Abstract
Studies of cellular mechanotransduction have converged upon the idea that cells sense extracellular matrix (ECM) elasticity by gauging resistance to the traction forces they exert on the ECM. However, these studies typically utilize purely elastic materials as substrates, whereas physiological ECM are viscoelastic, and exhibit stress relaxation, so that cellular traction forces exerted by cells remodel the ECM. Here we investigate the influence of ECM stress relaxation on cell behavior through computational modeling and cellular experiments. Surprisingly, both our computational model and experiments find that spreading for cells cultured on soft substrates that exhibit stress relaxation is greater than cells spreading on elastic substrates of the same modulus, but similar to that of cells spreading on stiffer elastic substrates. These findings challenge the current view of how cells sense and respond to the ECM.
INTRODUCTION
Mechanical properties of extracellular matrices (ECM) are thought to play an important role in regulating cell behaviors in development, tissue homeostasis, and disease 1–5. Studies investigating the influence of substrate elasticity on biological processes typically utilize 2D surfaces of collagen or fibronectin coated polyacrylamide gels as substrates for cell culture. These studies have found that cell spreading6,7, proliferation8, and nuclear localization of the transcriptional regulator YAP (Yes-associated protein) 9 are all suppressed on soft substrates. The mechanistic understanding is that cells sense substrate elasticity by gauging resistance to the traction forces the cells exert on the substrate10,11. However, the covalent crosslinking of these polyacrylamide hydrogels results in purely elastic substrates with a time independent storage or elastic modulus12. Correspondingly, deformations of the polymer matrix are elastic, not plastic, so that resistance to traction forces exerted by cells is constant over time, and elastic energy is stored in the substrate13. In contrast, reconstituted extracellular matrices, such as collagen14, or fibrin15, and many tissues 16–18, exhibit stress relaxation, or a decrease in the storage or elastic modulus over time when a constant strain is applied. On such matrices, the resistance to cellular traction forces is expected to be relaxed over time due to flow and remodeling of the matrix, dissipating the energy that cell-generated forces imparted into the material. While broadly presented to cells under physiological conditions, the influence of substrate stress relaxation on cell behavior is unknown.
Here we investigate the role of substrate stress relaxation on cell spreading. First, computational modeling predicts that cell spreading is enhanced for cells adhering to viscoelastic substrates that exhibit stress relaxation relative to elastic substrates at low initial elastic moduli and high ligand densities. Next, cell spreading experiments using purely elastic or viscoelastic alginate hydrogels as cell adhesion substrates confirm these predictions, finding that cells even spread on soft viscoelastic substrates to a similar extent as cells spread on stiffer elastic substrates. These results challenge the current view that cells sense ECM elasticity simply by gauging resistance to the traction forces they exert on the ECM
RESULTS
Model Predicts impact of stress relaxation on cell spreading
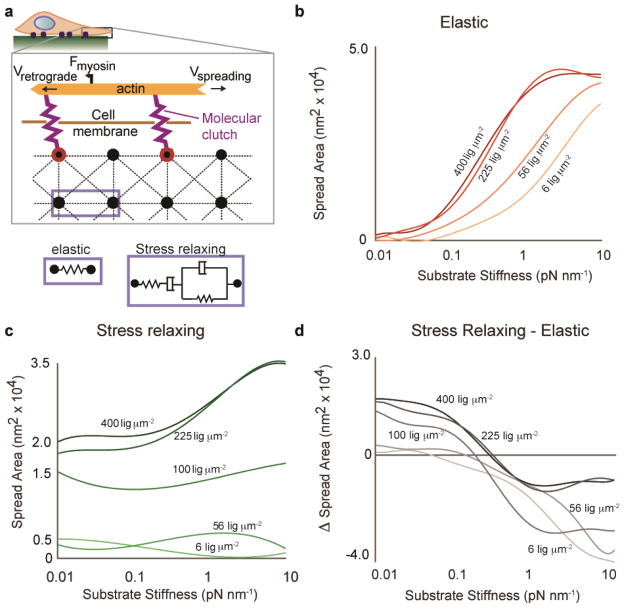
We first adapted a published model to predict the impact of stress relaxation on cell spreading. Since the time dependent elastic modulus of substrates with stress relaxation decreases over time when strained by cell traction forces, the intuitive expectation is that cells would integrate the modulus over time, and thus respond to substrates with stress relaxation as if they were substrates with an effectively lower elastic modulus. If this were the case, cell spreading would be attenuated in all cases on substrates with stress relaxation relative to elastic substrates, given the same initial elastic modulus. A simple stochastic lattice spring model was developed to test this intuitive expectation on the very early stages of cell spreading (Fig 1a, Supplementary Note 1, Supplementary Fig. 1). Following a recent model of filopodial protrusion19,20, this model considered cell spreading to be driven by polymerization of actin that was coupled to the surface of a substrate through molecular clutch adhesions, the key parameters relevant to the early stages of cell spreading, but additionally incorporated substrate stress relaxation and cell adhesion ligand density (Fig. 1a). The substrate was modeled as a series of nodes connected by either Hookean springs, representing an elastic substrate, or 4-element Burgers models, representing a viscoelastic substrate that exhibits stress relaxation. Simulations of cell spreading based on this model were run on either elastic substrates or substrates with stress relaxation, and cell spreading area was predicted as a function of cell adhesion ligand density and initial stiffness (Fig. 1b-c). On both types of substrates, cell spreading increased as a function of stiffness, consistent with previous experimental findings. At high stiffness, the model predicts only small differences in spreading between elastic and relaxing substrates. Surprisingly, though, the simulations reveal a regime, at low stiffness and higher ligand densities, in which cell spreading can be significantly enhanced on substrates with stress relaxation relative to elastic substrates (Fig. 1c), counter to the intuitive expectation. In the limit when the Burgers element becomes either a Maxwell element or a Voigt element, we find that the enhanced cell spreading on substrates that exhibit stress relaxation is better predicted in the case of Voigt element (Supplementary Fig. 2). While this model does not consider longer timescales and more complex feedback mechanisms21, it predicts an essential effect of substrate viscoelasticity when cells bind to substrates through molecular clutches.
Figure 1.
Stochastic lattice spring model predicts increased cell spreading at low initial stiffness on viscoelastic substrates that exhibit stress relaxation relative to elastic substrates. (a) Schematic depicting model of cell spreading on elastic or viscoelastic substrates. Actin polymerization at the leading edge of the cell is coupled to the substrate through molecular clutches, and these clutches inhibit retrograde flow of the actin driven by myosin motors. The substrate is modeled as an array of nodes connected by either Hookean springs, representing an elastic substrate, or Burgers model elements, representing a viscoelastic substrate exhibiting stress relaxation. (b) Simulation results for cell spreading area on elastic substrates as a function of initial substrate stiffness and adhesion ligand density. (c) Simulation results for cell spreading area on substrates with stress relaxation as a function of initial substrate stiffness and adhesion ligand density. Voigt damping coefficient, η1, was 5x10−13, and Maxwell damping coefficient, η2, was 1x10−13 for this set of simulations (See Supplementary Information). (d) Difference in cell spreading area for cells on substrates with stress relaxation relative to elastic substrates. Greater spreading on substrates with stress relaxation is observed for all conditions at low substrate stiffness.
Cell adhesion substrates with and without stress relaxation
In order to test the predictions of the model, we investigated cell spreading on alginate substrates with and without stress relaxation. Alginate is a polysaccharide derived from seaweed. It presents no intrinsic integrin binding sites and minimal protein absorption, but cell adhesion can be promoted through covalent coupling of the RGD cell adhesion peptide22. RGD-coupled alginate can be crosslinked into a hydrogel and used as a substrate for cell adhesion and spreading23. While recent work has highlighted the impact of the mode of ECM molecule tethering to substrates on cell behavior10, the use of short adhesion peptides covalently coupled to the substrate avoids this potential complication as the peptide attachment to the alginate is uniform and the peptides are homogenously distributed. Importantly, covalently crosslinking the alginate itself led to elastic substrates that had little stress relaxation (Fig. 2a, Supplementary Fig. 3). Some stress relaxation was measured at longer timescales, but this was previously found to be due to water migration out of the alginate gels during bulk compression, and is likely not relevant at the length-scale of a cell24. This interpretation was confirmed by the frequency independence of the shear storage modulus (Fig. 2b, Supplementary Fig. 3). Alternatively, crosslinking the alginate ionically, with divalent cations such as Ca2+, led to substrates that were viscoelastic and exhibited stress relaxation (Fig. 2a, Supplementary Fig. 3) due to matrix reorganization over time24. This was confirmed by the frequency dependence of the shear storage modulus (Fig. 2b), and occurs because ionic crosslinks are reversible, and can unbind and rebind when stresses are applied to the hydrogels24. Crosslinking was adjusted so that the initial Young’s moduli of the covalently and ionically crosslinked hydrogels, as measured by atomic force microscope indentation, could be matched for various values (Fig. 2c).
Figure 2.
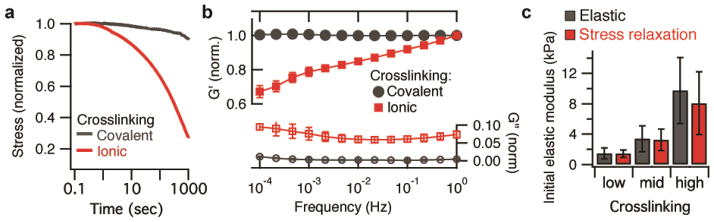
Crosslinking alginate gels covalently or ionically leads to substrates that are either purely elastic or exhibit stress relaxation, respectively. (a) Stress relaxation of covalently and ionically crosslinked alginate gels. Covalent crosslinking led to elastic gels that show little stress relaxation. Ionically crosslinked gels were viscoelastic and exhibited stress relaxation over time under a constant strain. (b) Shear storage and shear loss modulus as a function of frequency for ionically and covalently crosslinked gels. All values are normalized by the shear storage modulus at 1 Hz for that gel. Data are shown as mean +/− s.d., n = 3 for each data point. (c) Initial Young’s modulus of covalently (elastic) or ionically (stress relaxing) crosslinked alginate gels as measured by atomic force microscopy for different concentrations of crosslinker (see Supplementary Table 1). Data are shown as mean +/− s.d., n = 3 independent gels tested for each measurement.
Substrate stress relaxation regulates cell spreading
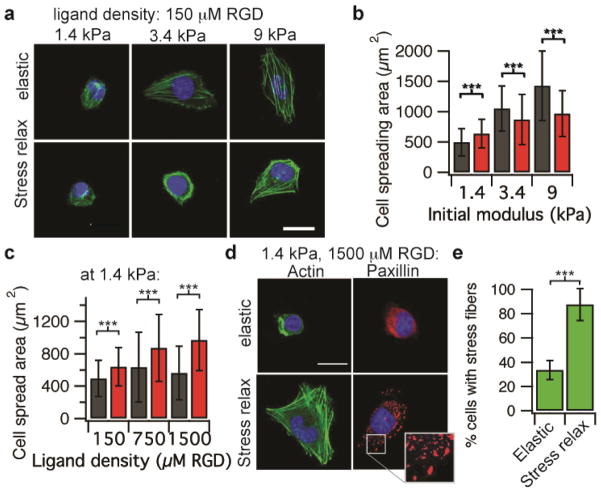
With these set of substrates, U2OS cells were plated on substrates with various, matched initial elasticities that were crosslinked ionically or covalently. These studies revealed that cell spreading area increased as the initial Young’s modulus was increased from 1.4 – 9 kPa on both ionically and covalently crosslinked gels (Fig. 3a-b, Spearman’s rank correlation p < 0.001), consistent with previous work and the simulations. Interestingly, at a low modulus of 1.4 kPa greater cell spreading area and stress fiber formation were observed on substrates that exhibit stress relaxation compared to purely elastic substrates, consistent with the model prediction. Strikingly, this effect was enhanced as ligand density was increased (Spearman’s rank correlation, p < 0.0001), as cells spreading increased monotonically as a function of increased ligand density on substrates that exhibit stress relaxation (Fig. 3c-d). In contrast, as the ligand density was increased on purely elastic substrates, a slight increase in cell spreading was observed at intermediate ligand densities, followed by a decrease in cell spreading at higher ligand densities, consistent with previous results6 (Fig 3c,3d). At high ligand densities, greater cell focal adhesion formation was observed, as indicated by the punctate localization of paxillin, on substrates with stress relaxation compared to purely elastic substrates (Fig. 3d). In addition, the number of cells that formed stress fibers increased significantly on substrates that exhibited stress relaxation relative to elastic substrates (Fig. 3e). Similarly, enhanced cell spreading on stress relaxing substrates relative to elastic substrates at lower elasticities and higher ligand densities was found for spreading of 3T3 mouse fibroblasts (Supplementary Fig. 4). While recent work has proposed a connection between the ratio of G′ to G″ and movement of epithelial cell sheets25, no correlation between G″, or the ratio of G′/G″ with cell spreading was found here (Supplementary Fig. 5).
Figure 3.
Cell spreading enhanced for cells on substrates that exhibit stress relaxation at a low initial elasticity and high ligand density. (a) Representative images of cells plated on the indicated conditions, taken after 20 hours. Actin is depicted in green; nucleus is depicted in blue. (b) Quantification of cell spreading area as a function of initial modulus for cells on elastic (grey) or stress relaxing (maroon) substrates with RGD density 150 μM. n = 38 – 81 cells analyzed for each data point. (c) Quantification of cell spreading area as a function of RGD ligand density for cells on elastic (grey) or stress relaxing (maroon) substrates. The initial modulus is 1.4 kPa for all substrates. n = 61 – 108 cells analyzed for each condition. (d) Representative image of cells plated on substrates with an initial modulus of 1.4 kPa, RGD density 1500 μM, and the indicated stress relaxation behavior stained for either actin or paxillin. Inset image is a zoom-in of the boxed region of the Paxillin stain of a cell on a substrate exhibit stress relaxation. Punctate localization of paxillin indicates formation of focal adhesions. (e) Quantification of the number of cells forming stress fibers on elastic and stress relaxing substrates with an initial modulus of 1.4 kPa, RGD density 1500 μM. n = 5 independent gels for each condition. Data are shown as mean +/− s.d. for all panels, and *** indicates p <0.001 (student’s t-test). Scale bars are all 25 μm.
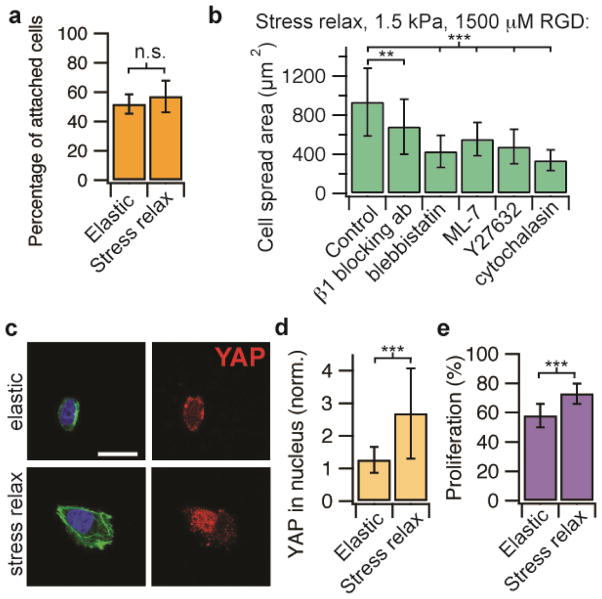
Next, the mechanistic basis for enhanced cell spreading on soft stress relaxing substrates relative to elastic substrates at low elasticities and high ligand densities was investigated. While the ligand density was the same for elastic and stress relaxing substrates since the same concentrations of RGD-alginate were used, it is in principle possible that the different modes of crosslinking might alter ligand presentation or accessibility. To test the possibility that RGD ligand presentation or accessibility is altered between elastic and stress relaxing substrates, cell adhesion studies were performed on soft gels at high ligand densities. Any difference in ligand accessibility would be expected to result in a difference in cell adhesion. No difference in the percentage of U2OS cells adhered to relaxing versus elastic substrates was found, indicating RGD presentation is similar between the substrates (Fig. 4a). Next, U2OS cell spreading on soft stress relaxing substrates with a high ligand density in the presence of various pharmacological inhibitors and blocking antibodies to mechanotransduction pathways was assessed. Cell spreading was significantly reduced in the presence of a β1 integrin blocking antibody, inhibitors of myosin based contractility (blebbistatin for myosin II ATPase, ML-7 for myosin light chain kinase), Rho associated kinase (Y-27632), and actin polymerization (Cytochalasin D) (Fig. 4b). These experiments together suggest that enhanced cell spreading on substrates with stress relaxation relative to purely elastic substrates at low elastic moduli and high ligand density is mediated through β1 integrin, actin polymerization, and actomyosin based contractility. This is consistent with the proposed model (Fig. 1a). Further, substrate stress relaxation led to increased nuclear translocation of the YAP transcriptional regulator (Fig. 4c-d), which is known to be the key transcriptional element mediating mechanotransduction for cells on 2D substrates9. Consistent with the result of enhanced nuclear translocation of YAP, proliferation is increased in this cancer cell line with substrate stress relaxation (Fig. 4e). Surprisingly, these findings demonstrate that cells are not simply integrating the modulus over time on substrates that exhibit stress relaxation. While it is currently thought that cell spreading and proliferation is suppressed on soft substrates, these results show unambiguously that substrate stress relaxation can directly compensate for the effect of decreased stiffness, as the effect of stress relaxation is mediated through some of the same pathways as stiffness: integrin adhesions, Rho activation, actomyosin based contractility, and nuclear translocation of YAP.
Figure 4.
Enhanced cell spreading on substrates with stress relaxation at a low initial elasticity is mediated through β1 integrin, actin polymerization, actomyosin contractility and associated with increased YAP nuclear localization and proliferation. (a) Quantification of the percentage of cells adhered to stress relaxing or elastic substrates (1.4 kPa, 1500 μM RGD) after 20 minutes. n= 5 gels, n.s. indicates that difference is not statistically significant by student’s t-test. (b) Quantification of cell spreading area on substrates with stress relaxation (initial Young’s modulus of 1.4 kPa and RGD density of 1500 μM), with and without addition of a β1 integrin blocking antibody, a myosin inhibitor (blebbistatin or ML-7), a ROCK inhibitor (Y27632), and an inhibitor of actin polymerization (Cytochalasin D). n = 11 – 88 cells from 3–4 gels analyzed for each condition. (c) Immunohistochemical stains of actin (green), nucleus (blue), and YAP (red) for cells on purely elastic or stress relaxing substrates with an initial Young’s modulus of 1.4 kPa, and a RGD density of 1500 μM. Scale bar is 25 μm. (d) Quantification of the ratio of nuclear YAP to cytoskeletal YAP for cells on substrates with an initial modulus of 1.4 kPa, and a RGD density of 1500 μM. n = 20 – 25 cells analyzed for each condition. (e) Quantification of proliferating cells after 20 hours in culture for cells on substrates with an initial modulus of 1.4 kPa and a RGD density of 1500 μM. n ≥ 26. Data are all shown as mean +/− s.d.. ** indicates p <0.01 and *** indicates p <0.001 (student’s t-test).
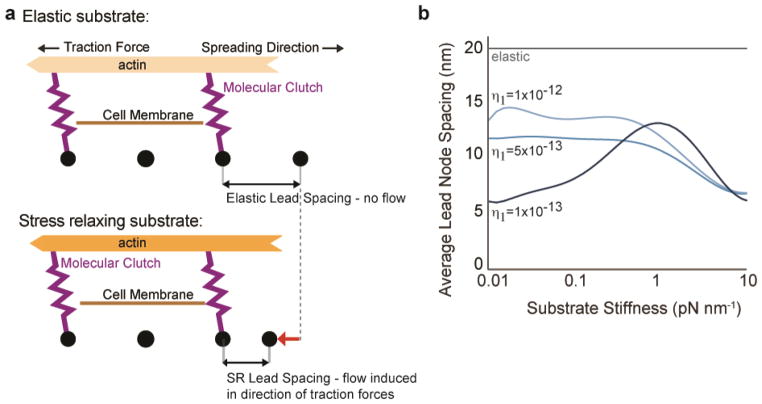
As cell traction forces will be relaxed by matrix reorganization and flow on substrates that exhibit stress relaxation, local matrix remodeling was examined both computationally and experimentally. Alteration of node spacing on substrates with stress relaxation was compared with node spacing on purely elastic substrates in the computational model. Substrate flow and plastic deformation was found to be associated with cell spreading on substrates the exhibit stress relaxation, but not with purely elastic substrates (Fig. 5a-b). While the density of RGD-alginate on the ionically crosslinked substrates is initially uniform, local clustering of RGD-alginate by cells has been reported previously23, and here was found to be greater than clustering of RGD-alginate on covalently crosslinked substrates (Supplementary Figure 6). These findings indicate that enhanced cell spreading on substrates that exhibit stress relaxation is associated with local substrate remodeling.
Figure 5.
Simulations predict that cell spreading on stress relaxing substrates is associated with flow and plastic deformation of the adhesion substrate. (a) Cartoon of cell spreading on elastic substrate or substrate with stress relaxation. (b) Simulation results for lead node spacing as a function of substrate stiffness for elastic substrates, and stress relaxing substrates with the indicated Burger’s model Maxwell damping coefficient (η1).
DISCUSSION
Taken together, these findings indicate that parallel to stiffness, substrate stress relaxation is a fundamental physical property of model ECM that has a substantial impact on cell behavior and function. The effect of stress relaxation was mediated through integrin adhesions and actomyosin based contractility, and increased stress relaxation drives nuclear translocation of YAP. This suggests that increased stress relaxation can compensate for matrices with a lower stiffness. Consistent with this idea, cell spreading and proliferation were increased on substrates with stress relaxation, compared to elastic substrates with the same initial elastic modulus. Reorganization of the actin cytoskeleton in response to mechanical cues initiates within seconds3, and, in the case of cell spreading, equilibrates over minutes to hours26 which is consistent with cells responding to stress relaxation on the order of minutes as observed here (Fig. 2a). Previous studies with cells cultured on purely elastic substrates have found that the stored elastic energy increases with the substrate modulus due to an increase in cellular traction stress 4,27, but it is unclear how this translates to viscoelastic substrates. One possibility is that the dissipation of energy through matrix yielding, and resultant decrease in stored energy, allow cells to generate more work on viscoelastic substrates relative to purely elastic substrates of the same elasticity, and this may enable spreading. Indeed the computational model predicts and experiments find that substrate flow and plastic deformation is associated with cell spreading on substrates the exhibit stress relaxation (Fig. 5a-b). Since RGD clustering is known to influence cell adhesion and spreading and promote integrin signaling, the ability to mechanical cluster ligands by cells on stress relaxing substrates may activate spreading behaviors in the cells28,29. Generally, the findings of our computational model suggest that this behavior may be an intrinsic, yet unexpected, result of molecular clutch based adhesions. A recent study showed that an increase in the loss modulus of polyacrylamide substrates led to increased cell spreading as well12. However, due to the covalent crosslinking and an associated time independent storage modulus of these substrates, as well as possible alterations in pore size arising from the method used to modulate the loss modulus10, it is likely that the mechanism for enhanced cell spreading differs in that study. Interestingly, our results might in part explain why high cell spreading was observed on soft but highly stress relaxing PDMS substrates in a recent paper10.
Overall, our results show that the hypothesis that mechanotransduction is solely mediated through cells sensing resistance to traction forces does not hold for cells on soft viscoelastic substrates. Since physiological extracellular matrices typically exhibit various degrees of stress relaxation, this finding highlights the importance of considering matrix stress relaxation as a fundamental property of the ECM that is critical to understanding the basics of cell-ECM interactions and the underlying biophysics of mechanotransduction,
METHODS
Alginate preparation
Sodium alginate rich in guluronic acid blocks and with a high molecular weight (280 kDa, LF20/40) was purchased from FMC Biopolymer. RGD-alginate was prepared by coupling the oligopeptide GGGGRGDSP (Peptides International) to the alginate using standard carbodiimide chemistry. In a typical reaction, 1 g of alginate was reconstituted at 1% wt/vol in MES Buffer (0.1M MES, 0.3 M NaCl, pH 6.5). For the highest ligand density RGD-alginate used in this study (corresponding to 1500 μM RGD condition), 274 mg of sulfo-NHS (Pierce), 484 mg of EDC (Sigma), and 112 mg of GGGGRGDSP peptide were added, and the reaction was allowed to proceed for 20 hours. For synthesis of RGD-alginate with other ligand densities, the quantities of sulfo-NHS, EDC, and RGD peptide were scaled accordingly. Concentrations of RGD were chosen such that 2, 10, or 20 RGD peptides were coupled to 1 alginate chain on average22. These correspond to densities of 150 μM, 750 μM, and 1500 μM RGD in a 2% wt/vol alginate gel. Alginate was dialyzed against deionized water for 2–3 days (molecular weight cutoff of 3.5 kDa), filtered with activated charcoal, sterile filtered, lyophilized, and then reconstituted in serum free DMEM (Life Technologies) for ionic crosslinking, or MES buffer for covalent crosslinking.
Mechanical characterization
AFM measurements of the Young’s modulus of the alginate gels were performed with an MFP-3D system (Asylum Research) using silicon nitride cantilevers (MLCT, Bruker AFM Probes). The stiffness was calibrated from the thermal fluctuations of the cantilever in air, and cantilevers with a stiffness of ~13 pN /nm were used. The cantilever was moved towards the stage at a rate of 1 μm/s and force indentation curves were interpreted using the Hertzian model for a pyramidal indentor30.
Rheology measurements were made with an AR-G2 stress controlled rheometer (TA Instruments). Alginate gels were deposited directly onto the surface plate of the rheometer immediately after mixing with the crosslinker. A 20 mm plate was immediately brought down, forming a 20 mm disk of gel with an average thickness of ~1.8 mm. The mechanical properties were then measured over time until the storage modulus reached an equilibrium value. The storage modulus at 0.5% strain and at 1 Hz was recorded periodically until the storage modulus reached its equilibrium value (2D studies), or after 45 minutes (3D studies). Then, a strain sweep was performed to confirm this value was within the linear elastic regime, followed by a frequency sweep. No prestress was applied to the gels for these measurements.
The stress relaxation properties of alginate gels were also measured from compression tests of the gel disks (15 mm in diameter, 2 mm thick, equilibrated in DMEM for 24 hr)24,31. The gel disks were compressed to 15% strain with a deformation rate of 1 mm/min using an Instron 3342 single column mechanical tester. Subsequently, the strain was held constant, while the load was recorded as a function of time.
Cell culture
U2OS and 3T3 fibroblasts (ATCC) were cultured in standard Dulbecco’s Modified Eagles Medium (DMEM, Invitrogen) with 10% Fetal Bovine Serum (Invitrogen) and 1% penicillin/streptomycin (Invitrogen).
Plating of cells on gels
1 mm thick alginate hydrogels were prepared24,31. Briefly, for ionically crosslinked gels, 0.8 mL RGD-alginate in serum free DMEM was rapidly mixed with 0.2 mL DMEM containing the appropriate amount of calcium sulfate (final concentrations detailed in Supplementary Table 1) using luerlock syringes (Cole-Parmer) and a female-female luerlock coupler (Value-plastics). Then, the mixed solution was quickly deposited between two glass plates spaced 1 mm apart. The solution was allowed to gel for 2 hours. For covalently crosslinked gels, 0.8 mL RGD-alginate in MES buffer was mixed with 0.2 mL MES buffer containing 2.5 mg/mL of 1-Hydroxybenzotriazole (HOBt, from Sigma), 50 mg/mL EDC, and the appropriate amount of adipic acid dihydrazide (AAD from Sigma, final concentrations detailed in Supplementary Table 1). Then, the mixed solution was quickly deposited between two glass plates spaced 1 mm apart. The solution was allowed to gel for 12 hours. Following gelation, 15 mm disks were punched out, and these disks were washed thoroughly 4 times over 2 days in serum free media. Disks were held fixed to the bottom of a well-plate by a customized plastic insert (so that they did not float when submerged in media), and cells were plated at a low density of 10,000 cells/cm2, so that cells did not contact other cells on average. Cells were allowed to spread for 20 hours, and then were fixed and stained for analysis. Only isolated cells, without any cell-cell contacts, were analyzed.
Immunohistochemistry
For immunohistochemical staining, media was first removed from the gels. The gels were then fixed with 4% paraformaldehyde in serum free DMEM at 37°C for 30 – 45 minutes. Gels were then washed 3 times in PBS containing calcium (cPBS). Gels were stained following standard immunohistochemistry protocols directly following washing. The following antibodies/reagents were used for immunohistochemistry: YAP antibody (Cell signaling, Catalogue # 4912:, dilution of 1:100), Paxillin antibody (Abcam, Catalogue # ab32084, dilution of 1:200), Prolong Gold antifade reagent with DAPI (Invitrogen), AF-488 Phalloidin to stain actin (Life Technologies, dilution of 1:80), Goat anti-Rabbit IgG AF 647 (Invitrogen, Catalogue # a21245, dilution of 1:500). The Click-IT EdU cell proliferation assay (Invitrogen) was used to identify proliferating cells.
Image analysis
For measurements of cell spreading area in 2D, images of phalloidin/DAPI stained cells were taken for the indicated conditions at 20X or 63X with a laser scanning confocal microscope (LSM 710, Zeiss). Only cells that did not exhibit any cell-cell contacts were considered in the analysis. Images of all single cells were then thresholded manually based on the actin stain, and the area of the thresholded cell body was determined using a custom macro in ImageJ (NIH).
For measurements of YAP nuclear localization, images of DAPI/phalloidin/YAP antibody stained cells were taken with an NA=1.40 63X PlanApo oil immersion objective with a laser scanning confocal microscope. Images were thresholded on each color channel to determine the nuclear area and cell/cytoskeleton area outside of the nucleus. The YAP nuclear localization ratio was then determined as the summed intensity of the YAP signal within the nucleus normalized by the nuclear area divided by the summed intensity of the YAP signal outside of the nucleus normalized by the non-nuclear cytoskeleton area.
Blocking antibody and inhibitor experiments
Cells were plated on hydrogel substrates at a density of 10,000 cells/cm2. Cells were allowed to spread for 20 hours at the presence of one of the following reagents: β1 integrin blocking antibody (5 μg/mL or dilution of 1:200, clone P5D2, EMD Millipore, Catalogue #MAB1959), blebbistatin (50 μM, Tocris Bioscience), ML-7 hydrochloride (25 μM, Tocris Bioscience), Y-27632 dihydrochloride (10 μM, Tocris Bioscience), or cytochalasin D (2 μM, Sigma-Aldrich). The cells were then fixed and stained for analysis. Only isolated cells, without any cell-cell contacts, were analyzed.
Cell adhesion experiments
Cells were plated on hydrogel substrates at a density of 20,000 cells/cm2. Cells were allowed to adhere for 20 minutes at 37 °C in a tissue culture incubator. All the media were then collected and the cells that didn’t adhere to the substrates were counted immediately with an automated cell counter (Countess cell counter, Life Technologies). The adhered cells were calculated by subtracting non-adhered cells from the total number of cells for each experiment.
RGD clustering experiments
Tetramethylrhodamine (TAMRA) labeled RGD peptide (GGGGRGDSPASSK-TAMRA, Peptides International) was coupled to alginate23,31. TAMRA labeled RGD-alginate was mixed with non-labeled RGD-alginate at a ratio of 1:9 or 1:19 before crosslinking, so the resulting hydrogel substrates have a fraction of fluorescently labeled RGD uniformly distributed in the gels. Cells were plated at a density of 10,000 cells/cm2 and were allowed to spread for 40 hours. Cells were then imaged live on the substrates with a confocal microscope.
Supplementary Material
Acknowledgments
The authors acknowledge the help of Sandeep Koshy, Manav Mehta, Catia Verbeke, Xuanhe Zhao (Duke University), and other members of the Mooney lab. The authors also thank the Weitz lab for use of the rheometer. This work was supported by an NIH F32 grant to OC (CA153802), NIH Grant to DJM (R01 DE013033), and DK is supported in part by ZonMW-VICI grant 918.11.635 (The Netherlands). This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNIN).
Footnotes
Author Contributions
OC, LG, DK, NH, and DM designed the experiments. OC, LG, DK, SB, and JW conducted experiments. MD developed the model and ran simulations. OC and LG analyzed the data. OC, LG, MD, and DM wrote the manuscript.
Competing Financial Interests. The authors declare no competing financial interests.
References
- 1.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Dev Camb Engl. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 4.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 5.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engler A, et al. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung T, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 8.Klein EA, et al. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol CB. 2009;19:1511–1518. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 10.Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 11.Swift J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32:5979–5993. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapp DM, et al. Rheology of reconstituted type 1 collagen gel in confined compression. J Rheol. 1997;41 [Google Scholar]
- 15.Janmey PA, Amis EJ, Ferry JD. Rheology of Fibrin Clots. VI. Stress Relaxation, Creep, and Differential Dynamic Modulus of Fine Clots in Large Shearing Deformations. J Rheol. 1983;27 [Google Scholar]
- 16.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Bilston L. On the viscoelastic character of liver tissue: experiments and modelling of the linear behaviour. Biorheology. 2000;37:191–201. [PubMed] [Google Scholar]
- 18.Geerligs M, Peters GWM, Ackermans PAJ, Oomens CWJ, Baaijens FPT. Linear viscoelastic behavior of subcutaneous adipose tissue. Biorheology. 2008;45:677–688. [PubMed] [Google Scholar]
- 19.Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- 20.Bangasser BL, Rosenfeld SS, Odde DJ. Determinants of maximal force transmission in a motor-clutch model of cell traction in a compliant microenvironment. Biophys J. 2013;105:581–592. doi: 10.1016/j.bpj.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zemel A, Rehfeldt F, Brown AEX, Discher DE, Safran SA. Optimal matrix rigidity for stress fiber polarization in stem cells. Nat Phys. 2010;6:468–473. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 23.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci U S A. 2005;102:4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, Huebsch N, Mooney DJ, Suo Z. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J Appl Phys. 2010;107:63509. doi: 10.1063/1.3343265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murrell M, Kamm R, Matsudaira P. Substrate viscosity enhances correlation in epithelial sheet movement. Biophys J. 2011;101:297–306. doi: 10.1016/j.bpj.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mooney DJ, Langer R, Ingber DE. Cytoskeletal filament assembly and the control of cell spreading and function by extracellular matrix. J Cell Sci. 1995;108(Pt 6):2311–2320. doi: 10.1242/jcs.108.6.2311. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh K, et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671–679. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold M, et al. Activation of Integrin Function by Nanopatterned Adhesive Interfaces. ChemPhysChem. 2004;5:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- 29.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113(Pt 10):1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 30.Bilodeau GG. Regular Pyramid Punch Problem. J Appl Mech. 1992;59:519. [Google Scholar]
- 31.Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.