Abstract
Mucormycosis is an opportunistic fungal infection, more commonly observed in immunocompromised patients. The mode of infection is via the inhalation route and infection begins initially in the nose and paranasal sinuses with subsequent invasion into the vascular tissue, eventually leading to thrombosis and necrosis of nearby hard and soft tissues. Here, we report a case of chronic osteomyelitis of the maxillary bone with fungal infection (mucormycosis) and extensive tissue necrosis in an uncontrolled diabetes mellitus patient.
Keywords: Mucormycosis, osteomyelitis, uncontrolled diabetes mellitus
INTRODUCTION
Mucormycosis is a rare opportunistic infection caused by a group of saprophytic fungi, belonging to the order — Mucorales and class — Zygomycetes.[1] The spores of these organisms are dispersed into the air from decaying material, and the mode of infection is via the inhalation route[2] and infection begins first in the nose and paranasal sinuses.[3] The organisms have a tendency for vascular invasion, forming thrombi within the blood vessels, leading to diminished blood supply and tissue necrosis. Generally, the disease may have various clinical presentations by systemic involvement-namely, rhinocerebral, pulmonary, gastrointestinal, cutaneous or disseminated forms.[4,5,6] The infection is more commonly observed in immunocompromised patients, such as those under long term steroid medication and uncontrolled diabetes mellitus patients.[7] A case of mucormycosis is presented here in an uncontrolled diabetes mellitus patient, involving right maxilla, zygomatic arch, pterygoid plates and maxillary sinus.
CASE REPORT
A 52-year-old male patient, who was moderately built and moderately nourished, was complaining of swelling in the right side of the face, involving middle third for the past 2 months. He gave a history of tooth pain in relation to upper right third molar before 3 months, for which he had previously visited a dentist. The pain was diagnosed to be due to acute periapical abscess, and the patient got the tooth extracted under local anesthesia. One month later, the patient developed pain and swelling in the middle third of the face, and he was referred to our institution by a private practitioner for further investigations.
During the day of the presentation, he had throbbing pain, moderate to severe in intensity, which was increasing during the night time. He also gave a history of watery discharge from the nose for the past 2-3 months. His medical history revealed that he was a known type II diabetes mellitus patient, and there was surgical history of appendecectomy 15 years back, without any complication.
On clinical examination, a diffuse swelling was noted extraorally, extending from nasolabial fold to pre auricular region in anterio-posterior direction and from infra orbital region to angle of mandible in superio-inferior direction. The overlying skin surface appeared smooth and shiny, with no ulceration or secondary changes, and bilateral submandibular lymph nodes were palpable. Intra orally, an oval shaped opening, with a diameter of approximately 1 cm was observed in the anterior palatal region [Figure 1]. The underlying bone was seen through the opening, and the surrounding palatal mucosa appeared edematous, with raised, irregular border. His right maxillary central incisor was missing due to mobility and the socket was continuous with the palatal opening. On palpation, foul smelling pus was oozing out from the labial and palatal gingival crevices of maxillary anterior teeth and on palatal mucosa near the opening. Nonhealing extraction socket, with visible underlying bone, was seen in relation to 18, with no bleeding or pus discharge. On radiographical examination, water's view [Figure 2] revealed haziness in the right maxillary region and perforation in the anterior palatal region. With these clinical details, provisional diagnosis of chronic suppurative osteomyelitis of right maxilla was given.
Figure 1.
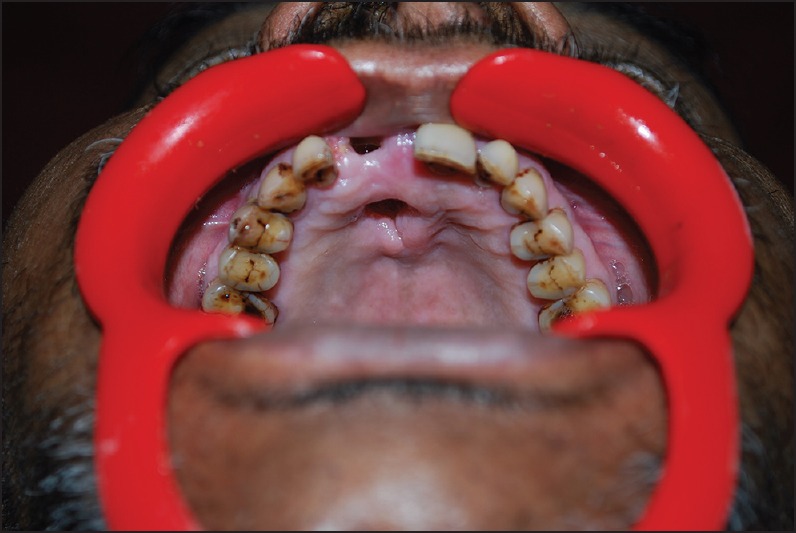
Intra oral photograph showing perforation of the palate
Figure 2.
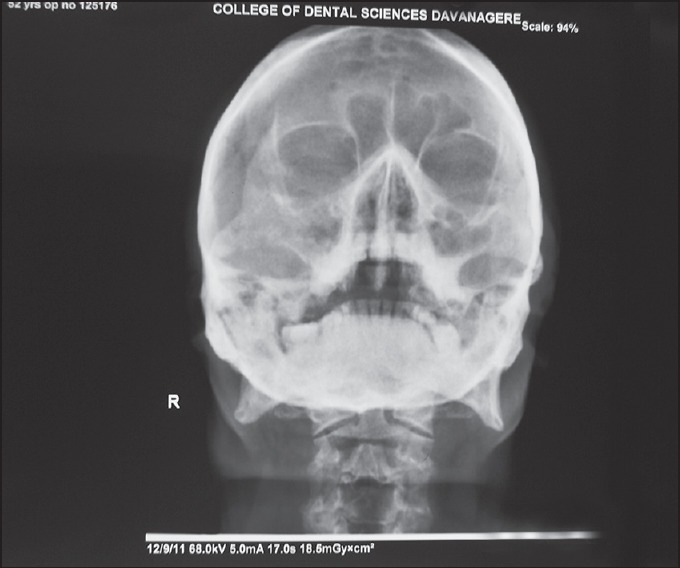
Water's view showing haziness in right maxillary sinus
The incisional biopsy specimen showed extensively necrotized tissue with no features of dysplasia under microscopic examination. Under general anesthesia, the lesion was treated surgically by hemimaxillectomy of the anterior palatal region, accompanied by debridement and curettage of the necrotized tissue. The excised specimens [Figure 3] were multiple in number, including hard and soft tissue components and were sent for histopathological examination. During grossing, the soft tissues were examined macroscopically, tissue processed, sectioned and stained with hematoxylin and eosin (H and E), periodic acid-Schiff (PAS), Gomori's methenamine silver (GMS) and Ziehl-Neelsen stain. The hard tissues, sent along with the soft specimens were subjected for decalcification and routinely processed, sectioned and stained with H and E stain. H and E stained sections of demineralized bone revealed bony trabeculae with fibrosis and inflamed bone marrow. The bony trabeculae were exhibiting many resting and reversal lines and the osteocytic lacunae were empty in numerous places. On soft tissue examination, highly necrotized tissue was seen under H and E staining. GMS staining revealed fungal organisms, branching at right angles, with large nonseptate hyphae [Figure 4a], along with round to ovoid spores. These hyphae were PAS-positive [Figure 4b]. No acid-fast organisms were seen on Ziehl–Neelsen staining. With these histopathological features, the final diagnosis of “chronic osteomyelitis of the maxillary bone with fungal infection (mucormycosis), causing extensive tissue necrosis” was made. The patient's blood glucose level was controlled and monitored by the physician, and antifungal treatment (amphotericin B) was included under medical regime.
Figure 3.
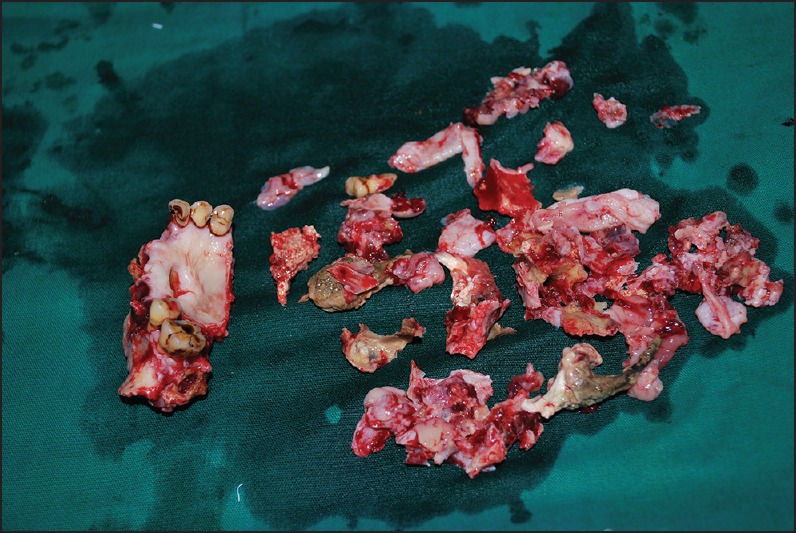
Surgically excised specimen showing anterior maxilla and multiple, necrotized hard and soft tissues
Figure 4.
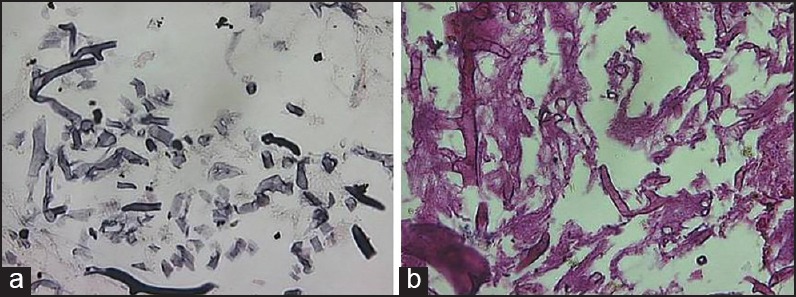
Fungal organism, identified by Gomary's methenamine silver stain (a) and periodic acid-Schiff stain (b)
DISCUSSION
Mucormycosis is an invasive fungal disease caused primarily by fungi belonging to the order Mucorales. This fungus usually acts as an opportunistic pathogen, seen in soil, decaying organic debris[8] and frequently occurs in the patients with compromised immune system. The leading predisposing factors for mucormycosis are uncontrolled diabetes mellitus, lymphomas, leukemias, renal failures, organ transplant, long-term intake of corticosteroids, immunosuppressive therapy and AIDS. Iron plays an important role in the growth of mucormycosis. Fungal hypae produce “rhizoferrin,” which binds iron fervently. This iron — Rhizoferrin complex is then taken up by the fungus and becomes available for its vital functions.[9] In the cases of diabetic ketoacidosis, the patients are at high risk of developing mucormycosis, due to an elevation in the available serum iron.[10] Clinically, mucormycosis has following six well-known forms, based on various systemic involvement-namely:
Rhino-orbito-cerebral,
Pulmonary,
Gastrointestinal,
Cutaneous,
Disseminated,
Miscellaneous.
Rhino-orbito-cerebral/rhinocerebral mucormycosis is the most common form and is noted commonly in uncontrolled diabetic patients.[8] Pulmonary form is seen in patients with leukemia, receiving chemotherapy. This form of mucormycosis may develop as a result of inhalation or by vascular mode. Gastrointestinal mucormycosis is rare, but it is believed to occur in extremely malnourished children. Cutaneous forms are noted in patients with disruption of the normal protective skin, especially in cases of burns. Dissemination form is seen more frequently in patients with pulmonary mucormycosis while it is less common in gastrointestinal or cutaneous form. The most common site for dissemination mucormycosis is brain, but is also noted in other parts of the body.[2,4,5] In the present case, the patient had uncontrolled diabetes mellitus, which is a well-known predisposing factor and also showed un-healing extraction site, which might have played a vital role, as the entrance point for infection. However, it is difficult to conclude, as no cases of mucormycosis had been reported so far in the literature with tooth extraction site as the portal of entry. The given diagnosis was confirmed histopathogically and by microbial studies. Microscopically, nonseptate hyphae, branching at right angles was evident. Tuberculosis infection, secondary to extraction is also possible but was ruled out by Ziehl-Neelsen staining. The treatment of mucormycosis was based on three vital measures namely, control of predisposing factors, antifungal therapy and surgical intervention. In the present case, the patient was monitored for controlled level of diabetes mellitus by his physician, followed by antifungal therapy (amphotericin B) and surgical intervention of involved maxilla was done by removing all necrotic bone and associated soft tissues. Mucormycosis, although rare in occurrence, it is an aggressive and potentially fatal disease in immunosuppressed patients. Careful clinical, radiographical examination, along with histopathological confirmation supported by special stains, are the key to early diagnosis of mucormycosis, leading to early and prompt treatment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Paulltauf A. Mycosis mucorina. Virchows Arch Pathol Anat. 1885;102:543–64. [Google Scholar]
- 2.Lee DG, Choi JH, Choi SM, Yoo JH, Kim YJ, Min CK, et al. Two cases of disseminated mucormycosis in patients following allogeneic bone marrow transplantation. J Korean Med Sci. 2002;17:403–6. doi: 10.3346/jkms.2002.17.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leitner C, Hoffmann J, Zerfowski M, Reinert S. Mucormycosis: Necrotizing soft tissue lesion of the face. J Oral Maxillofac Surg. 2003;61:1354–8. doi: 10.1016/s0278-2391(03)00740-7. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez CE, Couriel DR, Walsh TJ. Disseminated zygomycosis in a neutropenic patient: Successful treatment with amphotericin B lipid complex and granulocyte colony-stimulating factor. Clin Infect Dis. 1997;24:192–6. doi: 10.1093/clinids/24.2.192. [DOI] [PubMed] [Google Scholar]
- 5.Cuvelier I, Vogelaers D, Peleman R, Benoit D, Van Marck V, Offner F, et al. Two cases of disseminated mucormycosis in patients with hematological malignancies and literature review. Eur J Clin Microbiol Infect Dis. 1998;17:859–63. doi: 10.1007/s100960050207. [DOI] [PubMed] [Google Scholar]
- 6.Pagano L, Ricci P, Tonso A, Nosari A, Cudillo L, Montillo M, et al. Mucormycosis in patients with haematological malignancies: A retrospective clinical study of 37 cases. GIMEMA Infection Program (Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto) Br J Haematol. 1997;99:331–6. doi: 10.1046/j.1365-2141.1997.3983214.x. [DOI] [PubMed] [Google Scholar]
- 7.Mane RS, Watve JK, Mohite AA, Patil BC. Rhinocerebral mucormycosis: A deadly disease on the rise. Indian J Otolaryngol Head Neck Surg. 2007;59:112–5. doi: 10.1007/s12070-007-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martín-Moro JG, Calleja JM, García MB, Carretero JL, Rodríguez JG. Rhinoorbitocerebral mucormycosis: A case report and literature review. Med Oral Patol Oral Cir Bucal. 2008;13:E792–5. [PubMed] [Google Scholar]
- 9.Mathebula SD. Mucormycosis. S Afr Optom. 2008;67:36–41. [Google Scholar]
- 10.Dökmetas HS, Canbay E, Yilmaz S, Elaldi N, Topalkara A, Oztoprak I, et al. Diabetic ketoacidosis and rhino-orbital mucormycosis. Diabetes Res Clin Pract. 2002;57:139–42. doi: 10.1016/s0168-8227(02)00021-9. [DOI] [PubMed] [Google Scholar]


