Abstract
Medicinal properties of Asparagus racemosus (vernacular name: Shatavari) are attributed to its steroidal saponins called shatavarins. This plant is facing the threat of being endangered due to several developmental, seasonal constrains and malpractices involved in its collection and storage. To support its conservation, a tissue culture protocol is standardized which produces 20 fold higher levels of shatavarin. Here we evaluate the bioactivity and immunomodulatory potential of in vitro produced shatavarins from cell cultures of AR using human peripheral blood lymphocytes. In vitro produced shatavarin stimulated immune cell proliferation and IgG secretion in a dose dependent manner. It stimulated interleukin (IL)-12 production and inhibited production of IL-6. It also had strong modulatory effects on Th1/Th2 cytokine profile, indicating its potential application for immunotherapies where Th1/Th2 balance is envisaged. Our study demonstrating the bioactivity of tissue cultured AR extracts supports further in vivo evaluation of its immunomodulatory efficacy.
Keywords: Asparagus racemosus, immunomodulation, shatavarins, steroidal saponins, Th1/Th2 balance
INTRODUCTION
Asparagus racemosus (AR) root (local name Shatavari) is widely used for its steroidal saponins called shatavarins [Figure 1]. AR is of therapeutic interest due to its role as immunomodulant, galactogauge, adaptogen, antitusive, anticarcinogen, antioxidant, antidiarrheal and as a general tonic.[1,2,3,4,5,6] The plant is being endangered because of several seasonal and developmental constrains in the conventional multiplication strategies employed for its propagation. AR is conventionally propagated through seeds the efficiency of which is low,[7,8,9] which necessitates development of alternative methods of its propagation. Several tissue culture methods of AR were tried in 1980s[10,11] and recently, our group has reported a protocol for establishing callus and suspension cultures (SCs) of AR, which can be maintained easily under effecient laboratory conditions. These cultures stably produce high levels of saponins both intracellularly and extracellularly.[12,13] The applicability of a tissue culture protocol as a supportive conservation strategy will depend highly on the quality and quantity of shatavarins produced in vitro. The wild type AR root extract (WRE) is a well-known immunomodulator. Several studies have reported immunomodulatory effects of WRE. It is reported to restore lymphocyte and neutrophil counts.[6] It modulates macrophage activation[14] and delays tumour development in experimental animals.[15] Immunomodulatory effects of crude WRE extracts on tumor necrosis factor-alpha secretion and phagocytosis is also reported.[16] It also acts as an immunoadjuvent in animal and has strong impact on systemic Th1/Th2 immunity.[17,18,19] Hence in the current study we tested the immunomodulatory properties of in vitro produced saponins and compared it with WRE by evaluating IgG production, cell viability and proliferation, cytokine production using unstimulated and stimulated peripheral blood lymphocyte (PBLs).
Figure 1.
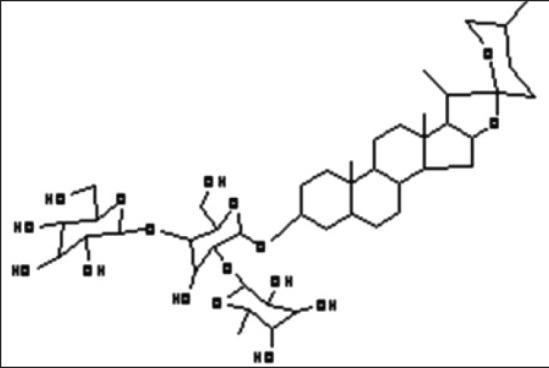
The structure of shatavarin IV: Steroidal saponin from Asparagus racemosus
MATERIALS AND METHODS
Plant material, callus and cell culture of Asparagus racemosus
The authors have reported a detailed methodology for the callus induction and cell culture of AR. Briefly, Field grown AR plants were first authenticated by Dr. Dongarwar, Taxonomist, Department of Botany, RTM Nagpur University, India. A Voucher specimen (Ac. No. 9869) was deposited at the University herbarium. Nodal explants of AR plants were grown on Murashige and Skoog medium with full concentrations of Vitamin and 3% sucrose,[20] supplemented with naphthalene acetic acid (1.0 mg/l), 2.4-dichlorophenoxyacetic acid (2,4-D, 1.0 mg/l) and 6-benzyl aminopurine (0.5 mg/l) was autoclaved at 121°C and 1.06 kg/cm2 for 20 min. Slants were prepared and explants were inoculated for raising callus cultures. Tubes were exposed to a 16 h photoperiod at 25°C ± 2°C and subcultured at regular intervals of 3 weeks. The above cultures were subsequently used to initiate cell SCs as reported previously.[12,13]
Extraction and analysis of shatavarin
Extraction and quantification of total shatavarins from the dried plant roots, callus and the spent media were performed as per the previously published method. Briefly, cells and the media were extracted separately with methanol (1:2) overnight and the procedure was repeated 4 times. The extracts were pooled and concentrated under reduced pressure on a rotary evaporator at 60°C to dryness. The dried residue was re-dissolved in 10 ml of H2O and extracted with n-butanol. The n-butanol fraction was concentrated, dried and re-dissolved in 5 ml of methanol. This reconstituted sample was stored till further analysis. Shatavarin IV (Natural Remedies, Bengaluru, India) was used as a marker for the quality control of the WRE; hence it was used as a standard for the assay of the in vitro cultures in this study. Stock solution of shatavarin IV (100 mg/ml) was prepared in methanol, and a series of standard shatavarin IV and sarsapogenin solutions with concentrations ranging from 10 mg/ml to 500 mg/ml were obtained by dilution of the stock solution by methanol (99.9%). The solutions were filtered through a 0.2 μm acrodisc and analysed by reversed-phase high-performance liquid chromatography as reported earlier.[12,13] High performance thin layer chromatography was carried out using linomat IV spotter and densitometer (CS-9301 PC, Shimadzu) and shatavarins from in vitro cultures were quantified.[21] WREs were prepared in a similar manner. 20 g of dried root powder was extracted with n-butanol and concentrates were then re-dissolved in methanol.
Isolation of peripheral blood lymphocyte and its culture
To isolate PBLs from human blood (after taking informed concent) was used. 5 ml of heparinised blood was diluted 1:1 with Roswell Park Memorial Institute Medium - 1640 (RPMI – 1640) medium containing 10% new-born calf serum and antibiotic-antimycotic solution (10,000 U penicillin, 10 mg streptomycin, amphotericin B - μg/ml in 0.09% NaCl) and layered carefully over 2.5 ml of histopaque-1077 in a 8 ml centrifuge tube. This was centrifuged at 1000 rpm for 20 min. Buffy coat containing lymphocytes was aspirated in a fresh tube, washed twice with RPMI medium and once with 0.87% ammonium chloride followed by washing with the medium.[22] The lymphocytes were pelleted and resuspended in 1 ml RPMI medium. This was kept in the CO2 incubator till further use. A part of it was used to test the viability using trypan blue staining.
Viability and proliferation assay
Peripheral blood lymphocytes were cultured in duplicate in 96 well microtiterplate (TPP, Europe/Switzerland) at a density of 2 × 105 cells/well with and without mitogens (Lipopolysaccharide [LPS] and Con A). The cells were grouped into two, namely unstimulated and stimulated. Lymphocytes were stimulated by mitogens: 10 ug/ml LPS (Sigma Co., St. Luis, MO, USA) for specifically enriching B-cells and with Con-A 10 ug/ml to enrich T-cells. Unstimulated lymphocytes were cultured without the addition of any mitogen and served as control. These two groups of cells were then cultured with or without the extracts (−500 μg/ml) and cells were incubated at 37°C in 5% CO2 for different time intervals (24, 48 and 72 h). After 72 h of incubation, 20 μl of XTT (2,3-bis [2-methoxy-4-nitro-5sulfophenyl ]-2H-tetrazolim-6-carboxanilideinnersalt;1 mg/m; Sigma, St. Louis, M.O.) containing Phenazine methosulfate (0.92 mg/ml XTT) (Sigma, St. Louis, M.O.) was added to the wells. After 4 h of incubation, plates were read at a test wavelength of 450 nm. Medium blanks were used to calculate the mean background values.
Assay for IgG production by enzyme linked immunosorbent assay
Enzyme linked immunosorbent assay (ELISA), procedure was used for the detection of IgG in the culture supernatant After 72 h of culture, PBLs from all the three experimental groups were centrifuged at 500 ×g, to obtain cell free supernatant.[23,24] 100 μl of the culture supernatants was coated on flat-bottom 96-well plates, for 3 h at 37°C. The wells were washed thrice with phosphate buffered saline (PBS), pH 7.4. Nonspecific sites were blocked by adding 100 μL of PBS containing 0.5% bovine serum albumin, at 37°C for 1 h. After blocking, wells were washed with PBS, followed by addition of 100 μL of affinity-purified horseradish peroxidase-conjugated rabbit antihuman IgG (1:10,000 diluted in PBS) (Genei, Banglore, India), for 1 h at 37°C. After 1 h of incubation, wells were again washed with five changes of PBS. 100 μl of 3,3’,5,5’-tetramethylbenzidine/H2O2 substrate solution was added into the wells and incubated at room temperature, for about 15 min. The reaction was stopped with 100 μl of 2.5 N sulphuric acid, and plates were read at 450 nm. Results are expressed in units/ml.
Cytokine assays: Interleukin (IL)-4, IL-6 and IL-12 were assayed in the culture supernatants using solid phase sandwich ELISA (Bender MedSystems). Assay was carried out as per the instruction manual of the kit.
Statistical analysis
Data is presented as mean ± standard error one-way analysis of variance was used for the statistical evaluation of the data. The results were considered significant at a value of P < 0.05.
RESULTS
A preliminary cell viability test was performed to confirm the use of methanol as a solvent, which showed that methanolic extracts did not have any adverse effect on the cell viability. Further experiments were performed using methanolic WRE and SCs of four selected concentrations, that is, 25 μg/ml (H1), 50 μg/ml (H2), 100 μg/ml (H3) and 500 μg/ml (H4). PBLs were divided into three groups, namely control (without extract and mitogen), unstimulated (with extracts and without mitogens) and stimulated (with extracts and mitogens), for studying the effects.
Effect on cell proliferation
Cell proliferation assay was carried out using XTT assay that correlates the number of viable cell directly to the colour intensity, which is measured by an ELISA reader. All the three groups of cells were treated with selected concentration of extracts and the observations were recorded after 24, 48 and 72 h respectively. Cells were viable and showed significant proliferation compared to the control group at all the tested concentrations of WRE and SCs. The viability peak was obtained at 50 μg/ml after 48 h in all the three experimental groups [Figure 2a–c].
Figure 2.
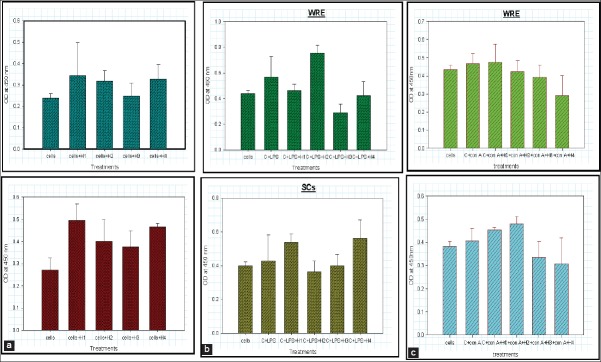
Effect of wild type Asparagus racemosus root extract (WRE) and suspension cultures (SC) on the proliferation of cells: (a) Unstimulated cells in presence of varied concentration of WRE and SC after 48 h. (b) lipopolysaccharide stimulated cells in presence of varied concentration of WRE and SCs after 48 h. (c) Con A stimulated cells in presence of varied concentration of WRE and SC after 48 h. (H1, H2, H3 and H4 are progressively higher concentrations of WRE and SC extracts)
Effect on B-cell mediated immunity
B-cell responses was measured by assay of secreted antibodies like IgG and IgM. For such assays, PBLs were first stimulated with 10 μg/ml of LPS and then cultured for 72 h in presence of extracts WRE and SCs [Figure 3a–c]. H4 concentration of both WRE and SCs induced maximum levels of IgG in LPS stimulated cells compared to the control groups. This effect was not observed at other concentrations (H1, H2 and H3). Hence indicating that both WRE and SCs have a stimulatory effect on B-cell mediated humoral immunity at a concentration of 500 μg/ml.
Figure 3.
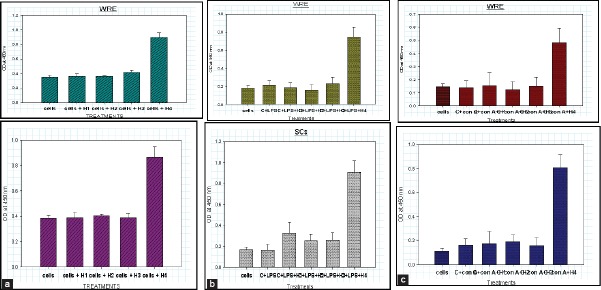
Effect on B-cell mediated immunity; (a) IgG production in unstimulated cells in presence of wild type Asparagus racemosus root extract (WRE) and SC after 72 h. (b) IgG production in lipopolysaccharide stimulated cells in presence of WRE and SC after 72 h. (c) IgG production in Con-A stimulated cells in presence of WRE and SC after 72 h (H1, H2, H3 and H4 are progressively higher concentrations of WRE and SC extracts)
EFFECT ON TH1/TH2 DIFFERENTIATION
In the current study stimulated and unstimulated PBLs were incubated for 24, 48 and 72 h with SCs and WRE. Culture supernatants were then assayed for IL-6 and IL-12. WRE and SCs had a mild stimulatory effect on IL-6 secretion, at H1 (50 ug/ml). However, they had a completely inhibitory effect at higher concentrations at all the observed time intervals. In stimulated and unstimulated group of cells a similar trend was observed. At higher concentrations both WRE and SCs inhibited IL-6 production.
WRE and SCs had a stimulatory effect on IL-12 production. Unstimulated cells were found to secrete IL-12 in at high concentrations (H4) after 72 h only. Among the stimulated groups, LPS induced cells failed to give IL-12 expression with WRE and SCs, but Con-A stimulated cells gave high IL-12 expression with lower concentrations of both WRE and SCs. These results indicate that the AR extracts (WRE and SCs) have inhibitory effect on IL-6 expression and a stimulatory effect on IL-12 expression [Figures 4a–c and 5a and b].
Figure 4.
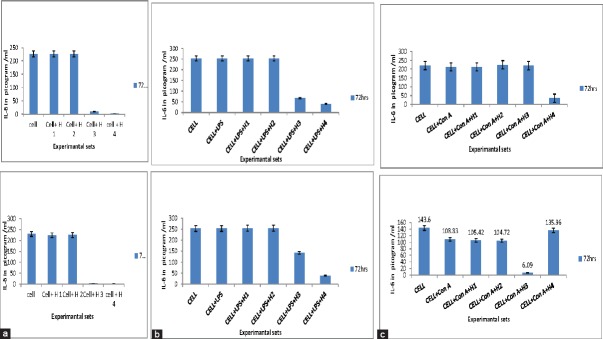
Secretion cytokines interleukin (IL)-6. (a) Secretion of IL-6 by unstimulated cells in presence of wild type Asparagus racemosus root extract (WRE) and suspension cultures (SC) after 72 h. (b) Secretion of IL-6 by lipopolysaccharide stimulated cells in presence of WRE and SC after 72 h. (c) Secretion of IL-6 by Con A stimulated cells in presence of WRE and SC after 72 h
Figure 5.
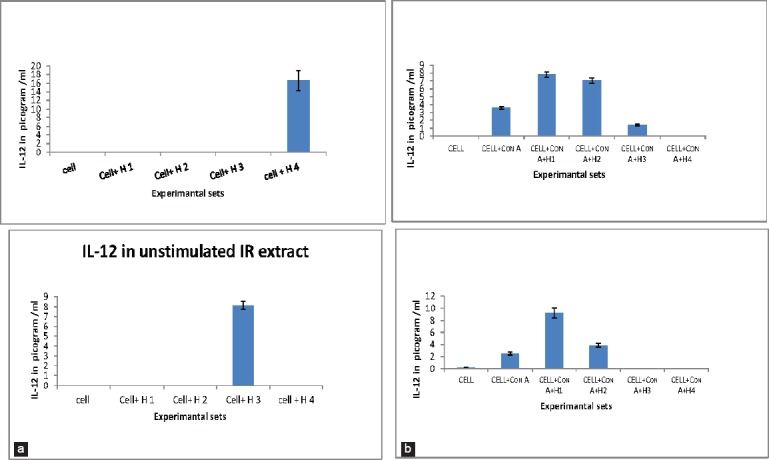
Secretion cytokines interleukin (IL)-12. (a) IL-12 production by unstimulated cells in presence of wild type Asparagus racemosus root extract (WRE) and suspension cultures (SC). (b) IL-12 production by Con A stimulated cells in presence of WRE and SC
DISCUSSION
Medicinal properties of AR are attirbuted to these shataverins. Wild type plant is a well-known immunomodulator and hence the current study was designed to test the immunomodulatory effects of saponins released in the SCs of AR and were compared to those displayed by WRE. In vitro produced steroidal saponins of AR had similar immunomodulatory properties to natural plant root extract. The in vitro produced shataverins stimulated immune cell proliferation and IgG secretion in a dose dependent manner. Many herbal products manifest their effect on immune system by affecting the lymphocyte differentiation pattern and can alter the cytokine profile or mimic the effects of cytokines.
Interleukin-6 is a pleotrophic cytokine produced by cells such as macrophages, dendritic cells, and B-cells and therefore can influence differentiation of various immune cells. It has both pro-and anti-immflamatory functions. In our study WRE and SCs were found to have inhibitory effect on IL-6 expression. IL-6 has two independent mechanisms to alter Th1/Th2 differentiation. Firstly, IL-6 stimulates production of IL-4 by naive CD4 (+) T cells and helps in their differentiation into effector Th2 cells. Secondly, IL-6 inhibits Th1 differentiation by up regulating suppressor of cytokine signaling-1 expression to interfere with interferon gamma signaling and the development of Th1 cells. Since both WRE and SCs had an inhibitory effect on IL-6, we suggest that these extracts do not drive Th2 differentiation. Our conclusion further gets strengthened from the findings that WRE and SCs stimulated expression of IL-12, which is a potent Th1 stimulator. Hence our study suggests that the extracts have strong modulatory effects on Th1/Th2 cytokine profile, which needs to be further explored using in vivo studies.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Gaitondé BB, Jetmalani MH. Antioxytocic action of saponin isolated from Asparagus racemosus Willd (Shatavari) on uterine muscle. Arch Int Pharmacodyn Ther. 1969;179:121–9. [PubMed] [Google Scholar]
- 2.Joglekar GV, Ahuja RH, Balwani JH. Galactogogue effect of Asparagus racemosus. Preliminary communication. Indian Med J. 1967;61:165. [PubMed] [Google Scholar]
- 3.Oketch-Rabah HA. Phytochemical Constituents of the Genus Asparagus and their biological activities. Hamdard. 1998;41:33–43. [Google Scholar]
- 4.Rao SB. Saponins (Sapogenins) from Indian medicinal plants: Part I Sapogenins from Asparagus. Indian J Pharm. 1952;14:131–2. [Google Scholar]
- 5.Rice G, editor. Vol. 2. Uenited Kingdom: Thompson and Morgan; 1988. Growing from Seed. [Google Scholar]
- 6.Thatte U, Chhabria S, Karandikar SM, Dahanukar S. Immunotherapeutic modification of Escherichia coli induced abdominal sepsis and mortality in mice by Indian medicinal plants. Indian Drug. 1987:95–7. [Google Scholar]
- 7.National Medicinal Plants Board, Ministry of Health and Family Welfare. Department of AYUSH. Govt. of India: Publication of Medicinal Plants Shatavari. [Last accessed on 2015 Apr 30]. Available from: http://nmpb.nic.in/WriteReadData/links/3733877856shatavari.pdf .
- 8.Saxena S, Bopana N. In vitro propagation of a high value medicinal plant: Asparagus racemosus Wild. J Ethanopharmacol. 2008;44:5–53. [Google Scholar]
- 9.Warner PK, Nambiar VP, Ganapathy PM. New Delhi, India: International Development Research Centre Artstock; 2001. Some Important Medicinal Plants of the Western Ghats, India: A profile; pp. 15–8. [Google Scholar]
- 10.Kar DK, Sen S. Organogenesis of diploid Asparagus racemosus through callus culture. Proc Indian Natl Sci Acad. 1984;B50:113–8. [Google Scholar]
- 11.Kar DK, Sen S. Propagation of Asparagus racemosus through tissue culture. Plant Cell Tissue Organ Cult. 1985;5:89–95. [Google Scholar]
- 12.Pise M, Rudra J, Begde D, Nashikkar N, Upadhyay A. Shatavarins production from in vitro cultures of Asparagus racemosus. J Med Plant Res. 2011;5:507–13. [Google Scholar]
- 13.Pise M, Rudra J, Bundale S, Begde D, Nashikkar N, Upadhyay A. Asparagus racemosus cell cultures: A source for enhanced production of shatavarins and sarsapogenin. In vitro Cell Dev Biol Plant. 2012;48:85–91. [Google Scholar]
- 14.Rege NN, Nazareth HM, Isaac A, Karandikar SM, Dahanukar SA. Immunotherapeutic modulation of intraperitoneal adhesions by Asparagus racemosus. J Postgrad Med. 1989;35:199–203. [PubMed] [Google Scholar]
- 15.Seena K, Kuttan G, Kuttan R. Antitumor activity of selected plant extracts. Amla Res Bull. 1993;13:41–5. [Google Scholar]
- 16.Bhatnagar M, Sisodia SS, Bhatnagar R. Antiulcer and antioxidant activity of Asparagus racemosus Willd and Withania somnifera Dunal in rats. Ann N Y Acad Sci. 2005;1056:261–78. doi: 10.1196/annals.1352.027. [DOI] [PubMed] [Google Scholar]
- 17.Gautam M, Diwanay S, Gairola S, Shinde Y, Patki P, Patwardhan B. Immunoadjuvant potential of Asparagus racemosus aqueous extract in experimental system. J Ethnopharmacol. 2004;91:1–5. doi: 10.1016/j.jep.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Gautam M, Saha S, Bani S, Kaul A, Mishra S, Patil D, et al. Immunomodulatory activity of Asparagus racemosus on systemic Th1/Th2 immunity: implications for immunoadjuvant potential. J Ethnopharmacol. 2009;121:241–7. doi: 10.1016/j.jep.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Diwanay S, Chitre D, Patwardhan B. Immunoprotection by botanical drugs in cancer chemotherapy. J Ethnopharmacol. 2004;90:49–55. doi: 10.1016/j.jep.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–97. [Google Scholar]
- 21.Satti NK, Suri KA, Dutt P, Suri OP, Musrat A, Qazi GN. Evaluation of Asparagus racemosus on the basis of immunomodulating sarasapogenin glycosides by HPTLC. J Liq Chromatogr Relat Technol. 2006;29:119–227. [Google Scholar]
- 22.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 23.Kashyap RS, Kainthla RP, Satpute RM, Chandak NH, Purohit HJ, Taori GM, et al. Demonstration of IgG antibodies to 30 Kd protein antigen in CSF for diagnosis of tuberculous meningitis by antibody-capturing ELISA. Neurol India. 2004;52:359–62. [PubMed] [Google Scholar]
- 24.Manjrekar PN, Jolly CI, Narayanan S. Comparative studies of the immunomodulatory activity of Tinospora cordifolia and Tinospora sinensis. Fitoterapia. 2000;71:4–7. doi: 10.1016/s0367-326x(99)00167-7. [DOI] [PubMed] [Google Scholar]


