Abstract
Background:
Use of opioids for perioperative analgesia is associated with sedation, respiratory depression and postoperative nausea and vomiting. N-methyl-D-aspartate receptor antagonist such as ketamine has both analgesic and antihyperalgesic properties. We studied the effect of intraoperative infusion of low-dose ketamine on postoperative analgesia and its management with opioids.
Materials and Methods:
A total of 80 patients scheduled for open cholecystectomy under general anesthesia were randomly allocated into two equal groups in a randomized double-blinded way. The general anesthetic technique was standardized in both groups. Group K patients (n = 40) received bolus of ketamine 0.2 mg/kg intravenously followed by an infusion of 0.1 mg/kg/h before skin incision, which was continued up to the end of surgery. Similar volume of saline was infused in Group C (n = 40). The pain score at different intervals and cumulative morphine consumption over 24 h was observed. Secondary outcomes such as hemodynamic parameters, patient satisfaction score and incidences of side effects were also recorded.
Results:
Intraoperative infusion of low-dose ketamine resulted in effective analgesia in first 6 h of the postoperative period, which was evident from reduced pain scores and reduced opioid requirements (P = 0.001). The incidence of side effects and patient satisfaction were similar in both groups.
Conclusion:
Intraoperative low-dose ketamine infusion provides good postoperative analgesia while reducing need of opioid analgesics, which must be considered for better management of postoperative analgesia.
Keywords: Ketamine, morphine, postoperative analgesia
INTRODUCTION
Pain triggers complex biochemical and physiological stress response leading to impairment of pulmonary, immunological and metabolic function,[1] hence use of analgesics postsurgery is a common practice. Opioids are the mainstay of postoperative pain relief however exposure to large doses of opioids can result in acute tolerance, hyperalgesia and side effects such as sedation, respiratory depression, nausea and emesis. Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist that has demonstrated excellent analgesic activity even at subanesthetic doses.[2] Recent studies have shown the role of NMDA-receptors in central nervous system facilitation of pain processing.[3] NMDA-receptor antagonist, such as ketamine when administered in subanesthetic doses prevents the development of central sensitization, hyperalgesia and opioid resistance.[4,5,6] Hence, we hypothesized that intraoperative low-dose ketamine infusion may have opioid sparing effect in the postoperative period and studied the impact of intraoperative low-dose ketamine at induction followed by infusion on postoperative pain and opioids consumption.
MATERIALS AND METHODS
Following approval from the Institutional Ethics Committee (Trg-2013/3575 Dated 30/01/14) and written informed consent, 80 patients of either sex belonging to American society of anesthesiologist physical status I and II, age 21-50 years, scheduled to undergo elective cholecystectomy under general anesthesia at our University Hospital were enrolled in the study. The study was designed as a prospective, randomized, double-blinded, placebo-controlled study. The exclusion criteria for enrolment in the study were patients on chronic analgesic medication, ketamine, morphine and tramadol or substance abuse, having allergy to drug used, with preexisting pulmonary, cardiac, hepatic, renal, neurological, endocrinal, psychological disorders, myopathy and pregnant women. During the preanesthetic assessment, patients were explained about visual analog scale (VAS) consisting of a 100-mm line with 0 equalling “no pain” and 100 equalling “worst possible pain.”
The patients were divided randomly into two groups of 40 patients each. Patients in Group K were administered ketamine infusion of 0.1 mg/kg/h following bolus 0.2 mg/kg of ketamine and patients in Group C were infused with normal saline in similar volume and rate. Continuous intravenous (IV) infusion of ketamine or normal saline following bolus dose was started before skin incision, intraoperatively and continued until completion of skin closure via infusion pump, infuser (Perfusor compact, Braun, Germany).
A computer-generated randomization list was created prior to commencement of the study. This list was enclosed in serially numbered, sealed envelopes. On the morning of surgery, one of the Anesthesiologists not involved with the study opened the sealed envelope and prepared the drug solution. The study drug was loaded into a 50 ml syringe (ketamine concentration was 2 mg/ml), the placebo syringe contained normal saline and was labeled similar to the study drug.
The patients were kept nil by mouth for at least 6 h prior to surgery and were monitored continuously by electrocardiogram, noninvasive blood pressure, and peripheral oxygen saturation and end tidal carbon di oxide using multipara monitor (Nihon kohden, Japan) throughout the surgical procedure. The IV access was secured with 18 G cannula on the dorsum of the hand for IV fluid management. All the patients were premedicated with glycopyrolate 0.2 mg IV. Induction was achieved using IV propofol (2 mg/kg) and tramadol (2 mg/kg). Atracurium besylate (0.5 mg/kg) was used to facilitate insertion of appropriate size of the endotracheal tube. Anesthesia was maintained with isoflurane and nitrous oxide in oxygen (FiO2 = 0.4). All patients received fluid in the form of Ringer lactate IV as per standardized calculation. The residual neuromuscular blockade was reversed using neostigmine (0.05 mg/kg) and glycopyrrolate (0.02 mg/kg), and intubation was removed upon complete recovery of the reflexes. All the investigators involved in patient management and data collection were unaware of the group to which the patient was assigned.
The patients were transferred to the postanesthesia unit and pain scores at rest were noted immediately on arrival and 2, 4, 6, 12 and 24 h postsurgery using VAS. The patients were observed in the postoperative care unit for 4 h, and rescue analgesia was provided in the form of IV morphine 0.05 mg/kg boluses if the pain score was >30. The total number of patients requiring morphine and the total amount of morphine given in 24 h were noted in both the groups. Thereafter, the patients were shifted to ward and were given acetaminophen 1 g IV t.i.d. Side effects such as nausea, vomiting, sedation, hallucination, respiratory depression (respiratory rate <10/min.) and emergence reaction were recorded. Nausea and vomiting were evaluated using categorical score (0 = none, 1 = slight, 2 = moderate, 3 = severe/request treatment) When moderate or severe nausea or vomiting was present, we administered 0.1 mg/kg of IV ondansetron. Patients were also asked to rate their overall satisfaction on a five-point scale (very satisfied, satisfied, neutral, dissatisfied, very dissatisfied) at 24 h postoperation. The coding was opened after completion of the study to compile results.
Statistical analysis
The results were analyzed using SPSS 11.5 software (IBM Corporation). During the planning stage of the study, the sample size was calculated with the help of power analysis. A sample size of 30 patients per group was estimated to demonstrate the significant difference of 35% in morphine consumption among the groups, at α = 0.05 with a power (1-β) of 80%. The inclusion of 40 patients in each group was done for better validation of results. All the data are represented as mean ± standard deviation or as the number of patients (%). Statistical significance was tested with the use of Student's t-test. P < 0.05 was considered as statistically significant.
RESULTS
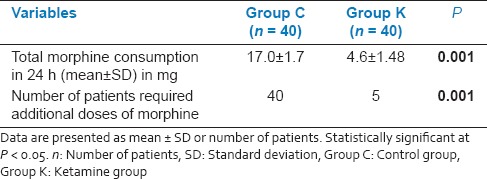
In the present study, a total of 80 patients out of 88 consecutive patients met the inclusion criteria and consented for the study. These 80 patients were randomized into two groups of 40 each [Figure 1]. There were no dropouts or loss to follow-up. The demographics and duration of surgery were comparable between the two groups [Table 1]. Comparison of hemodynamic parameters during the period of study drug infusion and intra-operative period between group K and group C at different time interval was statistically insignificant. At different time intervals patients in group K had less pain than group C when compared on VAS (P < 0.05), during the first 6 h after surgery. At the 12 h and 24 h, there was no significant difference between the two groups [Table 2]. In group K, only 5 patients (12.5%) required rescue analgesic (IV morphine) while in group C, 40 patients (100%) required additional dose of rescue analgesic (P = 0.001) [Table 3]. There was a statistically significant reduced consumption of morphine 4.6 ± 1.48 mg in group K compared with the Group C in which it was 17 ± 1.7 mg (P = 0.001) [Table 3].
Figure 1.
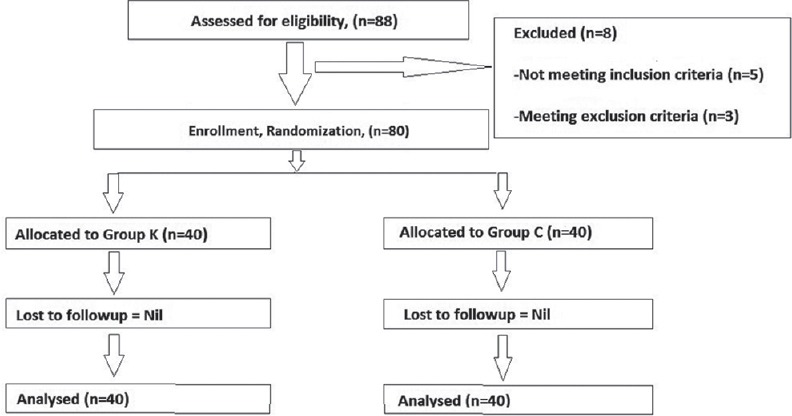
Patient flow (according the consort chart)
Table 1.
Demography
Table 2.
Assessment of pain in the postoperative period (VAS)
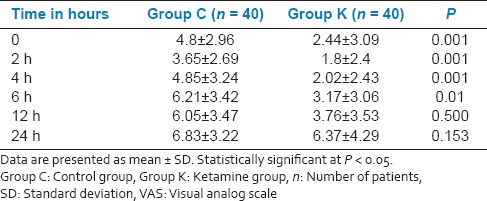
Table 3.
Postoperative analgesic requirements
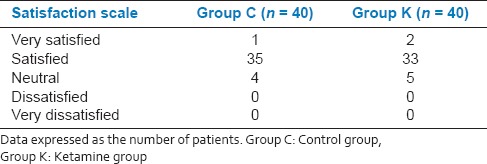
None of the patients experienced hallucination, sedation, headache, dizziness, respiratory depression and/or emergent reaction [Table 4]. Incidence of nausea, vomiting and patients satisfaction rate were similar in both groups [Tables 4 and 5].
Table 4.
Postoperative side effects
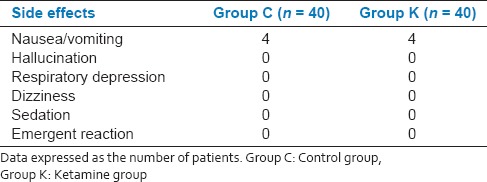
Table 5.
Patient satisfaction
DISCUSSION
Ketamine administered IV, is reported to produce analgesic effects by its action as a noncompetitive NMDA-receptor antagonist. However, it remains a controversial drug due to undesirable and unwanted adverse effects. It is mandatory to make a distinction between the high-dose ketamine as an anesthetic agent and low-dose ketamine as an antihyperalgesic agent. Low-dose ketamine is defined as a bolus dose of <2 mg/kg, when given intramuscularly or <1 mg/kg, when administered via the IV or epidural route. In continuous IV administration, low-dose ketamine is defined as a rate of ≤20 μg/kg/min.[7] Ketamine infusion used as a loading dose followed by an infusion rate of 1-6 μg/kg/min, was reported to provide antihyperalgesic, analgesic and opioid sparing effects.[8,9]
Low-dose ketamine infusion (0.1 mg/kg/h) following a loading dose of 0.2 mg/kg was previously tested perioperatively in spinal fusion surgery together with perioperative infusion of fentanyl.[10] In the present study, we tested similar dose of ketamine infusion intraoperatively in during abdominal surgery without intraoperative opioid infusion to avoid the masking of analgesic efficacy of ketamine. This significantly reduced postoperative pain scores and total morphine consumption, which is concurrent to previous reports.[6,11] Similar results are also reported with perioperative ketamine infusion after major abdominal and microdiscectomy surgery.[12,13] Interestingly effective postoperative analgesia was achieved with a combination of the intraoperative ketamine bolus of 0.5 mg/kg followed by 0.5 mg/kg/h and patient control epidural analgesia with morphine and bupivacaine. This also resulted in reduced analgesic consumption.[14]
In our study, the total quantity of morphine used within 24 h postsurgery was significantly lower in the group who received ketamine that is consistent with other reports.[15,16,17,18,19,20,21,22] However, a bolus of 1.5 mg ketamine plus 1.5 mg morphine in IV-patient-controlled analgesia failed to demonstrate the beneficial effect in morphine consumption and pain control after major orthopedic surgery.[23] Such lack of synergistic effects of ketamine and morphine in postoperative pain management is reported in several other studies, which warrants further analysis to address this discrepancy.[10,24,25] Nevertheless, low-dose ketamine is reported to be useful in the management of postsurgical acute pain.[21,26,27] Moreover, the opioid-sparing effect of ketamine in the clinical setting may still be useful for opioid intolerant patients, cancer patients or narcotic-tolerant patients who need a high-dose of opioids.[15]
Ketamine-related adverse effects are rare when the surgery is performed under general anesthesia.[28] In our study, all the surgeries were performed under general anesthesia and hence none of the patient complained of hallucination or nightmares. Large doses (>2 mg/kg, IV) and rapid administration of ketamine (>40 mg/min) predispose to this side effect whereas they are minimal at infusion rate of <2.5 mg/kg/min.[29] Some studies suggest that ketamine doesn’t have any effect on reducing the incidence of PONV;[11] however, this is contradicted.[17]
The limitation of our study is that we could not use higher doses of ketamine due to potential psychomimetic effects. However, further studies are needed to asses a clinically effective dose of ketamine. We conclude that IV infusion of low-dose ketamine in the intra-operative period has significant impact on reducing postoperative pain and cumulative analgesic consumption in patients undergoing cholecystectomy without any evidence of increased side effects pertaining to the drug.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Gehdoo RP. Postoperative pain management in paediatric patient. Indian J Anaesth. 2004;48:406–14. [Google Scholar]
- 2.De Kock M, Lavand’homme P, Waterloos H. ‘Balanced analgesia’ in the perioperative period: Is there a place for ketamine? Pain. 2001;92:373–80. doi: 10.1016/S0304-3959(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 3.De Kock MF, Lavand’homme PM. The clinical role of NMDA receptor antagonists for the treatment of postoperative pain. Best Pract Res Clin Anaesthesiol. 2007;21:85–98. doi: 10.1016/j.bpa.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Phillips WJ, Currier BL. Analgesic pharmacology: II. Specific analgesics. J Am Acad Orthop Surg. 2004;12:221–33. doi: 10.5435/00124635-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Visser E, Schug SA. The role of ketamine in pain management. Biomed Pharmacother. 2006;60:341–8. doi: 10.1016/j.biopha.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Carstensen M, Møller AM. Adding ketamine to morphine for intravenous patient-controlled analgesia for acute postoperative pain: A qualitative review of randomized trials. Br J Anaesth. 2010:401–6. doi: 10.1093/bja/aeq041. [DOI] [PubMed] [Google Scholar]
- 7.Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: A review of current techniques and outcomes. Pain. 1999:111–25. doi: 10.1016/S0304-3959(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 8.Owen H, Reekie RM, Clements JA, Watson R, Nimmo WS. Analgesia from morphine and ketamine. A comparison of infusions of morphine and ketamine for postoperative analgesia. Anaesthesia. 1987;42:1051–6. doi: 10.1111/j.1365-2044.1987.tb05167.x. [DOI] [PubMed] [Google Scholar]
- 9.Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiol Scand. 1997;41:1124–32. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- 10.Yeom JH, Chon MS, Jeon WJ, Shim JH. Peri-operative ketamine with the ambulatory elastometric infusion pump as an adjuvant to manage acute postoperative pain after spinal fusion in adults: A prospective randomized trial. Korean J Anesthesiol. 2012;63:54–8. doi: 10.4097/kjae.2012.63.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh B, Maliwad J, Shah VR. Preventive analgesia: Effect of small dose of ketamine on morphine requirement after renal surgery. J Anaesthesiol Clin Pharmacol. 2011;27:485–8. doi: 10.4103/0970-9185.86592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zakine J, Samarcq D, Lorne E, Moubarak M, Montravers P, Beloucif S, et al. Postoperative ketamine administration decreases morphine consumption in major abdominal surgery: A prospective, randomized, double-blind, controlled study. Anesth Analg. 2008;106:1856–61. doi: 10.1213/ane.0b013e3181732776. [DOI] [PubMed] [Google Scholar]
- 13.Hadi BA, Daas R, Zelkó R. A randomized, controlled trial of a clinical pharmacist intervention in microdiscectomy surgery - Low dose intravenous ketamine as an adjunct to standard therapy. Saudi Pharm J. 2013;21:169–75. doi: 10.1016/j.jsps.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kararmaz A, Kaya S, Karaman H, Turhanoglu S, Ozyilmaz MA. Intraoperative intravenous ketamine in combination with epidural analgesia: Postoperative analgesia after renal surgery. Anesth Analg. 2003;97:1092–6. doi: 10.1213/01.ANE.0000080205.24285.36. [DOI] [PubMed] [Google Scholar]
- 15.Guillou N, Tanguy M, Seguin P, Branger B, Campion JP, Mallédant Y. The effects of small-dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg. 2003;97:843–7. doi: 10.1213/01.ANE.0000075837.67275.36. [DOI] [PubMed] [Google Scholar]
- 16.Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology. 2005;102:211–20. doi: 10.1097/00000542-200501000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Kim SI, Ok SY, Park SY, Kim MG, Lee SJ, et al. Opioid sparing effect of low dose ketamine in patients with intravenous patient-controlled analgesia using fentanyl after lumbar spinal fusion surgery. Korean J Anesthesiol. 2013;64:524–8. doi: 10.4097/kjae.2013.64.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nesher N, Serovian I, Marouani N, Chazan S, Weinbroum AA. Ketamine spares morphine consumption after transthoracic lung and heart surgery without adverse hemodynamic effects. Pharmacol Res. 2008;58:38–44. doi: 10.1016/j.phrs.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Nesher N, Ekstein MP, Paz Y, Marouani N, Chazan S, Weinbroum AA. Morphine with adjuvant ketamine vs higher dose of morphine alone for immediate postthoracotomy analgesia. Chest. 2009;136:245–52. doi: 10.1378/chest.08-0246. [DOI] [PubMed] [Google Scholar]
- 20.Michelet P, Guervilly C, Hélaine A, Avaro JP, Blayac D, Gaillat F, et al. Adding ketamine to morphine for patient-controlled analgesia after thoracic surgery: Influence on morphine consumption, respiratory function, and nocturnal desaturation. Br J Anaesth. 2007;99:396–403. doi: 10.1093/bja/aem168. [DOI] [PubMed] [Google Scholar]
- 21.Cha MH, Eom JH, Lee YS, Kim WY, Park YC, Min SH, et al. Beneficial effects of adding ketamine to intravenous patient-controlled analgesia with fentanyl after the Nuss procedure in pediatric patients. Yonsei Med J. 2012;53:427–32. doi: 10.3349/ymj.2012.53.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollender Y, Bickels J, Stocki D, Maruoani N, Chazan S, Nirkin A, et al. Subanaesthetic ketamine spares postoperative morphine and controls pain better than standard morphine does alone in orthopaedic-oncological patients. Eur J Cancer. 2008;44:954–62. doi: 10.1016/j.ejca.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Sveticic G, Farzanegan F, Zmoos P, Zmoos S, Eichenberger U, Curatolo M. Is the combination of morphine with ketamine better than morphine alone for postoperative intravenous patient-controlled analgesia? Anesth Analg. 2008;106:287–93. doi: 10.1213/01.ane.0000289637.11065.8f. [DOI] [PubMed] [Google Scholar]
- 24.Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: A quantitative and qualitative systematic review. Anesth Analg. 2004;99:482–95. doi: 10.1213/01.ANE.0000118109.12855.07. [DOI] [PubMed] [Google Scholar]
- 25.Jensen LL, Handberg G, Helbo-Hansen HS, Skaarup I, Lohse T, Munk T, et al. No morphine sparing effect of ketamine added to morphine for patient-controlled intravenous analgesia after uterine artery embolization. Acta Anaesthesiol Scand. 2008;52:479–86. doi: 10.1111/j.1399-6576.2008.01602.x. [DOI] [PubMed] [Google Scholar]
- 26.Suppa E, Valente A, Catarci S, Zanfini BA, Draisci G. A study of low-dose S-ketamine infusion as “preventive” pain treatment for cesarean section with spinal anesthesia: Benefits and side effects. Minerva Anestesiol. 2012;78:774–81. [PubMed] [Google Scholar]
- 27.Min TJ, Kim WY, Jeong WJ, Choi JH, Lee YS, Kim JH, et al. Effect of ketamine on intravenous patient-controlled analgesia using hydromorphone and ketorolac after the Nuss surgery in pediatric patients. Korean J Anesthesiol. 2012;62:142–7. doi: 10.4097/kjae.2012.62.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elia N, Tramèr MR. Ketamine and postoperative pain – A quantitative systematic review of randomised trials. Pain. 2005;113:61–70. doi: 10.1016/j.pain.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 29.Reuben SS, Buvanendran A. Preventing the development of chronic pain after orthopaedic surgery with preventive multimodal analgesic techniques. J Bone Joint Surg Am. 2007;89:1343–58. doi: 10.2106/JBJS.F.00906. [DOI] [PubMed] [Google Scholar]


