Abstract
The etiology of gastric antral vascular ectasia (GAVE) syndrome or gastric hyperplastic polyps (HPs) is not fully understood. We report a case of gastric HP arising in a patient treated with argon plasma coagulation (APC) for GAVE syndrome. Despite unclear etiologic progression, this and previously reported cases suggest a temporal relationship between the treatment of GAVE and HP. A 68-year-old male with a history of coronary artery disease, congestive heart failure and diabetes type II who initially presented with symptomatic anemia 2 weeks after starting aspirin and clopidogrel therapy. Diagnostic esophagogastroduodenoscopy (EGD) demonstrated diffuse GAVE. He was treated with 5 APC treatments, at 6-week intervals, over a 30 weeks period. 16 months after the initial APC treatment, an EGD performed secondary to persistent anemia demonstrated innumerable, large, bleeding polyps in the gastric antrum. Biopsy performed at that time confirmed hyperplastic gastric polyps. It has been proposed that HPs are regenerative lesions that arise at sites of severe mucosal injury. Our patient's treatment of GAVE with APC created significant mucosal injury, resulting in HP. Technique and genetic factors may have promoted hyperplastic changes during the regeneration of mucosa, at sites previously treated with APC. This case highlights the potential progression of GAVE to HP in a patient with persistent anemia after APC therapy.
Keywords: Argon plasma coagulation, gastric antral vascular ectasia, gastric polyps, hyperplastic polyps
INTRODUCTION
The gastric antral vascular ectasia (GAVE) or “watermelon stomach” is a rare and often misdiagnosed cause of occult upper gastrointestinal bleeding. It was first described in 1953 by Rider et al. as a cause of massive gastric hemorrhage. Up to the present, there had been more data regarding of the epidemiology, pathology including the outcomes of variable treatment modalities for this condition. Treatment includes conservative measures such as transfusion and endoscopic therapy.[1,2] The etiology of GAVE syndrome or gastric hyperplastic polyps (HPs) are not fully understood. We report a case of gastroduodenal HP arising in a patient treated with argon plasma coagulation (APC) for GAVE syndrome. Despite the unclear etiologic overlap, this and previously reported cases suggest a relationship between GAVE and HP.
CASE REPORT
Our patient is a 68-year-old male with a history of coronary artery disease, congestive heart failure (CHF) and diabetes mellitus who presented with symptomatic anemia 2 weeks after being placed on aspirin and plavix following coronary artery stenting. Gastrointestinal bleeding was suspected based on reports of melena and subsequent guaiac-positive stools. Esophagogastroduodenoscopy (EGD) demonstrated diffuse GAVE syndrome, with multiple small subcutaneous nodules [Figure 1]. He was treated with five APC treatments, in 6-week intervals over a 30 weeks time span. Patient's gastrin and immunoglobulin levels were within normal limits. The ectasia were successfully obliterated in a linear pattern, from the antrum to the pyloric channel. During this time period, the patient continued to experience symptoms of anemia, receiving a multiple blood transfusions and weekly iron infusions. Serial EGDs demonstrated that the antral nodularities were progressively showing more signs of inflammatory changes. An EGD, performed 16 months after the initial APC treatment, demonstrated innumerable, large, bleeding polyps in the gastric antrum [Figure 2]. Pathology confirmed hyperplastic gastric polyps, with marked surface ulceration as well as regenerative epithelial changes [Figure 3]. There was no evidence of intestinal metaplasia, dysplasia or Helicobacter pylori infection.
Figure 1.
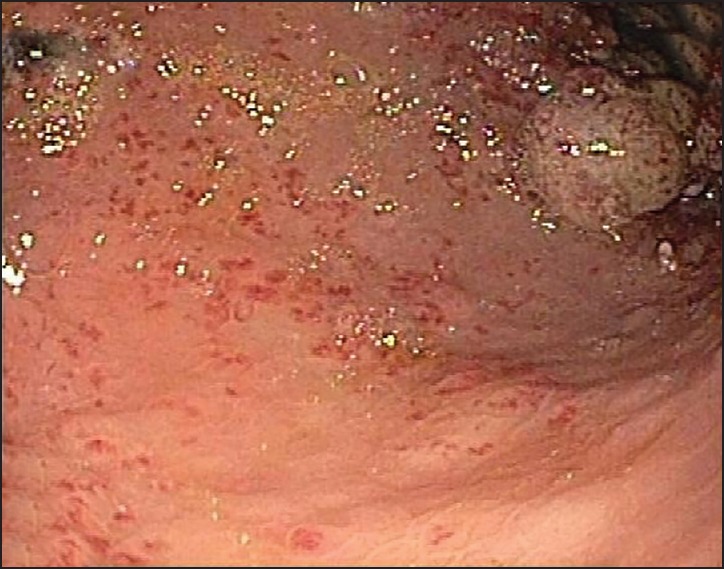
Gastric antral vascular ectasias in body of the stomach
Figure 2.
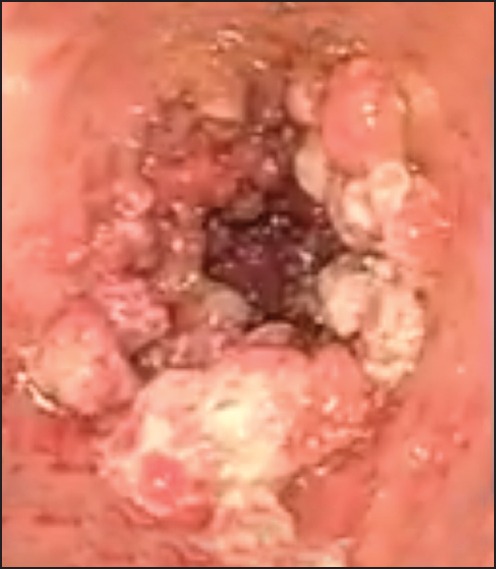
Multiple large polyps in the gastric antrum
Figure 3.
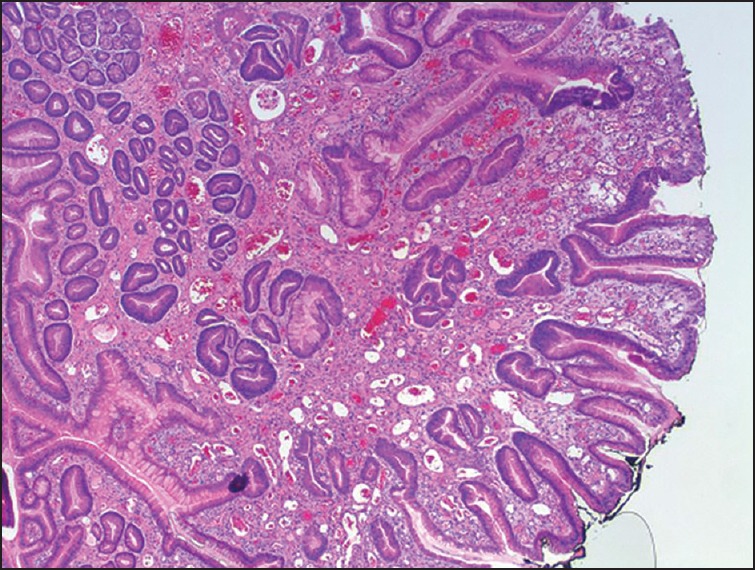
Hyperplastic gastric polyps histology showing marked surface ulceration as well as regenerative epithelial changes
DISCUSSION
Unlike gastric fundic gland polyps, which arise in otherwise normal gastric mucosa, HPs have been reported in association with various types of gastric injury, particularly autoimmune gastritis H. pylori gastritis and postantrectomy.[3,4] There is also reports been noted to arise after gastric ulcers and laser therapy for GAVE.[5] It has been proposed that HPs are regenerative lesions that arise at sites of severe mucosal injury. This is supported by reports of a high rate (up to 85%) of background mucosal disease in the stomachs of patients with HP.[7] Our case is consistent with other reports as well as a study by Chang et al., which correlate the treatment of GAVE with the subsequent development of HP. Baudet et al. also reported a similar case, particularly implicating APC in the development of HP.[9] We propose that our patient's treatment of GAVE syndrome with APC over the span of 30 weeks, created recurrent mucosal injury; caused to have HP.
Gastric antral vascular ectasia is an uncommon cause of upper gastrointestinal bleeding. The term “watermelon stomach” is derived from the characteristic endoscopic appearance of longitudinal rows of flat, reddish stripes radiating from the pylorus into the antrum, which resemble the stripes on a watermelon.[1,5,7] These red stripes represent ectatic and sacculated mucosal vessels. A punctate form has also been described and appears to be more common in patients with underlying cirrhosis. Histopathologically, GAVE is characterized by vascular ectasia, spindle cell proliferation, and fibro-hyalinosis. GAVE is thought to occur in an idiopathic fashion. However, it has been associated with cirrhosis, systemic sclerosis, bone marrow transplantation, CHF, end-stage renal disease. Bleeding is most often chronic, with patients presenting with occult blood in the stool, with or without iron deficiency anemia. Episodic transfusions are required in some chronic cases, but the bleeding is rarely acute and massive.[8,9,10]
Endoscopic coagulation with a heater probe, bipolar probe, argon plasma coagulator, laser therapy, or radiofrequency ablation obliterates the vascular ectasias and decreases the degree of bleeding.[9,10] APC is a noncontact technique of electrocoagulation in which energy is transmitted via ionized argon gas flow. APC has become increasingly popular for treating GAVE. Its main advantage is that coagulation is more superficial, thus reducing the risk of complications. Antrectomy prevents recurrent bleeding but is usually reserved for patients who fail endoscopic therapies. Importantly, portal decompression with a transjugular intrahepatic portosystemic shunt does not reliably reduce bleeding, underscoring the uncertain relationship of GAVE and portal hypertension.[10,11]
The etiology of gastric HP is also not known. HP commonly occur in the setting of chronic H. pylori infection, autoimmune/atrophic gastritis, elevated gastrin levels and at the periphery of ulcers/erosions, as well as near gastroenterostomy sites. However, any factor causing chronic gastritis including cytomegalovirus gastritis, amyloidopathy, Zollinger-Ellison syndrome, proton pump inhibitor therapy and the postantrectomy stomach may be predisposing conditions.[10,11,12]
Hyperplastic gastric polyps developing after electrocoagulation treatment of GAVE were first reported after endoscopic neodymium-doped yttrium aluminum garnet laser treatment. Hyperplastic gastric polyps occurring after APC treatment for GAVE are rare, however, have been reported following the treatment of GAVE with APC.[6,9,10,13] APC is a more superficial method of coagulation, implying lower thermal stresses. Several circumstances can increase thermal injury, such as repeat coagulation, the use of high-energy settings or, most importantly contact between the tip of the catheter and the mucosa during the procedure. Following mucosal injury or erosion, there is ongoing healing and a reparative response in the form of foveolar hyperplasia. HP syndrome is thought to result from excessive regeneration after mucosal damage. It is proposed that the loose connective tissue of the deeper muscularis propria serves as a reservoir for resting fibroblasts or myofibroblasts. Their function is to synthesize the extracellular matrix and participate in mucosal repair. When stimulated by mucosal injury, these cells differentiate, proliferate, and migrate toward the site in injury. This hyperplastic tissue can either disappear (in 24.2% of cases) or persists, and progress to a HP. HPs thus appears to be the result of over-vigorous regeneration that occurs in response to severe mucosal injury caused by penetration of the muscularis propria. Unlike polyps of the colon, gastric polyps are relatively uncommon, with an incidence of <2-3%.[10,11,12,13]
Hyperplastic polyps account for more than 50% of gastric polyps and are generally considered benign. Though their malignant potential is low, there is evidence supporting clonality and neoplastic potential of gastric HP particularly with polyps >5 mm. HP may present with anemia or mechanical obstruction. In addition, in patients with gastric polyps, other parts of the gastric mucosa should also be histologically evaluated by multiple biopsies for detection of any accompanying inflammatory changes or malignancy.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rider JA, Klotz AP, Kirsner JB. Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology. 1953;24:118–23. [PubMed] [Google Scholar]
- 2.Keohane J, Berro W, Harewood GC, Murray FE, Patchett SE. Band ligation of gastric antral vascular ectasia is a safe and effective endoscopic treatment. Dig Endosc. 2013;25:392–6. doi: 10.1111/j.1443-1661.2012.01410.x. [DOI] [PubMed] [Google Scholar]
- 3.Haruma K, Sumii K, Yoshihara M, Watanabe C, Kajiyama G. Gastric mucosa in female patients with fundic glandular polyps. J Clin Gastroenterol. 1991;13:565–9. doi: 10.1097/00004836-199110000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Geller A, Gostout CJ, Balm RK. Development of hyperplastic polyps following laser therapy for watermelon stomach. Gastrointest Endosc. 1996;43:54–6. doi: 10.1016/s0016-5107(96)70261-4. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka J, Fujimoto K, Iwakiri R, Koyama T, Sakata H, Ohyama T, et al. Hyperplastic polyps following treatment of acute gastric ulcers. Intern Med. 1994;33:366–8. doi: 10.2169/internalmedicine.33.366. [DOI] [PubMed] [Google Scholar]
- 6.Baudet JS, Salata H, Soler M, Castro V, Díaz-Bethencourt D, Vela M, et al. Hyperplastic gastric polyps after argon plasma coagulation treatment of gastric antral vascular ectasia (GAVE) Endoscopy. 2007;39(Suppl 1):E320. doi: 10.1055/s-2007-966802. [DOI] [PubMed] [Google Scholar]
- 7.Oberhuber G, Stolte M. Gastric polyps: An update of their pathology and biological significance. Virchows Arch. 2000;437:581–90. doi: 10.1007/s004280000330. [DOI] [PubMed] [Google Scholar]
- 8.Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the stomach: Associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol. 2001;25:500–7. doi: 10.1097/00000478-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Chang WH, Shih SC, Wang HY, Chang CW, Chen CJ, Chen MJ. Acquired hyperplastic gastric polyps after treatment of ulcer. J Formos Med Assoc. 2010;109:567–73. doi: 10.1016/S0929-6646(10)60093-9. [DOI] [PubMed] [Google Scholar]
- 10.Kwan V, Bourke MJ, Williams SJ, Gillespie PE, Murray MA, Kaffes AJ, et al. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: Experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol. 2006;101:58–63. doi: 10.1111/j.1572-0241.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 11.Wells CD, Harrison ME, Gurudu SR, Crowell MD, Byrne TJ, Depetris G, et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008;68:231–6. doi: 10.1016/j.gie.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Abraham SC. Gastric polyps: Classification and meaning. Pathol Case Rev. 2002;7:2–11. [Google Scholar]
- 13.Dijkhuizen SM, Entius MM, Clement MJ, Polak MM, Van den Berg FM, Craanen ME, et al. Multiple hyperplastic polyps in the stomach: Evidence for clonality and neoplastic potential. Gastroenterology. 1997;112:561–6. doi: 10.1053/gast.1997.v112.pm9024310. [DOI] [PubMed] [Google Scholar]


