Abstract
Cancer stem cells often have phenotypic and functional characteristics similar to normal stem cells including the properties of self-renewal and differentiation. Recent findings suggest that uncontrolled self-renewal may explain cancer relapses and may represent a critical target for cancer prevention. It is conceivable that the loss of regulatory molecules resulting from inappropriate consumption of specific foods and their constituents may foster the aberrant self-renewal of cancer stem cells. In fact, increasing evidence points to the network delivering signals for self-renewal from extracellular compartments to the nucleus including changes in stem cell environments, inducible expression of microRNAs, hyperplastic nuclear chromatin structures, and the on/off of differentiation process as possible sites of action for bioactive food components. Diverse dietary constituents such as vitamins A and D, genistein, (−)-epigallocatechin-3-gallate (EGCG), sulforaphane, curcumin, piperine, theanine, and choline have been shown to modify self-renewal properties of cancer stem cells. The ability of these bioactive food components to influence the balance between proliferative and quiescent cells by regulating critical feedback molecules in the network including dickkopf 1 (DKK-1), secreted frizzled-related protein 2 (sFRP2), B-cell-specific Moloney murine leukemia virus integration site 1 (Bmi-1), and cyclin-dependent kinase 6 (CDK6) may account for their biological response. Overall, the response to food components does not appear to be tissue or organ specific, suggesting there may be common cellular mechanisms. Unquestionably, additional studies are needed to clarify the physiological role of these dietary components in preventing the resistance of tumor cells to traditional drugs and cancer recurrence.
Keywords: Cancer Stem Cells, Vitamins A and D, Sulforaphane, Genistein, EGCG, Theanine, Curcumin, Piperin
Introduction
Cancer stem cells utilize several developmental mechanisms for self-renewal each of which appears to be fundamental to the initiation and relapse of tumors (1). Relevant examples include the loss of regulation in Wnt signaling pathways in colorectal cancer stem cells (2), reduced let-7 miRNAs (miRs) in breast tumor-initiating cells (3), and the ability of polycomb oncogene bmi-1 to maintain proliferative potential of leukemic stem cells (4). Furthermore, most cancer stem cells lose the self-renewal property as they differentiate into progeny and/or daughter cells that form the entire heterogeneous tumor cell population (5). Such information suggests a close relationship exists between self-renewal and differentiation. Evidence continues to arise that multi-layered regulatory networks that consist of extrinsic signaling pathways (6), genetic and epigenetic events (7, 8), and the cellular differentiating process (9) may be targets for blocking self renewal of cancer stem cells.
The cancer stem cell theory predicts that, even if “conventional” cancer cells are eliminated by drugs or radiation, it is only when the stem cells are destroyed that a full recovery is possible. Increasing evidence points to the ability of both drugs and bioactive food components to modify the self-renewal capabilities of cancer stem cells (10–16). Similar to drugs, diverse dietary constituents including vitamin A in liver, EGCG and theanine in tea, sulforaphane in cruciferous vegetables, vitamin D in fish, curcumin in curry spices, genistein in soy, and choline in eggs have been reported to influence the self-renewal of cancer stem cells (11–19). This review focuses on the mechanisms by which bioactive food components may modify one or more of molecular targets in self-renewal processes.
I. Evidence demonstrating bioactive food components influence niche signaling pathways
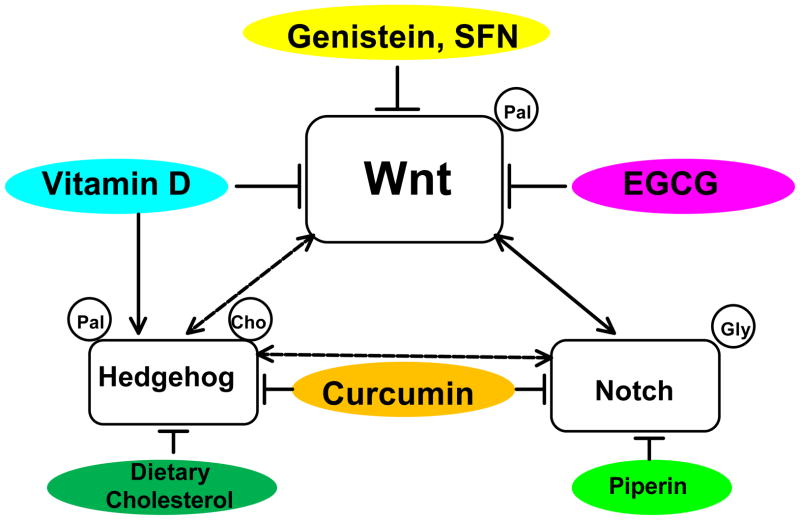
Uncontrolled self-renewal process may be initiated by deregulated developmental signals that arise from special extracellular microenvironments known as niches (1). The loss of tight regulation in self-renewal signals including Wnt, Notch, and Hedgehog pathways are considered as a bona fide characteristic of cancer stem cells (20). For example, the aberrant activation of Notch signaling together with the over-expression of Wnt1 transforms primary human mammary epithelial cells into tumor initiating cells (21). The next 3 sections highlight the ability of specific dietary components to modulate these niche signaling pathways and thereby influence the self-renewal process (Figure 1).
Figure 1.
Modulating effects of bioactive food components on niche signaling pathways and crosstalks among them in cancer stem cells. The self-renewal characteristic of stem cells is maintained by various niche signaling pathways including Wnt, Notch, and Hedgehog and modified by direct and/or indirect interactions among different signaling pathways in this regulation. A number of bioactive food components including genistein, sulforaphane, vitamin D, EGCG, curcumin, dietary cholesterol, and piperin are shown to inhibit the abnormally upregulated Wnt signaling pathway in cancer stem cells. Dotted lines indicate likely events without evidence yet. Arrows represent stimulation and lines with blunted ends indicate inhibition. Palmitic acid, cholesterol, and glycine shown inside the circle indicate the modification of ligands for the activation of the pathway. The left-side of ligands indicates the amino-terminal and the right-side, carboxyl terminal. SFN: sulforaphane, EGCG: (−)-epigallocatechin-3-gallate, pal: palmitic acid, gly: glycine, cho: cholesterol.
1. Dietary constituents modulate the Wnt pathway
Recent reports indicate that among the niche signals the Wnt pathway controls self-renewal of stem cells at various organ sites including embryonic, hematopoietic, colon, hepatic, breast, prostate, and skin in both normal and cancer stem cells (22). Although all these cells may utilize this pathway, activation of Wnt signaling is limited to a sub-population of cells that display cancer stem properties (23). Therefore, it is likely that cancer stem cells serve as key targets for modulating effects of dietary compounds on the Wnt pathway for cancer prevention.
Vitamin D increases Wnt inhibitor DKK-1 expression
Interestingly, the oncogenic effects of β-catenin observed in tumor cells consisting of both stem and non-stem cancer cells can be reduced by 1, 25-dihydroxyvitamin D3, possibly through regulatory processes that ultimately interfere with the aberrant activation of the Wnt pathway (Figure 2). For example, 2-day treatment with 1, 25-dihydroxyvitamin D3 (100 nM) has been shown to modulate the expression of Wnt signaling inhibitors including DKK-1 and DKK-4 in human SW480-ADH colon cancer cells. The former is commonly down-regulated and the latter is up-regulated in colon tumor cells (15, 16). Vitamin D in its active form 1, 25-dihydroxyvitamin D3 has been reported to increase tumor suppressive DKK-1 expression at the concentration of 100 nM (15) and decrease the oncogenic DKK-4 after as little as 1 nM exposure (16). Since physiological plasma 25-hydroxyvitamin D concentrations are approximately 100 nM (24), it is possible that normal dietary intake of this vitamin or sun light exposure may influence the activity of both genes and help harmonize to maintain the Wnt pathway under control.
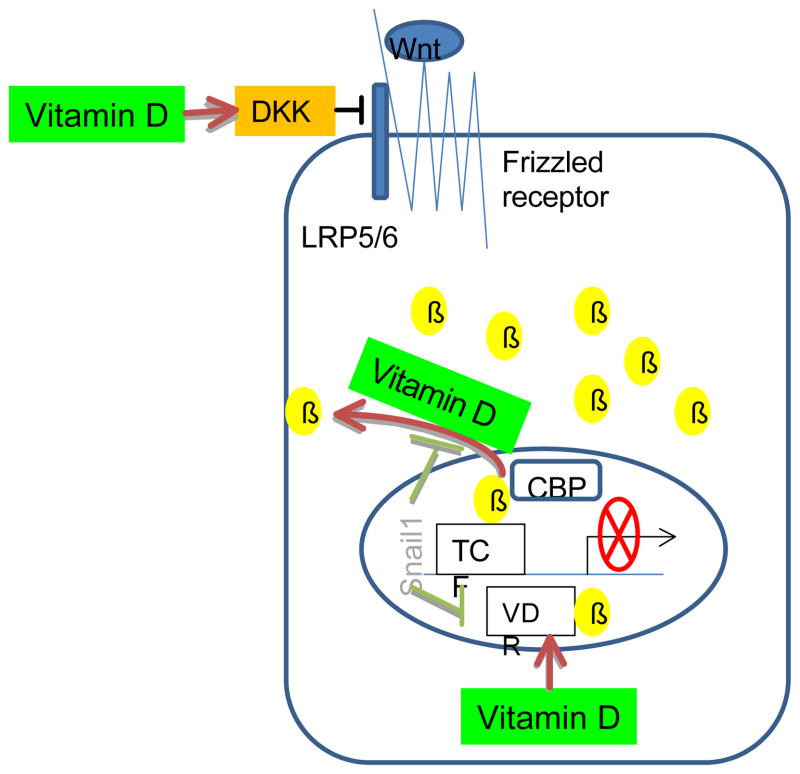
Figure 2.
Vitamin D suppresses the β-catenin-dependent canonical Wnt pathway in both stem and non-stem colon cancer cells. Vitamin D inhibits the aberrant activation of Wnt signals by stimulating Wnt repressor DKK, exporting β-catenin from nucleus to cytosol, and interefering with the TCF/ β-catenin mediated oncogene expression via the formation of VDR- β-catenin complex. The suppressive effects of vitamin D on the Wnt pathway may also arise from the indirect interference with Snail, a transcription factor that enhances Wnt signaling. DKK: dickkopf, β: β-catenin, CBP: CREB binding protein, TCF: T-cell factor, VDR: vitamin D receptor.
The same concentration 100 nM of 1,25-dihydroxyvitamin D3 has also been shown to promote the translocation of β-catenin from nucleus to plasma membrane and thereby inhibit the expression of β-catenin-responsive genes in colon cancer cells (25). The nuclear export of β-catenin induced by 1, 25-dihydroxyvitamin D3 can be abolished by the transcription factor Snail1 which causes a repression in VDR expression, at least in SW480-ADH colon cancer cells (Figure 2, 26). These results suggest that the anti-carcinogenic effects of 25-hydroxyvitamin D against colon cancer (27) may arise, at least in part, from its ability to suppress tumorigenic β-catenin actions in the Wnt pathway.
Finally, dietary vitamin D was shown to enhance the formation of the complex between vitamin D receptor (VDR) and β-catenin in the nucleus and thus compete with another transcription factor TCF4 for ligands thereby repressing β-catenin/TCF4-mediated gene transcription (28). In a primary keratinocyte stem cell model, the unliganded VDR was shown to be critical for the cooperative transcriptional interactions of β-catenin and Lef 1 (28). Furthermore, it appears that the level of VDR expression in the host influences the self-renewal property of kerotinocyte stem cells dramatically. Keratinocyte stem cells isolated from the VDR-null mice developed significantly fewer sphere forming colonies after 4 weeks of culture compared with those isolated from their wild-type littermates, suggesting the VDR is required for the self-renewal of bulge keratinocyte stem cells (28).
Wnt signaling antagonism may also be involved
Wnt signaling antagonists such as secreted frizzled-related protein 2 (sFRP2) have been reported to increase the self-renewal of mesenchymal stem cells and thereby lead to superior tissue engraftment and enhanced wound healing (29). Recent genome-wide expression profiling of rat mammary epithelial cells indicated that lifetime feeding of genistein (250 mg/kg/d), a bioactive constituent in soy, increased expression of sFRP2 (30) and thus might account for a reduction in stem cell self-renewal. Since mutations in APC or β-catenin that are key components of canonical Wnt pathways are rare in breast cancer that may be initiated by its cancer stem cells, we speculate that the constitutively activated Wnt signaling in breast tumors may be due to the lack of antagonistic regulation. The re-expression of sFRP2 by genistein is particularly important in the context that the down-regulation of sFRP2 is a frequent event in breast cancer (31, Figure 3).
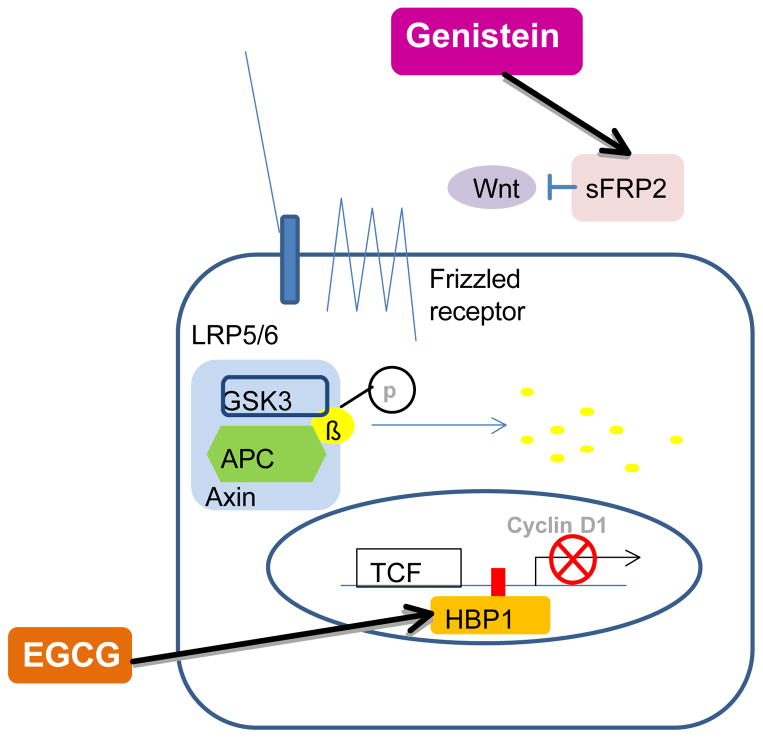
Figure 3.
The possible effects of soy isoflavone genistein and tea polyphenolic compound EGCG on the Wnt pathway in breast cancer stem cells. Genistein re-expresses sFRP2 that is a Wnt antagonist and EGCG promotes the expression of HBP1 transcription factor that blocks TCF mediated cyclin D1 expression in breast cancer cells. sFRP: secreted frizzled-related protein, EGCG: (−)-epigallocatechin-3-gallate, GSK: glycogen synthase kinase, APC: adenomatous polyposis coli, TCF: T-cell factor
It is also possible that the up-regulated Wnt pathways in breast cancer cells may follow β-catenin-independent non-canonical pathways that transduce the signals down to the Dishevelled- / Ca++-dependent cascades facilitating proliferation. Admittedly, the hypothesis that genistein influences a non-canonical Wnt pathway, which may contribute to the depression in self-renewal of breast cancer stem cells needs additional attention. Since the anti-carcinogenic effects of genistein have been shown to be dependent upon estrogen activity (32), and since the effects seem to be time-dependent (33, 34), multiple factors must be considered to delineate the interaction between genistein and breast cancer stem cells.
In fact, the involvement of genistein with stem cell behavior was observed in leukemic cells almost 20 years ago. Among multiple functions of genistein, its inhibitory effects on protein tyrosine kinases were examined in patients with chronic myelogenous leukemia (CML) that represents dysregulated tyrosine kinase activity in its hematopoietic stem cells. The treatment of long-term culture-initiating cells from CML with genistein (200 μmol/L for 18 hours) decreased the self-renewal of cancer stem cells, but not normal stem cells, by more than half (18). While the results suggest a significant role of genistein in reducing cancer stem cells, a couple of issues including the amount used (pharmacological vs. nutritional) and the unclarified mechanism(s) for its action remain to be addressed.
Wnt receptor Lrp5 plays a role in self-renewal
In mammary gland, knock-outs of Wnt receptor Lrp5 (Lrp5−/−) exhibit fewer terminal end buds and diminished side branching in mammary stem/progenitor cells suggesting this pathway is critical for maintaining the ductal stem cell activity (35). Recently, it has been shown that the Wnt pathway is blocked by EGCG in breast cancer cells (Figure 3). The addition of EGCG (25 to 100 μM) to cultured MDA-MB-231 human breast cancer cells suppressed Wnt in a dose-dependent manner (36). At 100 μM, over a 50% depression was observed according to the Wnt stimulated reporter assay. The reduction appeared to involve the induction of HMG box-containing protein 1 (HBP1) transcriptional factor which is a recognized suppressor of Wnt signaling. Quantative real-time RT-PCR analysis revealed that the endogenous HBP1 mRNA was induced 2- and 4-fold by 50 μM and 100 μM EGCG respectively. No changes were observed with either β-catenin or GSK-3β. The biological consequences of EGCG-induced HBP1 expression includes a suppression in c-myc that is a Wnt signaling target gene and has been shown to be constitutively up-regulated in breast cancer cells (36). A new study confirmed that the role of Wnt pathway in highly enriched mammary stem cells in culture is the maintenance of their self-renewing property (37). We speculate that effects of EGCG on Wnt stem pathway in breast cancer cells are associated with the modulation of the self-renewal property of mammary stem cells.
Other effects of EGCG on cancer stem cells were examined in a couple of sites including blood and neurons (38, 39). In circulating systems, the proliferation of leukemic blast cells isolated from acute myeloid leukemia (AML) patients which are premature and usually do not appear in normal conditions was significantly suppressed in response to 100 uM EGCG treatment (38). Likewise, the treatment of normal neural stem cells isolated from rat embryonic cortical neurons with 20μg/mL EGCG for 24 hrs dramatically inhibited their ability to attach and differentiate (39). Nevertheless, the specific mechanism involved with this response remains unresolved.
Sulforaphane, a cruciferous vegetable component, may eliminate breast cancer stem cells by modulating Wnt signaling
Sulforaphane, a promising dietary cancer preventive agent abundant in cruciferous vegetables and known to target antioxidant transcription factor Nrf2 (40), has been shown to suppress the mammosphere formation of stem cells isolated from estrogen receptor positive MCF-7 and estrogen receptor negative SUM159 breast cancer cells with an IC50 of approximately 0.5–1 μmol/L (41). This concentration is about 10 fold lower than that exihibiting its antiproliferative effects. Furthermore, when tumor bearing animals were supplemented with 50 mg/kg sulforaphane for 2 weeks, the tumor size was significantly reduced by half and that was correlated with the decreased stem population isolated using Aldefluor assay (41). The mechanism explaining how SFN suppresses breast cancer stem cells is unclear because with the same concentration, no changes in β-catenin or CDK1 were observed (41). Nevertheless, evidence exists that this level can be physiologically achievable. Plasma concentrations of sulforaphane equivalents appear to peak between 0.94 and2.27 μmol/L in humans within 1 hour after a single dose of 200 μmol broccoli sprout isothiocyanates (42).
Overall these investigations suggest that multiple sites of Wnt signaling pathway, especially antagonizing molecules, are modulated by various bioactive food components including vitamin D, genistein, EGCG, and sulforaphane that are commonly consumed in diet. However, most studies have dealt with the effects of these dietary components on the self-renewal property of cancer cells that have not been enriched with cancer stem cell population. Therefore, additional attention may be warranted to examine and/or validate the efficacy of dietary components on the self-renewal property in the isolated cancer stem cell population with the surrounding niche environment. Furthermore, the mechanism for the event with the same concentration needs to be revealed.
2. Dietary effects on the Hedgehog pathway
Increasing evidence exists that the regulation of self-renewal property of pancreatic, glioma, and leukemia cancer stem cells involves Hedgehog (Hh) signaling pathway (43–45). When human glioma-initiating cells were isolated on the basis of sphere formation and treated with inhibitors for Hedgehog, Notch, or Wnt, only the Hedgehog signaling inhibitors prevented the self-renewal of glioma-initiating cells (44). Likewise, it was reported that the loss of Hh receptor, Smo, impaired haematopoietic stem cell renewal and decreases induction of chronic myelogenous leukaemia (CML) by the BCR-ABL1 oncoprotein (45). The modification of these signals by lipid-related dietary components including cholesterol and curcumin is discussed below.
Oxidation of dietary cholesterol may positively regulate Hh signaling in mesenchymal stem cells
The activation of Hh ligands requires cholesterol at their carboxyl ends (46), which creates a plausible hypothesis that dietary cholesterol might regulate its signal-transduction. This hypothesis has been extensively investigated (47, 48) and reports that at least in the M2-10B4 pluripotent marrow stromal cell line (mesenchymal stem cells), specific derivatives of cholesterol such as 20-OH-cholesterol and 22-OH-cholesterol increase the expression of Hh target genes (49). While the mechanism explaining how these oxidative species stimulate Hh signaling remains unclear, the effects of cholesterol derivatives were shown to be abolished by the addition of 4 μM cyclopamine, an inhibitor of the Hh pathway, in culture (49). Since the oxidative status of cholesterol can be modulated by endogenous reactive oxygen species that are frequently altered by bioactive food components, the impact of diet on the Hh pathway in these cells and their self-renewal properties is warranted.
Cholecalciferol binds to Hedgehog (Hh) Smo receptor in culture but not in vivo
It has been suggested that one of the vitamin D isoforms, vitamin D3 (cholecalciferol), is an antagonist of the Hh signaling pathway (50). Vitamin D3 binding to the Smo receptor appears to result in down-regulation of Hh signaling pathways in both MDA-MB-231 breast tumour cells and the C3H/10T1/2 fibroblasts possibly explaining this change in signaling. Interestingly the magnitude of inhibition of oncogenic Smo activity conferred by 1 μM vitamin D3 was stronger than that of 10 μM cyclopamine in the Ptch1- transfected C3H/10T1/2 cells (51). However, with in vivo models vitamin D3 was not found to be effective against tumor cell growth (52). This discordance may stem from three possible causes. One, the concentration required for the changes in the Gli transcription factor to induce ductal hyperplasia is rather high, observed at 100 μM vitamin D3 (50). Healthy women typically have plasma 25-hydroxyvitamin D concentration in the 40 to 50 nM range (53). Thus, the physiological relevance of these high exposures remains questionable. The recent study reports the lack of tumor suppressive effects of vitamin D3 (10 μM) against pancreatic cancer in vivo but not in vitro serves as additional justification for further investigations (52). Second, the production of the Smo inhibitor demonstrated in an in vitro study (50) was reduced by pravastatin and rescued by mevalonate, which suggests that it could be an endogenously synthesized sterol derivative. Thus, vitamin D that must be derived from either nutritional sources or UV irradiation of 7-dehydrocholesterol in the skin, may not be the molecular species that caused Smo inhibition. Third, it is possible that Shh signaling may work through a paracrine mechanism (54). While it is true that pancreatic tumors overexpress Shh ligands, these may work indirectly by stimulating other growth factors or through stromal cells. At this point, the consequence of adding Shh inhibitors deserves additional attention.
Curcumin is shown to block Hedgehog (Hh) signaling in stem-like fractions of pancreatic and brain tumor cells
In addition to vitamin D, a few commonly found dietary polyphenolics including curcumin in tumerics were shown to inhibit Hedgehog signaling in transgenic adenocarcinoma of the mouse prostate as monitored by glioma associated 1 (Gli1) mRNA or Gli reporter activity with IC50 values ranging from <1 to 25 μM (55). These dietary components are known to reduce various cancer risks, which suggests that the inhibition of Hh pathway may serve at least partially as the mechanism for their suppressive effects on tumor cell growth.
One of these compounds, curcumin is well known for its poor bioavailability, administration of 3.6 g oral curcumin daily for 4 months resulted in about 10 nanomolar concentrations in the peripheral blood of advanced colon cancer patients (56). One approach taken to increase the bioavailability of curcumin was the reduction of the particle size to increase the surface area, which makes it more soluble. Investigators engineered encapsulated nanoparticle curcumin with more than 100 folds higher bioavailability than traditional curcumin and tested for its efficacy in xenograft models of human pancreatic cancer that overexpresses Hh pathway (57). In pancreatic cancer, cells enriched for cancer initiating stem cells exihibit drug resistance, metastatic properties, and more than 40-fold higher expression of Shh compared with normal pancreatic epithelial cells (58). A single administration of 25 mg/kg of nanoparticle curcumin combined with gemcitabine completely abrogated systemic metastases, which did not occur with a treatment using gemcitabine only (57). It also has been shown that the treatment of brain cancer stem cells with nanoparticle-encapsulated curcumin inhibited their growth, self-renewal, and clonogenicity by blocking Hh signaling (59). These results suggest that curcumin may suppress cancer stem properties of Hh signaling pathway that leads to drug-resistance and metastases. Future studies on the mechanism and the tissue specificity of curcumin effects on the Hh pathway are warranted.
3. Dietary influence on Notch signaling pathway
Notch is a receptor that is activated by removing its extracellular domain upon binding its ligands including Delta-1, -3, -4, and Jagged-1 and -2. The activated intracellular domain (NICD) migrates to the nucleus, binds to a transcription factor CSL (CBF1/RBPjk in vertebrates, suppressor of hairless in Drosophila, and LAG superfamily in C. elegans) and triggers the expression of Notch target genes such as Hairy Enhancer-of-Spirit (Hes) that activates the self-renewal of stem cells (60, 61). The critical role of Notch signaling in tumor initiating cells was demonstrated in breast cancer stem cells. Notch4 receptor activity was shown to be 8-fold higher in stem cell-enriched breast cancer cell population compared with differentiated cells (62). Furthermore, an inhibitor of its receptor, N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S- phenylglycine t-butyl ester (DAPT), was shown to reduce mammosphere formation in a pre-invasive ductal carcinoma in situ (61).
Curcumin and piperin modulate Notch
While evidence exists that activated Notch signaling impairs mammary stem cell self-renewal and promotes the proliferation of alveolar epithelial progenitors (63), the Notch alone does not seem to be sufficient. However, its activation together with the expression of Wnt1 is recognized to transform primary human mammary epithelial cells (64–66). In addition, the recent finding that there is a cross-talk between Notch and estrogen receptor-α or her2/neu (67) suggests that the development of human breast cancer may arise from the network that is wired with the cross-communication between and/or among individual signaling pathways. Recently, two dietary polyphenolics, curcumin and piperin, were found to inhibit the mammosphere formation of breast cancer cells by half when provided at 5 μM and completely at 10 μM (17). Nevertheless, it remains unclear if the suppressive effects of curcumin and piperin on the mammosphere formation come from the direct interaction with a specific pathway such as Notch or via the generation of secondary alterations in Wnt pathways.
II. Dietary modulation of self-renewal gene expression by epigenetic events
In mammalian cells, genes are surrounded in chromatin by various components including histones, associated proteins such as Polycomb group repressive complex (PRC), and miRNAs within an exclusive structure. These components are recognized to influence gene expression patterns without changing DNA base pairs, which is known as epigenetic programming. Interestingly, the chromatin of stem cells is extremely plastic and hyperdynamic compared to differentiated cells. It contains both activating and repressive types of histone modifications (68). Evidence exists that deregulation of epigenetic chromatin programming in normal stem cells influences their self-renewal property, which leads to tumor initiation and propagation (69). Those include changes in chromosomal structure and alterations in various miRs that are expressed in a cell-type and time-specific manner.
1. EGCG modulates chromosomal structure in cancer stem cells
PRCs consist of two distinct groups, PRC1 and PRC2, which work coordinately. PRC1 recruits histone deacetylase and thereby facilitates the methylation of K27 of histone H3. PRC2 subsequently binds to the methylated K27 of H3 and suppresses gene expression (70). Mice deficient in one of the PRC1 genes, Bmi-1, exhibited a post-natal self-renewal defect that led to the depletion of stem cells in blood, ovary, and neurons by early adulthood (71). Bmi-1 is highly expressed in cancer stem cells such as leukemia, neuroblastomas, and skin cancer, which is accompanied by the decreased expression of p16Ink4a and p19Arf tumor suppressor genes (72–74). Recently, Bmi-1 was reported to be influenced by (−)–epigallocatechin-3-gallate (EGCG), a bioactive food component in green tea (12). Treatment of a transformed cell line derived from squamous cell carcinoma (SCC-13 cells) with 40 μM EGCG for 24 hours reduced Bmi-1 levels that coincided with an inhibition of skin carcinogenesis (12). Human studies reveal that a plasma concentration higher than 7.5 μM of tea polyphenols including EGCG is required for antioxidant activity (75). Thus, it remains to be determined if the lower concentrations are sufficient to change Bmi-1 gene expression. The EGCG-induced decrease in Bmi-1 level was associated with reduced levels of cell cycle-dependent kinases 1, 2, and 4 and cyclins D1 and E (76), which suggests that this tea polyphenolic compound may suppress the accelerated cell cycle progression in these transformed cells.
2. Theanine in black tea may help maintain the self-renewal of human embryonic stem cells
Interestingly, high-throughput screening of undifferentiated human embryonic stem cells identified theanine, a specific amino acid in black tea, was highly effective in maintaining the self-renewal property of these cells (13). Human embryonic stem cells (hESCs) were seeded into the 384-well plates, 6000 cells per well, and the cells were imaged with automated microscopy at 7 days after plating. Oct4 expression was used as a biomarker for the stem cell property. hESCs stimulated by fibroblast growth factor 2 (FGF2), a key growth factor that maintains the balance between proliferation and self-renewal signals in these cells, were used as a positive control. This new technology provided evidence that the supplementation of culture media with10 μM theanine increased Oct4 expression to the level comparable to that achieved by FGF2. While these results suggest that a dietary component in commonly consumed black tea modulates the expression of a self-renewal regulatory gene Oct4, the mechanism of action including epigenetic modulation remains to be established.
3. Diet-induced rearrangements of microRNAs (miRs) in cancer stem cells
Stem cell self-renewal and differentiation are known to be associated with various miRs that are expressed in a cell-type- and time-specific manner (77). MiRs are non-coding RNAs that are short fragments between 18 and 25 nucleotides long and generated from the endogenous RNA containing a local hairpin structure (78). These RNAs are abundant in mammals and have various roles during developmental processes (79). Changes in miR levels are commonly observed in various cancer stem cells (80). For example, let-7 miRs are markedly reduced in cancer stem cells and increased following differentiation (3) suggesting that cancer stem cells may utilize miRs in controlling their cell identity. Recently, it has been shown that the inhibition of let-7 miR lead to the increased glucose metabolism and insulin sensitivity in mice (81).
MiR expression profiles can also be influenced by various dietary stimuli including high-fat, high-glucose, and methyl-deficiency (82–84). The expression of miR-143, a miR that plays a role in adipocyte differentiation, was up-regulated in high-fat diet-induced obese mice (82). Likewise, the expression of miR-30d that increases insulin gene expression was up-regulated in the presence of high glucose in the pancreatic β-cell line MIN6 (83). Finally, a folate deficiency in normal human lymphoblast cells TK6 was found to lead to lower S-adenosyl methionine levels, global genomic hypomethylation, and an increased, but reversible, expression of miR-222 (85). This change was confirmed in whole blood–derived RNA obtained from human subjects participated in a population-based case-control study of head and neck squamous cell carcinoma (86). The subjects in the lowest percentile of dietary folate intake had a significantly higher relative expression of this miR compared with those in the highest folate intake category. In rats, however, different species of miRs including let-7a and miR-17–92 responded to liver cancer induced by feeding a diet deficient in folic acid, methionine, and choline (84, 87). While the cause of these changes remains unknown, it is possible that dietary components may influence the developmental and/or tumorigenic processes involved with cancer stem cells and thereby bring about alterations in miR expression profiles. Since the changes induced by food components are highly dependent on the cell type examined, the functional relationship between diet-specific stimuli and the corresponding changes in miR expression profiles needs to be delineated.
III. Dietary components may modulate the differentiation of tumor initiating cells
1. The role of retinoic acids in stem cell differentiation
Retinoic acids were shown to directly interact with a developmental transcriptional factor Sox3 and thereby upregulate its expression that is critical determinant of neuronal differentiation (88). Human embryonal carcinoma cells that develop testicular germ cell tumors possess pluripotency and self-renewal ability. The removal of the stem properties by the addition of dietary component all-trans retinoic acid (10 μM) for 2 days results in a loss of tumorigenicity accompanied by G1 cell cycle arrest and the repressed expression of a cassette of genes on chromosome 12p in these cells (89). While the concentration used is pharmacological and the mechanism for these actions remains to be determined, it has been thought that the ability of vitamin A to induce differentiation as shown in NT2/D1 embryonal carcinoma cells (88) and suppress the self-renewal property involved with the lineage-specific transcription factor Nanog on chromosome 12p (89) may explain the observed outcome.
In culture of hematopoietic stem cells isolated using lin− c-kit+ sca-1+ markers, the effect of all-trans-retinoic acid on their self-renewal was examined (90). Supplementation of these primitive stem cells with 1 μM all-trans-retinoic acid enhanced their ability to repopulate and prevented their differentiation. Since all-trans retinoic acid can activate a number of nuclear receptors including retinoic acid receptors (RARs; -α, -β, and –γ), and peroxisome proliferator-activated receptor β/σ (PPARβ/σ), it remains unclear which of these participates in promoting the self-renewal property. The knockout mice of RARγ, but not RARα, exhibited markedly reduced numbers of hematopoietic stem cells in their bone marrow compared with wild-type mice (91). Furthermore, when the hematopoietic cells isolated from these mice were cultured in vitro with all-trans retinoic acid, they were no longer able to maintain the self-renewal. These results suggest that RARγ is critical for sustaining the ability of hematopoietic stem cells to maintain and self-renew. Considering the effects of vitamin A metabolites on developmental, differentiation, and self-renewal processes, the study examining the relationship between these dietary components and the core developmental transcription factors including Sox-3, Oct4, Nanog and GATA is warranted.
2. Choline may influence the differentiation of hepatic and neural stem cells
Choline is another dietary component that is involved with the differentiation of hepatic oval stem cells into the mesenchymal tumor tissue (92). The LE/6 hepatic stem cell line was derived from the liver of male Sprague-Dawley rats fed a choline-deficient diet and transplanted by subcutaneous injection into adult female nude mice. The results indicate that 22 out of 50 nude mice inoculated with LE/6 hepatic epithelial stem cells developed trans-differentiated mesenchymal tumors while the choline adequate did not develop any. These results suggest that choline may inhibit the differentiation process of hepatic oval stem cells from epithelial to mesenchymal and the choline deficiency may accelerate this process resulting in the tumor formation.
Choline is also known to be critical during fetal development, particularly during the differentiation of the brain (93). The area of the brain that seems to be the most sensitive to choline is reported to be the hippocampus where nerve cells continue to grow and differentiate into neurons throughout adulthood (94). Recently, it was shown that neurogenic zones could be reservoirs for brain tumor-initiating cells (95). In culture, choline deprivation caused decreased H3K9 methylation in E14 neural progenital cells (19). In animal models, choline deficiency interferes with nerve cell growth and neurongenesis, which results in decreased memory and learning ability (96). Choline deficiency during pregnancy was reported to be associated with the delivery of infants with neural tube defects, suggesting the significance of choline during this period of development (97). Overall, these findings suggest that investigations dealing with the effects of choline-deficiency on brain cancer stem cells are warranted.
3. Effects of dietary components on adipocyte differentiation
New adipocytes are considered to arise from the differentiation of mesenchymal stem cells (MSC), resident preadipocytes in adipocyte tissue, or bone marrow-derived circulating progenitor cells arrived at the adipose tissue. Interestingly, a couple of dietary constituents including fat and ceramide are shown to modulate the differentiation of adipocytes in various sites including adipose tissues or blood vessels (98–100). For example, high-fat feeding of wild-type C57BL/6 mice for 3 to 5 weeks was shown to promote the circulating adipocyte progenitor cells and their differentiation into adipocytes (98). High-fat (42% calories from fat) feeding of 8-week-old male C57BL/6J mice for 2 weeks also brought about the decreased differentiation and a heightened proinflammatory state in perivascular adipocytes, which was mediated via the activation of the nuclear hormone receptor PPAR-γ (99).
When murine mesenchymal stem cells were treated with low concentration of ceramide (10–25 μM), this dietary component was shown to inhibit the adipogenesis of mesenchymal stem cells (100). The critical role of ceramide in adipocyte differentiation was demonstrated in another study showing that the deprivation of ceramide caused increased formation of mesenchymal stem cells that can differentiate into adipocytes (101). Interestingly, these effects of ceramide on mesenchymal stem and adipocytes are shown to be reversible (102). Overall, the above mentioned results suggest that the differentiation of stem cells into adipocytes seems to be suppressed by an adequate amount of dietary ceramide and enhanced by a high-fat diet. Further research on the prevention of adipocyte differentiation/accumulation using dietary strategies should be a promising research frontier.
Conclusions
One of the potential targets for bioactive food components is that associated with self-renewal properties of cancer stem cells though the majority of support arises from cell culture studies at this point. While the origin of self-renewal properties remains unclear, evidence continues to surface that some bioactive food components can modulate various steps in the process and help prevent cancer regeneration/recurrence. The characteristic sites for these dietary constituents include Dkk-1 and sFRP2 that are Wnt antagonists, the activation of Hh ligands, Notch target gene expression, Bmi-1 that induces a suppressive histone modification, miRs that prevent the expression of self-renewal genes, and the EMT that determines the differentiation of mesenchymal stem cells, all of which can block the uncontrolled self-renewal of cancer stem cells. Adequate consumption of specific food items including vitamins A and D, genistein, EGCG, sulforaphane, theanine, curcumin, choline and possibly many others may suppress the chaotic property of cancer stem cells to self-renew. However, greater attention is needed to determine the minimum amounts required and specific circumstances that would derive the benefits from these agents. The identification of cancer stem cell models that provide clues about niche signaling as well as stem properties will assist in defining which dietary components are most important in modifying cancer stem cell self renewal. Likewise, a greater understanding of how cross talk among stem pathways influences the overall response and what quantities of food components are needed to elicit a biological response in these cells is urgently needed. Unquestionably, a diet-induced shift from deregulation to regulation in cancer stem cells could have profound influence on cancer relapses and therefore of immense societal importance.
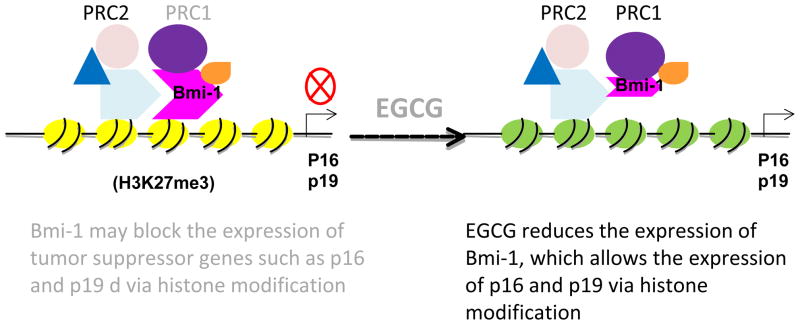
Figure 4.
EGCG reduces the expression of Polycomb group protein Bmi-1 and thereby may antagonize its oncogenic activity in skin cancer stem cells. Bmi-1 is a member of Polycomb repressive complex 2 (PRC2) that alters the histone structure into a suppressive form (H3K27me3), which down-regulates the expression of tumor suppressor genes including p16 and p19. EGCG reduces the expression of oncoprotein Bmi-1 and thus prevents the self-renewal of skin cancer stem cells. Yellow circles indicate suppressed histones and green, re-activated histones. PRC: polycomb repressive complex, Bmi-1: B-cell-specific moloney murine leukemia virus integration site 1 (Bmi-1), EGCG: (−)-epigallocatechin-3-gallate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katoh M. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 2007;3:30–8. doi: 10.1007/s12015-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 2.Haegebarth A, Clevers H. Wnt Signaling, Lgr5, and Stem Cells in the Intestine and Skin. Am J Pathol. 2009;174:715–21. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 4.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–60. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 5.Southall TD, Egger B, Gold KS, Brand AH. Regulation of Self-renewal and Differentiation in the Drosophila Nervous System. Cold Spring Harb Symp Quant Biol. 2008;73:523–8. doi: 10.1101/sqb.2008.73.051. [DOI] [PubMed] [Google Scholar]
- 6.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–4. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–53. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 8.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116–25. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, Allemand I, Riou L, Fouchet P. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–6. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 10.McCormack MP, Young LF, Vasudevan S, de Graaf CA, Codrington R, Rabbitts TH, Jane SM, Curtis DJ. The Lmo2 oncogene initiates leukemia in mice by inducing thymocyte self-renewal. Science. 2010;327:879–83. doi: 10.1126/science.1182378. [DOI] [PubMed] [Google Scholar]
- 11.Ginestier C, Wicinski J, Cervera N, Monville F, Finetti P, Bertucci F, Wicha MS, Birnbaum D, Charafe-Jauffret E. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle. 2009;8:3297–302. doi: 10.4161/cc.8.20.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balasubramanian S, Adhikary G, Eckert RL. The Bmi-1 polycomb protein antagonizes the (−)-epigallocatechin-3-gallate-dependent suppression of skin cancer cell survival. Carcinogenesis. 2010;31:496–503. doi: 10.1093/carcin/bgp314. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 13.Desbordes Sabrina C, Placantonakis Dimitris G, Ciro Anthony, Socci Nicholas D, Lee Gabsang, Djaballah Hakim, Studer Lorenz. High-Throughput Screening Assay for the Identification of Compounds Regulating Self-Renewal and Differentiation in Human Embryonic. Stem Cells Cell Stem Cell. 2008;2:602–612. doi: 10.1016/j.stem.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–90. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilera O, Peña C, García JM, Larriba MJ, Ordóñez-Morán P, Navarro D, Barbáchano A, López de Silanes I, Ballestar E, Fraga MF, Esteller M, Gamallo C, Bonilla F, González-Sancho JM, Muñoz A. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–84. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 16.Pendás-Franco N, García JM, Peña C, Valle N, Pálmer HG, Heinäniemi M, Carlberg C, Jiménez B, Bonilla F, Muñoz A, González-Sancho JM. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–77. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 17.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–85. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlo-Stella C, Dotti G, Mangoni L, Regazzi E, Garau D, Bonati A, Almici C, Sammarelli G, Savoldo B, Rizzo MT, Rizzoli V. Selection of myeloid progenitors lacking BCR/ABL mRNA in chronic myelogenous leukemia patients after in vitro treatment with the tyrosine kinase inhibitor genistein. Blood. 1996;88:3091–100. [PubMed] [Google Scholar]
- 19.Mehedint Mihai G, Niculescu Mihai D, Craciunescu Corneliu N, Zeisel Steven H. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010;24:184–195. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 21.Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP, Brisken C. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103:3799–804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–41. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korkaya H, Wicha MS. Cancer stem cells: nature versus nurture. Nat Cell Biol. 2010;12:419–21. doi: 10.1038/ncb0510-419. [DOI] [PubMed] [Google Scholar]
- 24.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 25.Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Muñoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–87. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larriba MJ, Valle N, Pálmer HG, Ordóñez-Morán P, Alvarez-Díaz S, Becker KF, Gamallo C, de Herreros AG, González-Sancho JM, Muñoz A. The inhibition of Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr Relat Cancer. 2007;14:141–51. doi: 10.1677/ERC-06-0028. [DOI] [PubMed] [Google Scholar]
- 27.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S, Tjønneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Kaaks R, Linseisen J, Boeing H, Bergmann MM, Trichopoulou A, Misirli G, Trichopoulos D, Berrino F, Vineis P, Panico S, Palli D, Tumino R, Ros MM, van Gils CH, Peeters PH, Brustad M, Lund E, Tormo MJ, Ardanaz E, Rodríguez L, Sánchez MJ, Dorronsoro M, Gonzalez CA, Hallmans G, Palmqvist R, Roddam A, Key TJ, Khaw KT, Autier P, Hainaut P, Riboli E. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci U S A. 2007;104:9428–33. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfaro MP, Vincent A, Saraswati S, Thorne CA, Hong CC, Lee E, Young PP. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J Biol Chem. 2010;285:35645–53. doi: 10.1074/jbc.M110.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su Y, Simmen FA, Xiao R, Simmen RC. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol Genomics. 2007;30:8–16. doi: 10.1152/physiolgenomics.00023.2007. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Toyota M, Carraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T, Mori M, Hirata K, Imai K, Shinomura Y, Baylin SB, Tokino T. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer. 2008;98:1147–56. doi: 10.1038/sj.bjc.6604259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;16:219–26. doi: 10.1007/s10787-008-8020-0. [DOI] [PubMed] [Google Scholar]
- 33.Messina M, Wu AH. Perspectives on the soy-breast cancer relation. Am J Clin Nutr. 2009;89:1673S–1679S. doi: 10.3945/ajcn.2009.26736V. [DOI] [PubMed] [Google Scholar]
- 34.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281:35081–7. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–75. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 37.Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asano Y, Okamura S, Ogo T, Eto T, Otsuka T, Niho Y. Effect of (−)-epigallocatechin gallate on leukemic blast cells from patients with acute myeloblastic leukemia. Life Sci. 1997;60:135–42. doi: 10.1016/s0024-3205(96)00603-0. [DOI] [PubMed] [Google Scholar]
- 39.Chen CN, Liang CM, Lai JR, Tsai YJ, Tsay JS, Lin JK. Capillary electrophoretic determination of theanine, caffeine, and catechins in fresh tea leaves and oolong tea and their effects on rat neurosphere adhesion and migration. J Agric Food Chem. 2003;51:7495–503. doi: 10.1021/jf034634b. [DOI] [PubMed] [Google Scholar]
- 40.Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–90. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 43.Hidalgo M, Maitra A. The hedgehog pathway and pancreatic cancer. N Engl J Med. 2009;361:2094–6. doi: 10.1056/NEJMcibr0905857. [DOI] [PubMed] [Google Scholar]
- 44.Takezaki T, Hide T, Takanaga H, Nakamura H, Kuratsu JI, Kondo T. Essential role of the Hedgehog signaling pathway in human glioma-initiating cells. Cancer Sci. 2011;102:1306–12. doi: 10.1111/j.1349-7006.2011.01943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, Munchhof M, VanArsdale T, Beachy PA, Reya T. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–9. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang X, Litingtung Y, Chiang C. Region-specific requirement for cholesterol modification of sonic hedgehog in patterning the telencephalon and spinal cord. Development. 2007;134:2095–105. doi: 10.1242/dev.000729. [DOI] [PubMed] [Google Scholar]
- 47.Eaton S. Multiple roles for lipids in the Hedgehog signalling pathway. Nat Rev Mol Cell Biol. 2008;9:437–45. doi: 10.1038/nrm2414. [DOI] [PubMed] [Google Scholar]
- 48.Steinhauer J, Treisman JE. Lipid-modified morphogens: functions of fats. Curr Opin Genet Dev. 2009;19:308–14. doi: 10.1016/j.gde.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282:8959–68. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 50.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:1397–1410.43. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bijlsma MF, Peppelenbosch MP, Spek CA. (Pro-)vitamin D as treatment option for hedgehog-related malignancies. Med Hypotheses. 2008;70:202–3. doi: 10.1016/j.mehy.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Brüggemann LW, Queiroz KC, Zamani K, van Straaten A, Spek CA, Bijlsma MF. Assessing the efficacy of the hedgehog pathway inhibitor vitamin D3 in a murine xenograft model for pancreatic cancer. Cancer Biol Ther. 2010;10:79–88. doi: 10.4161/cbt.10.1.12165. [DOI] [PubMed] [Google Scholar]
- 53.Markestad T. Plasma concentrations of vitamin D metabolites in unsupplemented breast-fed infant. Eur J Pediatr. 1983;141:77–80. 40. doi: 10.1007/BF00496794. [DOI] [PubMed] [Google Scholar]
- 54.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 55.Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382–90. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 56.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 57.Bisht S, Mizuma M, Feldmann G, Ottenhof NA, Hong SM, Pramanik D, Chenna V, Karikari C, Sharma R, Goggins MG, Rudek MA, Ravi R, Maitra A. Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol Cancer Ther. 2010;9:2255–64. doi: 10.1158/1535-7163.MCT-10-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelleher FC. Hedgehog signaling and therapeutics in pancreatic cancer. Carcinogenesis. 2011;32:445–51. doi: 10.1093/carcin/bgq280. [DOI] [PubMed] [Google Scholar]
- 59.Lim KJ, Bisht S, Bar EE, Maitra A, Eberhart CG. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther. 2011;11:464–73. doi: 10.4161/cbt.11.5.14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korkaya H, Wicha MS. HER-2, notch, and breast cancer stem cells: targeting an axis of evil. Clin Cancer Res. 2009;15:1845–7. doi: 10.1158/1078-0432.CCR-08-3087. [DOI] [PubMed] [Google Scholar]
- 61.Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev. 2007;3:169–75. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 62.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–18. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–41. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP, Brisken C. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103:3799–804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–7. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 66.Miele L. Rational targeting of Notch signaling in breast cancer. Expert Rev Anticancer Ther. 2008;8:1197–202. doi: 10.1586/14737140.8.8.1197. [DOI] [PubMed] [Google Scholar]
- 67.Rizzo P, Osipo C, Pannuti A, Golde T, Osborne B, Miele L. Targeting Notch signaling cross-talk with estrogen receptor and ErbB-2 in breast cancer. Adv Enzyme Regul. 2009;49:134–41. doi: 10.1016/j.advenzreg.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Tiwari VK, McGarvey KM, Licchesi JD, Ohm JE, Herman JG, Schübeler D, Baylin SB. PcG Proteins, DNA Methylation, and Gene Repression by Chromatin Looping. PLoS Biol. 2008;6:2911–27. doi: 10.1371/journal.pbio.0060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–9. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 70.Fischle W, Wang Y, Jacobs SA, Kim Y, Allis CD, Khorasanizadeh S. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev. 2003;17:1870–81. doi: 10.1101/gad.1110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grinstein E, Wernet P. Cellular signaling in normal and cancerous stem cells. Cell Signal. 2007;19:2428–33. doi: 10.1016/j.cellsig.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Mohty M, Szydlo RM, Yong AS, Apperley JF, Goldman JM, Melo JV. Association between BMI-1 expression, acute graft-versus-host disease, and outcome following allogeneic stem cell transplantation from HLA-identical siblings in chronic myeloid leukemia. Blood. 2008;112:2163–6. doi: 10.1182/blood-2008-04-148130. [DOI] [PubMed] [Google Scholar]
- 73.Cui H, Hu B, Li T, Ma J, Alam G, Gunning WT, Ding HF. Bmi-1 is essential for the tumorigenicity of neuroblastoma cells. Am J Pathol. 2007;170:1370–8. doi: 10.2353/ajpath.2007.060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang C, Toulev A, Kamarashev J, Qin JZ, Dummer R, Döbbeling U. Consequences of p16 tumor suppressor gene inactivation in mycosis fungoides and Sézary syndrome and role of the bmi-1 and ras oncogenes in disease progression. Hum Pathol. 2007;38:995–1002. doi: 10.1016/j.humpath.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 75.Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, Wang H, Go VL, Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–64. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 76.Balasubramanian S, Adhikary G, Eckert RL. The Bmi-1 polycomb protein antagonizes the (−)-epigallocatechin-3-gallate-dependent suppression of skin cancer cell survival. Carcinogenesis. 2010;31:496–503. doi: 10.1093/carcin/bgp314. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 77.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116–25. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–89. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shkumatava A, Stark A, Sive H, Bartel DP. Coherent but overlapping expression of microRNAs and their targets during vertebrate development. Genes Dev. 2009;23:466–81. doi: 10.1101/gad.1745709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zimmerman AL, Wu S. MicroRNAs, cancer and cancer stem cells. Cancer Lett. 2011;300:10–9. doi: 10.1016/j.canlet.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, Takeuchi A, Grasemann C, Rinn JL, Lopez MF, Hirschhorn JN, Palmert MR, Daley GQ. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet. 2010;42:626–30. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takanabe R, Ono K, Abe Y, Takaya T, Horie T, Wada H, Kita T, Satoh N, Shimatsu A, Hasegawa K. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem Biophys Res Commun. 2008;376:728–32. doi: 10.1016/j.bbrc.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 83.Tang X, Muniappan L, Tang G, Ozcan S. Identification of glucose-regulated miRNAs from pancreatic {beta} cells reveals a role for miR-30d in insulin transcription. RNA. 2009;15:287–93. doi: 10.1261/rna.1211209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tryndyak VP, Ross SA, Beland FA, Pogribny IP. Down-regulation of the microRNAs miR-34a, miR-127, and miR-200b in rat liver during hepatocarcinogenesis induced by a methyl-deficient diet. Mol Carcinog. 2009;48:479–87. doi: 10.1002/mc.20484. [DOI] [PubMed] [Google Scholar]
- 85.Marsit CJ, Eddy K, Kelsey KT. MicroRNA responses to cellular stress. Cancer Res. 2006;66:10843–8. doi: 10.1158/0008-5472.CAN-06-1894. [DOI] [PubMed] [Google Scholar]
- 86.Peters ES, McClean MD, Liu M, Eisen EA, Mueller N, Kelsey KT. The ADH1C polymorphism modifies the risk of squamous cell carcinoma of the head and neck associated with alcohol and tobacco use. Cancer Epidemiol Biomarkers Prev. 2005;14:476–82. doi: 10.1158/1055-9965.EPI-04-0431. [DOI] [PubMed] [Google Scholar]
- 87.Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, Jacob ST, Ghoshal K. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99:671–8. doi: 10.1002/jcb.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nikcević G, Savić T, Kovacević-Grujicić N, Stevanović M. Up-regulation of the SOX3 gene expression by retinoic acid: characterization of the novel promoter-response element and the retinoid receptors involved. J Neurochem. 2008;107:1206–15. doi: 10.1111/j.1471-4159.2008.05670.x. [DOI] [PubMed] [Google Scholar]
- 89.Giuliano CJ, Kerley-Hamilton JS, Bee T, Freemantle SJ, Manickaratnam R, Dmitrovsky E, Spinella MJ. Retinoic acid represses a cassette of candidate pluripotency chromosome 12p genes during induced loss of human embryonal carcinoma tumorigenicity. Biochim Biophys Acta. 2005;1731:48–56. doi: 10.1016/j.bbaexp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 90.Purton LE, Bernstein ID, Collins SJ. All-trans retinoic acid enhances the long-term repopulating activity of cultured hematopoietic stem cells. Blood. 2000;95:470–7. [PubMed] [Google Scholar]
- 91.Purton LE, Dworkin S, Olsen GH, Walkley CR, Fabb SA, Collins SJ, Chambon P. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med. 2006;203:1283–93. doi: 10.1084/jem.20052105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong H, Chen X, Xiang S, Liang H, Zhang W, Jing K, Zhang W, Zhang W, Chen L. The Epithelial-Mesenchymal Transition Promotes Trans-differentiation of Subcutaneously Implanted Hepatic Oval Cells into Mesenchymal Tumor Tissue. Stem Cells Dev. 2009;18:1293–8. doi: 10.1089/scd.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanders LM, Zeisel SH. Choline: Dietary Requirements and Role in Brain Development. Nutr Today. 2007;42:181–186. doi: 10.1097/01.NT.0000286155.55343.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeisel SH. Choline and brain development. In: Bowman BR, Russell RM, editors. Present Knowledge in Nutrition. 9. Vol. 1. ILSI Press; Washington, DC: 2006. pp. 352–360. [Google Scholar]
- 95.Kroonen J, Nassen J, Boulanger YG, Provenzano F, Capraro V, Bours V, Martin D, Deprez M, Robe P, Rogister B. Human glioblastoma-initiating cells invade specifically the subventricular zones and olfactory bulbs of mice after striatal injection. Int J Cancer. 2011;129:574–85. doi: 10.1002/ijc.25709. [DOI] [PubMed] [Google Scholar]
- 96.Mellott TJ, Kowall NW, Lopez-Coviella I, Blusztajn JK. Prenatal choline deficiency increases choline transporter expression in the septum and hippocampus during postnatal development and in adulthood in rats. Brain Res. 2007;1151:1–11. doi: 10.1016/j.brainres.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shaw GM, Finnell RH, Blom HJ, Carmichael SL, Vollset SE, Yang W, Ueland PM. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology. 2009;20:714–9. doi: 10.1097/EDE.0b013e3181ac9fe7. [DOI] [PubMed] [Google Scholar]
- 98.Crossno JT, Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–8. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–9. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu F, Yang CC, Gomillion C, Burg KJ. Effect of Ceramide on Mesenchymal Stem Cell Differentiation Toward Adipocytes. Appl Biochem Biotechnol. 2010;160:197–212. doi: 10.1007/s12010-008-8505-8. [DOI] [PubMed] [Google Scholar]
- 101.Guan F, Handa K, Hakomori SI. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc Natl Acad Sci U S A. 2009;106:7461–6. doi: 10.1073/pnas.0902368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guan F, Schaffer L, Handa K, Hakomori SI. Functional role of gangliotetraosylceramide in epithelial-to-mesenchymal transition process induced by hypoxia and by TGF-{beta} FASEB J. 2010;24:4889–903. doi: 10.1096/fj.10-162107. [DOI] [PMC free article] [PubMed] [Google Scholar]