Abstract
Background
Women exhibit an accelerated progression from first cannabis use to cannabis use disorder (CUD) and show pronounced negative clinical issues related to CUD relative to men. Whether sex-dependent differences in cannabis’ direct effects contribute to the heightened risk in women is unknown. This analysis directly compared cannabis’ abuse-related subjective effects in men and women matched for current cannabis use.
Methods
Data from four double-blind, within-subject studies measuring the effects of active cannabis (3.27–5.50% THC, depending on study) relative to inactive cannabis (0.00% THC) were combined for this analysis. Data from equal numbers of men and women from each study matched for current cannabis use were pooled (total n = 35 men; 35 women); cannabis’ effects were analyzed according to cannabis condition (active versus inactive) and sex.
Results
Active cannabis produced more robust subjective effects associated with abuse liability (‘Good,’ ‘Liking,’ ‘Take Again’) and intoxication (‘High,’ ‘Stimulated’) relative to inactive cannabis (p • 0.0001). Women reported higher ratings of abuse-related effects [‘Take Again’ and ‘Good’ (p • 0.05)] relative to men under active cannabis conditions but did not differ in ratings of intoxication. Active cannabis increased heart rate (p • 0.0001) equally for both sexes.
Conclusions
The results from this study suggest that when matched for cannabis use, women are more sensitive to the subjective effects related to cannabis’ abuse liability relative to men, which may contribute to the enhanced vulnerability to developing CUD. Thus, sex is an important variable to consider when assessing the development of CUD.
Keywords: Cannabinoids, Abuse liability, Sex-differences, Subjective effects
1. INTRODUCTION
Cannabis is the most widely used illicit drug worldwide (United Nations Office in Drugs and Crime, 2013), and has the highest rates of abuse in the United States relative to other illicit drugs, with 18.1 million people reporting use in the previous month, a number that has increased by over 20% since 2007 (Substance Abuse and Mental Health Services Administration, 2012a). In the United States, the number of people seeking treatment for cannabis use increased by 21% between 2000–2010 (Substance Abuse and Mental Health Services Administration, 2012b). With a growing number of states legalizing cannabis for medical and non-medical purposes (Hoffmann and Weber, 2010; National Conference of State Legislature, 2013), it is conceivable that increased access and legalization may lead to higher rates of use and increased risk of dependence. Elucidating the variables that contribute to the development of abuse and dependence can improve prevention and treatment for such disorders.
The estimated probability of initiating cannabis use and subsequently developing a cannabis use disorder (CUD) is higher in men (Wagner and Anthony, 2007). However, women show an accelerated progression from first use to CUD relative to men providing evidence for a ‘telescoping effect’ (Hernandez-Avila, 2004; Ehlers et al., 2010; Khan et al., 2013). Sex-differences have also been reported in the overall effects that daily cannabis use can have on physical and mental well-being; the quantity of cannabis smoked per day was shown to be negatively associated with self-reported measures of Quality of Life, an effect that was more pronounced in women than men (Lev-Ran et al., 2012). Additionally, men and women differ in the magnitude of withdrawal symptoms, with women exhibiting greater physiological symptoms associated with withdrawal relative to men (Copersino et al., 2010); this is a variable that is hypothesized to contribute to relapse in cannabis-dependent treatment seekers (Haney et al., 2013).
Assessing cannabis’ acute effects is an important determinant when investigating variables that contribute to continued cannabis use and the development of abuse and dependence. Preclinical studies with laboratory animals demonstrate that females are more sensitive to the behavioral and physiological effects of cannabinoids compared to males (for review, see Craft et al., 2013). Of note, female rats are more sensitive to the reinforcing effects of cannabinoids with faster acquisition of cannabinoid self-administration, higher rates of responding for cannabinoids (Fattore et al., 2007), and increased rates of cue and drug-induced reinstatement (Fattore et al., 2010).
Very little has been reported on sex-dependent differences in smoked cannabis’ effects in humans; our laboratory reported subtle differences in cannabis’ acute effects between men and women in a pattern that reflects preclinical studies, with women reporting higher ratings of cannabis’ positive subjective effects relative to men (Cooper and Haney, 2009). Females have also been reported to rate feeling ‘more dizzy’ after smoking cannabis or receiving intravenous THC; no sex-dependent differences were observed in cardiovascular endpoints or subjective ratings of intoxication (Mathew et al., 2003). However, sex-dependent effects of cannabinoids were not the primary focus of these study, therefore, they were not designed with adequate power to assess these effects. In addition to the small sample size, the men and women in these studies were not matched according to pattern of cannabis use, an important consideration when comparing the acute effects of cannabis between groups since tolerance to cannabis’ effects occurs with repeated use (Haney et al., 1997; Hart et al., 2002).
The objective of the current study was to directly compare cannabis’ subjective and cardiovascular effects in men and women matched for frequency and magnitude of current cannabis use. Data obtained from the above-mentioned cannabis-administration study and three subsequent controlled laboratory studies with similar designs yielded a sample with adequate power to assess differences in cannabis’ effects between men and women while matching for cannabis use. Because three of the four studies were designed to assess medication effects, only data obtained under placebo medication conditions were used for this analysis.
2. METHODS
Data from four outpatient studies carried out at New York State Psychiatric Institute were used for this analysis (total N = 146). These double-blind, within-subject studies were designed to assess cannabis’ effects in non-treatment seeking recreational (non-medicinal) cannabis smokers who were not interested in treatment, and measured the subjective ratings of drug quality, drug effect, mood, and physiological effects of a single strength of active cannabis (3.27–5.50% THC, strengths varied according to study) relative to inactive cannabis (0.00% THC). From each study, data from equal numbers of men and women matched for frequency of cannabis use (days/week) and amount smoked per day (joints/day) were pooled. Subjective and cardiovascular effects of cannabis smoked according to a controlled smoking procedure were analyzed according to cannabis condition (active and inactive) and sex.
2.1. Participants
Volunteers ages 21–50 were recruited through newspaper advertisements requesting volunteers to participate in research studying the effects of cannabis, and those who met inclusion/exclusion criteria after an initial telephone screen were invited to the laboratory for further screening. Prior to enrollment, participants gave written informed consent, received a psychiatric and medical evaluation, and provided a detailed drug use and medical history. Participants were accepted into the study if they were healthy, as determined by a physical examination, electrocardiogram, and urine and blood chemistries. All eligible participants currently smoked > 3 cannabis cigarettes at least four times a week for the previous four weeks before screening, based on self-report and clinical interviews, and tested positive on a cannabis urine toxicology screen. Participants were excluded if they repeatedly used other illicit drugs, as determined by urine toxicology and self-report, or met criteria for alcohol dependence. Urine toxicology screens were performed during every screening visit and before each session. Exclusion criteria included Axis I psychopathology (DSM-IV edition) as assessed by clinical interview, current use of over-the-counter or prescription medications, with the exception of oral contraceptives, pregnancy or nursing. Volunteers were told that the study objective was to determine cannabis’ effects on mood and physiology and that during each sessions they would take smoke a portion of a cannabis cigarette, but that the strength of the cannabis would vary. Participants were admitted into the studies only after written informed consent to participate was given and eligibility criteria were verified. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute and were in accord with the Declaration of Helsinki.
2.2. Design and procedures
The studies included 5–10 outpatient sessions over the course of 2–8 weeks at the New York State Psychiatric Institute. Sessions began around 9 AM, and were 6–7 hours in duration. Before study onset, participants were familiarized with computerized tasks and study procedures with 1–2 training sessions, during which time cannabis was not administered. Some of these studies included medication administration before cannabis smoking; only data from placebo medication conditions were utilized for this analysis. A within-subject design was used in which all participants received active and inactive cannabis and medication strengths. For studies testing medication effects, one capsule containing placebo or the test medication (naltrexone or dronabinol) was administered 45 minutes before cannabis was smoked. The order of cannabis and medication dosing was randomized across sessions. All studies included a minimum of 48 hours between test sessions, enough time to allow for drug clearance in medication administration studies to ensure no carryover effects of naltrexone or dronabinol from a previous session.
2.2.1 Experimental session
For all studies, participants were instructed not to smoke cannabis or cigarettes after midnight the night before each session and not to eat breakfast. Before the session, carbon monoxide levels were measured to confirm no recent smoking, breath alcohol levels were assessed, and use of illicit drugs other than cannabis was determined by a urine toxicology screen. If carbon monoxide levels indicated that the participant had smoked cannabis or a cigarette prior to arrival (> 8 ppm) the session was rescheduled. A standardized breakfast was provided to all participants prior to session onset.
Before capsule administration and cannabis smoking, baseline subjective-effects questionnaires were completed and heart rate and blood pressure were measured using a Sentry II vital signs monitor (Model 6100: NBS Medical Services, Costa Mesa CA). Participants smoked a cannabis cigarette according to a cued-smoking procedure shown to produce reliable increases in heart rate and plasma THC levels (Foltinet al., 1987): Investigators instructed participants to ‘inhale’ (5s), ‘hold smoke in lungs’ (10s) and ‘exhale’. Participants smoked according to this procedure with a 40-second interval between puffs until 50 or 75% of the cigarette was pyrolized depending on the study (3–7 puffs). Subjective ratings of drug effect and mood, heart rate, and blood pressure were assessed at set timepoints throughout the session. Cigarette smokers were permitted to smoke at predetermined intervals throughout the session in order to minimize nicotine withdrawal symptoms. At the end of each session (about 5 hours after smoking) participants were free to leave the laboratory after passing field sobriety tasks.
2.2.2 Subjective Drug-effects
Subjective drug effects were measured using visual analog scales, a series of 100-mm lines anchored with ‘not at all’ (0 mm) and ‘extremely’ (100 mm). Participants were instructed to indicate on the line how they felt at that moment.
2.2.3 Subjective Effect-Visual Analog Scale (SE-VAS)
Participants were asked to rate their mood and physical symptoms on a modified 44-item VAS intended to measure affective and physical subjective drug effects (see Haney et al., 1999 for description of the original 50-question version).
2.2.4 Cannabis Rating Form
Subjective cannabis-related drug effects were assessed using a 5-item VAS asking participants to rate the strength of the drug effect, good effect, bad effect, drug liking, and willingness to take the drug again. Participants were also asked to indicate whether they thought the cannabis was ‘placebo’ or ‘active.’
2.3 Drugs
Cannabis cigarettes (0.00, 3.27 – 5.50% THC; ca. 800 mg) were provided by the National Institute on Drug Abuse. Cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 hours prior to the session. For medication studies, capsules (size 00 opaque capsules with lactose filler) containing placebo or the test medication were prepared by the New York State Psychiatric Institute Research Pharmacy.
2.4. Data Analysis
Differences in demographics and cannabis use between men and women were determined by independent t-tests. Repeated measures analysis of variance with between-group analysis were used to determine sex differences in cannabis’ subjective and physiological effects across post-smoking time-points (15–195 minutes; 6 time-points for subjective effects, 7 time-points for cardiovascular effects). Differences between men and women for the ratings of cannabis strength and quality as measured by the MRF (‘Liking,’ Strength,’ ‘Good,’ Take again’) and subjective ratings of cannabis intoxication (‘High’) and positive drug ratings that are indicative of cannabis’ effects (i.e., ‘Stimulated,’ ‘Content,’ ‘Social’; Haney et al., 1997, 1999, 2005) as measured by the SE-VAS were assessed as a function of cannabis condition (active versus inactive cannabis) and time-point. Sex-dependent cardiovascular effects of cannabis were also assessed according to cannabis condition and time-point. Results were considered statistically significant when p values were equal to or less than 0.05 using Huynh-Feldt corrections. Statistical analyses were performed with SPSS (IBM SPSS Statistics Version 20, IBM Corp.).
3. RESULTS
3.1. Demographics
Data obtained from 35 men and 35 women were used for the present analysis. Of these, 11 men and women participated in a study investigating the analgesic effects of cannabis relative to dronabinol (Cooper et al., 2013) and 7 men and women participated in a study assessing the effects of cannabis smoked as joints (cannabis rolled in cigarette paper) or blunts (cannabis rolled in a tobacco leaf; Cooper and Haney, 2009). Ten men and women participated in a study examining the effects of acute naltrexone administration on cannabis (Cooper and Haney, 2010); only data obtained from placebo naltrexone conditions were used for the current analysis. Finally, 7 men and women participated in a study investigating the effects of daily naltrexone administration on cannabis (Haney et al., in preparation). Only data from the first two laboratory sessions when cannabis effects were assessed prior to the onset of naltrexone administration were used for this analysis. Table 1 portrays the demographic information of the participants according to sex. Men and women did not differ in age, number of days cannabis was smoked per week, or number of cannabis cigarettes smoked per day. Men and women did not differ in number of weekly alcohol drinkers, tobacco cigarette smokers, or number of cigarettes smoked during sessions. However, men weighed significantly more than women.
Table 1.
Demographic Characteristics of Study Participants.
| Men (N = 35) | Women (N = 35) | |
|---|---|---|
|
| ||
| Age (years old) | 27 ± 5 | 27 ± 6 |
| Race (B/W/M) | 28 / 6 / 1 | 21 / 8 / 6 |
|
| ||
| Body Weight (kg) | 72.3 ± 8.7* | 65.4 ± 15.7 |
|
| ||
| Cannabis Use | ||
| Days/Wk | 6.9 ± 0.4 | 6.8 ± 0.5 |
| MJ Cigarettes/Day | 4.9 ± 2.9 | 6.3 ± 5.6 |
| $/Wk | 68.8 ± 68.2 | 62.3 ± 47.3 |
|
| ||
| Tobacco Smokers | ||
| # Daily Smokers | 24/35 | 20/35 |
| Cigs/Day | 6.8 ± 5.3 | 6.6 ± 4.9 |
|
| ||
| Alcohol | ||
| # Weekly Drinkers | 18/35 | 15/35 |
| Days/wk | 2.3 ± 1.3 | 1.8 ± 1.0 |
| Drinks/Day | 3.1 ± 2.3 | 2.4 ± 1.2 |
Note: Data are presented as means (± SD) or as frequency.
Race is indicated as Black (B), White (W), and Mixed (M).
Significant differences between men and women are indicated by a * = p ≤ 0.05.
3.2. Subjective Drug Effects
Figure 1 illustrates subjective ratings of active and inactive cannabis according to sex and session time-point as assessed by the MRF. Ratings of cannabis ‘Liking,’ ‘ Good,’ ‘ Take Again,’ and ‘Strong’ were higher for active cannabis relative to inactive (p ≤ 0.001), peak ratings occurred immediately after smoking and decreased over the course of the session (p ≤ 0.01). A between-groups effect was observed for ratings of ‘Good,’ Take Again, ‘ and ‘Strong.’ Under active cannabis conditions, compared to men, women reported higher ratings for ‘Good’ and ‘Take Again’ (p ≤ 0.05), and a trend was observed for higher ratings of ‘Strong’ (p ≤ 0.10). Men and women did not differ in these ratings under the inactive cannabis condition.
Figure 1.
Subjective ratings of cannabis quality (‘Good,’ ‘Liking,’ ‘Take Again,’ and ‘Strength) as measured by the MRF. Data are presented as mean (±SEM ) ratings as a function of cannabis condition and time-point. Refer to the results section for explanation of significant differences between cannabis conditions and sexes.
Figure 2 illustrates subjective ratings of active and inactive cannabis according to sex and session time-point as assessed by the SE-VAS. Of the items analyzed, only ‘Stimulated’ and ‘High’ varied according to cannabis condition; active cannabis increased ratings for both of these items relative to inactive cannabis (p ≤ 0.001), and peak ratings occurred immediately after smoking and decreased over the session (p ≤ 0.0001). Men and women did not differ in their ratings of ‘High’ or ‘Stimulated’ for active or inactive cannabis.
Figure 2.

Participant ratings of subjective drug effects for ‘High’ and ‘Stimulated,’ the two items that were significantly different between active and inactive cannabis, as measured by the SE-VAS. Data are presented as mean (±SEM) ratings according to sex as a function of cannabis condition and time-point. Refer to the results section for explanation of significant differences between cannabis conditions and sexes.
3.3. Cardiovascular Effects
Figure 3 portrays heart rate over the session as a function of sex and cannabis condition. Active cannabis significantly increased heart rate relative to inactive cannabis (p ≤ 0.0001). Heart rate peaked immediately after active cannabis was smoked and decreased over the course of the session (p ≤ 0.0001). The cardiovascular effects of active and inactive cannabis did not differ between men and women.
Figure 3.
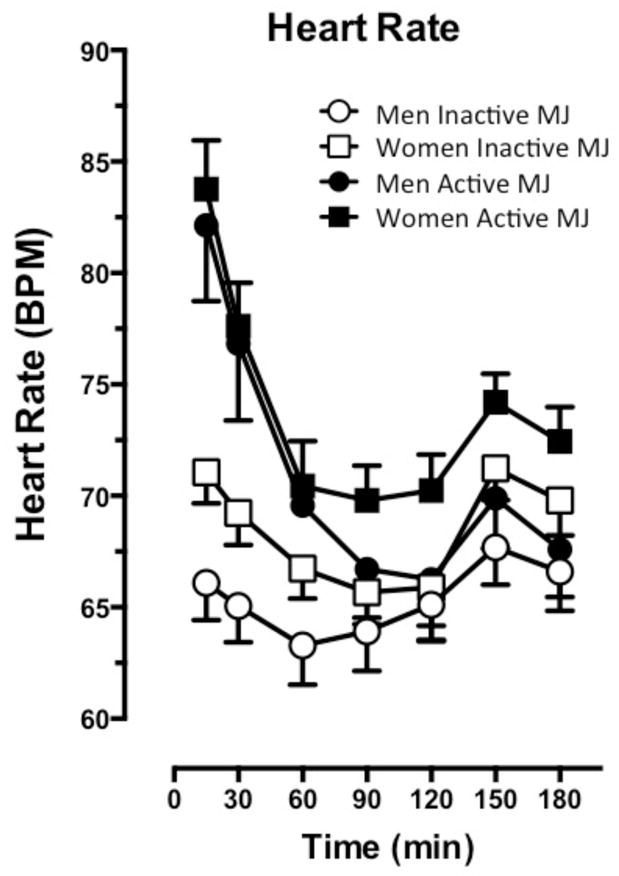
Cardiovascular effects of active and inactive cannabis presented as the average (±SEM) heart rate (BPM) according to sex for post-smoking time-points. Refer to the results section for explanation of significant differences between cannabis conditions and sexes.
4. DISCUSSION
The results from this study demonstrate that when cannabis smokers are matched for use, ratings of cannabis’ subjective effects that are associated with abuse liability are higher in women compared to men. Although men and women significantly differed in body weight, sex differences were not observed for all subjective effects, including ratings of cannabis intoxication. Also, cannabis’ cardiovascular effects did not differ between men and women. Thus, it is unlikely that the enhanced abuse-related ratings in women is due to the fact that they received a functionally higher ‘dose’ of THC relative to men. These findings indicate that among near-daily cannabis smokers, women appear to be more sensitive to the subjective effects that may contribute to maintaining cannabis smoking relative to men. As such, sex potentially contributes in a clinically meaningful manner to cannabis use trajectories and the development of cannabis use disorders.
CUD is more prevalent among men because cannabis smoking overall is more prevalent in men than women, with 51.4% of men reporting lifetime cannabis use versus 37.4% of women (SAMHSA, 2012). Yet among cannabis smokers, women have a faster trajectory to CUD, which the current findings might in part explain. If women who start smoking cannabis find that it produces greater positive subjective effects, this could lead to the faster rate of developing CUD and ultimately greater difficulty achieving and maintaining abstinence. In fact, preclinical studies show that female rodents have higher rates of drug self-administration and are more likely to reinstate cannabis self-administration (an preclinical model of relapse (Fattore et al., 2010 and 2007). Future studies are needed in humans to confirm how sex influences the reinforcing effects of cannabis.
Further, controlled studies assessing differences between men and women in cannabis self-administration during abstinence, or relapse to cannabis use during abstinence is an essential issue in determining the clinical significance of sex in CUD. It is hypothesized that withdrawal symptoms contribute to relapse (Haney et al., 2013), and women report more severe cannabis withdrawal symptoms (Copersino et al., 2010), although these findings came from a convenience sample of men and women who were not matched according to pre-quit cannabis use. Assessing cannabis withdrawal and relapse under controlled laboratory conditions would provide critical data to determine the clinical significance of sex in cannabis use disorders.
The neurobiological mechanism for sex-dependent effects of cannabinoids may arise from sexual dimorphism of the endocannabinoid system and/or the direct effects of fluctuating reproductive hormones on the endocannabinoid system (Craft et al., 2013). Thus, an important aspect of assessing sex-dependent effects is taking into account menstrual-cycle phase, a variable that could not be feasibly explored in the current experimental designs, i.e., studies ran across 4 to 8 weeks, with dosing conditions counter-balanced across sessions. Across species, the fluctuation of reproductive hormones across the menstrual cycle has been shown to alter the effects of psychoactive drugs including cocaine (Sofuoglu et al., 1999, 2002; Evans et al., 2002; Evans and Foltin, 2006; Mello et al., 2007; Lynch, 2008) and opioids (see Craft, 2003 for review). During the follicular, or estrus, phase when progesterone is low and estradiol is high, females exhibit higher rates of cocaine and opioid self-administration relative to males (Evans and Foltin, 2006; Lynch, 2008). The behavioral effects of cannabinoids are similarly modulated by fluctuating endogenous ovarian hormones with a positive association between estradiol levels and cannabinoid self-administration (Fattore et al., 2010). In humans, the neurobiological basis of sex-dependent effects of cannabinoids is more speculative, and there are little data from studies investigating phase-dependent effects of the psychoactive effects of cannabinoids. A self-report study found that among 30 cannabis-smoking females ranging from daily to once a week smokers, only 1 participant showed consistent fluctuations in use across the phases over 3 cycles (Griffin et al., 1986). However, another study by the same group found that increased cannabis self-administration in the laboratory was associated with severity of premenstrual dysphoria (Mello and Mendelson, 1985) suggesting that phase may indeed be an important variable to consider. Further, there is evidence in peripheral lymphocytes, levels of the endogenous anandamide and its degrading enzyme, fatty acid amide hydrolase (FAAH) fluctuate according to menstrual cycle phase (Lazzarin et al., 2004). These phase-dependent changes in the endocannabinoid system may alter the psychoactive effects of cannabinoids. The current analysis did not control for menstrual cycle phase, but detected robust differences between men and women for subjective effects associated with cannabis’ abuse liability. Investigating phase-dependent effects would determine to what extent these differences are attributed to fluctuating reproductive hormones across the menstrual cycle.
Another important aspect of this analysis to consider is that the population studied was comprised of near-daily cannabis users. The differences between men and women observed may not be generalized to lighter users. Investigating cannabis’ sex-dependent effects in non-daily, or recreational users could help provide insight into the more rapid progression of first cannabis use to abuse or dependence observed in female smokers (Kahn et al., 2013). Sex-dependent differences in the rate at which tolerance develops to cannabinoid effects may also play a role in the trajectory from first use to dependence. Although little is known about the differences between men and women and tolerance to cannabis’ effects, preclinical studies report that tolerance to cannabinoid effects seems to develop in female rodents to a greater extent than males (Wiley, 2003; Wiley et al., 2007, 2011).
When matched for current cannabis use, women report enhanced subjective rating for cannabis’ effects associated with abuse liability relative to men. These findings underscore the importance of including sex as a primary variable when designing studies and clinical trials geared towards understanding cannabinoid effects, dependence, and potential pharmacotherapies for CUD.
Acknowledgments
This research was supported by US National Institute on Drug Abuse Grant DA19239, DA09236, and DA02775. The authors acknowledge and appreciate the exceptional assistance of Divya Lakhaney, Elyssa Berg, Michael Harakas, Sarah Badach, Bennett Wechsler, and Ursula Rogers in data collection. ZDC and MH designed the study; ZDC performed analysis and wrote manuscript. MH provided guidance in analysis and composition and reviewed the submitted version of the manuscript.
Footnotes
AUTHOR DISCLOSURES
The authors have no financial disclosures or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cooper ZD, Comer SD, Haney M. Analgesic effects of smoked and oral THC in healthy marijuana smokers. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.97. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Comparison of the subjective, physiologic, and pharmacokinetic effects of marijuana smoked in cigarette paper (joints) versus cigar paper (blunts) Drug Alcohol Depend. 2009;103:107–13. doi: 10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Opioid antagonism enhances marijuana’s abuse liability in heavy marijuana smokers. Psychopharmacology. 2010;211:141–148. doi: 10.1007/s00213-010-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Sociodemographic characterstics of cannabis smokers and the experience of cannabis withdrawal. Drug Alcohol Depend. 2010;111:120–127. doi: 10.3109/00952990.2010.503825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–481. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Vieten C, Gilder DA, Stouffer GM, Lau P, Wilhelmsen KC. Cannabis dependence in the San Francisco D=Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritiability. Addict Behav. 2010;35:102–110. doi: 10.1016/j.addbeh.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoids-seeking behavior in male and female rats: influence of ovarian hormones. Br J Pharmacol. 2010;160:724–735. doi: 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Brady JV, Fischman MW, Emurian CS, Dominitz J. Effects of smoked marijuana on social interaction in small groups. Drug Alcohol Depend. 1987;20:87–93. doi: 10.1016/0376-8716(87)90079-2. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Mendelson JH, Mello NK, Lex BW. Marihuana use across the menstrual cycle. Drug Alcohol Depend. 1986;18:213–224. doi: 10.1016/0376-8716(86)90053-0. [DOI] [PubMed] [Google Scholar]
- Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, Foltin RW. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol Psychiatry. 2013;73:242–248. doi: 10.1016/j.biopsych.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8:101–112. [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007;45:545–554. doi: 10.1097/QAI.0b013e31811ed205. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002;67:301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hoffmann DE, Weber E. Medical marijuana and the law. N Engl J Med. 2010;362:1453–1457. doi: 10.1056/NEJMp1000695. [DOI] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Pérez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: results form the National Epidemiological Survey of Alcohol and Related Conditions. Drug Alcohol Depend. 2013;130:101–108. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarin N, Valensise H, Bari M, Ubaldi F, Battista N, Finazzi-Agrò A, Maccarrone M. Fluctuations of fatty acid amide hydrolase and anandamide levels during the human ovulatory cycle. Gynecol Endocrinol. 2004;18:212–218. doi: 10.1080/09513590410001692492. [DOI] [PubMed] [Google Scholar]
- Lev-Ran S, Imtiaz S, Taylor BJ, Shield KD, Rehm J, Le Foll B. Gender differences in health-related quality of life among cannabis users: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2012;123:190–200. doi: 10.1016/j.drugalcdep.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, Malison RT. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addict Biol. 2008;13:403–410. doi: 10.1111/j.1369-1600.2008.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Davis R. Postural syncope after marijuana: a transcranial Doppler study of the hemodynamics. Pharmacol Biocehm Behav. 2003;75:309–318. doi: 10.1016/s0091-3057(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology. 2007;32:1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Operant acquisition of marihuana by women. J Pharmacol Exp Ther. 1985;235:162–171. [PubMed] [Google Scholar]
- National Conference of State Legislature. [accessed on July 16, 2013];State Medical Marijuana Laws. 2013 http://www.ncsl.org/issues-research/health/state-medical-marijuana-laws.aspx.
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2012a. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. State Admissions to Substance Abuse Treatment Services. Rockville, MD: 2012b. Treatment Episode Data Set (TEDS): 2000–2010. DASIS Series: S-63, HHS Publication No. SMA-12-4729. [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report. Division for Policy Analysis and Public Affairs United Nations Office; Vienna, Austria: 2013. [Google Scholar]
- Wagner FA, Anthony JC. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug Alcohol Depend. 2007;86:191–198. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wiley JL. Sex-dependence effects of delta 9-tetraydrocannabinol on locomotor activity in mice. Neursci Lett. 2003;352:77–80. doi: 10.1016/j.neulet.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Evans RL, Grainger DB, Nicholson KL. Locomotor activity changes in female adolescent and adult rats during repeated treatment with cannabinoid or club drug. Pharmacol Rep. 2011;63:1085–1092. doi: 10.1016/s1734-1140(11)70627-2. [DOI] [PubMed] [Google Scholar]
- Wiley JL, O’connell MM, Tokarz ME, Wright MJ., Jr Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]



