Abstract
Background:
Gastric ulcer is one of the most serious diseases. Most classic treatment lines produce adverse drug reactions. Therefore, this study aimed to investigate the protective effects of two natural extracts, namely ginger and marshmallow extracts, on indomethacin-induced gastric ulcer in rats.
Materials and Methods:
Animals were divided into five groups; a normal control group, an ulcer control group, and three treatment groups receiving famotidine (20 mg/kg), ginger (100 mg/kg), and marshmallow (100 mg/kg). Treatments were given orally on a daily basis for 14 days prior to a single intra-peritoneal administration of indomethacin (20 mg/kg).
Results:
Indomethacin administration resulted in significant ulcerogenic effect evidenced by significant elevations in ulcer number, ulcer index, and blood superoxide dismutase activity accompanied by significant decreases in gastric mucosal nitric oxide and glutathione levels. In addition, elevations in gastric mucosal lipid peroxides and histamine content were observed. Alternatively, pretreatment with famotidine, ginger or marshmallow significantly corrected macroscopic and biochemical findings, supported microscopically by results of histopathological study.
Conclusion:
These results demonstrate that administration of either ginger or marshmallow extract could protect against indomethacin-induced peptic ulcer in rats presumably via their antioxidant properties and inhibition of histamine release.
Keywords: Famotidine, ginger, indomethacin, marshmallow, peptic ulcer
INTRODUCTION
Peptic ulcer is one of the world's major gastrointestinal disorders, embracing both gastric and duodenal ulcers, and affecting 10% of the world population.[1] Peptic ulcer disease is a complex and multicausal disease that occurs when biological balance between defense and aggressive factors in gastrointestinal tract is disturbed.[2] Among aggressive factors are endogenous factors like gastric acid and pepsin secretion,[3] active free radicals and oxidants, leukotrienes,[4] and endothelins[3] as well as exogenous factors like ethanol[5] or nonsteroidal anti-inflammatory drugs (NSAIDs).[6] In contrast, gastric mucus, bicarbonate,[7] normal blood flow,[3] prostaglandins (PGs), nitric oxide (NO), and antioxidant enzymes like catalase (CAT), or antioxidant peptides like glutathione (GSH) work as defensive barriers.[8]
There are many drugs that are used in the treatment of peptic ulcer. Until now, there is no one drug without a side effect or that gives 100% curative rate or complete cure.[9]
Famotidine is an H2 receptor antagonist that inhibits acid production by reversibly competing with histamine for binding with histamine H2 receptors that are located at the basolateral membrane of the parietal cells.[10] Histamine H2 receptor antagonists not only inhibit acid secretion induced by histamine, gastrin and cholinergic stimulation, but also promote healing of the ulcers.[11]
Various medicinal plants were used traditionally in the treatment of peptic ulcer. Plants and phytomedicines exhibit their action by various mechanisms like antioxidant, cytoprotective or antisecretory actions.[1] Plants possessing active principles such as flavonoid, tannins, or terpenoids usually show antiulcer activity.[12]
Ginger (Zingiber officinale Roscoe, Family: Zingiberaceae) is a herbal drug. Some active components of ginger are reported also to stimulate digestion and absorption and to relieve constipation and flatulence by increasing muscular activity in the digestive tract.[13,14]
In folk medicine and literature, marshmallow (Althaea officinalis L., Family: Malvaceae) was used in gastrointestinal disorders.[15] Aqueous marshmallow flower extract demonstrated protection against ethanol-induced gastric ulcer in some nations. It has been shown that mucilage and flavonoids have the property of covering and protecting gastric mucosa, thereby reducing the incidence of gastric ulcer.[16]
Based on the abovementioned data, the aim of the present study was to investigate the putative protective values and mechanisms of ginger and marshmallow extracts in indomethacin-induced peptic ulcer in rats.
MATERIALS AND METHODS
Animals
Adult male Wistar rats, weighing 200-250 g, were used as experimental animals in the present investigation. Animals were obtained from the animal house of Nahda University, Beni-Suef. They were kept under observation for about 15 days before the onset of the experiment to exclude any inter-current infection. The chosen animals were housed in plastic cages with good aerated covers at 25°C ± 0.5°C as well as 12 h light/dark cycles. Animals were allowed free access to water and were supplied daily with standard forage ad libitum. All animal housing and handling were conducted in accordance with the research protocols established by the Animal Care Committee of the National Research Center (Cairo, Egypt) which followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Plant materials
Ginger and marshmallow were purchased from Haraz Company, Cairo, Egypt and were identified phytochemically by staff members of the Department of Pharmacognosy, Faculty of Pharmacy, Nahda University. Alcoholic extract of ginger was prepared by cutting rhizomes into small pieces that were completely dried in shed up for 3-4 days. Powder (1 kg) was obtained with the help of a mixer, and then extraction was done using 50% ethanol (v/v). The homogenate was concentrated on rotavapor (IKA® RV 10, digital, 20-270 rpm - IKA® HB 10, basic, 0-180°C - made in Germany). The residue was designated as ethanol extract (11.5 g). The extract was presolubilized in distilled water for the in vivo studies.[17] The residue was dissolved in normal saline, 100 mg/mL, and prepared for oral use. Aqueous extract of marshmallow was prepared by soaking the dried flowers of marshmallow (1 kg) in hot water (85-90°C) for ½ h, followed by filtration and drying of the filtrate under reduced pressure, with a final yield of about 11.8%. The residue was dissolved in normal saline in a concentration of 100 mg/mL, and kept for oral administration.[16]
Drugs, chemicals, and reagent kits
All chemicals used in the study were of analytical grade. Famotidine was obtained as a gift from Amoun Pharmaceutical Industries Company “APIC,” Cairo, Egypt. Indomethacin was obtained as a gift from Sigma Pharmaceutical Company, Egypt. Histamine reagent kit was obtained from Oxford Biomedical Research, Inc., USA. Malondialdehyde (MDA) reagent kit was obtained from Cell Biolabs, Inc., USA. NO reagent kit was obtained from Assay Designs, Inc., USA. GSH reagent kit was obtained from Cell Biolabs, Inc., USA. Superoxide dismutase (SOD) reagent kit was obtained from Cell Biolabs, Inc., USA.
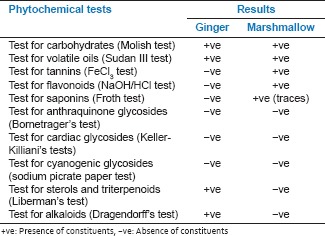
Preliminary phytochemical study
The extracts were subjected to preliminary phytochemical screening.[18,19] Results of screening are shown in the following table:
Experimental design
Rats were randomly allocated into five groups, each consisting of 6-8 rats, where test agents or saline were administrated by oral feeding tube once daily for consecutive 14 days. Groups 1 and 2 received normal saline (10 ml/kg/day, p.o.) and served as normal control and peptic ulcer control groups, respectively. Group 3 received famotidine (20 mg/kg/day, p.o.)[20] and served as standard treatment group. Group 4 received ginger (100 mg/kg/day, p.o.),[17,21] while Group 5 received marshmallow (100 mg/kg/day, p.o.),[16] and served as study treatment groups. On 15th day, all animals were subjected to a single intra-peritoneal administration of indomethacin (20 mg/kg, i.p.),[22] except for the normal control group, after 24 h of starvation. Three hours after injection of indomethacin or saline, animals were sacrificed by cervical dislocation under anesthesia by urethane (1.25 g/kg, i.p.). The blood samples were collected from the retino-orbital sinus before rats were sacrificed. Stomachs were isolated and opened along the greater curvature. The stomachs were washed with ice-cold saline and the glandular portion was then exposed and examined for ulceration. Ulcer index was determined and following gastric ulcer assessment, gastric mucosal homogenates were prepared in saline for biochemical estimations.
Assessment of gross mucosal damage
The gastric mucosal layer was carefully inspected for the occurrence of ulcers and their numbers were counted with the aid of an illuminated magnifying lens (10x).[23] The sum of the total length of long ulcers and hemorrhagic spots in each group of rats was divided by the number of animals to calculate the ulcer index (mm). Ulcer index was calculated according to the method described by.[24] The preventive index was calculated according to the method previously described.[25]

Biochemical estimations
Histamine content of the gastric mucosa was determined according to the method described earlier[26] using enzyme immunoassay at 650 nm spectrophotometrically. Gastric mucosal GSH was determined in stomach homogenate using a chromogen that reacts with the thiol group of GSH to produce a colored compound that absorbs at 405 nm spectrophotometrically.[27] Lipid peroxide formation was determined in gastric mucosal homogenate according to the method described previously[28] at 532 nm spectrophotometrically. Total NO concentration was determined in gastric mucosal homogenate according to the method previously described[29] at 540 ± 20 nm spectrophotometrically. SOD activity was determined in blood according to the method described previously[30] at 490 nm spectrophotometrically.
Histopathological study
Hematoxylin and eosin (H and E) was used for histological examination of the general structure of the stomach.[31] The effect of drugs was evaluated through assessment of the inflammatory and necrotic changes in the mucosal tissue. Briefly, gastric tissue samples from each group were fixed in 10% formalin for 24 h. The formalin fixed specimens were embedded in paraffin and sectioned (3-5 mm) and stained with H and E dye. The histochemical sections were evaluated by light microscope and photographed.
Statistical analysis
Statistical analysis and the significance of the difference between group means were determined using one-way ANOVA test followed by Tukey-Kramer multiple comparisons test, using GraphPad Instat computer software, San Diego, USA. Graphs and tables were performed using Microsoft Excel 2010 computer program (Microsoft Office Privacy, Microsoft Corporation, One Microsoft Way, Redmond, WA, USA).
RESULTS
Ulcer number, ulcer index, and preventive index [Figure 1]
Figure 1.
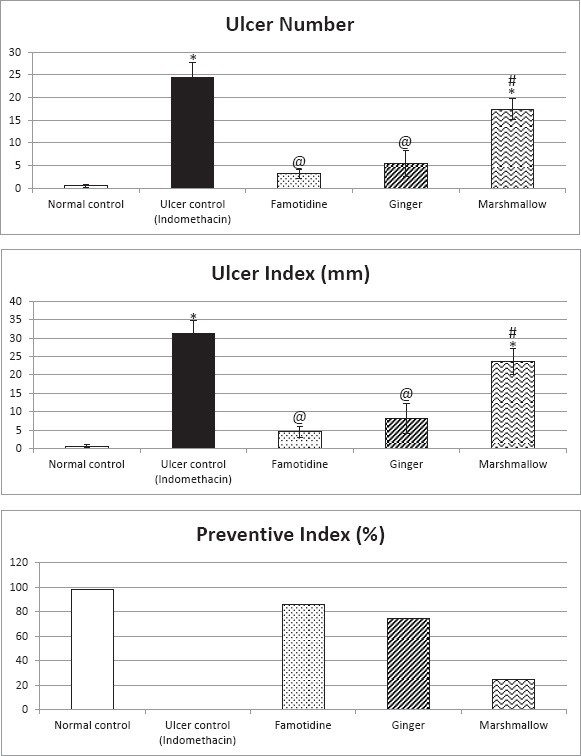
Protective effects of 14 days daily treatment by ginger, marshmallow and famotidine on ulcer number, ulcer index, and preventive index in indomethacin-induced gastric ulceration in rats. Each value represents the mean of 6-8 animals ± standard error of the mean. Statistical analysis was determined using one-way ANOVA test followed by Tukey-Kramer multiple comparisons test. *Significantly different from normal control group value at P < 0.05. @Significantly different from the ulcer control group value at P < 0.05. #Significantly different from famotidine (standard drug) treated group value at P < 0.05
Rats subjected to indomethacin administration showed significant ulceration in the glandular area of their stomachs compared to normal control rats. Pretreatment with famotidine and ginger significantly reduced ulcer number and ulcer index and significantly prevented the incidence of ulceration as compared to ulcer control group. In addition, marshmallow pretreatment did not significantly affect the ulcer number or ulcer index but significantly prevented the incidence of ulceration as compared to ulcer control group and significantly increased the ulcer number and ulcer index as compared to famotidine (standard drug) treated group.
Blood superoxide dismutase activity [Figure 2]
Figure 2.
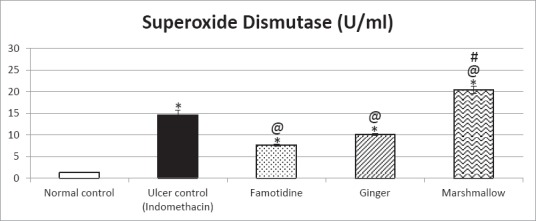
Protective effects of 14 days daily treatment by ginger, marshmallow and famotidine on superoxide dismutase in indomethacininduced gastric ulceration in rats. Each value represents the mean of 6-8 animals ± standard error of the mean. Statistical analysis was determined using one-way ANOVA test followed by Tukey-Kramer multiple comparisons test. *Significantly different from normal control group value at P < 0.05. @Significantly different from the ulcer control group value at P < 0.05. #Significantly different from famotidine (standard drug) treated group value at P < 0.05.
Rats subjected to indomethacin administration (ulcer control rats) showed a significant increase in SOD activity as compared to normal control rats. Pretreatment with famotidine and ginger significantly decreased SOD activity as compared to ulcer control rats. In addition, marshmallow pretreatment significantly increased SOD activity as compared to ulcer control rats, being significantly better than famotidine (standard drug) treated group.
Tissue nitric oxide, glutathione, malondialdehyde, and histamine [Table 1]
Table 1.
Protective effects of 14 days daily treatment by ginger, marshmallow, and famotidine on NO, GSH, MDA and histamine in indomethacin-induced gastric ulceration in rats
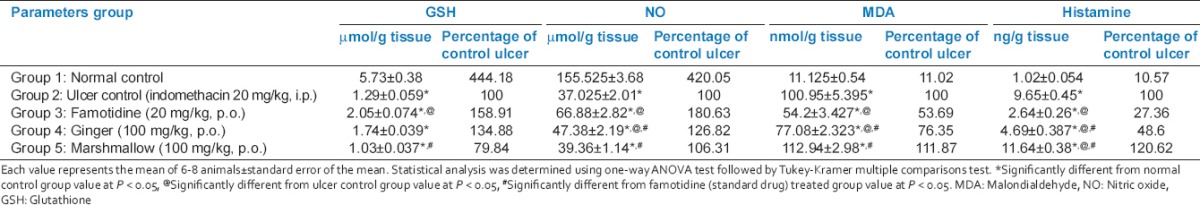
Rats subjected to indomethacin administration (ulcer control rats) showed significantly decreased GSH and NO content and significantly increased MDA and histamine contents as compared to normal control rats. Pretreatment with famotidine significantly increased GSH and NO contents and significantly decreased MDA and histamine contents as compared to ulcer control rats. Ginger pretreatment significantly increased NO content as compared to ulcer control group. On the other hand, it did not significantly affect GSH content as compared to ulcer control group. Ginger pretreatment significantly decreased MDA and histamine content as compared to ulcer control rats. In addition, marshmallow pretreatment did not significantly affect GSH or NO contents as compared to ulcer control rats. Marshmallow also did not significantly affect MDA content but significantly increased histamine content as compared to ulcer control rats.
Histopathological examination [Figure 3a–e]
Figure 3.
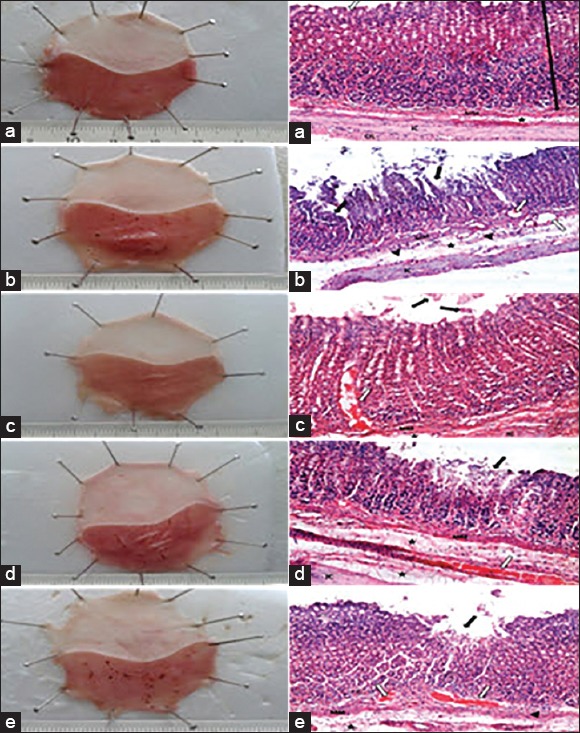
Images from stomachs and histopathological examination of each group. (a) Image from stomach and a photomicrograph of the fundus of Group 1 normal control group. (b) Image from stomach and a photomicrograph of the fundus of Group 2-ulcer control group. (c) Image from stomach and a photomicrograph of the fundus of Group 3-famotidine (20 mg/kg, p.o.). (d) Image from stomach and a photomicrograph of the fundus of Group 4-ginger (100 mg/kg, p.o.). (e) Image from stomach and a photomicrograph of the fundus of Group 5-Marshmallow (100 mg/kg, p.o.). Where (white arrow) is gastric pits or congested blood vessels, (black arrow) is the glandular mucosa, (arrow heads) is cellular infiltration, (ê) is the submucosal layer, MM is the muscularis mucosa, IC is the inner circular layer of musculosa and OL is outer longitudinal layer
Histopathological examination of normal control rats’ stomachs showed normal basic layers of the fundus. The glandular mucosa appears darker because of nuclei of epithelial and connective tissue. It contains thin pink strip marks in the muscularis mucosa [Figure 3a]. Rats subjected to indomethacin showed severe disruption in the glandular epithelium with exfoliation of cells. Edema of submucosa, cellular infiltration, and congested blood vessels were also evident [Figure 3b]. Pretreatment with famotidine limited the damage to the superficial epithelium with detachment of few cells [Figure 3c]. Ginger-pretreated rat sections showed mild disruption to the glandular epithelium, with some edema of the submucosal layer and intact muscularis mucosa [Figure 3d]. Marshmallow pretreatment showed disruption to the glandular epithelium with exfoliation of cells, associated with edema of the submucosal layer, thickened muscularis mucosa, congested blood vessels, and cellular infiltration [Figure 3e].
DISCUSSION
The present investigation aims to evaluate the possible protective effects of ginger and marshmallow on indomethacin-induced gastric ulcer in rats. Results of the current investigation revealed that indomethacin administration caused significant ulceration in the glandular area of the rat stomach as seen in histopathological examination, associated with marked elevation in ulcer number and ulcer index. These results are in agreement with previous investigations demonstrating that anti-inflammatory drug like indomethacin can produce visible gastric ulcers in experimental animals.[19,22,32] In the present investigation, indomethacin administration significantly decreased gastric GSH content compared to normal rats, again in harmony with previous studies demonstrating that indomethacin administration produced significant reduction in GSH contents in gastric mucosa.[33] The decreased concentration of GSH is typically associated with increased lipid peroxidation, evident in the present study as increased MDA level. In agreement, antioxidant markers have been shown to be reduced in stomach tissue damaged by indomethacin in a previous study.[34] NSAID administration was reported to cause suppression of antioxidant defense and to initiate lipid peroxidation in stomach tissue, resulting in gastric damage.[35] According to our results, indomethacin administration significantly decreased NO content. Similarly, NO levels were shown to be reduced in stomach tissue damaged by indomethacin.[34] In addition, it was demonstrated that indomethacin application significantly decreased levels of endogenous antioxidants GSH and NO.[33,36,37] A well-known mechanism responsible for gastric damage induced by indomethacin is the inhibition of cycloxygenase, a rate limiting enzyme in the synthesis of PGs. However, recent studies have suggested that NSAIDs such as indomethacin have pro-oxidant activity and initiate lipid peroxidation by generating reactive oxygen species, thereby interfering with endogenous antioxidant systems of the mucosa cells.[33,38,39] Increased oxidative stress is an important cause of NSAIDs’ damaging effect on stomach tissue.[38,39] Our results showed that indomethacin administration significantly increased gastric tissue histamine content. These results are in harmony with previous investigations concluding that PGs are extremely potent inhibitors of mast cell degranulation, and mast cells are capable of releasing a variety of mediators, like leukotriene C4 and platelet activating factor, that can contribute to mucosal injury. In addition, NSAIDs could reduce gastric mucosal blood flow and thus contribute to the pathogenesis of ulcer disease.[40] According to our work results, indomethacin administration significantly increased gastric SOD activity, which was in agreement with previous reports.[41] The relationship between SOD activity and PG synthesis is suggested as a possible mechanism of indomethacin-induced ulcers.[42,43]
On the other hand, mechanisms for such actions of NSAIDs seem to be complex and multifactorial, including the inhibition of PG synthesis, induction of apoptosis and necrosis of gastric mucosal cells,[44,45] neutrophil penetration, dysfunction of microvessels, reduced secretion of bicarbonate and mucus, and increased gastric motility.[46] However, the actual mechanisms by which NSAIDs produce acute and chronic gastro-duodenal mucosal injury are not completely understood.[47] However, recent studies have focused on the role of oxidative stress in mediating NSAID-induced microvascular disturbance associated with gastric mucosal injury.[48] Indomethacin produced more severe gastric damage in rats than did other non-NSAIDs.[49]
Results of the present study revealed that famotidine protected animals from indomethacin-induced gastric ulceration as manifested by significantly reduced ulcer number and ulcer index. Similar results were obtained by previous authors[39] showing high antiulcer activity of famotidine on indomethacin-induced ulcers. Oral administration of H2 antagonists significantly reduced ulcer index after indomethacin administration.[37]
Famotidine significantly increased GSH content compared to ulcer control rats. Previous investigations reported that famotidine administration significantly increased GSH content in indomethacin-treated animals.[39] In addition, famotidine significantly increased gastric GSH and NO contents after indomethacin administration, in harmony with previous investigations.[34,39] In the present study, famotidine significantly decreased MDA content after indomethacin administration. This significant reduction in MDA levels along with significant increase in CAT level suggested antioxidant activity of H2 antagonists.[37]
According to our study, ginger extract significantly reduced the ulcer number and ulcer index after indomethacin administration. Earlier, ethanolic extract of ginger produced a significant decrease in the intensity of ulceration induced by indomethacin and aspirin.[50] Data of the current work also demonstrated that ginger extract did not change gastric GSH content, again in agreement with previous studies.[51] Ginger administration significantly increased NO content after indomethacin administration. Similar results have been reported demonstrating that ginger was shown to be a potent inhibitor of NO synthesis in activated macrophages.[52] Ginger extract administration also decreased MDA after indomethacin administration. These results are in harmony with that obtained reporting an increase in MDA level could be prevented by orally treating the ulcer-induced animals with ginger extract as ginger extract caused a reduction in MDA concentration in the ulcerated gastric mucosa.[51] Data of the current work demonstrated that ginger extract significantly decreased histamine content. Similar results were obtained demonstrating that both ginger extract as well as 6-gingerol could inhibit production of pro-inflammatory cytokines from macrophages.[53] In addition, ginger extract significantly decreased SOD activity after indomethacin administration. Pretreatment with ginger for 2 weeks reversed these oxidative changes with concomitant decrease in SOD level, thereby suppressing most of the biochemical adverse effects induced by indomethacin. It has been reported that ginger had antioxidant activity and could improve enzymatic antioxidant activity.
The key of using ginger as a potential anti-ulcer agent originated from the finding that two of its active constituents, 6-gingerol and the terpenoid zingiberene, can ameliorate gastric lesion formation.[51] Surprisingly, some constituents of ginger have been shown to inhibit the synthesis of PGs.[54] It is therefore not understood how crude extracts of ginger can antagonize the effect of NSAIDs compounds.[50]
Results of the present study revealed that marshmallow extract did not change the ulcer number and ulcer index, and did not significantly affect GSH, NO or MDA contents after indomethacin administration. However, marshmallow extract significantly increased histamine content after indomethacin administration. Similar results have been reported demonstrating that marshmallow has immunostimulant activity where aqueous extracts of the roots stimulated phagocytosis and release of oxygen radicals and leukotrienes from human neutrophils in vitro.[55] The aqueous extract also induced the release of cytokines, interleukin-6, and tumor necrosis factor from human monocytes in vitro. In addition, marshmallow extract significantly increased SOD activity after indomethacin administration. Marshmallow was reported to promote antioxidant activity, in a neotetrazolium model of injury.[56]
Therefore, the anti-ulcer effect of marshmallow could be attributed to the reduction of oxidative stress and histamine release. The gastroprotective effect of marshmallow observed could be attributed to active compounds found in the extract such as flavonoids and mucilage polysaccharides.[8,16]
CONCLUSION
The present study demonstrates that administration of either ginger or marshmallow extract could protect against indomethacin-induced peptic ulcer in rats, presumably via their antioxidant properties and inhibition of histamine release.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rao CV, Venkataramana K. A Pharmacological review on natural antiulcer agents. J Glob Trends Pharm Sci. 2013;4:1118–31. [Google Scholar]
- 2.Sumbul S, Ahmad MA, Mohd A, Mohd A. Role of phenolic compounds in peptic ulcer: An overview. J Pharm Bioallied Sci. 2011;3:361–7. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14:581–91. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Wallace JL, McKnight GW, Keenan CM, Byles NI, MacNaughton WK. Effects of leukotrienes on susceptibility of the rat stomach to damage and investigation of the mechanism of action. Gastroenterology. 1990;98:1178–86. doi: 10.1016/0016-5085(90)90331-t. [DOI] [PubMed] [Google Scholar]
- 5.Soll A, Graham D. Peptic ulcer disease. In: Yamada T, editor. Text Book of Gastroenterology. 5th ed. USA: Blackwell Publication Ltd; 2009. pp. 936–41. [Google Scholar]
- 6.Takeuchi K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotility. World J Gastroenterol. 2012;18:2147–60. doi: 10.3748/wjg.v18.i18.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen A, Garner A. Mucus and bicarbonate secretion in the stomach and their possible role in mucosal protection. Gut. 1980;21:249–62. doi: 10.1136/gut.21.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farzaei MH, Rahimi R, Abbasabadi Z, Abdollahi M. An Evidence-Based review on medicinal plants used for the treatment of peptic ulcer in traditional Iranian medicine. Int J Pharmacol. 2013;9:108–24. [Google Scholar]
- 9.Mills SY. The Essential Book of Herbal Medicine. New York: Viking Arkana; 1991. Out of the earth; pp. 544–7. [Google Scholar]
- 10.Ahirrao VK, Pawar RP. Simultaneous quantification of famotidine and ibuprofen in pharmaceutical dosage by using validated stability indicating LC method. Res J Pharm Sci. 2013;2:1–9. [Google Scholar]
- 11.Qin Z, Chen C. Synergistic action of famotidine and chlorpheniramine on acetic acid-induced chronic gastric ulcer in rats. World J Gastroenterol. 2005;11:7203–7. doi: 10.3748/wjg.v11.i45.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad A, Kumar V, Maurya SK. Natural antiulcer agents: A pharmacological review. Int J Res Pharm Biomed Sci. 2013;4:535–41. [Google Scholar]
- 13.Stewart JJ, Wood MJ, Wood CD, Mims ME. Effects of ginger on motion sickness susceptibility and gastric function. Pharmacology. 1991;42:111–20. doi: 10.1159/000138781. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee S, Mullick HI, Banerjee J. Zingiber officinale: A natural gold. Int J Pharm Biol Sci. 2011;2:283–94. [Google Scholar]
- 15.Deters A, Zippel J, Hellenbrand N, Pappai D, Possemeyer C, Hensel A. Aqueous extracts and polysaccharides from Marshmallow roots (Althea officinalis L.): Cellular internalisation and stimulation of cell physiology of human epithelial cells in vitro . J Ethnopharmacol. 2010;127:62–9. doi: 10.1016/j.jep.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 16.Hage-Sleiman R, Mroueh M, Daher CF. Pharmacological evaluation of aqueous extract of Althaea officinalis flower grown in Lebanon. Pharm Biol. 2011;49:327–33. doi: 10.3109/13880209.2010.516754. [DOI] [PubMed] [Google Scholar]
- 17.Arun K, Ch VR, Vijayakumar M, Ayaz A, Naiyer S, Irfan KM. Anti-ulcerogenic and ulcer healing effects of Zingiber officinale (L.) on experimental ulcer models: Possible mechanism for the inhibition of acid formation. Int J Pharm Res. 2010;1:75–85. [Google Scholar]
- 18.Harborne JB. London: Chapman and Hall; 2007. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 19.Singh AP, Shukla V, Khare P. Effects of Plumeria obtusa Linn. in peptic ulcer induced by pylorus ligation & indomethacin. J Pharm Sci Innov. 2012;1:26–32. [Google Scholar]
- 20.Bharti S, Wahane VD, Kumar VL. Protective effect of Calotropis procera latex extracts on experimentally induced gastric ulcers in rat. J Ethnopharmacol. 2010;127:440–4. doi: 10.1016/j.jep.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 21.El-Abhar HS, Hammad LN, Gawad HS. Modulating effect of ginger extract on rats with ulcerative colitis. J Ethnopharmacol. 2008;118:367–72. doi: 10.1016/j.jep.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Huligol SV, Kumar VH, Narendar K. Evaluation of gastroprotective role of alpha-tocopherol in indomethacin induced peptic ulcer in albino rats. Int J Pharmacol Clin Sci. 2012;1:39–44. [Google Scholar]
- 23.Khayyal MT, el-Ghazaly MA, Kenawy SA, Seif-el-Nasr M, Mahran LG, Kafafi YA, et al. Antiulcerogenic effect of some gastrointestinally acting plant extracts and their combination. Arzneimittelforschung. 2001;51:545–53. doi: 10.1055/s-0031-1300078. [DOI] [PubMed] [Google Scholar]
- 24.Cho CH, Ogle CW. Cholinergic-mediated gastric mast cell degranulation with subsequent histamine H1-and H2-receptor activation in stress ulceration in rats. Eur J Pharmacol. 1979;55:23–33. doi: 10.1016/0014-2999(79)90144-4. [DOI] [PubMed] [Google Scholar]
- 25.Hano J, Bugajski J, Danek L, Wantuch C. The effect of neuroleptics on the development of gastric ulcers in rats exposed to restraint-cold stress. Pol J Pharmacol Pharm. 1976;28:37–47. [PubMed] [Google Scholar]
- 26.Demoly P, Lebel B, Messaad D, Sahla H, Rongier M, Daurès JP, et al. Predictive capacity of histamine release for the diagnosis of drug allergy. Allergy. 1999;54:500–6. doi: 10.1034/j.1398-9995.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- 27.Halliwell B, Gutteridge JM. 3rd ed. New York: Oxford University Press; 1999. Free Radicals in Biology and Medicine. [Google Scholar]
- 28.Brindeiro CM, Lane PH, Carmines PK. Tempol prevents altered K+ channel regulation of afferent arteriolar tone in diabetic rat kidney. Hypertension. 2012;59:657–64. doi: 10.1161/HYPERTENSIONAHA.111.184218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kendrick KM, Guevara-Guzman R, Zorrilla J, Hinton MR, Broad KD, Mimmack M, et al. Formation of olfactory memories mediated by Nitric oxide. Nature. 1997;388:670–4. doi: 10.1038/41765. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Zhang W, Jung DY, Ko HJ, Lee Y, Friedline RH, et al. TRPM2 Ca 2+ channel regulates energy balance and glucose metabolism. Am J Physiol Endocrinol Metab. 2012;302:E807–16. doi: 10.1152/ajpendo.00239.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drury RA, Wallington EA. 6th ed. London: Oxford University Press; 1980. Carleton's Histological Techniques; p. 183. [Google Scholar]
- 32.Heeba GH, Hassan MK, Amin RS. Gastroprotective effect of simvastatin against indomethacin-induced gastric ulcer in rats: Role of Nitric oxide and prostaglandins. Eur J Pharmacol. 2009;607:188–93. doi: 10.1016/j.ejphar.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Olaleye SB, Farombi EO. Attenuation of indomethacin - And HCL/Ethanol-induced oxidative gastric mucosa damage in Rats by kolaviron, a natural biflavonoid of Garcinia kola seed. Phytother Res. 2006;20:14–20. doi: 10.1002/ptr.1793. [DOI] [PubMed] [Google Scholar]
- 34.Dursun H, Bilici M, Albayrak F, Ozturk C, Saglam MB, Alp HH, et al. Antiulcer activity of fluvoxamine in rats and its effect on oxidant and antioxidant parameters in stomach tissue. BMC Gastroenterol. 2009;9:36. doi: 10.1186/1471-230X-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chattophadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induced reactive oxygen mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006;40:1397–408. doi: 10.1016/j.freeradbiomed.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Motawi TK, Abd-Elgawad HM, Shahin NN. Gastroprotective effect of leptin in indomethacin-induced gastric injury. J Biomed Sci. 2008;15:405–12. doi: 10.1007/s11373-007-9227-6. [DOI] [PubMed] [Google Scholar]
- 37.Abdallah IZ, Khattab HA, Heeba GH. Gastroprotective effect of Cordia myxa L. fruit extract against indomethacin-induced gastric ulceration in rats. Life Sci J. 2011;8:433–45. [Google Scholar]
- 38.Naito Y, Yoshikawa T, Yoshida N, Kondo M. Role of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injury. Dig Dis Sci. 1998;43(9 Suppl):30S–4. [PubMed] [Google Scholar]
- 39.Suleyman H, Cadirci E, Albayrak A, Polat B, Halici Z, Koc F, et al. Comparative study on the gastroprotective potential of some antidepressants in indomethacin-induced ulcer in rats. Chem Biol Interact. 2009;180:318–24. doi: 10.1016/j.cbi.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Wallace JL. How do NSAIDs cause ulcer disease? Baillieres Best Pract Res Clin Gastroenterol. 2000;14:147–59. doi: 10.1053/bega.1999.0065. [DOI] [PubMed] [Google Scholar]
- 41.Shetty BV, Arjuman A, Jorapur A, Samanth R, Yadav SK, Valliammai N, et al. Effect of extract of Benincasa hispida on oxidative stress in rats with indomethacin induced gastric ulcers. Indian J Physiol Pharmacol. 2008;52:178–82. [PubMed] [Google Scholar]
- 42.El-Missiry MA, El-Sayed IH, Othman AI. Protection by metal complexes with SOD-mimetic activity against oxidative gastric injury induced by indomethacin and ethanol in rats. Ann Clin Biochem. 2001;38(Pt 6):694–700. doi: 10.1258/0004563011900911. [DOI] [PubMed] [Google Scholar]
- 43.Bandyopadhyay SK, Pakrashi SC, Pakrashi A. The role of antioxidant activity of Phyllanthus emblica fruits on prevention from indomethacin-induced gastric ulcer. J Ethnopharmacol. 2000;70:171–6. doi: 10.1016/s0378-8741(99)00146-4. [DOI] [PubMed] [Google Scholar]
- 44.Hoshino T, Tsutsumi S, Tomisato W, Hwang HJ, Tsuchiya T, Mizushima T. Prostaglandin E2 protects gastric mucosal cells from apoptosis via EP2 and EP4 receptor activation. J Biol Chem. 2003;278:12752–8. doi: 10.1074/jbc.M212097200. [DOI] [PubMed] [Google Scholar]
- 45.Redlak MJ, Power JJ, Miller TA. Role of mitochondria in aspirin-induced apoptosis in human gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G731–8. doi: 10.1152/ajpgi.00150.2005. [DOI] [PubMed] [Google Scholar]
- 46.Jiang GL, Im WB, Donde Y, Wheeler LA. EP4 agonist alleviates indomethacin-induced gastric lesions and promotes chronic gastric ulcer healing. World J Gastroenterol. 2009;15:5149–56. doi: 10.3748/wjg.15.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaarin K, Gapor MT, Nafeeza MI, Fauzee AM. Effect of various doses of palm Vitamin E and tocopheerol on aspirin-induced gastric lesions in rats. Int J Exp Pathol. 2002;83:295–302. doi: 10.1046/j.1365-2613.2002.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dengiz GO, Gürsan N. Effects of Momordica Charantia L. (Cucurbitaceae) on indomethacin-induced ulcer model in rats. Turk J Gastroenterol. 2005;16:85–8. [PubMed] [Google Scholar]
- 49.Süleyman H, Akçay F, Altinkaynak K. The effect of nimesulide on the indomethacin-and ethanol-induced gastric ulcer in rats. Pharmacol Res. 2002;45:155–8. doi: 10.1006/phrs.2001.0933. [DOI] [PubMed] [Google Scholar]
- 50.Al-Yahya MA, Rafatullah S, Mossa JS, Ageel AM, Parmar NS, Tariq M. Gastroprotective activity of ginger (Zingiber officinale Rosc) in albino rats. Am J Chin Med. 1989;17:51–6. doi: 10.1142/S0192415X89000097. [DOI] [PubMed] [Google Scholar]
- 51.Ko JK, Leung CC. Ginger extract and polaprezinc exert gastroprotective actions by anti-oxidant and growth factor modulating effects in rats. J Gastroenterol Hepatol. 2010;25:1861–8. doi: 10.1111/j.1440-1746.2010.06347.x. [DOI] [PubMed] [Google Scholar]
- 52.Tchombé NL, Louajri A, Benajiba MH. Therapeutic effects of ginger (Zingiber officinale) Isesco J Sci Technol. 2012;8:64–9. [Google Scholar]
- 53.Tripathi S, Maier K, Bruch D, Kittur D. Ginger and its active ingredient 6-gingerol down regulate pro-inflammatory cytokine release by macrophages. J Surg Res. 2006;130:318. [Google Scholar]
- 54.Kiuchi F, Shibuya M, Sankawa U. Inhibitors of prostaglandin biosynthesis from ginger (Zingiber officinale) Chem Pharm Bull (Tokyo) 1982;30:754–7. doi: 10.1248/cpb.30.754. [DOI] [PubMed] [Google Scholar]
- 55.Shah SM, Akhtar N, Akram M, Shah PA, Saeed T, Ahmed K, et al. Pharmacological activity of Althaea officinalis L. J Med Plants Res. 2011;5:5662–6. [Google Scholar]
- 56.Al-Snafi AE. The pharmaceutical importance of Althaea officinalis and Althaea rosea: A review. Int J Pharm Technol Res. 2013;5:1378–85. [Google Scholar]


