swab/saliva was used to culture and smear preparation for Gram stain and periodic acid schiff (PAS) stain.
Culture for Candida
Samples were taken from the oral cavity (dorsum of tongue) for each individual using a sterile cotton wool swab. Sabouraud's dextrose agar (SDA) slope with antibiotic (gentamicin/actidione) was used for the isolation of Candida. Antibiotics were used in order to eliminate bacterial contamination. If colonies were white or cream to yellow, dull/shiny, pasty yeast like a smear was prepared from the colony, with a drop of saline. Smear was then air-dried, heat fixed, and stained with crystal violet (simple stain) to confirm the presence of yeast. The isolates were then subjected to the following tests for identification of various species of Candida:
Germ tube test
Morphology on corn meal agar — for chlamydospore formation.
Carbohydrate fermentation test
Carbohydrate assimilation test
CHROMagar Candida culture characteristics
Smear and Gram stain
Smears taken from the oral cavity (tongue) were air-dried, heat fixed, and stained.
RESULTS
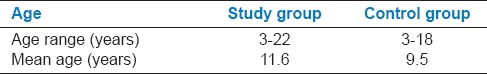
The study included a total of 100 (50 Down syndrome, 50 controls) subjects of both sexes. The study group of 50 Down syndrome individuals comprised of 37 males and 13 females in the age range of 3-22 years and the mean age of 11.6 years. The control group of 50 normal individuals comprised of 34 males and 16 females in the age range of 3-18 years and a mean age of 9.5 years [Tables 1 and 2].
Table 1.
Distribution of patients according to sex
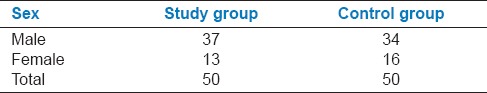
Table 2.
Distribution of patients according to age
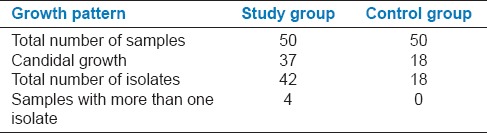
Of the 50 study group samples, which were cultured, 37 (74%) showed growth of Candida colonies, whereas in the 50 control samples only 18 (36%) were positive for Candida growth. In 4 SDA culture slopes of the study group, more than one morphological type of colonies were observed [Figure 1], and the colonies were considered as different isolates and subjected to further testing. A total of 42 isolates (1 isolate in 33 samples, 2 isolates in 3 samples, and 3 isolates in 1 sample) were obtained from the 37 culture positive samples, in the study group and 18 isolates were obtained from the 18 culture positive samples in the control group (1 isolate each in 18 samples) [Table 3].
Figure 1.

Sabouraud's dextrose agar slope showing more than one type of colony (white and cream)
Table 3.
Candidal growth pattern in the study group and control group
Smears taken from the oral cavity (tongue), for both the study group (50) and the control group (50) were stained with Gram stain [Figure 2] and PAS stain [Figure 3]. A tenfold increase in the positive staining for Candida was observed in the study group when compared to the control group [Table 4].
Figure 2.
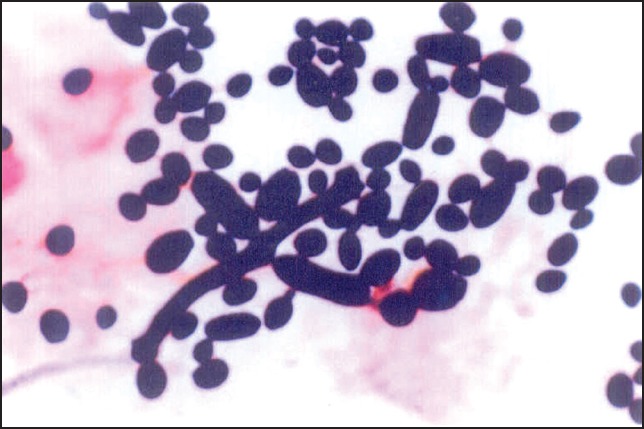
Gram-stained smear showing yeasts and pseudohyphae
Figure 3.
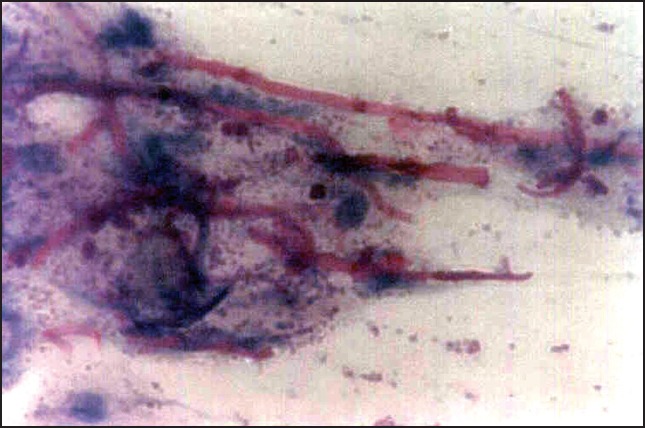
Periodic acid schiff stained smear showing candidal hyphae and yeasts
Table 4.
Distribution of positive candidal staining in study group and control group

Of the 42 isolates from the 37 culture positive samples, 31 were germ tube positive, whereas all the 18 culture positive samples of the control group were positive for germ tube formation [Figure 4]. Hence, 31 of the 42 isolates were Candida albicans in the study group, and all 18 isolates of the control group were C. albicans. This was further confirmed by chlamydospore formation on corn meal agar [Figure 5]. All the isolates that were germ tube positive produced chlamydospores on corn meal agar further confirming C. albicans. Of the 42 isolates and 37 culture positive samples, 31 isolates were C. albicans, 6 were Candida Tropicalis, and 5 were either Candida parapsilosis/Candida krusei/Candida glabrata. Of the 18 control samples, which were culture positive, all 18 were C. albicans. Further confirmation and speciation was done by the carbohydrate assimilation test and supplemented with growth on CHROMagar Candida.
Figure 4.

Germ tube formation by Candida albicans
Figure 5.
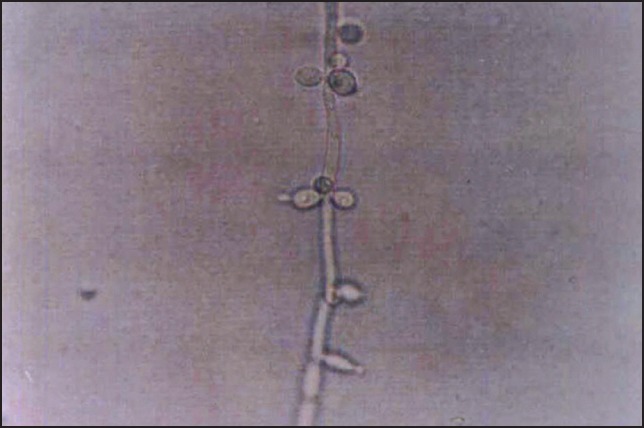
Chlamydospore formation by Candida albicans
Carbohydrate assimilation test
This test was done with sugars: Glucose, maltose, sucrose, lactose, galactose, cellulose, and trehalose [Table 5].
Table 5.
Carbohydrate assimilation reactions for different Candida species
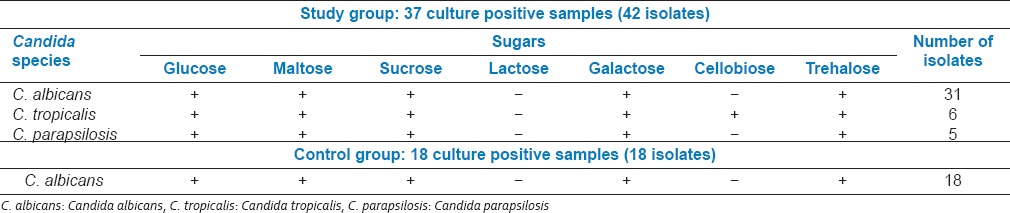
Of the 37 samples that were culture positive in the study group, 31 (84%) samples had isolates of C. albicans, 6 (16%) samples had isolates of C. tropicalis and 5 (14%) had isolates of C. parapsilosis. 4 samples of the study group were colonized with more than one type of species. Of the 18 control samples that were culture positive all 18 isolates were C. albicans.
Chromagar Candida
Growth of all culture positive samples on CHROMagar Candida was observed for the characteristic colony color [Table 6].
Table 6.
CHROMagar Candida culture characteristics for different Candida species
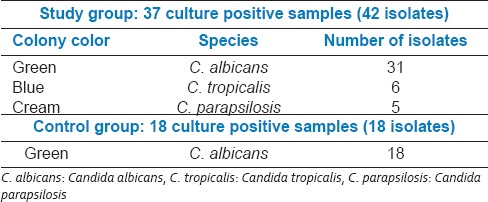
In the CHROMagar Candida, study sample showed 31 isolates of C. albicans, 6 of C. tropicalis, and 5 of C. parapsilosis [Figure 6]. In the control samples, all 18 of the isolates were C. albicans, thus confirming the earlier results.
Figure 6.
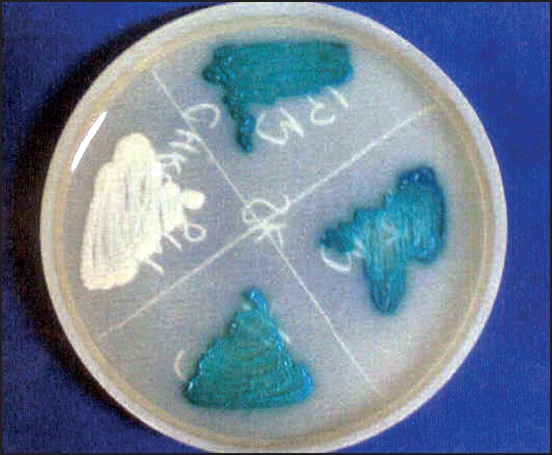
CHROMagar Candida showing green colonies of Candida albicans and cream colonies of Candida parapsilosis
Culture for identification of streptococci
Saliva samples collected from both the study group (50%) and the control group (50) after inoculation on chocolate agar, and incubation at 37°C for 24 h, the growth of alpha-hemolytic colonies were identified [Figure 7]. The colonies were checked for optochin resistance to classify it as Streptococcus viridans group [Table 7].
Figure 7.
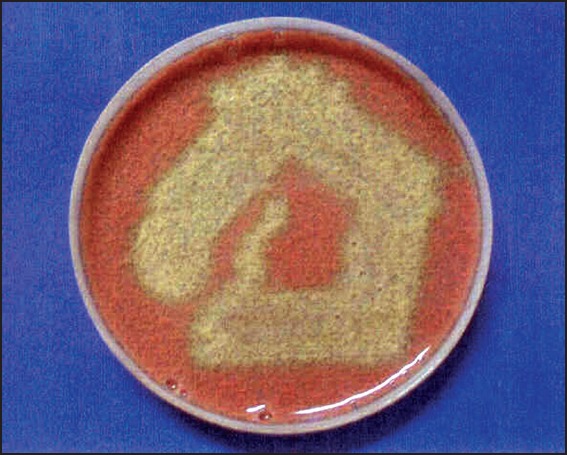
Primary culture showing predominantly alpha hemolytic colonies
Table 7.
Growth pattern of Streptococcus

The growth of alpha hemolytic S. viridans colonies in the primary culture was classified as in Table 8.
Table 8.
Distribution of viridans group of Streptococci in control group and study group
Twenty-three out of 50 samples from study group had S. viridans colonies. In the 23 samples positive for Streptococci 16 had many streptococcal colonies, and 7 had few streptococcal colonies in the primary culture. Of the 50 control samples, 32 had S. viridans colonies. In the 32 samples, which were positive for Streptococci, 29 had predominantly streptococcal colonies while 3 had few streptococcal colonies in the primary culture.
Association of Candida with streptococci
The presence of Candida with Streptococci was compared in both the study group and the control group [Table 9].
Table 9.
Comparison of candidal and streptococcal growth patterns in study group and control group
Candidal and streptococcal colonization was seen in 14 (28%) individuals in the study group and 6 (12%) individuals in the control group. Candida colonization alone in the absence of streptococcal colonization was seen in 23 (46%) individuals of the study group and 12 (24%) of the control group. Thus showing that candidal colonization occurred to a greater extent in the absence of Streptococci (P < 0.01, z = 2.96).
Our results showed that in the study group (74%) Candida colonization was comparatively higher when compared, with the control group (36%). Of the 37 culture-positive samples, 42 isolates were obtained, four samples had more than one isolate (one sample with three isolates, three samples with two isolates). The species isolated in the study group out of the 37 culture-positive samples were predominantly C. albicans (84%) followed by C. tropicalis (16%) and C. parapsilosis (14%). In the control group, only C. albicans were isolated. Thus, out of the total 50 Down syndrome subjects, 31 (62%) showed colonization with C. albicans, 6 (12%) with C. tropicalis, and 5 (10%) with C. parapsilosis. In the comparative study of Candida and Streptococci, Candida colonization was predominantly seen in the absence of Streptococci.
Oral manifestations
The oral findings of all the children and adolescents of the study group were recorded [Figures 8–10]. Table 10 shows in an ascending order the frequency of various findings.
Figure 8.
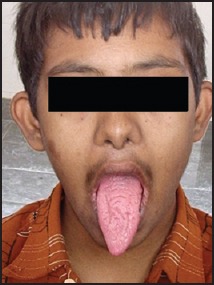
12-year-old male showing fissured tongue
Figure 10.
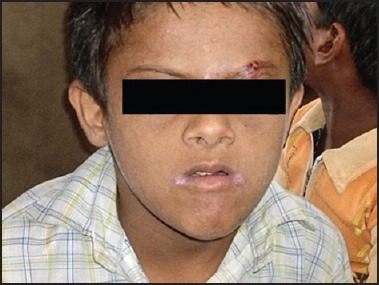
Angular cheilitis with prognathic mandible
Table 10.
Distribution of oral findings in down syndrome subjects
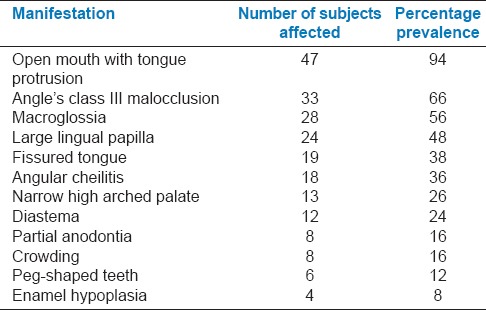
Figure 9.
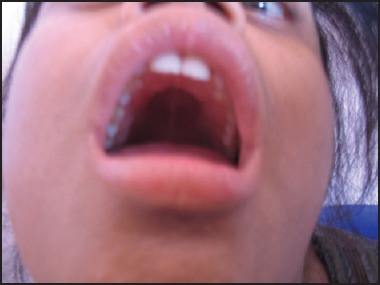
High arched palatal vault
In the 50 Down syndrome individuals, 17 (34%) of them showed an erythematous or a pseudomembranous lesion on the tongue (12 - erythematous, 5 - pseudomembranous), indicating possible oral candidiasis.
DISCUSSION
Down syndrome individuals are reported to exhibit an increased susceptibility to infections due to various factors such as increased activity of superoxide dismutase,[5,6] increased activity of glutathione peroxidase,[6] decreased levels of myeloperoxidase in leukocytes,[7] defective neutrophil chemotaxis,[8] abnormal serum immunoglobulin patterns[9] and disturbed cell-mediated and humoral immunity.[10]
The most common opportunistic infection affecting the oral cavity is candidiasis. The present study determined the prevalence of oral Candida carriage in Down syndrome children and adolescents. Of the 37 individuals in the study group who exhibited Candida colonization, only 14 showed colonization with Streptococci while 23 remained negative and showed no streptococcal colonization. Thus, we concluded that the majority of the individuals showed Candida colonization when there was no concomitant streptococcal colonization. The increased Candida carriage in Down syndrome individuals could probably be attributed to the relatively lower streptococcal levels. The increased susceptibility to Candida infection may be a result of the disturbance in the microbial homeostasis. However, other factors such as a compromised immune system, poor oral hygiene, malnourishment and the like cannot be completely ruled out and are also expected to play a significant role. It may be also possible that the Candida and Streptococci may produce mutually inhibitive factors in the form of toxins or enzymes, thus producing this inverse relationship.
The most common predisposing factor resulting in candidiasis is the presence of a suppressed immune status, thus this infection is seen in a higher frequency in Down syndrome subjects, where a compromised immune status is the hallmark.[11] In addition to this, a variety of local factors may also play a role, the most common being an altered intra oral milieu. As observed in this study, it may be assumed that the presence of microbial imbalance in the oral cavity and the occurrence of fissured tongue could be some major local factors contributing to the increased Candida carriage in Down syndrome individuals.
CONCLUSION
The oral cavity is an environment heavily colonized by microorganisms, which are generally in harmony with each other. This relationship seems to be related to the immune status of the individual. The Down syndrome subjects run a greater risk of having opportunistic infections with a possibility of systemic spread, and the associated morbidity and mortality is also higher. Hence, to improve the quality-of-life of an individual with Down syndrome, it becomes necessary to diagnose and treat these infections when they still remain localized.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Regezi JA, Sciubba JJ, Jordan RC. 4th ed. USA: Elsevier Science; 2003. Oral Pathology: Clinical Correlations; pp. 358–9. [Google Scholar]
- 2.Berdicevsy I, Ben-Aryeh H, Sazargel R. Oral Candida in children. Oral Surg Oral Med Oral Pathol. 1980;57:37–40. doi: 10.1016/0030-4220(84)90257-3. [DOI] [PubMed] [Google Scholar]
- 3.Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002;78:455–9. doi: 10.1136/pmj.78.922.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SR, Kwon HK, Song KB, Choi YH. Dental caries and salivary immunoglobulin A in Down syndrome children. J Paediatr Child Health. 2004;40:530–3. doi: 10.1111/j.1440-1754.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- 5.Mattei JF, Baeteman MA, Baret A, Ardissone JP, Rebuffel P, Giraud F. Erythrocyte superoxide dismutase and redox enzymes in trisomy 21. Acta Paediatr Scand. 1982;71:589–91. doi: 10.1111/j.1651-2227.1982.tb09479.x. [DOI] [PubMed] [Google Scholar]
- 6.Sinet PM, Michelson AM, Bazin A, Lejeune J, Jerome H. Increase in glutathione peroxidase activity in erythrocytes from trisomy 21 subjects. Biochem Biophys Res Commun. 1975;67:910–5. doi: 10.1016/0006-291x(75)90763-9. [DOI] [PubMed] [Google Scholar]
- 7.Björkstén B, Marklund S, Hägglöf B. Enzymes of leukocyte oxidative metabolism in Down's syndrome. Acta Paediatr Scand. 1984;73:97–101. doi: 10.1111/j.1651-2227.1984.tb09905.x. [DOI] [PubMed] [Google Scholar]
- 8.Izumi Y, Sugiyama S, Shinozuka O, Yamazaki T, Ohyama T, Ishikawa I. Defective neutrophil chemotaxis in Down's syndrome patients and its relationship to periodontal destruction. J Periodontol. 1989;60:238–42. doi: 10.1902/jop.1989.60.5.238. [DOI] [PubMed] [Google Scholar]
- 9.Avanzini MA, Söderström T, Wahl M, Plebani A, Burgio GR, Hanson LA. IgG subclass deficiency in patients with Down's syndrome and aberrant hepatitis B vaccine response. Scand J Immunol. 1988;28:465–70. doi: 10.1111/j.1365-3083.1988.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 10.Annerén G, Magnusson CG, Lilja G, Nordvall SL. Abnormal serum IgG subclass pattern in children with Down's syndrome. Arch Dis Child. 1992;67:628–31. doi: 10.1136/adc.67.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scully C. Down's syndrome and dentistry. Dent Update. 1976;3:193–6. [PubMed] [Google Scholar]


