Abstract
Current product labels for maraviroc and raltegravir provide no dosing guidance for patients with end-stage liver disease and worsening renal function. We describe a 41-year-old man with human immunodeficiency virus (HIV) infection and rapidly progressive liver failure and vanishing bile duct syndrome at presentation. Despite discontinuation of all potential offending drugs, the patient’s liver function continued to deteriorate. To achieve and maintain HIV suppression while awaiting liver transplantation, a regimen consisting of maraviroc, raltegravir, and enfuvirtide was started. These agents were chosen because the patient was not exposed to them before the onset of liver failure. While receiving product label–recommended twice-daily dosing of these drugs, he achieved and maintained HIV suppression. During a complicated and prolonged hospitalization, the patient also developed renal dysfunction. As hepatic metabolism is the primary route of clearance of maraviroc and raltegravir, we predicted that using approved doses of these drugs could result in significant drug accumulation. Since the safety profiles of supratherapeutic concentrations of these agents are not well defined, we chose to use therapeutic drug monitoring to guide further dosing. The reported concentrations showed severely impaired metabolic clearance of both drugs, with markedly prolonged elimination half-lives of 189 hours for maraviroc and 61 hours for raltegravir. Previously reported half-lives for maraviroc and raltegravir in HIV-infected patients with normal hepatic and renal function are 14–18 hours and 9–12 hours, respectively. Based on these results, the dosing intervals were extended from twice/day to twice/week for maraviroc and every 48 hours for raltegravir. Unfortunately, the patient’s clinical condition continued to deteriorate, and he eventually died of complications related to end-stage liver disease. This case illustrates the difficulties in managing antiretroviral therapy in an HIV-infected patient with combined severe liver and renal failure. Prolonged excessively high exposure to maraviroc and raltegravir is likely to result in some level of concentration-dependent toxicity. Until more data are available, therapeutic drug monitoring remains the only evidence-based approach to optimize dosage selection of these drugs in this patient population.
Keywords: maraviroc, raltegravir, human immunodeficiency virus, HIV, liver failure, renal failure, pharmacokinetics, therapeutic drug monitoring, TDM
Hepatic metabolism is the primary route of systemic clearance for many antiretroviral drugs; as such, severe liver dysfunction can impair drug clearance and may result in significant drug accumulation and toxicities. Dosing recommendations for antiretroviral agents are based on safety and pharmacokinetic data from subjects with normal hepatic and renal function, with some guidance for dosage adjustment in patients with mild-to-moderately compromised organ function. In patients with severe liver disease (Child-Pugh score > 9), hepatically metabolized antiretroviral drugs are frequently contraindicated or not recommended. Such patients represent a clinical conundrum for human immunodeficiency virus (HIV) care practitioners attempting to select a safe and effective antiretroviral regimen. We describe the utility and challenges of therapeutic drug monitoring to guide maraviroc and raltegravir dosing in a patient with end-stage liver disease and compromised renal function.
Case Report
A 41-year-old African-American man was referred to the National Institutes of Health (NIH) Clinical Center for assessment of progressive liver failure. He had received a diagnosis of HIV type 1 infection 20 years earlier and had a long history of antiretroviral nonadherence until approximately 8 months before presentation, when he finally achieved and maintained viral suppression while receiving a regimen of ritonavir-boosted atazanavir, tenofovir, and didanosine. His CD4+ count was 304 cells/mm3. He was well until 2 months before coming to the NIH (early December 2009), when he presented with icteric sclera, nausea, diarrhea, and dark urine. Laboratory findings were consistent with acute cholestatic liver failure. All antiretroviral drugs were stopped while assessing the etiology of his liver disease.
At presentation to the NIH, the patient had laboratory findings consistent with acute liver failure: total bilirubin 24.9 mg/dl (normal range 0.1–1.0 mg/dl), alkaline phosphatase 1098 U/L, alanine aminotransferase 229 U/L, γ-glutamyl transferase 511 U/L, and albumin 3.3 g/dl. His serum creatinine concentration was normal at this time (1.06 mg/dl). Extensive laboratory and imaging studies did not reveal a cause of his liver failure. Hepatitis A, B, and C serology and viral load assessment showed no evidence of active viral hepatitis. He denied any recent travel history, alcohol or illicit drug use, or use of herbal products. A liver biopsy specimen showed preserved hepatic architecture, but lacked identifiable bile duct in all portal areas.
Despite discontinuation of all drug therapy, the patient’s liver function continued to decline. At this point, the patient’s only management option for clinical recovery and survival was liver transplantation. In the interim, the primary therapeutic goal was to achieve and maintain virologic suppression and optimize immunologic function before the transplant procedure.
Taking care to avoid antiretroviral drugs that the patient was receiving during the onset of liver dysfunction, we instituted a regimen consisting of raltegravir, etravirine, enfuvirtide, and lamivudine 1 month after presentation. After 1 week of therapy, the patient was transferred to a liver transplant center for evaluation, and his antiretroviral drugs were discontinued by the center’s providers. The patient returned to the NIH for further follow-up, and as his liver function remained relatively stable, raltegravir and enfuvirtide were restarted. Maraviroc 300 mg twice/day was added 2 weeks later, after tropism assay confirmed chemokine receptor 5 (CCR5)-tropic HIV. All antiretroviral drugs were prescribed at the product-label–recommended dosages.1,2
The patient’s hospital course was further complicated by pneumococcal bacteremia, intussusception requiring surgical intervention, worsening anemia, thrombocytopenia, and coagulopathy. During this period, the patient’s liver function continued to worsen, and he began to develop renal insufficiency, with his serum creatinine concentration peaking at 4.6 mg/dl. Table 1 shows the changes in laboratory parameters during his hospital course.
Table 1.
Pertinent Laboratory Findings
| Date | Total Bilirubin/Direct Bilirubin (mg/dl) | ALP(U/L) | ALT(U/L) | AST (U/L) | INR | Albumin (g/dl) | Scr (mg/dl) | CD4+ Count (cells/mm3) | HIV RNA (copies/ml) |
|---|---|---|---|---|---|---|---|---|---|
| 2009 | |||||||||
| September 4 | 1.1/NA | 77 | 21 | 21 | NA | 4.2 | 1.22 | 304 | < 75 |
| December 4a | 4.7/NA | 1051 | 439 | 438 | 1.4 | NA | 1.32 | NA | NA |
| December 11 | 14.3/NA | 1049 | 312 | 283 | NA | 3.4 | 1.32 | NA | NA |
| 2010 | |||||||||
| January 8 | 16.7/15.6 | 1474 | 228 | 293 | 1.5 | 3.1 | NA | 167 | 50283 |
| January 28 | 24.5/19.9 | 1702 | 277 | 476 | 1.4 | 1.9 | 1.06 | 164 | 33945 |
| February 25 | 14.4/11.9 | 924 | 86 | 148 | 1.34 | 1.4 | 3.09 | NA | NA |
| March 29b | 17.2/13.5 | 2757 | 169 | 308 | 1.27 | 1.8 | 1.31 | 192 | 53853 |
| April 9c | 25.3/21.1 | 1290 | 120 | 201 | 1.54 | 1.4 | 2.52 | 101 | 52 |
| April 16 | 23.0/17.9 | 1948 | 159 | 538 | 1.42 | 1.2 | 2.19 | 126 | < 50 |
| May 3 | 40.5/31.8 | 1087 | 204 | 314 | NA | 2.5 | 2.61 | NA | NA |
| May 18 | 31.1/27.2 | 601 | 142 | 248 | 1.67 | 2.1 | 4.60 | 336 | < 50 |
| June 1 | 45.7/40.5 | 1138 | 106 | 233 | 1.29 | 2.7 | 4.23 | NA | < 50 |
ALP alkaline phosphatase; ALT alanine aminotransferase; AST aspartate aminotransferase; INR international normalized ratio, Scr = serum creatinine; HIV = human immunodeficiency virus; NA = not available.
Normal ranges are as follows: total bilirubin 0.1–1.0 mg/dl; direct bilirubin 0.0–0.2 mg/dl; ALP 37–116 U/L; ALT 6–41 U/L; AST 9–34 U/L; INR albumin 3.7–4.7 g/dl; Scr 0.56–1.16 mg/dl; CD4+ count 334–1556 cells/mm3.
First evaluation of liver dysfunction; all antiretroviral drugs discontinued.
Raltegravir + enfuvirtide started.
Maraviroc started.
Given the patient’s worsening hepatic and renal function, and the potential for excessive accumulation of raltegravir and maraviroc, serum samples were sent to assess the concentration of each drug. After receiving raltegravir 400 mg twice/day for 24 days and maraviroc 300 mg twice/day for 9 days, predose concentrations were reported as 1130 ng/ml and 1590 ng/ml, respectively (both assays performed at the University of Florida Infectious Diseases Pharmacokinetics Laboratory [IDPL] using high-performance liquid chromatography assay). Considering that the reference serum concentration range for raltegravir 400 mg twice/day in HIV-positive patients is 30–120 ng/ml (according to the IDPL), and the mean minimum concentration for maraviroc given as 300 mg twice/day ranged from 33.6–60 ng/ml,1 the concentrations we observed in our patient exceeded those previously reported by 10-fold for raltegravir and 25-fold for maraviroc. Although therapeutic and toxic threshold concentrations have not been established for these agents, these predose concentrations were well above those reported in HIV-positive and HIV-negative subjects with normal hepatic and renal function.3,4 The time from blood sampling to reporting the results was more than 1 week.
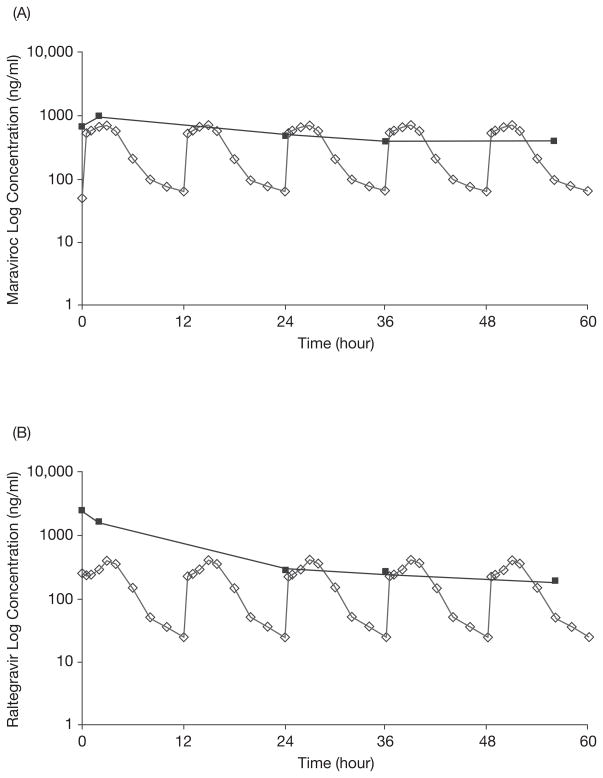
Based on these results, maraviroc and raltegravir were withheld for 2 days and then restarted on an every-48-hour schedule. Serial serum samples were obtained just before and at several time points after the second dose after starting this new schedule. The predose maraviroc and raltegravir concentrations were 670 ng/ml and 2250 ng/ml, respectively, indicating significant drug accumulation despite withholding treatment for 2 days and restarting the doses at 25% of the original doses. As noted in Figure 1, the postdose concentrations at 2, 24, 36, and 56 hours showed a rapid early-phase decline for both drugs, followed by a much slower terminal elimination phase.
Figure 1.
(A) Steady-state maraviroc log concentration–time curve after a 300-mg dose (given at time 0) in the case patient (solid squares) compared with previously reported steady-state maraviroc log concentration–time curves after repeated maraviroc doses of 300 mg every 12 hours in subjects with normal renal and hepatic function (open diamonds).3 (B) Steady-state raltegravir log concentration–time curve after a 400-mg dose (given at time 0) in the case patient (solid squares) compared with previously reported steady-state raltegravir log concentration–time curves after repeated raltegravir doses of 400 mg every 12 hours in subjects with normal renal and hepatic function (open diamonds).5
With use of WinNonlin Professional computer program, version 5.0 (Pharsight Corp., Mountain View, CA), elimination rate constants (λZ) were determined for maraviroc and raltegravir by calculating the absolute value of the slope of the log-linear regression using multiple time points (24, 36, and 56 hrs) on the plasma concentration–time plots. Half-life was defined as ln2/λZ. The values for half-life were found to be 189 hours for maraviroc and 61 hours for raltegravir, both of which were markedly prolonged compared with those reported in patients with normal renal and hepatic function (14–18 hrs for maraviroc and 9–12 hrs for raltegravir).3–5 Based on these therapeutic drug monitoring results, the dosing interval for both drugs was further extended to twice/week, which, in retrospect, was too infrequent for raltegravir, as a 96-hour postdose concentration was below the limit of quantitation for the assay (< 40 ng/ml). Conversely, the maraviroc concentration at 96 hours after dose was 1340 ng/ml, much higher than its previous postdose measurement, indicating that while raltegravir concentrations eventually diminished over time, maraviroc continued to accumulate.
Shortly thereafter, the patient was transferred to a liver transplant center. Unfortunately, his condition continued to deteriorate and he was discharged to hospice care, where he died several weeks later.
Discussion
This case illustrates several significant challenges involved in the management of antiretroviral therapy in an HIV-infected patient with end-stage liver disease and progressively worsening renal function. As current antiretroviral therapy improves patient survival, liver-related morbidities and mortality are becoming more frequent,6,7 and more cases like our patient’s may not be an uncommon event in the future. Studies addressing antiretroviral dosing in patients with severe liver and/or renal dysfunction are needed to guide clinicians in clinical practice.
The product labels for maraviroc and raltegravir provide no dosage guidance for such patients, making accurate dosage selection a challenge for clinicians. Several clinical trials are under way to evaluate the pharmacokinetics of raltegravir in HIV-infected patients with end-stage liver disease, in liver transplant recipients, or in hepatitis C (HCV)-coinfected patients with serious liver dysfunction (ClinicalTrials.gov identifier numbers NCT01022476 and NCT 01289951).8,9 Results from these studies will provide further guidance for the management of patients with liver diseases.
Maraviroc is primarily metabolized by cytochrome P450 (CYP) 3A4. In patients with normal organ function, renal clearance accounts for approximately 23% of total drug clearance in HIV-negative volunteers.10 This is consistent with a recent labeling change that suggests maraviroc concentrations may be increased in patients with renal impairment, which may increase the risk of postural hypotension—a dose-dependent adverse effect noted in a pre-marketing dose-ranging study.11 In our patient, the half-life of maraviroc when given at 400 mg every 48 hours was found to be 189 hours, much longer than the 14–18 hours reported in subjects with normal liver and renal function.
Raltegravir was studied at doses higher than the approved dose of 400 mg twice/day. It was well tolerated at 800 mg twice/day for 10 days12 and 600 mg twice/day for 24 weeks.13 Unlike maraviroc, raltegravir primarily undergoes glucuronidation in the liver by using the uridine diphosphate glucuronosyltransferase (UGT) 1A1 enzyme, with UGT1A3 and UGT1A9 playing lesser roles.2 No significant changes in raltegravir pharmacokinetics were observed after a single 400-mg dose was given to subjects with moderate hepatic insufficiency or severe renal impairment.14 In a report of five HIV-HCV–coinfected patients receiving a raltegravir-containing regimen, all patients had higher trough concentrations than those of 24 HIV-monoinfected patients (637 vs 221 ng/ml); the concentrations were also higher in the two HIV-HCV–coinfected patients with cirrhosis compared with the three without cirrhosis (665 vs 581 ng/ml).15 Raltegravir has not been associated with hepatotoxicity, although in one report, liver enzyme level elevations were more frequent in HIV-HCV–coinfected patients compared with HIV-monoinfected patients.16 There is no significant pharmacokinetic interaction between raltegravir and maraviroc in either HIV-positive17 or HIV-negative18 subjects with normal hepatic and renal function.
When dosed every 48 hours in our patient, maraviroc had a much more prolonged half-life than raltegravir. The reasons for this are likely multifactorial and relate to one or more of the following: maraviroc’s elimination is affected by both hepatic and renal dysfunction, and the renal function of our patient had progressively worsened over time; the involvement of multiple metabolic pathways for raltegravir (UGT1A1, UGT1A3, and UGT1A9) may allow for greater conservation of metabolic function compared with maraviroc, which is metabolized by a single CYP isoform (CYP3A4); and although it cannot be substantiated, it is possible that hepatic failure in this patient resulted in a greater reduction in CYP3A activity (phase 1 metabolism) versus glucuronidation (a phase 2 metabolic reaction).
In HIV-infected patients with advanced liver disease, the benefits of virologic control and resultant immune reconstitution must be balanced against the risk of untoward toxicities due to supratherapeutic antiretroviral drug exposure. As there were limited dosing recommendations to guide therapy, we used therapeutic drug monitoring with the goal of achieving plasma concentrations similar to those reported in subjects with normal organ function receiving approved raltegravir and maraviroc dosages. With the multiple comorbidities in this patient, it was not possible to determine whether high drug concentrations contributed to the progressive worsening of liver and renal function, or other clinical events such as grade 4 thrombocytopenia (nadir platelet count 14.0 × 103/mm3); however, the possibility exists.
In the United States, therapeutic drug monitoring of antiretroviral drugs is not performed routinely in clinical practice; thus, most serum samples are sent to outside reference laboratories. It is typically reserved for select populations at risk for altered drug absorption, metabolism, or distribution secondary to an altered physiologic state (e.g., organ dysfunction, pregnancy, advanced age). Therapeutic drug monitoring may also be used to manage complex drug interactions involving multiple drugs. One key to the successful implementation of therapeutic drug monitoring is to have rapid turnaround time to allow clinicians to make timely dosage adjustments, thereby avoiding the continued administration of an inappropriate dose while awaiting drug concentration data. In our case, however, it took more than 7 days for us to receive the final reports, which hampered our ability to act expeditiously and resulted in greater drug exposure. To this end, laboratories that perform commercial antiretroviral assays should continue to strive for quicker turnaround time while maintaining a high level of accuracy and precision.
Conclusion
This case illustrates the difficulties in managing antiretroviral therapy in a patient with combined severe liver and renal failure. Even though maraviroc and raltegravir are not typically associated with severe adverse effects, prolonged excessively high exposure to these agents is likely to result in some level of toxicity. In fact, it is plausible that persistently elevated concentrations of maraviroc and raltegravir are associated with clinically relevant toxicities heretofore unreported. Until more data are available, therapeutic drug monitoring remains the only evidence-based approach to optimize dosage selection of maraviroc and raltegravir in patients with severe hepatic and/or renal dysfunction. Use of this approach may prevent drug-related toxicity associated with prolonged overexposure to these drugs.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and in part with federal funds from the National Cancer Institute, National Institutes of Health (contract no. HHSN261200800001E). The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade.
Footnotes
For reprints, visit https://caesar.sheridan.com/reprints/redir.php?pub=10089&acro=PHAR. For questions or comments, contact Alice K. Pau, Pharm.D., National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 10, Room 11C103, MSC 1880, 10 Center Drive, Bethesda, MD 20892; apau@niaid.nih.gov.
References
- 1.Pfizer Inc. Selzentry (maraviroc) package insert. New York, NY: 2010. [Google Scholar]
- 2.Merck & Co., Inc. Isentress (raltegravir) package insert. Whitehouse Station, NJ: 2011. [Google Scholar]
- 3.Markowitz M, Morales-Ramirez JO, Nguyen BY, et al. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2006;43:509–15. doi: 10.1097/QAI.0b013e31802b4956. [DOI] [PubMed] [Google Scholar]
- 4.Abel S, Back DJ, Vourvahis M. Maraviroc: pharmacokinetics and drug interactions. Antivir Ther. 2009;14:607–18. [PubMed] [Google Scholar]
- 5.Jackson A, D’Avolio A, Watson V, et al. Pharmacokinetics and safety of the co-administration of the antiretroviral raltegravir and the lipid lowering drug ezetimibe in healthy volunteers. J Antimicrob Chemother. 2011;66:885–9. doi: 10.1093/jac/dkq546. [DOI] [PubMed] [Google Scholar]
- 6.Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 7.Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198–209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- 8.French National Agency for Research on AIDS and Viral Hepatitis. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine; [Accessed July 28, 2011]. Raltegravir in patients with end stage liver disease and in transplant recipients (LIVERAL) Available from http://clinicaltrials.gov/ct2/show/NCT01022476?term=NC-T01022476&rank=1. [Google Scholar]
- 9.Fundacion para la Investigacion Biomedica del Hospital Universitario Ramon y Cajal. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine; [Accessed July 28, 2011]. Pharmacokinetic study of raltegravir in human immunodeficiency virus/hepatitis C virus (HIV/VHC) coinfected patients with advanced (Child-Pugh C) hepatic cirrhosis (LIVERAL) Available from http://clinicaltrials.gov/ct2/show/NCT01289951?term=NCT01289951&rank=1. [Google Scholar]
- 10.Abel S, Russell D, Whitlock LA, Ridgway CE, Nedderman AN, Walker DK. Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects. Br J Clin Pharmacol. 2008;65(suppl 1):60–7. doi: 10.1111/j.1365-2125.2008.03137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abel S, van der Ryst E, Rosario MC, et al. Assessment of the pharmacokinetics, safety and tolerability of maraviroc, a novel CCR5 antagonist, in healthy volunteer. Br J Clin Pharmcol. 2008;65(suppl 1):5–18. doi: 10.1111/j.1365-2125.2008.03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto M, Wenning LA, Petry AS, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008;83:293–9. doi: 10.1038/sj.clpt.6100281. [DOI] [PubMed] [Google Scholar]
- 13.Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–9. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto M, Hanley WD, Petry AS, et al. Lack of a clinically important effect of moderate hepatic insufficiency and severe renal insufficiency on raltegravir pharmacokinetics. Antimicrob Agents Chemother. 2009;53:1747–52. doi: 10.1128/AAC.01194-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tommasi C, Nicastri E, Gallo AL, et al. Raltegravir and darunavir plasma pharmacokinetic in HIV-1 infected patients with advanced liver disease [abstract]. Presented at the 11th international workshop on clinical pharmacology and HIV therapy; Sorrento, Italy. April 7–9, 2010; p. abstract 10. [Google Scholar]
- 16.Vispo E, Mena A, Maida I, et al. Hepatic safety profile of raltegravir in HIV-infected patients with chronic hepatitis C. J Antimicrob Chemother. 2010;65:543–7. doi: 10.1093/jac/dkp446. [DOI] [PubMed] [Google Scholar]
- 17.Baroncelli S, Villani P, Weimer LE, et al. Raltegravir plasma concentrations in treatment-experienced patients receiving salvage regimens based on raltegravir with and without maraviroc coadministration. Ann Pharmacother. 2010;44:838–43. doi: 10.1345/aph.1M688. [DOI] [PubMed] [Google Scholar]
- 18.Andrews E, Glue P, Fang J, Crownover P, Tressler R, Damle B. Assessment of the pharmacokinetics of co-administered maraviroc and raltegravir. Br J Clin Pharmacol. 2010;69:51–7. doi: 10.1111/j.1365-2125.2009.03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]