Abstract
Background:
Predentin, the unmineralized organic matrix is important in maintaining the integrity of dentin. It is usually thick where active dentinogenesis occurs. A wide variation in its thickness is reported. Hence, we determined the variation in predentin thickness at various sites of different age groups.
Materials and Methods:
60 freshly extracted teeth (maxillary and mandibular first premolars) were divided into three groups with 20 teeth in each as, Group 1 - teeth with incomplete root formation (age <16 years), Group 2 - teeth with complete root formation (aged between 16 and 30 years), Group 3 - teeth of patients aged above 30 years. The teeth were fixed, decalcified and sections of 6 μ thickness were obtained, and stained with hematoxylin and eosin. The distance between the odontoblastic cell layers of the pulp to the border line of the dentin was considered for the measurement of the predentin thickness. A total of nine sites were considered for each specimen.
Results:
The present study revealed varied mean predentin thickness at all nine sites in all three age groups. Maximum and minimum thickness was observed at the apex and pulp floor respectively in all three groups. There was a statistical significant difference in predentin thickness between groups 1 and 3 and 2 and 3.
Conclusion:
The predentin thickness in the first group gradually increased toward the growing end near the apex, while it was relatively constant in the second group and increased overall thickness at all the sites in the third group. A notable finding was a linear increase with age in width of the predentin and the thickness vary as a function of odontoblastic activity during different stages of tooth development.
Keywords: Age groups, dentinogenesis, odontoblasts, predentin thickness
INTRODUCTION
Dentin is an avascular mineralized tissue that forms the bulk of the tooth. It is a live tissue, not normally exposed to the oral environment. It provides the general form of the tooth since it forms slightly before enamel.[1,2] Dentin is sensitive and is formed throughout life increasing its thickness at the expense of the dental pulp. This is reflected in the presence of an unmineralized layer of dentin matrix at the pulpal surface known as predentin.[3] Predentin is the newly formed dentin before calcification and maturation. It is the innermost portion of dentin and is located adjacent to pulpal tissues. It is usually thick where active dentinogenesis occurs. Its presence is necessary for maintaining the integrity of the dentin since its absence appears to leave the mineralized dentin vulnerable to resorption by odontoclasts.[4] However, there is a wide variation in the reported thickness of the predentin.[2,3,4,5] The thickness also varies in systemic conditions such as Vitamin D, calcium deficiency, hypophosphatemic Vitamin D resistant rickets and in persons suffering from chronic renal failure.[6,7,8] Variation is also noted with varying age and site. As dentin is formed throughout the life of an individual, the predentin thickness might also undergo various changes at different stages of life. The present study was designed to determine the thickness of predentin at various sites of the tooth at three age intervals and to compare the differences between them if any.
MATERIALS AND METHODS
The study sample consisted 60 vital maxillary and mandibular first premolars, extracted for various reasons other than dental caries. The sample was further divided into three groups with 20 teeth in each as shown. Group 1: Teeth with incomplete root formation (age <16 years), Group 2: Teeth with complete root formation (aged between 16 and 30 years), Group 3: Teeth of patients aged above 30 years.
The freshly extracted teeth were rinsed in normal saline solution and were preserved in 10% neutral buffered formalin. Holes were drilled at cementoenamel junction (CEJ) using aerator hand piece to allow formalin solution to enter the pulp cavity of the tooth in order to fix the pulp tissue. Formalin fixation was done for 24-48 h. The teeth were then decalcified using mixture of equal amounts of 10% formic acid and 10% nitric acid (decalcifying agent) for a minimum of 8 days. After the teeth were decalcified they were trimmed carefully on the mesial and distal aspects parallel to the estimated mid sagittal line of the root using B P blade, thus providing a site reference for parallel longitudinal section in the bucco lingual direction. The decalcified teeth were then processed and embedded in paraffin. Four to five serial sections of 6 μ thickness were taken from each paraffin embedded tissue block using soft tissue microtome. These consecutive sections of each decalcified tooth were then stained with hematoxylin and eosin (H and E) stain to determine the predentin thickness. The odontoblastic cell layer is very essential for the presence of predentin. Maintaining the vitality of teeth was of major consideration without which the predentin could not be appreciated. Hence, the study included only the teeth with vital pulp.
Method of determining the predentin thickness
The stained sections were viewed under Trinocular research microscope (Olympus BX51). In each slide, nine different sites were identified and numbered [Figure 1]. This was done to represent all the areas of the tooth. Images of the predentin width were captured using a 3 chip CCD camera (Proview, Media Cybernetics) with ×40 apochromatic objective. All captured images were stored in a hard disk, and measurements (in microns) were carried out on these images using the tools of the Image – Proplus software Version 4.1.0.0 (Media Cybernetics USA). For measurements the distance between the odontoblastic cell layers of the pulp to the borderline of the dentin was considered [Figure 2].
Figure 1.
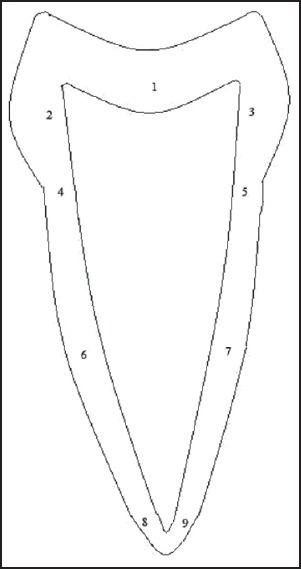
Illustrating nine different sites of measurement. 1 – Pulp floor 2 – Coronal buccal wall, 3 – Coronal lingual wall, 4 – CEJ of buccal wall, 5 – CEJ of lingual wall, 6 – Radicular buccal wall which is equidistant from both CEJ and apex 7 – Radicular lingual wall which is also equidistant from both CEJ and apex, 8 – Apical area on the buccal wall, 9 – Apical area on the lingual wall
Figure 2.
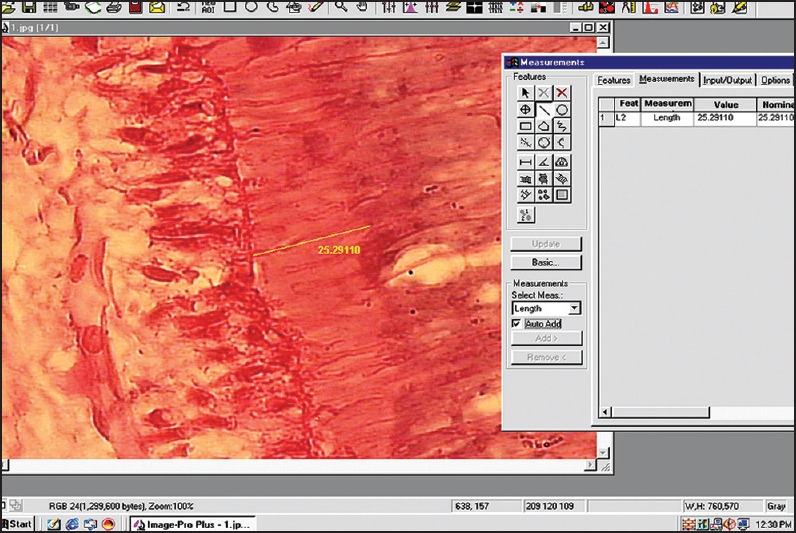
Photograph of measured predentin thickness as seen on image analysis software window
Statistical analysis
Statistical constants like mean, standard deviation and range values of all nine measurements of three different groups were first computed. One-way ANOVA was used for simultaneous multiple group comparison followed by Student's t-test for group wise comparisons. P = 0.05 or less was considered for statistical significance.
RESULTS
Predentin thickness at specific sites in various age groups is summarized in Graph 1.
Graph 1.
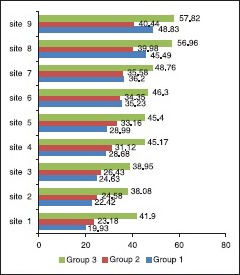
Mean predentin thickness at nine sites
Pulpal floor (site 1); mean values (μm) with standard deviation of all three groups was – group I (19.93 ± 3.5), group 2 (23.18 ± 4.6), group 3 (41.90 ± 18.5). The measurements ranged between 14.87-27.28, 11.19-31.39 and 21.88-86.51 among groups 1, 2 and 3 respectively.
Coronal buccal wall (site 2); the mean values (μm) with standard deviation was – group I (22.42 ± 3.7), group II (24.58 ± 5.4), group III (38.08 ± 11.7). The measurements ranged between 16.52-29.96, 11.25-36.92 and 20.01-57.51 among groups 1, 2 and 3 respectively.
Coronal lingual wall (site 3); mean values (μm) with standard deviation was – group I (24.63 ± 6.0), group II (26.43 ± 6.4), group III (38.95 ± 13.9). The measurements ranged between 16.14-38.75, 11.46-39.23 and 19.25-69.55 among groups 1, 2 and 3 respectively.
Buccal wall - CEJ (site 4); the mean values (μm) with standard deviation of all three groups was – group I (28.68 ± 4.8), group II (31.12 ± 8.2), group III (45.17 ± 11.6). The measurements ranged between 21.90-43.06, 20.60-57.35 and 20.60-66.10 among groups 1, 2 and 3, respectively.
Lingual wall - CEJ (site 5); mean values (μm) with standard deviation was – group 1 (28.99 ± 4.5), group 2 (33.16 ± 6.5), group 3 (45.40 ± 10.9). The measurements ranged between 21.90-43.06, 20.60-57.35 and 20.60-66.10 among groups 1, 2 and 3 respectively.
Radicular buccal wall, equidistant from both CEJ and apex (site 6); mean values (μm) with standard deviation was – group 1 (35.23 ± 2.4), group 2 (34.35 ± 6.5), group 3 (46. 30 ± 12.5). The measurements ranged between 21.90-43.06, 20.60-57.35 and 20.60-66.10 among groups 1, 2 and 3 respectively.
Radicular lingual wall, equidistant from both CEJ and apex (site 7); the mean values (μm) with standard deviation of all three groups was group 1 (36.20 ± 4.8), group 2 (35.58 ± 7.3), group 3 (48.76 ± 13.4). The measurements ranged between 21.90-43.06, 20.60-57.35 and 20.60-66.10 among groups 1, 2 and 3 respectively.
Apical area on the buccal wall (site 8); the mean values (μm) with standard deviation was – group 1 (45.49 ± 7.6), group 2 (39.98 ± 8.0), group 3 (56.96 ± 16.1). The measurements ranged between 21.90-43.06, 20.60-57.35 and 20.60-66.10 among groups 1, 2 and 3 respectively.
Apical area on the lingual wall (site 9); mean values (μm) with standard deviation was – group 1 (48.83 ± 6.8), group 2 (40.44 ± 8.4), group 3 (57.82 ± 16.2). The measurements ranged between 21.90-43.06, 20.60-57.35 and 20.60-66.10 among groups 1, 2 and 3 respectively.
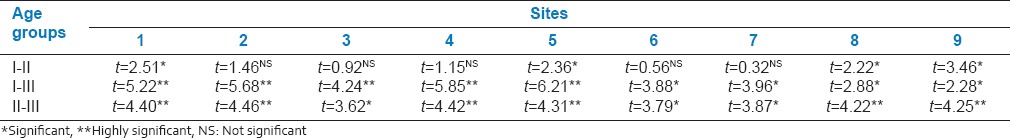
When the difference in predentin thickness between groups 1 and 2 were compared to all the specified sites, a statistically significant difference (P < 0.05) was noted at pulpal floor (site 1), CEJ of lingual wall (site 5) and apical area of both buccal and lingual wall (sites 8 and 9 respectively). When the difference in predentin thickness between groups 1 and 3 and between 2 and 3 groups were compared, a statistically significant difference was noted at all the nine sites. The mean predentin thickness Increased in thickness progressively from the crown to the apex, with a maximum thickness at the apical area and with age.
DISCUSSION
Predentin is the innermost portion of the dentin, which is not mineralized and located adjacent to pulp tissues. It is regarded as a zone of formation and maturation of the scaffolding collagen web of the dentin organic matrix.[9] Predentin in H and E stained sections is similar to osteoid in bone and stains less intensely than mineralized dentin. It appears pale owing to the differences in the composition of the matrix.[2] Various pathological, systemic and metabolic conditions do affect the predentin thickness. Factors like caries,[10] High sucrose diet[11] trauma, extreme heat produced by cutting dentin alter the thickness.[12] The predentin differs from mineralized dentin which contains exclusively type I collagen, whereas predentin contains type I, type III and type V collagen.[12] It is regarded as the metabolic equivalent of osteoid in bone.[8] Its presence is necessary for maintaining the integrity of the dentin since its absence appears to leave the mineralized dentin vulnerable to resorption by odontoclasts, which leads to internal resorption.[4]
Predentin thickness of various age groups
Statistically significant variation in predentin thickness was observed at all nine sites among all the three age groups. Previous studies have indicated highest thickness at the growing end next to the apex (mean value 40.4 μm) and we observed a similar finding in group I (teeth with incomplete root formation), wherein the widening of predentin occurred at the apical region compared with other sites (mean value 45.49 μm), which could be due to elevated odontoblastic activity during dentinogenesis.[13] However at the coronal region, where the primary dentin is completely formed the predentin width is reduced (mean value of 14.8 μm), which is consistent with out observations of mean predentin thickness of 19.93 μm at the pulpal floor.
The predentin thickness remained constant in group 2 (teeth with complete root formation), which is concurrent to previous report.[14]
In group 3 (above 30 years) the maximum thickness was found at the apical area (mean value 56.96 μm), which was in contrast to previous study reporting the widening of predentin only at the cervical and central areas particularly after the age of 39 years.[14]
Comparison of predentin thickness between different age groups
Comparing groups 1 and 2 statistically significant difference in predentin thickness was observed only at the pulpal floor, CEJ of lingual wall and at the apical areas of both buccal and lingual wall. The predentin thickness was relatively constant at all nine sites in group 2, whereas in group 1 the width varied, with increase in thickness towards apical region [Table 1]. When compared between groups 1 and 3 a statistically significant difference in predentin thickness was observed in all the nine sites. In both the groups the maximum thickness was at the apical region (mean value 45.49 μm in group 1 and 56.96 μm in group 3). Elevated odontoblastic activity due to active dentinogenesis could be the reason for differences in-group 1 while diminished rate of calcification was the cause in group 3. However, there was increase in overall mean value of predentin thickness at all nine sites in group 3 [Table 1]. Groups 2 and 3, when compared showed a statistically significant difference in predentin thickness at all nine sites, with a constant thickness in second group and an overall increase in thickness in third group.
Table 1.
Comparison of predentin thickness between three age groups
CONCLUSION
Our study showed the mean predentin thickness at specific sites in three age groups, and the difference in width between all the three groups, which to best of our knowledge has not been reported previously. A notable finding was the linear increase in the width of the predentin with age, although it always remained wider at the apical area. It could be concluded that the thickness of the predentin layer varies as a function of dentinogenic activity during different stages of development of teeth.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Avery JK. 3rd ed. Germany: Library of Congress Cataloging– in– Publication Data; 2002. Oral Development and Histology; pp. 90–1. [Google Scholar]
- 2.Avery JK. Dentin. In: Bhaskar SN, editor. Orban's Oral Histology and Embryology. 11th ed. Singapore: Harcourt Asia Pte Ltd; 2001. p. 110. [Google Scholar]
- 3.Berkovitz BK, Holland GR, Moxham BJ. 3rd ed. Edinburgh: Mosby International Limited; 2002. Oral Anatomy, Embryology and Histology. [Google Scholar]
- 4.Torneck CD. Dentin pulp complex. In: Tencate AR, editor. Oral Histology. 5th ed. Singapore: Harcourt Asia Pte Ltd; 1999. p. 152. [Google Scholar]
- 5.Ruch JV. Tooth morphogenesis and differentiation. In: Linde A, editor. Dentin and Dentinogenesis. Boca Raton: CRC Press; 1984. [Google Scholar]
- 6.Engström C, Linde A, Magnusson BC. Odontoblast metabolism in rats deficient in vitamin D and calcium I: A histochemical survey. J Oral Pathol. 1977;6:359–66. doi: 10.1111/j.1600-0714.1977.tb01801.x. [DOI] [PubMed] [Google Scholar]
- 7.Larmas M, Hietala EL, Similä S, Pajari U. Oral manifestations of familial hypophosphatemic rickets after phosphate supplement therapy: A review of the literature and report of case. ASDC J Dent Child. 1991;58:328–34. [PubMed] [Google Scholar]
- 8.Wysocki GP, Daley TD, Ulan RA. Predentin changes in patients with chronic renal failure. Oral Surg Oral Med Oral Pathol. 1983;56:167–73. doi: 10.1016/0030-4220(83)90284-0. [DOI] [PubMed] [Google Scholar]
- 9.Linde A. Dentin mineralization and the role of odontoblasts in calcium transport. Connect Tissue Res. 1995;33:163–70. doi: 10.3109/03008209509016997. [DOI] [PubMed] [Google Scholar]
- 10.Mjor IA. China: Quintessence Publishing Co., Inc; 2002. Pulp-Dentin Biology in Restorative Dentistry; p. 62. [PubMed] [Google Scholar]
- 11.Autio J, Hietala EL, Larmas M. The effect of two sucrose diets on formation of dentin and predentin in growing rats. Acta Odontol Scand. 1997;55:292–5. doi: 10.3109/00016359709114966. [DOI] [PubMed] [Google Scholar]
- 12.Okiji T. Pulp as a connective tissue. In: Hargreaves KM, Goodis HE, editors. Seltzer and Bender's Dental Pulp. Chicago: Quintessence Publishing Co., Inc; 2002. p. 100. [Google Scholar]
- 13.Couve E. Changes in the predentin thickness and mineralization front configuration in developing human premolars. Acta Anat (Basel) 1987;130:324–8. doi: 10.1159/000146464. [DOI] [PubMed] [Google Scholar]
- 14.Nitzan DW, Michaeli Y, Weinreb M, Azaz B. The effect of aging on tooth morphology: A study on impacted teeth. Oral Surg Oral Med Oral Pathol. 1986;61:54–60. doi: 10.1016/0030-4220(86)90203-3. [DOI] [PubMed] [Google Scholar]


